Abstract
Streptococcus pneumoniae strains lacking capsular polysaccharide have been increasingly reported in carriage and disease contexts. Since most cases of otitis media involve more than one bacterial species, we aimed to determine the capacity of a nonencapsulated S. pneumoniae clinical isolate to induce disease in the context of a single-species infection and as a polymicrobial infection with nontypeable Haemophilus influenzae. Using the chinchilla model of otitis media, we found that nonencapsulated S. pneumoniae colonizes the nasopharynx following intranasal inoculation, but does not readily ascend into the middle ear. However, when we inoculated nonencapsulated S. pneumoniae directly into the middle ear, the bacteria persisted for two weeks post-inoculation and induced symptoms consistent with chronic otitis media. During coinfection with nontypeable H. influenzae, both species persisted for one week and induced polymicrobial otitis media. We also observed that nontypeable H. influenzae conferred passive protection from killing by amoxicillin upon S. pneumoniae from within polymicrobial biofilms in vitro. Therefore, based on these results, we conclude that nonencapsulated pneumococci are a potential causative agent of chronic/recurrent otitis media, and can also cause mutualistic infection with other opportunists, which could complicate treatment outcomes.
Keywords: coinfection, nonencapsulated, Haemophilus, pneumococcus
Pneumococcal variants lacking capsular polysaccharide are increasingly common in carriage and in some disease states; in this study we show that one such clone can persist and cause chronic otitis media.
Graphical Abstract Figure.
Pneumococcal variants lacking capsular polysaccharide are increasingly common in carriage and in some disease states; in this study we show that one such clone can persist and cause chronic otitis media.
INTRODUCTION
Streptococcus pneumoniae causes a broad spectrum of human disease when it disseminates from the nasopharynx, including pneumonia, sepsis, sinusitis and otitis media (OM) (Kadioglu et al. 2008). OM, which occurs when bacteria in the nasopharynx ascend through the Eustachian tubes to colonize the middle ear, affects most children by the age of seven and is the leading cause of pediatric antibiotic prescription (Klein 2000; Gonzales et al. 2001).
S. pneumoniae and nontypeable Haemophilus influenzae (NTHi) are the two most commonly isolated bacterial species from individuals with OM (Laufer et al. 2011). Since the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), non-PCV7 pneumococcal serotypes have increased in prevalence, including nonencapsulated strains (Sa-Leao et al. 2009). The majority of S. pneumoniae involved in OM are encapsulated, but nonencapsulated S. pneumoniae strains have been isolated from both carriage and disease contexts (Carvalho et al. 2003; Martin et al. 2003; Berron et al. 2005; Hanage et al. 2006; Weatherholtz et al. 2010; Xu et al. 2011; Scott et al. 2012). Nonencapsulated S. pneumoniae is frequently detected living commensally in the nasopharynx (Hanage et al. 2006), but has been implicated in invasive disease (Weatherholtz et al. 2010; Scott et al. 2012), as well as in localized infections that include conjunctivitis (Carvalho et al. 2003; Martin et al. 2003; Berron et al. 2005; Hanage et al. 2006) and OM (Xu et al. 2011). The pneumococcal capsule is a critical factor for colonization in encapsulated S. pneumoniae (Magee and Yother 2001; Nelson et al. 2007); pathogenic nonencapsulated isolates have been reported to compensate for loss of capsule via expression of novel surface proteins such as PspK (Park et al. 2012; Keller et al. 2013).
The majority of middle ear infections involve more than one species (Jacoby et al. 2007; Laufer et al. 2011). S. pneumoniae and NTHi have been detected within polymicrobial biofilm communities in nasopharyngeal and middle ear clinical tissue samples (Hall-Stoodley et al. 2006; Hoa et al. 2009). Experimentally, NTHi and S. pneumoniae have alternatively been shown to compete or coexist. In a chinchilla model of OM, NTHi and S. pneumoniae formed polymicrobial biofilms and persisted together for at least three weeks (Weimer et al. 2010). Further, coinfection with NTHi promoted biofilm formation by S. pneumoniae, resulting in decreased systemic pneumococcal infection (Weimer et al. 2010). In contrast, in a mouse infection model, NTHi promoted clearance of S. pneumoniae during intranasal coinfection (Lysenko et al. 2005).
Both S. pneumoniae and NTHi are capable of forming surface-attached communities called biofilms (Murphy and Kirkham 2002; Domenech, Garcia and Moscoso 2012). Biofilm formation by S. pneumoniae occurs during both asymptomatic carriage (Munoz-Elias, Marcano and Camilli 2008; Marks, Parameswaran and Hakansson 2012) and disease (Reid et al. 2009), and S. pneumoniae forms surface-attached communities in the chinchilla model of OM (Reid et al. 2009). Interactions between multiple species in a biofilm can affect the efficacy of antibiotics. For example, NTHi can provide passive antibiotic protection to other species in vitro as mixed-species biofilm cultures and in vivo during polymicrobial OM (Armbruster et al. 2010; Weimer et al. 2011).
In this study, we tested the capacity of a nonencapsulated S. pneumoniae clinical isolate to induce disease in the chinchilla middle ear. We show that nontypeable S. pneumoniae (NTSp) can induce OM in a chinchilla model, both as a single-species infection and as a mixed-species infection with NTHi. We found that coinfection of nonencapsulated S. pneumoniae with NTHi has implications for both species, as coinfection promoted increased growth of NTHi in the middle ear, and NTHi provided protection against antibiotic treatment to S. pneumoniae in vitro.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Nonencapsulated S. pneumoniae strain MNZ1113 is a nasopharyngeal isolate from a child with OM (Hiller et al. 2010). Bacteria were grown on trypticase soy agar (BD) supplemented with 5% sheep's blood (Hemostat Laboratories) and 4 μg ml−1 of gentamicin (Sigma).
NTHi strain 86-028NP is a pediatric OM isolate (Bakaletz et al. 1989) that has been fully sequenced (Munson et al. 2004; Harrison et al. 2005) and induces OM in the chinchilla model (Bakaletz et al. 1997). Bacteria were grown on supplemented brain heart infusion (sBHI) agar (BD), which has been supplemented with hemin (MP Biochemicals), nicotinamide adenine dinucleotide (Sigma) and 3 μg ml−1 vancomycin (Sigma).
Chinchilla infection studies
Chinchillas were purchased from Rauscher's Chinchilla Ranch and allowed to acclimate in the facility for at least one week prior to infection. Chinchillas were evaluated by veterinary staff and found to have no overt illnesses. Infection studies were performed as previously described (Hong et al. 2007a,b). Briefly, NTHi grown overnight on agar plates was resuspended in phosphate buffered saline with 0.04% gelatin (PBSG) to a concentration of ∼108 cfu ml−1, and then diluted to the target inoculum. S. pneumoniae was diluted from frozen stocks of known concentration into PBSG to the desired concentration. Chinchillas were anesthetized via isoflurane inhalation and then inoculated through one of two routes. For intranasal infection experiments, S. pneumoniae was delivered into the nasal cavity by pipetting 100 μl of a 4 × 107 cfu ml−1 suspension into each nostril, for a total inoculum of 8 × 106 cfu. Transbullar infections were performed by delivering 100 μl of bacterial suspension into each middle ear cavity by puncturing the bullae with a 25 gauge needle. For single-species infections, chinchillas were inoculated with nonencapsulated S. pneumoniae at an inoculum of 3 × 108 or 4 × 105 cfu for the one-week experiment, and 4 × 106 cfu for the two-week experiment. For the mixed-species infection, chinchillas were inoculated via transbullar injection with 103 cfu of NTHi and either 105 or 107 cfu of nonencapsulated S. pneumoniae strain MNZ1113. Adult chinchillas (∼800 g body weight) were used for the first infection study we performed, the one-week transbullar infection; two to three month old chinchillas were used for all subsequent experiments. The switch to young chinchillas was done for the purposes of our intranasal infection study; we have previously found that young chinchillas are slightly more prone to ascension by encapsulated S. pneumoniae than are adult chinchillas (data not published).
Animals were monitored daily for signs of disease, including ataxia, head tilt and lethargy. Otoscopy was performed once every 48 h to evaluate the extent of ear disease. Each ear was scored on a 0 to 3 scale, using a rubric system accounting for erythema, tympanic membrane discoloration and opacity, fluid behind the tympanic membrane and tympanic membrane rupture (Hong et al. 2007a,b).
Animals were euthanized at designated time points following inoculation, and bacterial loads were quantitated. Fluid in the middle ear cavity was aspirated using a 21 gauge needle and the middle ear cavity was lavaged with 1 ml of PBS; these fluids were combined, serially diluted and plated on blood agar and/or sBHI for quantitation of S. pneumoniae and NTHi, respectively. Nasopharyngeal, Eustachian tube and middle ear bullae gross tissue were aseptically excised and placed in phosphate buffered saline (15, 1.5 and 10 ml, respectively), and then homogenized using a Power Gen 1000 tissue homogenizer (Fisher Scientific). Homogenized tissue suspensions were serially diluted and plated on blood agar and/or sBHI.
All animal infection studies were approved by the Wake Forest Institutional Animal Care and Use Committee.
Amoxicillin protection assay
Static biofilm cultures were established in 24-well culture plates by seeding nonencapsulated S. pneumoniae and NTHi at 106 cfu each into 1 ml of sBHI broth media supplemented with 10% horse serum and 300 μg ml−1 of catalase. Cultures were then incubated for 24 h in a 5% CO2 incubator at 37°C. At the 24 h mark, media was carefully removed and replaced with fresh media containing 125 μg ml−1 amoxicillin. Cultures were incubated for an additional 24 h. Bacteria in biofilm culture were quantitated by carefully removing culture supernatant, thoroughly scraping and resuspending surface-attached bacteria in phosphate buffered saline, and serially diluting and plating suspensions onto selective media.
RESULTS
Nonencapsulated S. pneumoniae colonization in the chinchilla nasopharynx
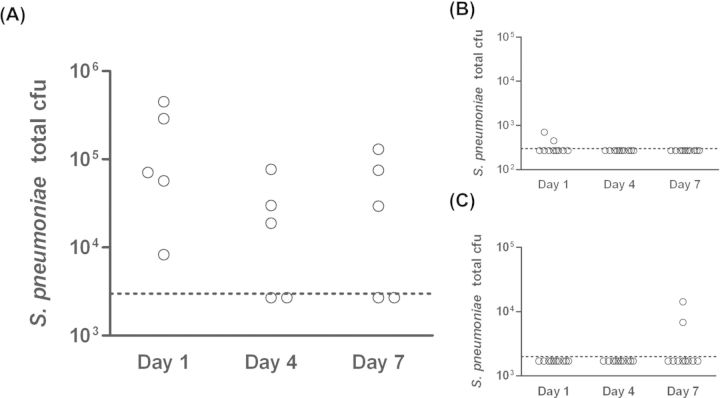
Nonencapsulated S. pneumoniae strain MNZ1113 was isolated from the nasopharynx of a child with acute OM (Hiller et al. 2010). We sought to determine the capacity of NTSp to colonize the healthy nasopharynx in a chinchilla model. Following intranasal inoculation, pneumococci were detected in the nasal cavity of most animals through seven days post-infection (Fig. 1A). We also quantified bacterial loads in the Eustachian tubes and middle ears, to determine if nonencapsulated S. pneumoniae ascends into the middle ear during the course of nasopharyngeal colonization. Pneumococcal counts predominantly fell below the limit of detection in the Eustachian tubes and middle ear tissues (Fig. 1B and C). Only two animals had detectable pneumococcal loads in their Eustachian tubes at any given time point, both unilaterally and at day 1 post-inoculation; neither of these animals had pneumococcal loads in their middle ears. A single animal was culture-positive for S. pneumoniae in the middle ear (bilateral) at seven days post-infection. However, no animals from the study, including the middle ear culture-positive animal, displayed clinical signs of OM during the course of the study. Based on these results, we conclude that nonencapsulated strain MNZ1113 colonizes the nasopharynx but does not readily ascend through the Eustachian tubes to induce OM in healthy chinchillas.
Figure 1.
NTSp colonizes the chinchilla nasopharynx with minimal ascension into the middle ear. Chinchillas were inoculated intranasally with NTSp strain MNZ1113. Pneumococcal bacterial loads were quantitated at one, four and seven days post-inoculation in the nasopharynx (A), Eustachian tubes (B) and middle ear bullae (C). Dotted line indicates limit of detection.
Nonencapsulated S. pneumoniae induces OM in the chinchilla infection model
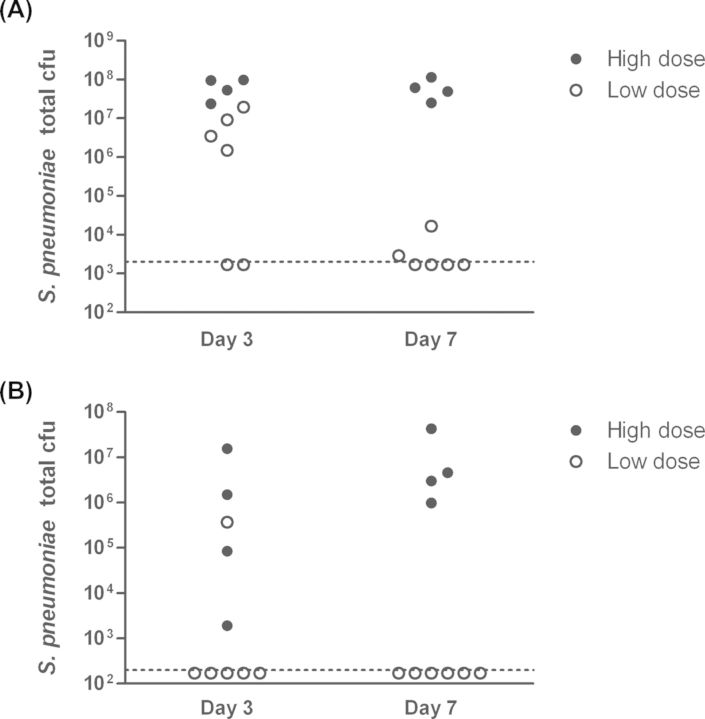
Bacterial ascension through the Eustachian tubes often requires other factors, such as viral upper respiratory infection, compromised immune function or other causes of Eustachian tube dysfunction (Bluestone et al. 2005; Bakaletz 2010; Coticchia et al. 2013). Therefore, in order to study the ability of nonencapsulated S. pneumoniae to induce disease in the context of a middle ear infection, we used a transbullar inoculation model. Healthy adult chinchillas were inoculated with one of two doses of strain MNZ1113 and pneumococcal bacterial loads were quantitated at three and seven days post-inoculation. Infection outcome was dose-dependent: approximately half of animals inoculated with the low dose of S. pneumoniae strain MNZ1113 cleared the infection within the first three days, while those inoculated with the high dose were consistently colonized (Fig. 2). S. pneumoniae was recovered from both homogenized middle ear tissue (Fig. 2A) and middle ear effusion and lavage fluid (Fig. 2B). Middle ear effusion fluid was present in about half of the chinchilla ears of either inoculum dose at three days post-inoculation, but was found only in the ears of the high-dose group at seven days post-inoculation (data not shown). Similarly, while biofilms of varying sizes were observable in all ears at three days post-inoculation, only the ears of the high-dose group had visible biofilms at seven days post-inoculation. In an extended time course experiment, we found that nonencapsulated S. pneumoniae strain MNZ1113 continued to persist through two weeks post-inoculation (Fig. 3). Beginning at seven days post-inoculation, pneumococci were detected only in homogenized tissue and not from middle ear fluid (Fig. 3A and B). Otoscopic monitoring of the infected animals indicated the presence of moderate inflammation around the tympanic membrane, along with fluid in the middle ear space in most animals during the first week of infection (Fig. 3C). During the second week of infection, the otoscopy scores became more heterogeneous as visible inflammation subsided in some ears but persisted in others. Interestingly, all animals had similar loads of S. pneumoniae within the middle ear at two weeks post-inoculation, independent of the disease severity.
Figure 2.
NTSp colonizes the chinchilla middle ear and induces otitis media. Chinchillas were inoculated via transbullar injection with a high- or low-dose S. pneumoniae strain MNZ1113. At three and seven days post-inoculation, pneumococcal bacterial loads were quantitated in homogenized middle ear bullae (A) and middle ear fluid (B). The dashed line indicates the bacterial limit of detection.
Figure 3.
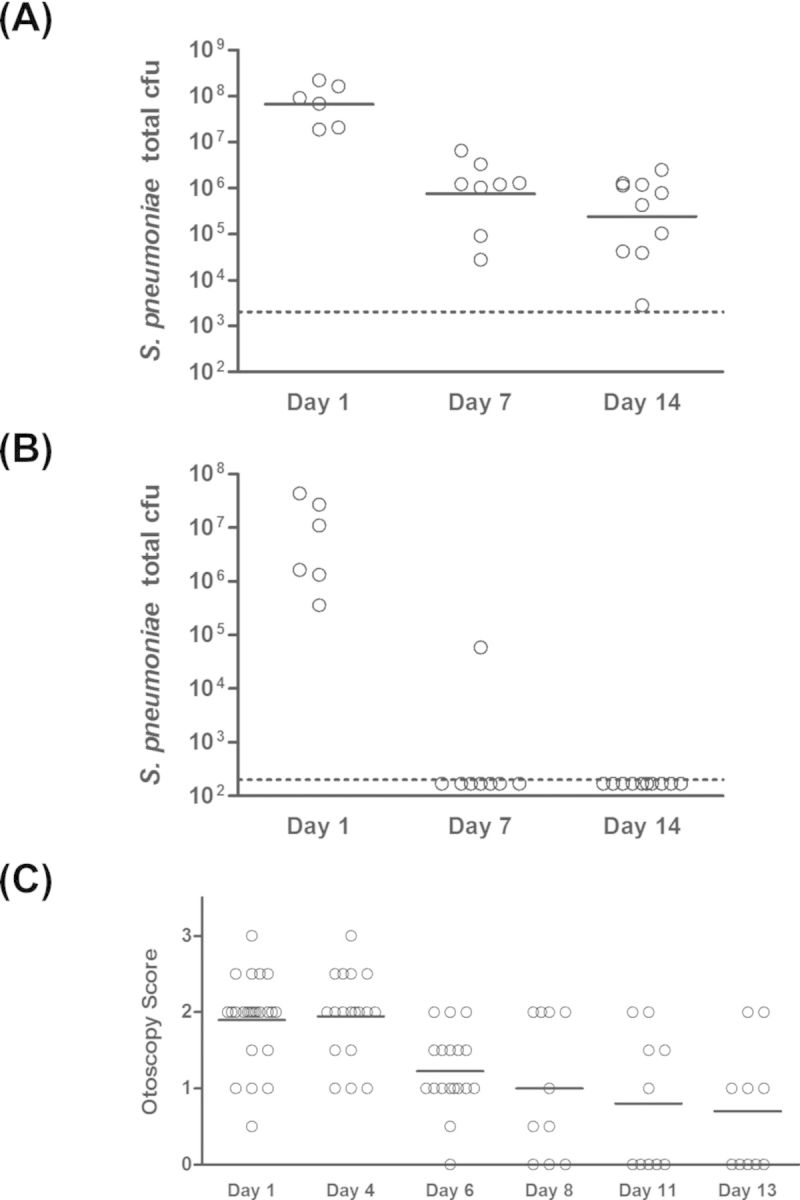
NTSp persists through two weeks in the chinchilla middle ear. Chinchilla middle ears were inoculated via transbullar injection of NTSp strain MNZ1113. Total pneumococcal cfu were quantitated at 1, 7 and 14 days post-inoculation in homogenized middle ear bullae (A) and middle ear fluid (B). Disease was assessed by via otoscopic examination (C). The solid horizontal lines indicate geometric mean (A) or mean (C). The dashed lines indicate the bacterial limit of detection.
Nonencapsulated S. pneumoniae and NTHi induce polymicrobial OM
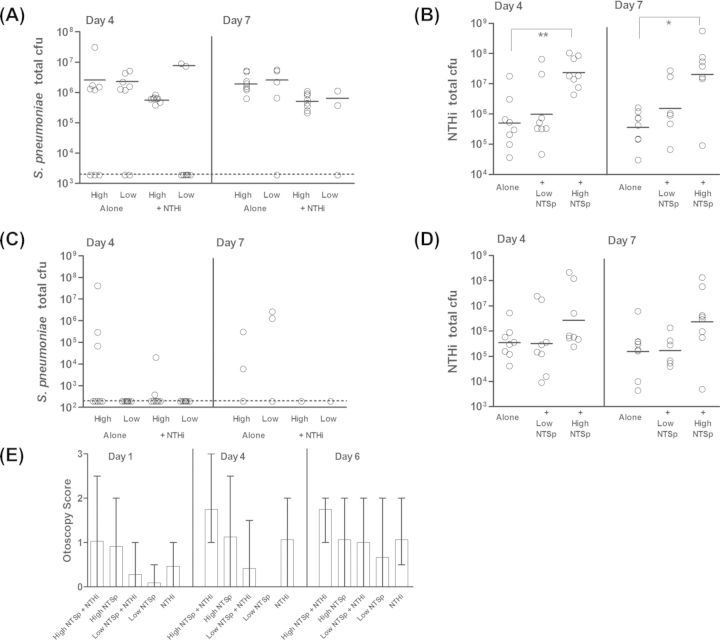
The majority of cases of OM are polymicrobial in nature (Jacoby et al. 2007; Laufer et al. 2011). NTHi and S. pneumoniae have been detected together in the nasopharynx (Jacoby et al. 2007; Casey, Adlowitz and Pichichero 2010) and middle ear (Elliott et al. 1999; Hall-Stoodley et al. 2006) of individuals with OM. In a chinchilla model, encapsulated S. pneumoniae and NTHi can form polymicrobial biofilms and induce polymicrobial OM (Weimer et al. 2010). It is unknown whether loss of the pneumococcal capsule affects the nature of the relationship with NTHi. We therefore sought to determine the capacity of nonencapsulated S. pneumoniae to live within a polymicrobial community with NTHi and how this affected disease. Chinchillas were inoculated via transbullar injection with one of two doses of nonencapsulated S. pneumoniae strain MNZ1113, alone or in combination with NTHi. At four and seven days post-inoculation, bacteria were quantitated in middle ear fluid and homogenized bullae. The quantity of S. pneumoniae in the middle ear homogenate was not affected by the presence of NTHi (Fig. 4A), but pneumococci were rarely detected in the middle ear fluid of single-species-infected or coinfected animals (Fig. 4C). The low dose of nonencapsulated S. pneumoniae was cleared from the ears of some animals in both the single-species-infected and coinfected groups. In contrast, NTHi was not cleared from any ears, and was consistently detected in the middle ear fluid (Fig. 4D). Interestingly, NTHi bacterial loads in homogenized middle ear tissue were significantly greater at both time points in the experimental group coinfected with 107 cfu of nonencapsulated S. pneumoniae, as compared to animals inoculated with NTHi alone (Fig. 4B, P < 0.05). There was not a significant difference between the geometric mean cfu of NTHi in animals coinfected with the low dose of S. pneumoniae and animals infected with NTHi alone. However, it should be noted that there was a bimodal distribution of NTHi bacterial counts in these coinfected animals at both time points, and that the ears with NTHi counts between 107 and 108 cfu were ears in which S. pneumoniae was still present. In contrast, ears in which S. pneumoniae was cleared had NTHi counts ranging from 104 to ∼106 cfu. Otoscopy evaluations were scored as an indicator of disease severity (Fig. 4E). Statistical analysis was not performed on these data due the qualitative nature of the scoring system (see the section ‘Materials and Methods’). The highest disease scores were observed in coinfected animals.
Figure 4.
NTSp and NTHi persist together in the chinchilla middle ear. Chinchillas were inoculated via transbullar injection with a high or low dose of NTSp, alone or in combination with NTHi (see the section ‘Materials and Methods’). At four and seven days post-inoculation, animals were euthanized and bacterial loads were assessed for S. pneumoniae (A and C) and NTHi (B and D) in homogenized middle ear tissue (A and B) and middle ear fluid (C and D). The geometric mean (solid horizontal line) was calculated for each group and did not include values below the bacterial limit of detection (dashed horizontal lines). Inflammation was evaluated by otoscopic examination at one, four and six days post-inoculation (E); graph shows mean otoscopy scores, with error bars denoting the range of scores in each group. *P < 0.05; **P < 0.01.
NTHi protects nonencapsulated S. pneumoniae against amoxicillin in vitro
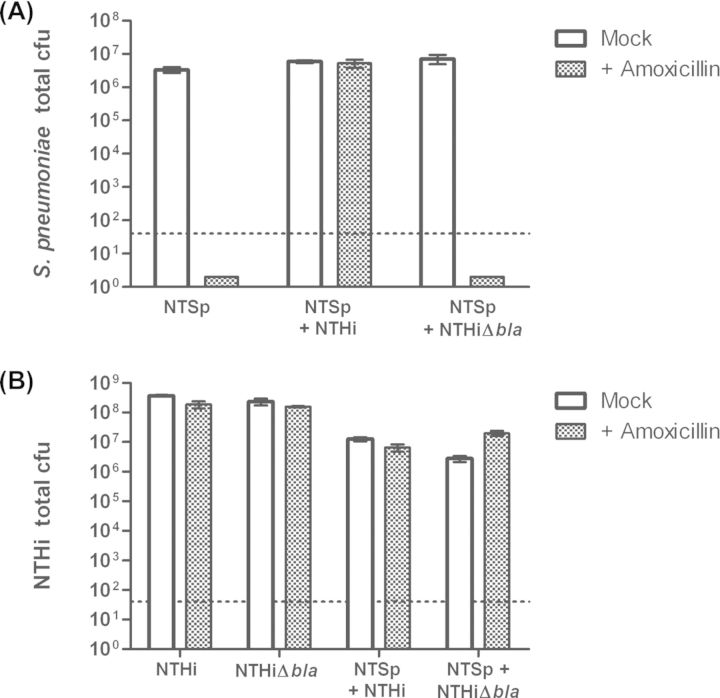
Bacterial growth as multi-species communities can affect responses to antibiotic treatment. In static biofilm culture, nonencapsulated S. pneumoniae strain MNZ1113 and NTHi grew as polymicrobial surface-attached cultures (data not shown), reflecting their ability to survive together in vivo. It has previously been shown that encapsulated S. pneumoniae can be protected from killing by amoxicillin when co-cultured with NTHi, due to beta-lactamase production by NTHi (Weimer et al. 2011). We sought to determine if nonencapsulated S. pneumoniae similarly benefitted from this interaction with NTHi. Briefly, nonencapsulated S. pneumoniae and NTHi were seeded alone or in combination with 24-well plates to establish static biofilm cultures, and then treated with amoxicillin (see the section ‘Materials and Methods’). Amoxicillin treatment completely killed MNZ1113 in single-species culture, but did not affect viable counts when MNZ1113 was co-cultured with NTHi (Fig. 5A). Co-culture with the beta-lactamase-deficient NTHi mutant 86-028NPΔbla did not provide any protection against amoxicillin treatment (Fig. 5A), indicating that protection was dependent on beta-lactamase production by NTHi.
Figure 5.
NTHi protects NTSp against beta-lactam antibiotics in vitro. NTSp and NTHi were grown as static biofilm cultures, alone or in co-culture. At 24 h, cultures were treated with 125 μg ml−1 of amoxicillin and cultures were incubated for an additional 24 h. Surface-attached bacteria were removed and plated on selective media to quantitate NTSp (A) and NTHi (B). Graphs indicate mean bacterial cfu; error bars denote 95% confidence interval. The dashed lines denote the bacterial limit of detection.
DISCUSSION
In this study, we demonstrate that a nonencapsulated clinical isolate of S. pneumoniae is capable of inducing OM in the chinchilla model, as both a single-species and polymicrobial infection. Using the transbullar route of inoculation, we found that nonencapsulated S. pneumoniae induced signs of inflammation during the first week of infection, but disease presentation differed among animals beginning in the second week of infection, independent of pneumococcal loads in the middle ear. The disparity in middle ear disease presentation may be a result of using the chinchilla as a model for OM, since only outbred lineages are available; this heterogeneity similarly reflects the range of differences in human disease. Notably, these results are similar to those in a recent report from Keller et al. who showed that nonencapsulated pneumococci can establish a persistent OM infection in the chinchilla model that is influenced by the PspK surface protein (Keller et al. 2014).
Following intranasal inoculation, nonencapsulated S. pneumoniae strain MNZ1113 colonized the chinchilla nasopharynx, the natural reservoir for bacterial otopathogens prior to ascension into the middle ear. Despite persisting in the nasopharynx through the seven-day time course, nonencapsulated S. pneumoniae was rarely detected in the middle ear. A single animal experienced bilateral middle ear colonization by nonencapsulated S. pneumoniae, but this resulted in no observable disease. There are several possibilities for the low incidence of middle ear infection following intranasal inoculation. First, the bacteria may ascend into the middle ear but fail to establish a viable infection. As seen in the transbullar inoculation experiments detailed in this study, a lower dose of nonencapsulated S. pneumoniae was more likely to result in clearance from the middle ear; therefore, if a limited number of bacteria ascend into the middle ear following intranasal inoculation, they may be easily cleared by host defenses. The pneumococcal capsular polysaccharide provides resistance to complement deposition (Melin et al. 2009; Sabharwal et al. 2009), so nonencapsulated S. pneumoniae may be more susceptible to opsonophagocytosis in the middle ear than encapsulated strains. Another possible explanation for the low incidence of middle ear infection by nonencapsulated S. pneumoniae is that the bacteria do not successfully ascend through the Eustachian tubes. This possibility is supported by the low detection rate of nonencapsulated S. pneumoniae in the Eustachian tubes of chinchillas following intranasal inoculation (Fig. 1A). The Eustachian tubes normally prevent bacterial ascension into the middle ear via mucociliary clearance, but dysfunction can occur following inflammatory events such as allergy or viral upper respiratory tract infection (Bakaletz 2010).
During coinfection with NTHi, nonencapsulated S. pneumoniae and NTHi persisted together during the one-week experimental time course. NTHi bacterial loads were significantly increased in animals coinfected with the high dose of pneumococci. A similar observation was reported in a neonatal rat nasal colonization model, in which Haemophilus influenzae type b had higher colonization levels in rats pre-colonized with S. pneumoniae (Margolis, Yates and Levin 2010). In contrast, nonencapsulated S. pneumoniae counts were slightly lower (not significant; P > 0.05) in coinfected animals compared to S. pneumoniae-alone infected animals at seven days post-inoculation. Additionally, animals inoculated with the low dose of nonencapsulated S. pneumoniae cleared the pneumococcal infection more frequently when coinfected with NTHi. Together, these observations indicate that NTHi may outcompete pneumococci. H. influenzae was reported to outcompete S. pneumoniae in a mouse intranasal infection model, by promoting enhanced opsonophagocytic clearance of S. pneumoniae (Lysenko et al. 2005). In contrast, Weimer et al. (2010) observed that NTHi and encapsulated S. pneumoniae were persisting together in a biofilm community within the chinchilla middle ear for up to three weeks post-inoculation. To better address the extent of competition between nonencapsulated S. pneumoniae and NTHi, an extended time course coinfection with the two species would allow us to determine if nonencapsulated S. pneumoniae is ultimately cleared while NTHi persists. Coinfection with NTHi could negatively impact the efficacy of antibiotic treatment against nonencapsulated S. pneumoniae, as indicated by our in vitro observations of NTHi-mediated protection of nonencapsulated S. pneumoniae against beta-lactam antibiotics.
Acknowledgments
The authors gratefully acknowledge Moon Nahm (University of Alabama Birmingham) for kindly providing nonencapsulated S. pneumoniae strain MNZ1113.
FUNDING
This work was supported by RO1 DC007444 and RO1 DC010051 (WES), and KAM was supported as a trainee on T32 AI07401.
Conflict of interest. None declared.
REFERENCES
- Armbruster CE, Hong W, Pang B, et al. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio. 2010;1:e00102–10. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukocyte Biol. 2010;87:213–22. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO, Leake ER, Billy JM, et al. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine. 1997;15:955–61. doi: 10.1016/s0264-410x(96)00298-8. [DOI] [PubMed] [Google Scholar]
- Bakaletz LO, Tallan BM, Andrzejewski WJ, et al. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins of nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3226–9. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron S, Fenoll A, Ortega M, et al. Analysis of the genetic structure of nontypeable pneumococcal strains isolated from conjunctiva. J Clin Microbiol. 2005;43:1694–8. doi: 10.1128/JCM.43.4.1694-1698.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone CD, Hebda PA, Alper CM, et al. Recent advances in otitis media. 2. Eustachian tube, middle ear, and mastoid anatomy; physiology, pathophysiology, and pathogenesis. Ann Oto Rhinol Laryn. 2005;194:16–30. doi: 10.1177/00034894051140s105. [DOI] [PubMed] [Google Scholar]
- Carvalho MG, Steigerwalt AG, Thompson T, et al. Confirmation of nontypeable Streptococcus pneumoniae-like organisms isolated from outbreaks of epidemic conjunctivitis as Streptococcus pneumoniae. J Clin Microbiol. 2003;41:4415–7. doi: 10.1128/JCM.41.9.4415-4417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coticchia JM, Chen M, Sachdeva L, et al. New paradigms in the pathogenesis of otitis media in children. Front Pediatr. 2013;1:52. doi: 10.3389/fped.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech M, Garcia E, Moscoso M. Biofilm formation in Streptococcus pneumoniae. Microb Biotechnol. 2012;5:455–65. doi: 10.1111/j.1751-7915.2011.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JA, Facklam RR, Nathan C, et al. Coisolation of Streptococcus pneumoniae and Hameophilus influenzae from middle ear fluid and sputum: effect on MIC results. J Clin Microbiol. 1999;37:277. doi: 10.1128/jcm.37.1.277-277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–62. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–11. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanage WP, Kaijalainen T, Saukkoriipi A, et al. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J Clin Microbiol. 2006;44:743–9. doi: 10.1128/JCM.44.3.743-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Dyer DW, Gillaspy A, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–36. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller NL, Ahmed A, Powell E, et al. Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection. PLoS Pathog. 2010;6:e1001108. doi: 10.1371/journal.ppat.1001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa M, Tomovic S, Nistico L, et al. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int J Pediatr Otorhi. 2009;73:1242–8. doi: 10.1016/j.ijporl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Hong W, Mason K, Jurcisek J, et al. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect Immun. 2007a;75:958–65. doi: 10.1128/IAI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Pang B, West-Barnette S, et al. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol. 2007b;189:8300–7. doi: 10.1128/JB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby P, Watson K, Bowman J, et al. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine. 2007;25:2458–64. doi: 10.1016/j.vaccine.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Weiser JN, Paton JC, et al. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- Keller LE, Friley J, Dixit C, et al. Nonencapsulated Streptococcus pneumoniae cause acute otitis media in the chinchilla that is enhanced by pneumococcal surface protein K. Open Forum Infect Dis. 2014;1:1–8. doi: 10.1093/ofid/ofu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LE, Jones CV, Thornton JA, et al. PspK of Streptococcus pneumoniae increases adherence to epithelial cells and enhances nasopharyngeal colonization. Infect Immun. 2013;81:173–81. doi: 10.1128/IAI.00755-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JO. The burden of otitis media. Vaccine. 2000;19:S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- Laufer AS, Metlay JP, Gent JF, et al. Microbial communities of the upper respiratory tract and otitis media in children. mBio. 2011;2:e00245. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko ES, Ratner AJ, Nelson AL, et al. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–61. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E, Yates A, Levin B. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol. 2010;10:59. doi: 10.1186/1471-2180-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LR, Parameswaran GI, Hakansson AP. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun. 2012;80:2744–60. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Turco JH, Zegans ME, et al. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N Engl J Med. 2003;348:1112–21. doi: 10.1056/NEJMoa022521. [DOI] [PubMed] [Google Scholar]
- Melin M, Jarva H, Siira L, et al. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun. 2009;77:676–84. doi: 10.1128/IAI.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Elias EJ, Marcano J, Camilli A. Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect Immun. 2008;76:5049–61. doi: 10.1128/IAI.00425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson RS, Jr, Harrison A, Gillaspy A, et al. Partial analysis of the genomes of two nontypeable Haemophilus influenzae otitis media isolates. Infect Immun. 2004;72:3002–10. doi: 10.1128/IAI.72.5.3002-3010.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Kirkham C. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2002;2:7. doi: 10.1186/1471-2180-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Roche AM, Gould JM, et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Kim KH, Andrade AL, et al. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. mBio. 2012;3:e00035–46. doi: 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SD, Hong W, Dew KE, et al. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199:786–94. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- Sa-Leao R, Nunes S, Brito-Avo A, et al. Changes in pneumococcal serotypes and antibiotypes carried by vaccinated and unvaccinated day-care centre attendees in Portugal, a country with widespread use of the seven-valent pneumococcal conjugate vaccine. Clin Microbiol Infec. 2009;15:1002–7. doi: 10.1111/j.1469-0691.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Sabharwal V, Ram S, Figueira M, et al. Role of complement in host defense against pneumococcal otitis media. Infect Immun. 2009;77:1121–7. doi: 10.1128/IAI.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Hinds J, Gould KA, et al. Nontypeable pneumococcal isolates among navajo and white mountain apache communities: are these really a cause of invasive disease? J Infect Dis. 2012;206:73–80. doi: 10.1093/infdis/jis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–46. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- Weimer KED, Armbruster CE, Juneau RA, et al. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis. 2010;202:1068–75. doi: 10.1086/656046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer KED, Juneau RA, Murrah KA, et al. Divergent mechanisms for passive pneumococcal resistance to Î2-lactam antibiotics in the presence of Haemophilus influenzae. J Infect Dis. 2011;203:549–55. doi: 10.1093/infdis/jiq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Kaur R, Casey JR, et al. Nontypeable Streptococcus pneumoniae as an otopathogen. Diagn Micr Infec Dis. 2011;69:200–4. doi: 10.1016/j.diagmicrobio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]