Abstract
Inflammatory cytokines activate tissue collagenases such as matrix metalloproteinases (MMPs). MMPs are antagonized by tissue inhibitors of metalloproteinases (TIMPs) that attempt to regulate excessive collagenase activity during inflammatory conditions. During chronic inflammatory conditions, induction of endotoxin tolerance negatively regulates the cytokine response in an attempt to curtail excessive host tissue damage. However, little is known about how downregulation of inflammatory cytokines during endotoxin tolerance regulates MMP activities. In this study, human monocyte-derived macrophages were either sensitized or further challenged to induce tolerance with lipopolysaccharide (LPS) from Porphyromonas gingivalis (PgLPS) or Escherichia coli (EcLPS). Inflammatory cytokines, such as TNF-α and IL-1β, and levels of MMP9 and TIMP1 were analyzed by a combination of cytometric bead array, western blot/gelatin zymography and real-time RT-PCR. Functional blocking with anti-TLR4 but not with anti-TLR2 significantly downregulated TNF-α and IL-1β. However, MMP9 levels were not inhibited by toll-like receptor (TLR) blocking. Interestingly, endotoxin tolerance significantly upregulated TIMP1 relative to MMP9 and downmodulated MMP9 secretion and its enzymatic activity. These results suggest that regulatory mechanisms such as induction of endotoxin tolerance could inhibit MMP activities and could facilitate restoring host tissue homeostasis.
Keywords: Endotoxin tolerance, Toll like receptors, MMP, TIMP, Macrophages, Lipopolysaccharide
Host tissue destruction during inflammatory response is regulated by balancing tissue destructive enzymes and their antagonists and this study explores how this balance is maintained during sustained microbial challenge.
Graphical Abstract Figure.
Host tissue destruction during inflammatory response is regulated by balancing tissue destructive enzymes and their antagonists and this study explores how this balance is maintained during sustained microbial challenge.
INTRODUCTION
Host tissue homeostasis is regulated by the levels of tissue collagenases such as matrix metalloproteinases (MMPs). During inflammatory conditions such as arthritis and periodontitis (Kinane 2000; Grayson et al., 2003), the levels of MMPs increase and if uncontrolled could disrupt the homeostasis associated with matrix remodeling (Parks, Wilson and Lopez-Boado 2004). Toll-like receptors (TLRs) of the innate immune system recognize a broad category of microbial pathogen-associated molecular patterns (Janeway 1989, 1992). For instance, TLR2 recognizes structures from gram-positive bacteria such as peptidoglycan and lipoteichoic acid (Schwandner et al., 1999) and TLR4 recognizes lipopolysaccharide (LPS) from gram-negative bacteria (Beutler et al., 2003). Inflammatory cytokines such as TNF-α (Zhou et al., 2003) and IL-1β (Wu et al., 2004) secreted through TLR signaling induce and activate latent forms of MMPs. Moreover, the conversion of biologically active forms of inflammatory cytokines such as IL-1β can be induced through certain MMPs such as MMP2 and MMP9 (Lefebvre, Peeters-Joris and Vaes 1991).
MMPs are secreted as inactive proenzymes and are activated by the cleavage of propeptide domain by proteolytic enzymes such as stromelysin-1 and MMPs such as MMP2. MMP activity is counteracted by the endogenous tissue inhibitors of metalloproteinases (TIMPs) (Verstappen and Von den Hoff 2006). MMPs such as MMP9 are secreted in conjunction with TIMP1 that control proteolytic activity. An imbalance in the levels of MMPs and TIMPs is implicated in neoplasms (Bramhall et al., 1997; Brehmer, Biesterfeld and Jakse 2003), cancer metastasis (Ueda et al., 1996; Ogata et al., 2001; Zhang et al., 2003), ulcerative conditions of gastric mucosa (Rautelin et al., 2009) and corneal mucosa (Gabison et al., 2005), neural (Mun-Bryce et al., 2004) and cardiovascular (Squire et al., 2004) injury and inflammatory conditions such as arthritis (Kanyama et al., 2000; Tchetverikov et al., 2004) and periodontitis (Kubota et al., 2008). Although increased levels of TIMPs are implicated during inflammatory conditions such as periodontitis, these levels are considered to be insufficient to inhibit activated MMPs (Garlet et al., 2004). Interestingly, periodontal pathogens have the ability to increase the tissue destruction by inactivating TIMPs (Grenier and Mayrand 2001).
MMP activity is tightly regulated at RNA transcription, protein synthesis, extracellular localization and activation (Page-McCaw, Ewald and Werb 2007). Moreover, the expression of TIMPs and alpha 2-macroglobulin provides additional regulatory measures to establish tissue homeostasis. Inflammatory conditions if not regulated lead to host tissue damage. Induction of endotoxin tolerance is one of the adaptive measures to curtail excessive host inflammatory response during chronic inflammatory conditions such as periodontitis (Muthukuru, Jotwani and Cutler 2005; Muthukuru and Cutler 2006). During the induction of endotoxin tolerance, the inflammatory cytokines are downmodulated in an effort to attain host tissue homeostasis. However, little is known how negative regulation of inflammatory response mediated by endotoxin tolerance affects the balance of MMPs and TIMPs and thereof regulates host tissue homeostasis.
In this study, concentrations of LPS from Porphyromonas gingivalis (PgLPS) and Escherichia coli (EcLPS) were optimized based on equivalent NF-κB activation. TLR2 and TLR4 functional blocking antibodies were titrated for optimal TLR blocking. Human monocyte-derived macrophages (MΦ) were either stimulated once (sensitized) or stimulated and further challenged (to induce tolerance) with 1000 ng/ml of PgLPS or 100 ng/ml of EcLPS. The data suggest that PgLPS and EcLPS significantly induced the secretion of TNF-α and IL-1β relative to unstimulated controls. Relative to TLR2, blocking TLR4 significantly downmodulated secretion of TNF-α and IL-1β when MΦ were stimulated with either PgLPS or EcLPS. However, levels of MMP9 were not affected with either TLR2 or TLR4 blocking but interestingly, induction of endotoxin tolerance downregulated the secretion of enzymatically active MMP9. This could be attributed to elevated levels of TIMP1 that were significantly upregulated during endotoxin tolerance. Induction of endotoxin tolerance that negatively regulated MMP9 could minimize tissue destruction during chronic inflammatory conditions.
MATERIALS AND METHODS
LPS isolation
LPS was isolated from either P. gingivalis strain 381 (PgLPS) or type ATCC strain E. coli 25922 (EcLPS) by hot phenol–water extraction followed by isopycnic density gradient centrifugation and was further purified of contaminating nucleic acids, proteins and lipoproteins. In addition, some of the LPS preparations (purified identically) were a gift of T. E. Van Dyke, Boston University, Goldman School of Dental Medicine. The purity of LPS was confirmed by gel electrophoresis, which detected visible LPS ladder staining pattern with silver staining and no visible proteins analyzed by Coomassie blue staining (data not shown).
Human peripheral blood monocyte-derived MΦ cultures
Monocytes were isolated from mononuclear fractions of peripheral blood of healthy donors by means of adherence to polystyrene culture flasks as previously described (Muthukuru et al., 2005; Muthukuru and Cutler 2008). Briefly, whole peripheral blood was centrifuged on Ficoll, and the mononuclear cell fraction pelleted and resuspended in RPMI 1640 (Invitrogen) with 10% heat-inactivated fetal calf serum (FCS) (Sigma, St. Louis, MO). Mononuclear cells (∼2.7 × 108) were seeded on 150 ml polystyrene culture flasks and incubated for 2 h at 37°C in a 5% CO2 incubator. After the non-adherent cells were washed off, the adherent monocytes were retrieved from the culture dishes by using 1× trypsin-EDTA solution. Monocytes were cultured in RPMI 1640 with 10% heat-inactivated FCS. The percentages of viable monocytes (typically >90% after LPS stimulation) were monitored by trypan blue exclusion. Human blood-derived monocytes were cultured in the presence of macrophage (MΦ) colony-stimulating factor (M-CSF) for 5 to 7 days as previously reported (Muthukuru and Cutler 2008). Briefly, the differentiation of monocytes into MΦ was confirmed by the uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (Dil)-conjugated labeled low-density lipoproteins (LDL) by day 7 of culture and by high constitutive expression of HLA-DR by MΦ relative to monocytes. The percentages of MΦ (typically >95%) were determined by counterstaining the nuclei with DAPI (4′,6′-diamidino-2-phenylindole) and calculating the percentages of Dil-LDL+ cells over the total DAPI+ cells per ×20-magnification microscopic field (data not shown).
In vitro LPS stimulation and challenge: induction of endotoxin tolerance
For the purpose of LPS dose responses, PgLPS or EcLPS were employed at the concentrations of 10, 100 and 1000 ng/ml. MΦ were stimulated with these concentrations of LPS for 24 h. The in vitro design for induction of endotoxin tolerance has previously been described in detail previously (Muthukuru et al., 2005; Muthukuru and Cutler 2006, 2008). Briefly, human MΦ were incubated for 24 h either with no LPS stimulus (control) or with initial sensitization with 1000 ng/ml of P. gingivalis or 100 ng/ml of E. coli LPS for 24 h followed by a challenge with the same LPS at the same initial dosage for a further duration of 24 h. The cells were then pelleted and washed with cold phosphate-buffered saline three times to be employed for subsequent experiments as described below.
Quantification of NF-κB activity
NF-κB activity was analyzed by performing quantification of active p65 by employing DNA-binding ELISA-based TransAM kits from Active Motif according to manufacturer's instructions. Nuclear extracts from MΦ cell cultures were added and the activated p65 was allowed to bind to oligonucleotides at its consensus binding site and was quantified using the prediluted antibody and generating a standard curve from recombinant p65 protein. All analyses were performed in triplicate.
Luciferase assay
Human embryonic kidney (HEK) 293 cells with NF-κB reporter construct (ELAM-1 firefly luciferase), the β-actin-Renilla luci-ferase reporter construct and the modified pDisplay expression vector were kindly provided by Dr Richard Darveau (University of Washington, Seattle, WA). These cells were cloned with the expression constructs for human TLR4 (phuTLR4), mCD14 (phumCD14) and MD2 (cells designated as HEK-TLR4) or with human TLR1 (phuTLR1) and TLR2 (phuTLR2) (cells designated as HEK-TLR2) into the modified pDisplay expression vector. HEK 293 cells were transfected by calcium phosphate precipitation in a 96-well plate assay format as previously described (Hajjar et al., 2002). Cells were washed twice with medium 3 h after transfection and stimulated for 24 h with either 1μg/ml of PgLPS or EcLPS. Cells were cultured, transfected and stimulated in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 10% human AB serum at 37°C. Cells were washed with phosphate-buffered saline and lysed with 50 μl of passive lysis buffer (Promega, Madison, WI). Reporter gene expression in each lysate (10 μl) was measured using the Dual Luciferase reporter assay system (Promega, Madison, WI). Data are expressed as fold increase in relative light units (representing the ratio of ELAM luciferase to β-actin-Renilla luciferase expression) relative to that of unstimulated controls. All experiments were performed in triplicate.
Quantification of cytokines
Cell culture supernatants were collected after stimulation and challenge with PgLPS and EcLPS for 24 h. Culture supernatants were analyzed for secretion of TNF-α and IL-1β by flow cytometry using cytometric bead array (CBA Kit, BD Biosciences). Concentrations of cytokines were calculated based on a standard curve for each cytokine. The CBA software calculated cytokine levels in pg/ml.
Real-time RT-PCR analysis
RNA extraction and cDNA synthesis: Unstimulated MΦ or PgLPS/EcLPS sensitized or tolerized MΦ from in vitro cultures were placed in RNAlater RNA stabilizing reagent (Qiagen) and frozen at –80°C for later use. Frozen cells were subject to lysis/homogenization and total RNA was extracted using Qiagen RNeasy mini kits as specified by the manufacturer. Avian reverse transcriptase (RT) first-strand kits (Sigma) were used to synthesize cDNA from total RNA. The concentration of total RNA was determined at OD260. The purity of total RNA was determined by analysis of the OD260/OD280 ratio. Small discrepancies in the total RNA concentration were rectified by loading the same concentration of RNA for cDNA synthesis.
Primers for PCR: Nucleotide sequences were determined from PubMed (National Center for Biomedical Information) and the primers were custom designed using primer3 software. Presented in Table 1 are the sequences of the primers and product sizes of RT-PCR.
Real-time RT-PCR quantification: Real-time RT-PCR analysis was performed using the iCycler (Bio-Rad, Hercules, Calif.) with SYBR Green kits (Bio-Rad) and mRNA quantification was performed by standard curve method as previously described (Muthukuru et al., 2005; Muthukuru and Cutler 2006). For each transcript analyzed, a standard curve with predetermined concentrations and serial diluted respective PCR amplification products from 0.1 to 0.00 001 ng was constructed. This approach allows the standards to be amplified in the same way as the template cDNA in the unknown samples since the product sequence and size are identical. Levels of ß-actin mRNA served as an internal control to normalize samples for variations in sample volume loading, presence of inhibitors, and nucleic acid recovery during extraction and cDNA synthesis procedures. All analyses were performed in triplicate.
Table 1.
Primer sequences and size of PCR products.
| Gene | Primer sequence (5′ to 3′) | Size of product (bp) | |
|---|---|---|---|
| Left primer | Right primer | ||
| MMP9 | GACACCTCTGCCCTCACC | ACTCTCCACGCATCTCTGC | 207 |
| TIMP1 | AATTCCGACCTCGTCATCAG | TGCAGTTTTCCAGCAATGAG | 230 |
| Beta-actin | ACTCTTCCAGCCTTCCTTCC | GTTGGCGTACAGGTCTTTGC | 204 |
Western blot analysis of MMP-9 secretion
The total protein concentration from the culture supernatant of the MΦ cultures as described above was quantitated using protein assay kits (Bio-Rad). Culture supernatants were then analyzed for MMP9 secretion. Aliquots of cell culture supernatants containing equal amount of total protein (including FCS in the medium) were quantitated by generating a standard curve with bovine serum albumin and were suspended in equal amounts of Laemmle sample buffer (Bio-Rad) and subjected to SDS-PAGE (8% gel) at 120 V, transferred to PVDF membrane at 25 V overnight and blocked with 5% blocking agent (Amersham Bioscience–ECL blocking agent) in PBS and 0.1% Tween-20. Primary anti-human MMP-9 (mouse anti-human immunoglobulin IgG1, clone GE-213, from Research Diagnostics, Inc., NJ, USA) was used at 1:15 000, followed by incubation with horseradish peroxidase-conjugated sheep anti-mouse IgG (Amersham Bioscience) at 1:50 000 dilutions. As an internal control, peroxidase conjugated goat anti-bovine polyclonal antibody (Rockland antibodies and assays) was employed to determine the globulin fraction in the culture supernatants that were supplemented with bovine serum. The proteins were detected using an enhanced chemiluminescence's detection system (Amersham Bioscience).
Gelatin zymography
Culture media containing equal amount of total protein (including FCS in the media) was added to an equal volume of sample buffer [0.5 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate (SDS), 0.005% bromophenol blue, 20% glycerol] (Bio-Rad, USA), incubated at room temperature for 10 min and separated on 10% SDS-polyacrylamide electrophoresis (Page-McCaw et al., 2007) gels containing 0.1% gelatin (Bio-Rad, USA). The gels were denatured in denaturing buffer (2.5% Triton-X-100) (Bio-Rad, USA) in water for 30 min at room temperature and then incubated at 37°C overnight in developing buffer (50 mM Tris, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij 35, pH 7.6) (Bio-Rad, USA). After incubation, gels were stained with 0.25% Coomassie Brilliant Blue R-250 for 4 h at room temperature and destained in distilled water containing 30% methanol and 10% glacial acetic acid to reveal zones of lysis within the gelatin matrix.
RESULTS
PgLPS is less potent relative to EcLPS in inducing NF-κB
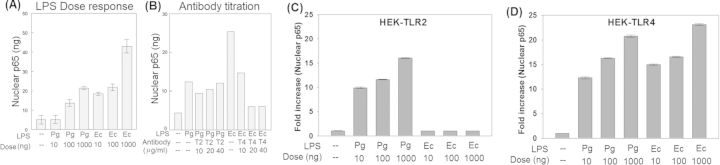
The lipid A structure of P. gingivalis LPS purified through conventional methods is a weak inducer of host inflammatory response relative to the canonical E. coli lipid A (Dixon and Darveau 2005). Stimulation with LPS activates TLR signaling culminating in NF-κB activation (Mancuso et al., 2005). MΦ were stimulated with PgLPS or EcLPS with concentrations of 10, 100 or 1000 ng/ml. A dose dependent increase in NF-κB activation with either LPS is noted in Fig. 1A. However, NF-κB levels were unaltered (relative to unstimulated controls) when stimulated with 10 ng/ml of PgLPS but 1000 ng/ml of PgLPS and 100 ng/ml of EcLPS induced equivalent NF-κB activation (Fig. 1A). Titration of anti-TLR2 and anti-TLR4 blocking antibodies demonstrated optimal blocking at antibody concentration of 20 μg/ml (Fig. 1B). In subsequent experiments shown in Fig. 2, 1000 ng/ml of PgLPS or 100 ng/ml of EcLPS and 20 μg/ml of either anti-TLR2 or anti-TLR4 functional blocking antibodies were employed. To determine how PgLPS and EcLPS preparations interact with TLR2 and TLR4, HEK cells (expressing NF-κB and the β-actin-Renilla) were transiently transfected with either TLR1 and TLR2 (HEK-TLR2) or with TLR4, CD14 and MD2 (HEK-TLR4) as described in the section ‘Materials and Methods’. These cells were stimulated with PgLPS or EcLPS at doses of 10, 100 or 1000 ng/ml for 24 h, washed and lysed. Dual Luciferase reporter assay system was employed to determine NF-κB expression relative to that of β-actin. The data suggest that relative to unstimulated controls, PgLPS upregulated NF-κB expression in both HEK-TLR2 (Fig. 1C) and HEK-TLR4 cells (Fig. 1D). EcLPS induced NF-κB in HEK-TLR4 (Fig. 1D) but not HEK-TLR2 cells (Fig. 1C).
Figure 1.
PgLPS is less potent relative to EcLPS in inducing NF-κB. MΦ were stimulated with PgLPS or EcLPS at concentrations of 10, 100 or 1000 ng/ml. Activation of NF-κB was determined by analyzing the nuclear p65 component of NF-κB (A). 1000 ng/ml of PgLPS and 100 ng/ml of EcLPS induced equivalent NF-κB activation. Anti-TLR2 and anti-TLR4 functional antibodies were titrated for optimal blocking by determining NF-κB activity. 20 μg/ml induced optimal downregulation of p65 component of NF-κB (B). PgLPS upregulated NF-κB in HEK cells transiently transfected with TLR1 and TLR2 (HEK-TLR2) and TLR4, CD14 and MD2 (HEK-TLR4) and EcLPS specifically targeted TLR4 but not TLR2 (C and D).
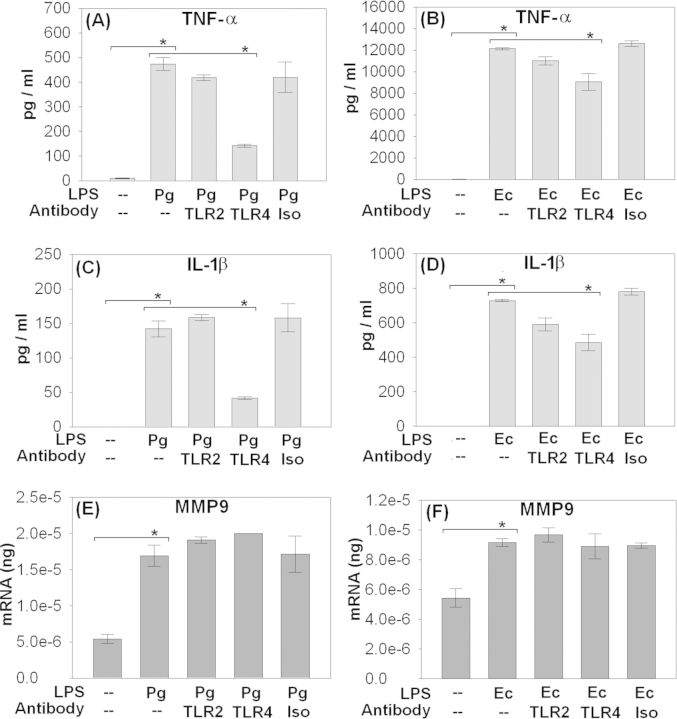
Figure 2.
TLR blocking inhibits inflammatory cytokines but not MMP9. Monocyte-derived MΦ were cultured as described in materials and methods. MΦ were stimulated with 1000 ng/ml of PgLPS or 100 ng/ml of EcLPS. 20 μg/ml of anti-TLR2 or anti-TLR4 functional blocking antibodies were employed. Secretion of TNF-α (A, B) and IL-1β (C, D) was analyzed through CBA and regulation of MMP9 (E, F) was analyzed through real-time RT-PCR as described in materials and methods. PgLPS and EcLPS significantly upregulated TNF-α, IL-1β and MMP9. Also blocking with anti-TLR4 significantly downregulated TNF-α and IL1β but not MMP9. (*P < 0.001 analyzed by all pairwise multiple comparison performed through Holm–Sidak method).
TLR blocking inhibits inflammatory cytokines but not MMP9
TLR signaling results in secretion of inflammatory cytokines such as TNF-α and IL-1β (Ozinsky et al., 2000). Subsequently, these cytokines provide the signals for MMP secretion and also mediate their activation (Zhou et al., 2003; Wu et al., 2004). MΦ were stimulated with 1000 ng/ml of PgLPS or 100 ng/ml of EcLPS. Anti-TLR2 and anti-TLR4 functional blocking antibodies were employed at 20 μg/ml. Secretion of TNF-α (Fig. 2A and B) and IL-1β (Fig. 2C and D) was significantly induced by PgLPS (Fig. 2A and C) or EcLPS (Fig. 2B and D) relative to unstimulated controls. However, EcLPS (Fig. 2B and D) was more potent in inducing TNF-α and IL-1β relative to PgLPS (Fig. 2A and C). Blocking with anti-TLR4 antibody (Fig. 2A–D) significantly reduced the secretion of TNF-α (Fig. 2A and B) and IL-1β (Fig. 2C and D) secretion when MΦ were stimulated with either PgLPS (Fig. 2A and C) or EcLPS (Fig. 2B and D). Anti-TLR2 functional blocking was not potent in downregulating these cytokines (Fig. 2A–D). MΦ stimulated with either PgLPS (Fig. 2E) or EcLPS (Fig. 2F) significantly upregulated the expression of MMP9. Moreover, PgLPS was potent relative to EcLPS in inducing MMP9. Interestingly, blocking with either anti-TLR2 or anti-TLR4 antibodies did not downregulate the induction of MMP9 transcripts (Fig. 2E and F).
Induction of endotoxin tolerance downregulates the secretion of MMP9
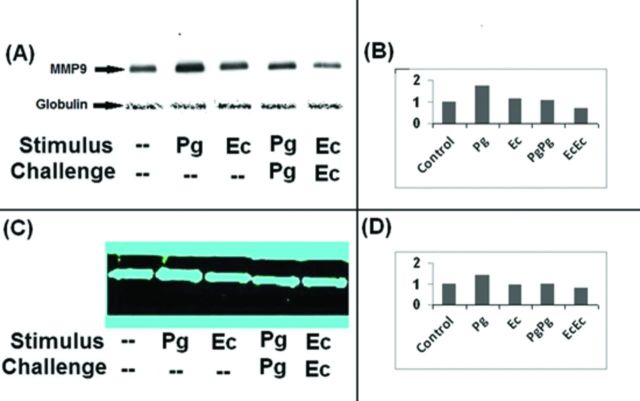
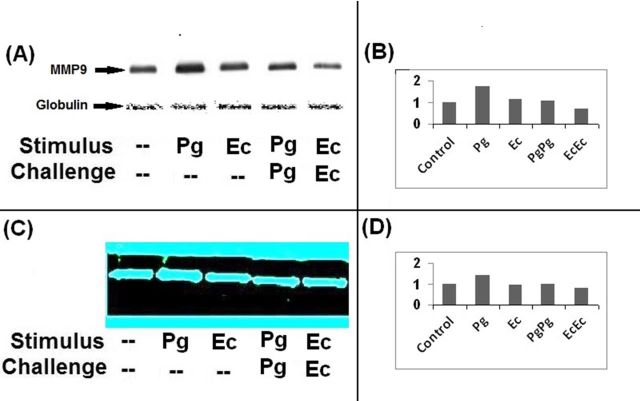
Inflammatory cytokines secreted in response to TLR signaling induce the expression of MMPs and also activate the latent forms of MMPs (Zhang et al., 2003; Zhou et al., 2003; Wu et al., 2004). Induction of endotoxin tolerance is implicated in reprogramming TLR signaling and downmodulating cytokine secretion (Muthukuru et al., 2005; Muthukuru and Cutler 2008). However, little is known how negative regulation of inflammatory response during endotoxin tolerance influences the expression of MMPs. MΦ were sensitized or further challenged to induce tolerance with either PgLPS or EcLPS as described in the section ‘Materials and Methods’. Culture supernatants were analyzed for MMP9 levels through western blot analysis (Fig. 3A and B) and MMP9 activity through gelatin zymography (Fig. 3C and D). When MΦ were sensitized with PgLPS or EcLPS, the levels of MMP9 were upregulated relative to that of the unstimulated MΦ. However, MMP9 was highly induced with PgLPS relative to stimulation with EcLPS. Induction of endotoxin tolerance with either PgLPS or EcLPS resulted in downregulation of MMP9 secretion relative to either PgLPS or EcLPS sensitization. Secretion of MMP9 analyzed through western blot (Fig. 3A and B) correlated with the gelatinolytic activity as analyzed by zymography (Fig. 3C and D).
Figure 3.
Induction of endotoxin tolerance downregulates the secretion of MMP9. MΦ were either unstimulated (controls) or sensitized with 1000 ng/ml of P. gingivalis (PgLPS) or 100 ng/ml of E. coli LPS (EcLPS) for 24 h or sensitized and challenged with the same LPS at the same initial dosage for a further duration of 24 h to induce endotoxin tolerance as described in materials and methods. Culture supernatants were collected and MMP9 secretion was analyzed through western blot analysis (A) and gelatin zymography (C). Bovine globulin fraction in the culture supernatants served as internal control for western blot analysis (A). The intensities of the bands from western blot (B) and zymography (D) were quantitated by using image-q software from National Institute of Health. Stimulation with PgLPS and EcLPS upregulated MMP9 relative to unstimulated controls. Induction of endotoxin tolerance by stimulation and subsequent challenge with either PgLPS or EcLPS downregulated MMP9 secretion.
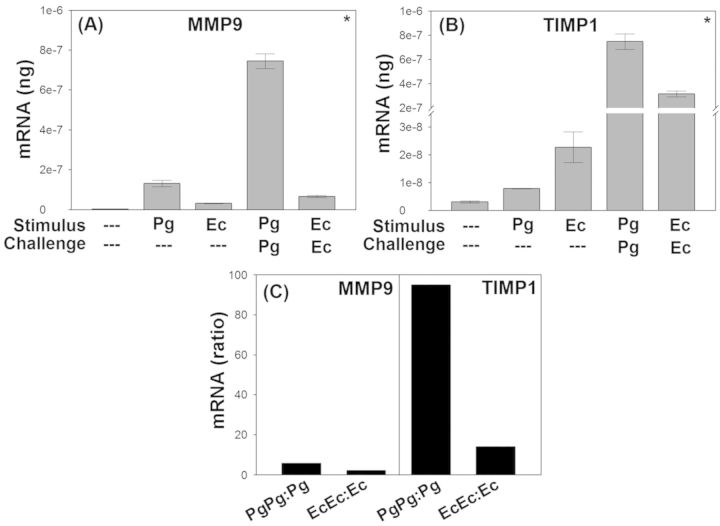
Disproportionate upregulation of TIMP1 relative to MMP9 during induction of endotoxin tolerance
In order to determine the underlying mechanism of downregulation of MMP9 during endotoxin tolerance, we analyzed the expression of TIMP1, an inhibitor of MMP9 activity along with the expression of MMP9 through quantitative real-time RT-PCR as described in materials and methods. Stimulation with PgLPS or EcLPS significantly increased the mRNA expression of MMP9 (Fig. 4A) and TIMP1 (Fig. 4B) relative to the unstimulated controls. Contrary to downregulation of MMP9 protein secretion (Fig. 3), the transcript levels were upregulated by ∼ 3 times during the induction of endotoxin tolerance with either PgLPS or EcLPS (Fig. 4A). Interestingly, induction of tolerance through PgLPS or EcLPS significantly upregulated TIMP1 by 93 and 15 times respectively relative to sensitization with either LPS (Fig. 4B). This disproportionate upregulation of TIMP1 (Fig. 4B) relative to MMP9 (Fig. 4A) is presented as a ratio of PgLPS or EcLPS challenged (tolerance) to sensitized groups (Fig. 4C).
Figure 4.
Disproportionate upregulation of TIMP1 relative to MMP9 during induction of endotoxin tolerance. MMP9 and TIMP1 transcripts were analyzed through quantitative real-time RT-PCR as described in the section ‘Materials and Methods’. Stimulation with PgLPS or EcLPS upregulated MMP9 (A) and TIMP1 (B) relative to unstimulated controls. Stimulus and challenge (i.e. induction of endotoxin tolerance) with either PgLPS or EcLPS further induced a 3-fold upregulation of MMP9 relative to initial sensitization. Induction of endotoxin tolerance through sensitization and challenge with either PgLPS or EcLPS significantly upregulated TIMP1 by 93 and 15 times respectively relative to initial sensitization with PgLPS and EcLPS. Shown in C is the ratio of PgLPS or EcLPS challenged to sensitized groups. (*P < 0.05 analyzed by all pairwise multiple comparison performed through Student–Newman–Keuls method).
DISCUSSION
Our results indicate that unlike inflammatory cytokines such as IL-1β and TNF-α, MMP9 and TIMP1 transcripts are resistant to downregulation during endotoxin tolerance. However, a disproportionate upregulation of TIMP1 relative to MMP9 could downregulate MMP9 protein activity. MMPs belong to the superfamily of endopeptidases that comprise more than 25 distinct proteins (Brinckerhoff and Matrisian 2002). MMPs facilitate trafficking of antigen presenting cells (APC) that patrol, capture the invading pathogens and subsequently migrate toward the lymphoid organs for antigen presentation (Osman et al., 2002). Also, MMPs play a crucial role during physiological conditions such as uterine tissue resorption, ovulation (Weeks, Halme and Woessner 1976) and during endochondral bone morphogenesis (Ortega et al., 2003, Ortega, Behonick and Werb 2004). The MMP activities during these physiological conditions are tightly regulated and are counteracted by TIMPs (Verstappen and Von den Hoff 2006). The balance between MMPs and TIMPs plays an important role in maintaining tissue homeostasis. However, only four TIMPs are currently known that counteract the MMP activities.
E. coli is a gut commensal whose LPS can provoke septic shock syndrome (Poli-de-Figueiredo et al., 2008). Chronic periodontitis is an inflammatory disease of the oral mucosa (Seymour 1991) mediated by periodontal pathogens such as P. gingivalis (Kroes, Lepp and Relman 1999) that share the common property of being gram negative, i.e. producing LPS (Socransky et al., 1998; Ezzo and Cutler 2003; Cohen, Morisset and Emilie 2004). During these inflammatory conditions, LPS triggers inflammatory cytokine secretion and MMPs are induced and become activated by these inflammatory cytokines (Liacini et al., 2003; Cox et al., 2006). The lipid A component of LPS is the biologically active moiety that contributes to septic shock syndrome and inflammatory conditions such as periodontitis. The bi-phosphorylated, hexa-acylated lipid A structure from E. coli is the most potent in provoking the host inflammatory response (Dixon and Darveau 2005). Other lipid A structures have variability and P. gingivalis lipid A structure which is mono-phosphorylated and penta-acylated closely resembles that of Bacteroides (Kumada et al., 1995; Al-Qutub et al., 2006). These deviations from the canonical lipid A of E. coli are less potent and profoundly impact the host innate immune response (Dixon and Darveau 2005).
In this study, we show a dose-dependent activation of NF-κB in MΦ when stimulated with either PgLPS or EcLPS (Fig. 1A). However, PgLPS induced equivalent NF-κB activation at 10 times higher concentration relative to stimulation with EcLPS. Based on these data, 1000 ng/ml of PgLPS and 100 ng/ml of Ec LPS were employed in subsequent studies. Anti-TLR2 and anti-TLR4 blocking antibodies were titrated (Fig. 1B) and 20 μg/ml of these antibodies optimally blocked NF-κB activation. Although LPS is primarily recognized by TLR4, LPS structures from oral mucosal pathogens such as P. gingivalis (Ezzo and Cutler 2003) are recognized by TLR4 (Darveau et al., 2002; Coats et al., 2003) and also by TLR2 (Hirschfeld et al., 2001; Hajishengallis et al., 2002; Kido, Kido and Suryono 2003; Martin et al., 2003). To further determine how our LPS preparations interact with TLR2 and TLR4, we employed HEK cells expressing NF-κB and β-actin-Renilla that were transiently transfected with either TLR1/TLR2 (HEK-TLR2) or with TLR4, CD14 and MD2 (HEK-TLR4). It should be noted that TLR1 or TLR6 function as co-receptors for TLR2 signaling and CD14, MD2 and TLR4 complex effectively recognizes LPS structures (Beutler et al., 2003). The data suggest that stimulating with PgLPS resulted in upregulation of NF-κB in both HEK-TLR2 and HEK-TLR4 cells but EcLPS specifically targeted TLR4 but not TLR2 (Fig. 1C and D).
Recognition of microbial structures through TLRs leads to secretion of inflammatory cytokines (Ozinsky et al., 2000; Uwiera et al., 2001; Blander and Medzhitov 2004). Subsequently, the secretion of cytokines such as TNF-α and IL-1β leads to induction and activation of latent forms of MMPs. (Liacini et al., 2003; Cox et al., 2006). Anti-TLR2 and anti-TLR4 functional blocking antibodies were employed to determine how TLRs mediate the secretion of TNF-α, IL-1β and MMP9 when stimulated with PgLPS and EcLPS stimulation. MΦ stimulated with either PgLPS or EcLPS significantly induced the secretion of TNF-α, IL-1β and MMP9 relative to unstimulated controls (Fig. 2A–D). However, EcLPS was a potent inducer of TNF-α and IL-1β relative to PgLPS. Interestingly, PgLPS (Fig. 2E) was a potent inducer of MMP9 relative to stimulation with EcLPS (Fig. 2F). Due to structural mono-phosphorylation of PgLPS (relative to bi-phosphorylated EcLPS), and that PgLPS targets TLR2, the commensal receptor, the inflammatory response induced by PgLPS is relatively weaker than that of the putative EcLPS. Although PgLPS can target TLR4, structural variations of the biologically active lipid A component of PgLPS can also antagonize TLR4 activity (Liu et al., 2008). These results support our previous findings that EcLPS is a potent inducer of inflammatory cytokines such as TNF-α and IL-1β (Muthukuru et al., 2005) and on the contrary, PgLPS strongly upregulates MMP9 (Jotwani et al., 2010). Anti-TLR4 blocking antibody reduced the secretion TNF-α and IL-1β (Fig. 2A–D). Interestingly, either TLR2 or TLR4 blocking antibodies did not affect the levels of MMP9 (Fig. 2E and F). These data suggest that MMP9 may not be exclusively controlled by TLR signaling but could be influenced by downstream events such as cytokines secretion in response to TLR activation.
Immunosurveillance and immunoregulation are important in mucosal linings such as oral mucosa due to enormous microbial complexity and diversity (Paster et al., 2001). Induction of immune tolerance toward commensals combined with responsiveness to pathogens is essential to sustaining immune homeostasis while preventing life threatening infections (Yilmaz, Watanabe and Lamont 2002). We have previously reported through in situ studies and with in vitro models (Muthukuru et al., 2005) that APCs, such as Mϕ, downregulate the expression of TLRs and inflammatory cytokines such as IL-1β, IL-6 and TNF-α when induced to a state of endotoxin tolerance. In the present study, we show that secretion of MMP9 is downmodulated by induction of endotoxin tolerance mediated by either PgLPS or EcLPS (Figs 3 and 4). Despite downregulation of TNF-α and IL-1β with TLR blocking, levels of MMP9 were not affected. This suggests that low levels of cytokines could be sufficient in the induction of MMP9.
MMP9 is one of the two gelatinase (92 kDa; gelatinase B) that has two conserved motifs, the prodomain and the catalytic domain (Sorsa et al., 2006). Similar to other collagenases, the conserved cysteine residue in the prodomain binds to zinc in the catalytic domain. The cleavage of the prodomain is required for activation of MMP9. In the latent form, proMMP9 binds to TIMP1 and can be activated by serine proteinase (Sorsa et al., 2006). In an effort to determine how MMP9 secretion was downregulated during the induction of endotoxin tolerance (Fig. 3), the expression of TIMP1 and MMP9 transcripts was analyzed through real-time RT-PCR (Fig. 4). The data suggest that sensitization and challenge with PgLPS or EcLPS and induction of endotoxin tolerance induced a 3-fold upregulation of MMP9 transcripts relative to sensitization (Fig. 4A). However, the expression of TIMP1 was significantly upregulated by 93 and 15 times during the induction of endotoxin tolerance with PgLPS and EcLPS, respectively. These data suggest that upregulation of TIMP1 expression during endotoxin tolerance could counteract MMP9 activities.
Although the induction and activation of MMPs are well understood, the regulation of TIMPs is less explored. TGF-β has multiple functions in facilitating tissue homeostasis. TGF-β has been implicated in downmodulating MMPs and inducing TIMPs during invasive breast cancer (Gomes et al., 2012). On the contrary, blocking TGF-β activity is implicated in upregulation of MMP3 in the gut mucosa (Di Sabatino et al., 2008). Src homology 2 (SH2) domain-containing 5′-inositol phosphatase (SHIP) is one of inhibitory phosphatases (Harder et al., 2004) that is upregulated during the induction of endotoxin tolerance and during inflammatory conditions such as chronic periodontitis (Muthukuru and Cutler 2006). The expression of SHIP can be induced through autocrine action of TGF-β (Sly et al., 2004). In this context, TGF-β could play a common role in the induction of tolerance and upregulation of TIMP1. Also, anti-inflammatory cytokines such as IL-10 have been demonstrated to be relatively unaltered during the induction of endotoxin tolerance when compared to downregulation of pro-inflammatory cytokines (Muthukuru et al., 2005). Interestingly, IL-10 has been shown to downmodulate MMP2/9 (John et al., 2002) and also induce the expression of TIMP1 (Stearns et al., 1997, 1999). IL-10 being relatively resistant to downmodulation (relative to pro-inflammatory cytokines such as IL-1β) during endotoxin tolerance and also, IL-10 being able to downregulate MMP9 but upregulate TIMP1 could provide mechanistic clues as to how MMP9 activity could be downmodulated during endotoxin tolerance. Apart from the data we have presented here, no other studies are currently available that have explored the regulation of MMP/TIMP activities during the induction of endotoxin tolerance.
Regulation of MMP9 is implicated as a potential therapeutic target during chronic obstructive pulmonary disease and mitral stenosis (Muroski et al., 2008). Also, TGF-β may inhibit the growth of melanomas by specifically decreasing plasmin activity of tumor cells and could play a protective role during the early stages of tumor progression (Ramont et al., 2003). Understanding the signaling networks of MMPs, TIMPs and TGF-β pathways and exploration of negative inducers of inflammation such as induction of endotoxin tolerance could further facilitate therapeutic interventions.
Acknowledgments
These studies were supported by R-01 DE14328.
Conflict of interest. None declared.
REFERENCES
- Al-Qutub MN, Braham PH, Karimi-Naser LM, et al. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 2006;74:4474–85. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Hoebe K, Du X, et al. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukocyte Biol. 2003;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Neoptolemos JP, Stamp GW, et al. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347–55. doi: 10.1002/(SICI)1096-9896(199707)182:3<347::AID-PATH848>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Brehmer B, Biesterfeld S, Jakse G. Expression of matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer P D. 2003;6:217–22. doi: 10.1038/sj.pcan.4500657. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Bio. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Coats SR, Reife RA, Bainbridge BW, et al. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71:6799–807. doi: 10.1128/IAI.71.12.6799-6807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N, Morisset J, Emilie D. Induction of tolerance by Porphyromonas gingivalis on APCS: a mechanism implicated in periodontal infection. J Dent Res. 2004;83:429–33. doi: 10.1177/154405910408300515. [DOI] [PubMed] [Google Scholar]
- Cox SW, Eley BM, Kiili M, et al. Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis. 2006;12:34–40. doi: 10.1111/j.1601-0825.2005.01153.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Arbabi S, Garcia I, et al. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect Immun. 2002;70:1867–73. doi: 10.1128/IAI.70.4.1867-1873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Pickard KM, Rampton D, et al. Blockade of transforming growth factor beta upregulates T-box transcription factor T-bet, and increases T helper cell type 1 cytokine and matrix metalloproteinase-3 production in the human gut mucosa. Gut. 2008;57:605–12. doi: 10.1136/gut.2007.130922. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res. 2005;84:584–95. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- Ezzo PJ, Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003;32:24–35. doi: 10.1046/j.0906-6713.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- Gabison EE, Mourah S, Steinfels E, et al. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: role in epithelio-stromal interactions and matrix metalloproteinase induction. Am J Pathol. 2005;166:209–19. doi: 10.1016/S0002-9440(10)62245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Fonseca BA, et al. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004;31:671–9. doi: 10.1111/j.1600-051X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- Gomes LR, Terra LF, Wailemann RA, et al. TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson R, Douglas CW, Heath J, et al. Activation of human matrix metalloproteinase 2 by gingival crevicular fluid and Porphyromonas gingivalis. J Clin Periodontol. 2003;30:542–50. doi: 10.1034/j.1600-051x.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- Grenier D, Mayrand D. Inactivation of tissue inhibitor of metalloproteinases-1 (TIMP-1) by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;203:161–4. doi: 10.1111/j.1574-6968.2001.tb10835.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Martin M, Schifferle RE, et al. Counteracting interactions between lipopolysaccharide molecules with differential activation of toll-like receptors. Infect Immun. 2002;70:6658–64. doi: 10.1128/IAI.70.12.6658-6664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, Ernst RK, Tsai JH, et al. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–9. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- Harder KW, Quilici C, Naik E, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–10. doi: 10.1182/blood-2003-12-4396. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Sym. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–6. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- John M, Oltmanns U, Fietze I, et al. Increased production of matrix metalloproteinase-2 in alveolar macrophages and regulation by interleukin-10 in patients with acute pulmonary sarcoidosis. Exp Lung Res. 2002;28:55–68. doi: 10.1080/019021402753355535. [DOI] [PubMed] [Google Scholar]
- Jotwani R, Eswaran SV, Moonga S, et al. MMP-9/TIMP-1 imbalance induced in human dendritic cells by Porphyromonas gingivalis. FEMS Immunol Med Mic. 2010;58:314–21. doi: 10.1111/j.1574-695X.2009.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyama M, Kuboki T, Kojima S, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J Orofac Pain. 2000;14:20–30. [PubMed] [Google Scholar]
- Kido J, Kido R, Suryono Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-Toll-like receptor-nuclear factor kappaB pathway. J Periodontal Res. 2003;38:557–63. doi: 10.1034/j.1600-0765.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontol 2000. 2000;24:215–25. doi: 10.1034/j.1600-0757.2000.2240110.x. [DOI] [PubMed] [Google Scholar]
- Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci USA. 1999;96:14547–52. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Itagaki M, Hoshino C, et al. Altered gene expression levels of matrix metalloproteinases and their inhibitors in periodontitis-affected gingival tissue. J Periodontol. 2008;79:166–73. doi: 10.1902/jop.2008.070159. [DOI] [PubMed] [Google Scholar]
- Kumada H, Haishima Y, Umemoto T, et al. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol. 1995;177:2098–106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Peeters-Joris C, Vaes G. Production of gelatin-degrading matrix metalloproteinases (‘type IV collagenases’) and inhibitors by articular chondrocytes during their dedifferentiation by serial subcultures and under stimulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta. 1991;1094:8–18. doi: 10.1016/0167-4889(91)90020-x. [DOI] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208–17. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- Liu R, Desta T, Raptis M, et al. P. gingivalis and E. coli lipopolysaccharides exhibit different systemic but similar local induction of inflammatory markers. J Periodontol. 2008;79:1241–7. doi: 10.1902/jop.2008.070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso G, Midiri A, Biondo C, et al. Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4. Infect Immun. 2005;73:5620–7. doi: 10.1128/IAI.73.9.5620-5627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Schifferle RE, Cuesta N, et al. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–25. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Wilkerson A, Pacheco B, et al. Depressed cortical excitability and elevated matrix metalloproteinases in remote brain regions following intracerebral hemorrhage. Brain Res. 2004;1026:227–34. doi: 10.1016/j.brainres.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Muroski ME, Roycik MD, Newcomer RG, et al. Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr Pharm Biotechno. 2008;9:34–46. doi: 10.2174/138920108783497631. [DOI] [PubMed] [Google Scholar]
- Muthukuru M, Cutler CW. Upregulation of immunoregulatory Src homology 2 molecule containing inositol phosphatase and mononuclear cell hyporesponsiveness in oral mucosa during chronic periodontitis. Infect Immun. 2006;74:1431–5. doi: 10.1128/IAI.74.2.1431-1435.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukuru M, Cutler CW. Antigen capture of Porphyromonas gingivalis by human macrophages is enhanced but killing and antigen presentation are reduced by endotoxin tolerance. Infect Immun. 2008;76:477–85. doi: 10.1128/IAI.00100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukuru M, Jotwani R, Cutler CW. Oral mucosal endotoxin tolerance induction in chronic periodontitis. Infect Immun. 2005;73:687–94. doi: 10.1128/IAI.73.2.687-694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y, Miura K, Ohkita A, et al. Imbalance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinases 1 expression by tumor cells implicated in liver metastasis from colorectal carcinoma. Kurume Med J. 2001;48:211–8. doi: 10.2739/kurumemedj.48.211. [DOI] [PubMed] [Google Scholar]
- Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega N, Behonick D, Stickens D, et al. How proteases regulate bone morphogenesis. Ann NY Acad Sci. 2003;995:109–16. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Osman M, Tortorella M, Londei M, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases define the migratory characteristics of human monocyte-derived dendritic cells. Immunology. 2002;105:73–82. doi: 10.1046/j.0019-2805.2001.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Smith KD, Hume D, et al. Co-operative induction of pro-inflammatory signaling by Toll-like receptors. J Endotoxin Res. 2000;6:393–6. [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Bio. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, et al. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–9. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- Ramont L, Pasco S, Hornebeck W, et al. Transforming growth factor-beta1 inhibits tumor growth in a mouse melanoma model by down-regulating the plasminogen activation system. Exp Cell Res. 2003;291:1–10. doi: 10.1016/s0014-4827(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Rautelin HI, Oksanen AM, Veijola LI, et al. Enhanced systemic matrix metalloproteinase response in Helicobacter pylori gastritis. Ann Med. 2009;41:208–15. doi: 10.1080/07853890802482452. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Seymour GJ. Importance of the host response in the periodontium. J Clin Periodontol. 1991;18:421–6. doi: 10.1111/j.1600-051x.1991.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Sly LM, Rauh MJ, Kalesnikoff J, et al. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–39. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- Squire IB, Evans J, Ng LL, et al. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004;10:328–33. doi: 10.1016/j.cardfail.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Stearns ME, Fudge K, Garcia F, et al. IL-10 inhibition of human prostate PC-3 ML cell metastases in SCID mice: IL-10 stimulation of TIMP-1 and inhibition of MMP-2/MMP-9 expression. Invas Metast. 1997;17:62–74. [PubMed] [Google Scholar]
- Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5:189–96. [PubMed] [Google Scholar]
- Tchetverikov I, Ronday HK, Van El B, et al. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–3. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Imai K, Tsuchiya H, et al. Matrix metalloproteinase 9 (gelatinase B) is expressed in multinucleated giant cells of human giant cell tumor of bone and is associated with vascular invasion. Am J Pathol. 1996;148:611–22. [PMC free article] [PubMed] [Google Scholar]
- Uwiera RR, Gerdts V, Pontarollo RA, et al. Plasmid DNA induces increased lymphocyte trafficking: a specific role for CpG motifs. Cell Immunol. 2001;214:155–64. doi: 10.1006/cimm.2001.1899. [DOI] [PubMed] [Google Scholar]
- Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. J Dent Res. 2006;85:1074–84. doi: 10.1177/154405910608501202. [DOI] [PubMed] [Google Scholar]
- Weeks JG, Halme J, Woessner JF., Jr Extraction of collagenase from the involuting rat uterus. Biochim Biophys Acta. 1976;445:205–14. doi: 10.1016/0005-2744(76)90173-x. [DOI] [PubMed] [Google Scholar]
- Wu CY, Hsieh HL, Jou MJ, et al. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–88. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4:305–14. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li L, Lin JY, et al. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroentero. 2003;9:899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zhang Y, Ardans JA, et al. Interferon-gamma differentially regulates monocyte matrix metalloproteinase-1 and -9 through tumor necrosis factor-alpha and caspase 8. J Biol Chem. 2003;278:45406–13. doi: 10.1074/jbc.M309075200. [DOI] [PubMed] [Google Scholar]