Abstract
Background:
Pancreatic tumors cause changes in whole-body metabolism, but whether prediagnostic circulating metabolites predict survival is unknown.
Methods:
We measured 82 metabolites by liquid chromatography–mass spectrometry in prediagnostic plasma from 484 pancreatic cancer case patients enrolled in four prospective cohort studies. Association of metabolites with survival was evaluated using Cox proportional hazards models adjusted for age, cohort, race/ethnicity, cancer stage, fasting time, and diagnosis year. After multiple-hypothesis testing correction, a P value of .0006 or less (.05/82) was considered statistically significant. Based on the results, we evaluated 33 tagging single-nucleotide polymorphisms (SNPs) in the ACO1 gene, requiring a P value of less than .002 (.05/33) for statistical significance. All statistical tests were two-sided.
Results:
Two metabolites in the tricarboxylic acid (TCA) cycle—isocitrate and aconitate—were statistically significantly associated with survival. Participants in the highest vs lowest quintile had hazard ratios (HRs) for death of 1.89 (95% confidence interval [CI] = 1.06 to 3.35, P trend < .001) for isocitrate and 2.54 (95% CI = 1.42 to 4.54, P trend < .001) for aconitate. Isocitrate is interconverted with citrate via the intermediate aconitate in a reaction catalyzed by the enzyme aconitase 1 (ACO1). Therefore, we investigated the citrate to aconitate plus isocitrate ratio and SNPs in the ACO1 gene. The ratio was strongly associated with survival (P trend < .001) as was the SNP rs7874815 in the ACO1 gene (hazard ratio for death per minor allele = 1.37, 95% CI = 1.16 to 1.61, P < .001). Patients had an approximately three-fold hazard for death when possessing one or more minor alleles at rs7874851 and high aconitate or isocitrate.
Conclusions:
Prediagnostic circulating levels of TCA cycle intermediates and inherited ACO1 genotypes were associated with survival among patients with pancreatic cancer.
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States (1). Among patients with pancreatic adenocarcinoma, less than 5% will survive five years after diagnosis, and most patients live fewer than 12 months (2). The length of patient survival is associated with disease stage, but few other prognostic markers have been characterized.
Recent studies indicate that prediagnostic obesity (3,4) and diabetes (5–7) adversely impact survival in patients with pancreatic cancer, but the biologic mechanisms underlying these survival differences are unclear. Furthermore, cancer cells have different metabolic needs than normally differentiated cells, exhibiting altered metabolism to support inappropriate cell division (8). As a consequence of these metabolic changes, specific metabolites have been identified that support cancer growth, and some cancer cells demonstrate dependencies upon particular metabolic pathways (9–11).
Pancreatic cancer cells exist in a harsh local environment, surrounded by dense connective tissue and poor vascularization. This hypoxic and nutrient-depleted microenvironment, along with the hallmark KRAS oncogene mutation, reprograms pancreatic cancer cell metabolism and promotes tumor growth (11–15). Nevertheless, circulating markers of metabolism that may predict patient outcomes have not been systemically explored. We measured prediagnostic circulating metabolites using liquid chromatography–mass spectroscopy (LC-MS) to identify markers of altered metabolism associated with survival among patients with pancreatic cancer.
Methods
Study Population
Participants with pancreatic cancer and banked prediagnostic blood were included from four prospective cohort studies: Health Professionals Follow-up Study (HPFS), Nurses’ Health Study (NHS), Physicians’ Health Study (PHS), and Women’s Health Initiative–Observational Study (WHI). HPFS was initiated in 1986 when 51 529 US men age 40 to 75 years working in health professions completed a mailed biennial questionnaire (16). NHS was established in 1976 when 121 700 female nurses age 30 to 55 years completed a mailed biennial questionnaire (17). PHS is a completed trial initiated in 1982 of aspirin and β-carotene among 22 071 male physicians, age 40 to 84 years. After trial completion in 1995, participants were followed as an observational cohort (18). WHI consists of 93 676 postmenopausal women age 50 to 79 years enrolled from 1994 to 1998 at 40 US clinical centers (19). Participants completed a baseline clinic visit and annual mailed questionnaires. This study was approved by Human Research Committee at Brigham and Women’s Hospital (Boston, MA), and participants provided informed consent.
We identified 488 incident pancreatic adenocarcinoma case patients diagnosed through 2010 with available plasma. Deaths were ascertained from next-of-kin, the postal service, and the National Death Index, which captures more than 98% of deaths (20). Records were reviewed by study physicians blinded to exposure data, and diagnoses were confirmed by medical records, death certificates, and/or tumor registry data. Four case patients with unclear date of diagnosis were excluded.
Blood Collection and Metabolite Profiling
Blood samples in EDTA tubes were collected from 18 225 men in HPFS (1993–1995), 14 916 men in PHS (1982–1984), and 93 676 women in WHI (1994–1998), and in heparin tubes from 32 826 women in NHS (1989–1990). Samples in HPFS and NHS were collected by participants, mailed overnight on cold-packs, and spun to collect plasma (delayed processing) while PHS and WHI participants’ whole blood was separated immediately into plasma and stored. Plasma metabolites were measured as peak areas by targeted LC-MS at the Broad Institute of MIT and Harvard University (Cambridge, MA). Blood processing and metabolite profiling methods have been described previously (21).
We measured 133 metabolites, and 82 were included in survival analyses (Supplementary Figure 1, available online). In pilot studies (22), 32 metabolites had poor reproducibility in samples with delayed processing, so were excluded. Three heparin quality control (QC) plasma pools (57 total QC samples) and three EDTA QC plasma pools (128 total QC samples) were randomly interspersed among participant samples. We calculated mean coefficients of variation (CVs) for each metabolite across QC plasma pools and set an a priori threshold of 25% or less for satisfactory reproducibility; 13 metabolites with a mean CV greater than 25% were excluded. Six metabolites were excluded for undetectable levels in greater than 10% of case patients. We evaluated 10 volunteers with plasma collected simultaneously in heparin and EDTA tubes. Spearman correlation coefficients between Heparin and EDTA samples were 0.70 for isocitrate, 0.84 for aconitate, and 0.89 for citrate.
Single-Nucleotide Polymorphism Selection and Genotyping
We selected 33 tagging single-nucleotide polymorphisms (SNPs) in the ACO1 gene +/-20kb using the tagger algorithm in Haploview with cutoffs at an r 2 of 0.8 and a minor allele frequency (MAF) of 5% or higher in whites from the HapMap Project database, forcing in two SNPs related to melanoma risk (rs7855483 and rs10813813) (23). From 387 of the case patients, DNA was extracted from archived buffy coat with QIAGEN QIAmp and whole-genome-amplified with GE Healthcare Genomiphi. All genotyping was performed at Partners HealthCare Center for Personalized Genetic Medicine using a custom-designed Illumina Golden Gate genotyping assay. Two tagging SNPs were not supported by the Golden Gate platform and could not be genotyped. Replicate samples included for QC (n = 44 sample groups) had mean genotype concordance of 97.7% across the 33 SNPs. No SNPs deviated from Hardy-Weinberg Equilibrium at a P value of less than .01. Most case patients from the cohorts (n = 371) were evaluated in a recently completed genome-wide association study (GWAS) of pancreatic cancer (PanScan) (24). To obtain a denser map of SNPs at ACO1 (+/-20kb) and investigate SNPs at mitochondrial aconitase ACO2 (+/-20kb), we investigated genotyped or imputed SNPs from the HPFS, NHS, PHS, and WHI case patients included in PanScan with MAFs of 5% or greater (Supplementary Table 1, available online). Genotyping and imputation methods have been described previously (24). For more details, see the Supplementary Methods (available online).
Covariate Data
Age, race/ethnicity, smoking status, physical activity, body mass index (BMI), alcohol intake, and history of diabetes were obtained from baseline questionnaires in PHS and WHI and questionnaires prior to blood collection in HPFS and NHS (25). Date of cancer diagnosis and pancreatic cancer stage at diagnosis were obtained from medical record review, as described previously (21). For more details, see the Supplementary Methods (available online).
Statistical Analysis
Overall survival time was calculated from date of pancreatic cancer diagnosis to date of death or date of last follow-up, whichever came first. Metabolites were log-transformed to improve normality and included as continuous variables in Cox proportional hazards regression models adjusted for age at diagnosis (years, continuous), cohort (HPFS, NHS, PHS, WHI; which also adjusts for sex), race/ethnicity (white, black, other, missing), stage at diagnosis (localized, locally advanced, metastatic, unknown), fasting time (<4, 4–8, 8–12, ≥12 hours, missing), and year of diagnosis (1984–1995, 1996–2005, 2006–2010). Using a conservative Bonferroni correction for multiple hypothesis testing (26), metabolites with a P trend of .0006 or less (.05/82) were considered statistically significant. In secondary analyses, we also adjusted for time between blood collection and cancer diagnosis (0-<5, 5-<10, ≥10 years), BMI (World Health Organization categories), and history of diabetes (yes, no).
To evaluate effect magnitude, statistically significant metabolites were examined in Cox regression models after pooled categorization into quintiles defined by fasting status (≥8 hours, <8 hours since last meal). Hazard ratios (HRs) and 95% confidence intervals (CIs) were also calculated per standard deviation (SD) change in log-transformed metabolite levels. We calculated median survival time for subjects in each quintile adjusted for covariates using direct adjusted survival estimation (27,28). This method uses proportional hazards models to estimate probabilities of survival at each follow-up timepoint for individual subjects and averages them to obtain an overall survival estimate. Partial Spearman correlation coefficients were calculated for metabolites, adjusted for cohort and fasting time.
We assessed heterogeneity of metabolite associations with pancreatic cancer survival across cohorts using Cochran’s Q-statistic (29). We also conducted analyses stratified by time interval between blood collection and cancer diagnosis, sex, cancer stage, fasting status, and BMI. Statistical interactions were assessed by entering into models the main effect terms and cross-product terms of metabolites and stratification variables, evaluating likelihood ratio tests.
We examined tagging SNPs in ACO1 and survival by including each three-level genotype as a continuous variable (additive model) in multivariable-adjusted Cox regression models. SNPs were considered statistically significant if P values were less than .002 (.05/33 genotyped variants). The same approach was followed for SNPs genotyped or imputed at ACO1 and ACO2 in the PanScan GWAS. Survival curves by genotypes were generated using the Kaplan-Meier method, and statistical significance measured using the log-rank test. We identified SNPs highly correlated with our most statistically significant tagged SNPs (r2 > 0.7 in 1000G CEU data) and used HaploReg v2 (30) to explore noncoding functional annotation. We also assessed cis associations of these SNPs and expression of nearby genes in peripheral tissues from subjects of European descent (31–33).
The proportionality of hazards assumption was satisfied (P > .05) for Cox proportional hazards models by evaluating a time-dependent variable, which was the cross-product of time and metabolite or genotype. All analyses were performed with SAS 9.3 statistical package. All P values were two-sided.
Results
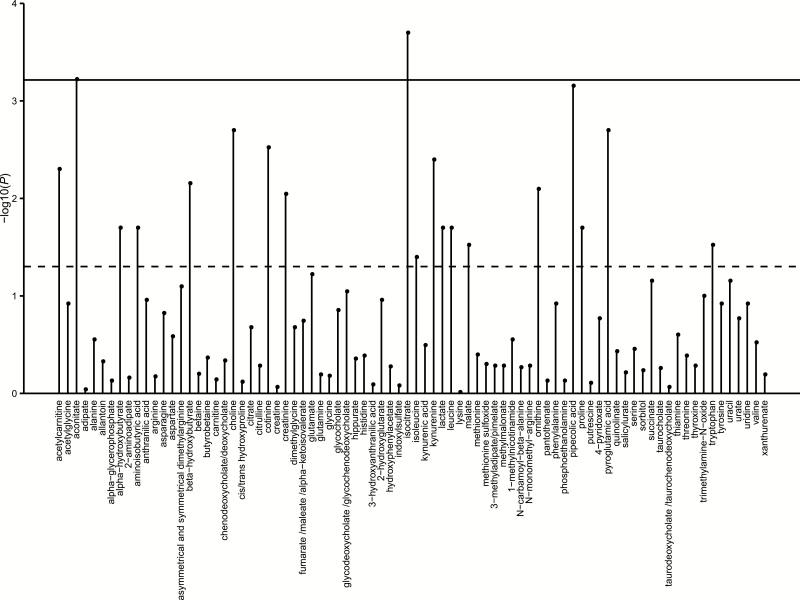
Characteristics of 484 pancreatic cancer case patients are shown in Table 1 and by cohort in Supplementary Table 2 (available online). The median time between blood collection and cancer diagnosis was 8.0 years. Among the case patients with known stage, 16.8% had localized disease, 28.7% had locally advanced disease, and 54.5% had metastatic disease. The median survival by cancer stage was 16 months for those with localized disease, 10 months for those with locally advanced disease, and three months for those with metastatic disease. By the end of follow-up, 448 (93%) patients were deceased. In Cox regression models, two metabolites were statistically significant to P values of .0006 or less, the predefined statistical significance threshold after Bonferroni correction (Figure 1; Supplementary Table 3, available online).
Table 1.
Baseline characteristics of patients with pancreatic cancer
| Characteristic* | Pancreatic cancer cases (n = 484) |
|---|---|
| Age at blood collection, Mean (SD), y | 63.3 (8.4) |
| Age at cancer diagnosis, mean (SD), y | 72.2 (8.0) |
| Female sex, No. (%) | 322 (66.5) |
| Race/ethnicity, No. (%) | |
| White | 430 (88.8) |
| Black | 16 (3.3) |
| Other | 14 (2.9) |
| Missing | 24 (5.0) |
| Body mass index, mean (SD), kg/m2 | 26.4 (4.8) |
| Physical activity, mean (SD), MET-h/wk | 20.2 (32.9) |
| History of diabetes mellitus, No. (%) | 28 (5.8) |
| Tobacco use, No. (%) | |
| Never | 206 (42.6) |
| Past | 216 (44.6) |
| Current | 58 (12.0) |
| Missing | 4 (0.8) |
| Alcohol (≥1 drink/day), No. (%) | 120 (24.8) |
| Median time from blood draw to cancer diagnosis, y | 8.0 |
| Fasting (≥ 8h) at blood collection, No. (%) | 355 (73.3) |
| Diagnosis period, No. (%) | |
| 1984–1995 | 45 (9.3) |
| 1996–2005 | 370 (76.4) |
| 2006–2010 | 69 (14.3) |
| Cancer stage, No. (%) | |
| Localized | 65 (13.4) |
| Locally advanced | 111 (22.9) |
| Metastatic | 211 (43.6) |
| Unknown | 97 (20.0) |
| Median survival time, mo | |
| All patients | 6 |
| By stage | |
| Localized | 16 |
| Locally advanced | 10 |
| Metastatic | 3 |
| Unknown | 6 |
* Continuous variables reported as mean (SD) and categorical variables reported as No. (%) at time of blood collection, unless otherwise noted.
Figure 1.
Association of prediagnostic levels of plasma metabolites with overall survival among pancreatic cancer case patients. Two-sided P value of the log-transformed metabolite as a continuous variable in Cox regression models adjusted for age at diagnosis (years, continuous), cohort (Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative; also adjusts for sex), race/ethnicity (white, black, other, missing), stage at diagnosis (localized, locally advanced, metastatic, unknown), fasting time (<4, 4–8, 8–12, ≥12 hours, missing), and year of diagnosis (1984–1995, 1996–2005, 2006–2010). Solid line indicates the statistically significant P value threshold after Bonferroni correction for multiple-hypothesis testing (P value ≤ .0006, .05/82). Dashed line indicates P value of .05.
The two statistically significant plasma metabolites after multiple-hypothesis correction were intermediates of the tricarboxylic acid (TCA) cycle: isocitrate and aconitate (Table 2). Compared to case patients in the bottom quintile, those in the top quintile had hazard ratios for death of 1.89 (95% CI = 1.06 to 3.35, P trend < .001) for isocitrate and 2.54 (95% CI = 1.42 to 4.54, P trend < .001) for aconitate, corresponding to a reduction in median survival of four to six months. Within the oxidative TCA cycle, isocitrate is generated from citrate with aconitate as an intermediate step, and plasma levels of isocitrate and aconitate were highly correlated in our patients (ρ = .82, P < .001) (Supplementary Table 4, available online). Thus, we examined the ratio of citrate to the sum of aconitate plus isocitrate and noted a hazard ratio for death of 0.46 (95% CI = 0.27 to 0.79, P trend < .001) comparing extreme quintiles (Table 2). No statistically significant effect modification was identified in stratified analyses by cohort, cancer stage, fasting status at blood collection, or BMI (Supplementary Table 5, available online).
Table 2.
Hazard ratios for death among pancreatic cancer case patients by prediagnostic levels of plasma metabolites
| Model | Quintile of plasma metabolite | P* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Isocitrate | ||||||
| Person-mo | 1078 | 1145 | 1341 | 1195 | 884 | |
| Cases/deaths | 96/92 | 97/89 | 97/89 | 97/84 | 97/94 | |
| Median survival, mo | 9 | 8 | 7 | 6 | 5 | |
| HR (95% CI)† | 1 | 1.16 (0.73 to 1.85) | 1.27 (0.72 to 2.23) | 1.61 (0.90 to 2.85) | 1.89 (1.06 to 3.35) | <.001 |
| HR (95% CI)‡ | 1 | 1.17 (0.73 to 1.86) | 1.38 (0.78 to 2.42) | 1.65 (0.93 to 2.94) | 1.96 (1.10 to 3.48) | <.001 |
| HR (95% CI)§ | 1 | 1.14 (0.71 to 1.82) | 1.30 (0.73 to 2.29) | 1.56 (0.87 to 2.80) | 1.77 (0.98 to 3.19) | .002 |
| Aconitate | ||||||
| Person-mo | 1113 | 1294 | 1195 | 1020 | 1021 | |
| Cases/deaths | 96/92 | 97/88 | 97/91 | 97/89 | 97/88 | |
| Median survival, mo | 11 | 8 | 6 | 5 | 5 | |
| HR (95% CI)† | 1 | 1.49 (0.93 to 2.37) | 2.15 (1.21 to 3.80) | 2.33 (1.31 to 4.14) | 2.54 (1.42 to 4.54) | <.001 |
| HR (95% CI)‡ | 1 | 1.53 (0.96 to 2.45) | 2.26 (1.27 to 4.01) | 2.39 (1.34 to 4.26) | 2.70 (1.50 to 4.86) | <.001 |
| HR (95% CI)§ | 1 | 1.47 (0.91 to 2.37) | 2.10 (1.17 to 3.77) | 2.25 (1.25 to 4.05) | 2.44 (1.33 to 4.48) | .003 |
| Citrate/(isocitrate+ aconitate) | ||||||
| Person-mo | 875 | 875 | 1460 | 1260 | 1173 | |
| Cases/deaths | 96/94 | 97/91 | 97/84 | 97/85 | 97/94 | |
| Median survival, mo | 5 | 5 | 6 | 9 | 9 | |
| HR (95% CI)† | 1 | 0.88 (0.65 to 1.19) | 0.69 (0.50 to 0.94) | 0.45 (0.32 to 0.64) | 0.46 (0.27 to 0.79) | <.001 |
| HR (95% CI)‡ | 1 | 0.90 (0.66 to 1.22) | 0.73 (0.53 to 1.00) | 0.45 (0.32 to 0.64) | 0.47 (0.27 to 0.80) | <.001 |
| HR (95% CI)§ | 1 | 0.90 (0.66 to 1.22) | 0.74 (0.54 to 1.02) | 0.48 (0.34 to 0.69) | 0.50 (0.29 to 0.87) | <.001 |
* P trend calculated by entering the log-transformed metabolite as a continuous variable in Cox regression models. All P values are two-sided. CI = confidence interval; HR = hazard ratio.
† Hazard ratios (95% confidence intervals) from Cox regression models adjusted for age at diagnosis (years, continuous), cohort (Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative; also adjusts for sex), race/ethnicity (white, black, other, missing), stage at diagnosis (localized, locally advanced, metastatic, unknown), fasting time (<4, 4–8, 8–12, ≥12 hours, missing), and year of diagnosis (1984–1995, 1996–2005, 2006–2010).
‡ Model further adjusted for time between blood collection and cancer diagnosis (0-<5, 5-<10, ≥10 years).
§ Model further adjusted for body mass index (World Health Organization categories) and history of diabetes (yes, no).
Because cancer diagnoses occurred at varying timepoints after blood collection, we performed secondary analyses adjusting for this time in our multivariable models and noted little change in the hazard ratios for death for isocitrate, aconitate, and their ratio with citrate (Table 2). We also examined survival among patients stratified by time between blood collection and cancer diagnosis. The strongest associations were seen among case patients with blood collected within 10 years of diagnosis (Table 3). Interestingly, it has been estimated that eight to 10 years elapse from the formation of the initial invasive founder cell within the pancreas to a patient’s diagnosis (34). Thus, we also analyzed the association of all metabolites with survival among the 305 case patients with blood collected within 10 years of diagnosis (Supplementary Table 6, available online). Aconitate (P trend < .001) and isocitrate (P trend < .001) remained strongly associated with survival, with proline also reaching statistical significance (P trend < .001) in this exploratory analysis. Further adjustment of multivariable models by BMI and diabetes history demonstrated similar results (Table 2).
Table 3.
Hazard ratios for death among pancreatic cancer case patients by prediagnostic levels of plasma metabolites stratified by time between blood collection and cancer diagnosis
| Blood collection to cancer diagnosis | HR (95% CI)* | ||||||
|---|---|---|---|---|---|---|---|
| Isocitrate | Aconitate | Citrate/(isocitrate+aconitate) | |||||
| No. of cases | Extreme quartiles | Per SD | Extreme quartiles | Per SD | Extreme quartiles | Per SD | |
| 0-<5 y | 143 | 2.32 (1.29 to 4.18) | 1.40 (1.11 to 1.78) | 2.73 (1.49 to 5.00) | 1.49 (1.15 to 1.93) | 0.26 (0.14 to 0.51) | 0.69 (0.55 to 0.88) |
| P† | .004 | .002 | .002 | ||||
| 5-<10 y | 162 | 2.32 (1.06 to 5.09) | 1.42 (1.04 to 1.94) | 2.45 (1.10 to 5.49) | 1.62 (1.18 to 2.24) | 0.48 (0.21 to 1.08) | 0.76 (0.57 to 1.02) |
| P† | .02 | .003 | .05 | ||||
| ≥10 y | 179 | 1.58 (0.71 to 3.50) | 1.14 (0.82 to 1.61) | 1.11 (0.48 to 2.56) | 0.98 (0.64 to 1.50) | 1.01 (0.47 to 2.17) | 0.87 (0.60 to 1.25) |
| P† | .28 | .87 | .38 | ||||
* Hazard ratios (95% confidence intervals) for the comparison of the fourth quartile to the first quartile (referent) or per standard deviation change of the metabolite from Cox regression models adjusted for age at diagnosis (years, continuous), cohort (Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative; also adjusts for sex), race/ethnicity (white, black, other, missing), stage at diagnosis (localized, locally advanced, metastatic, unknown), fasting time (<4, 4–8, 8–12, ≥12 hours, missing), and year of diagnosis (1984–1995, 1996–2005, 2006–2010). CI = confidence interval; HR = hazard ratio.
† P trend calculated by entering the log-transformed metabolite as a continuous variable in Cox regression models. All P values are two-sided.
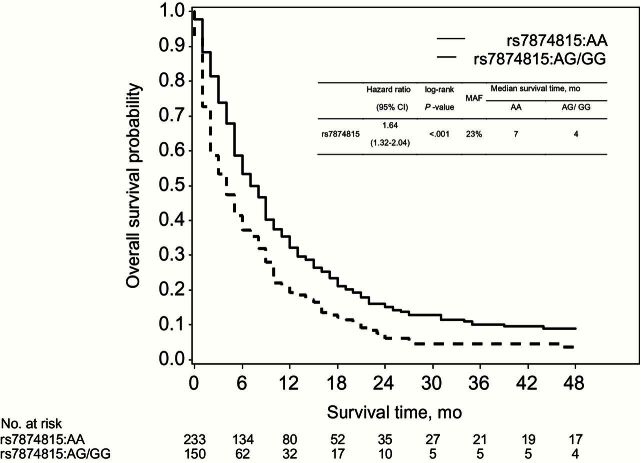
Within the cytosol of cells, the enzyme aconitase 1, encoded by ACO1, catalyzes interconversion of citrate and isocitrate via aconitate. Thus, we examined genotypic variation in ACO1 in relation to patient survival. Seven tagging SNPs in ACO1 were associated with survival to P values of less than .05 in an additive model of inheritance (Supplementary Table 7, available online). One SNP met the predefined statistical significance threshold after multiple-hypothesis correction, rs7874815, with a per-allele hazard ratio for death of 1.37 (95% CI = 1.16 to 1.61, P < .001) that was similar across cohorts (P heterogeneity = .26) (Supplementary Figure 2, available online). In a dominant model, the hazard ratio for death was 1.64 (95% CI = 1.32 to 2.04) comparing case patients with the AG/GG genotypes to those with the AA genotype with median overall survival times of four months and seven months, respectively (log-rank P < .001) (Figure 2). In linear regression models, we did not observe statistically significant associations between rs7874815 genotype and metabolite levels (Supplementary Table 8, available online). When considered together, patients with at least one minor allele at rs7874815 and higher levels of aconitate or isocitrate had an approximate three-fold hazard for death (Table 4), although P values for interaction were not statistically significant (P > .05). Using ACO1 genotypes generated in the PanScan GWAS in the same patient population, rs7874815 and several highly correlated SNPs remained most strongly associated with survival (Supplementary Figure 3, available online). Bioinformatic analyses for rs7874815 and highly correlated SNPs using HaploReg (30) demonstrated histone marks that tag enhancers in multiple tissue types, indicating possible regulatory elements in the region encompassing rs7874815 (Supplementary Table 9, available online). No statistically significant eQTLs were noted for these SNPs in MuTHER or blood eQTL databases (31–33). We also investigated genotypes at ACO2, which encodes mitochondrial aconitase, in the PanScan population (Supplementary Figure 4, available online). The SNP most statistically significantly associated with survival was rs112622778 (MAF = 0.05, per-allele HR = 1.98, 95% CI = 1.34 to 2.91, P < .001), intronic to ACO2.
Figure 2.
Overall survival among pancreatic cancer case patients by rs7874815 genotypes in ACO1. A table of the numbers of patients at risk in each group at various timepoints is given below the curves. Survival curves were generated by the Kaplan-Meier method, and the two-sided P value calculated using the log-rank test. CI = confidence interval; HR = hazard ratio; MAF = minor allele frequency.
Table 4.
Hazard ratios (95% CIs) for death among pancreatic cancer case patients by combined categories of plasma metabolite levels and rs7874815 genotypes in ACO1*
| Metabolite | Tertiles | rs7874815 | |
|---|---|---|---|
| AA HR (95% CI) | AG/GG HR (95% CI) | ||
| Isocitrate | 1 | 1.0 | 1.47 (1.00 to 2.16) |
| 2 | 1.31 (0.84 to 2.05) | 2.42 (1.50 to 3.91) | |
| 3 | 1.64 (1.05 to 2.58) | 2.72 (1.65 to 4.49) | |
| Aconitate | 1 | 1.0 | 1.67 (1.14 to 2.45) |
| 2 | 1.16 (0.73 to 1.83) | 1.74 (1.08 to 2.80) | |
| 3 | 1.44 (0.92 to 2.25) | 2.67 (1.62 to 4.39) | |
| Citrate/ (isocitrate + aconitate) | 3 | 1.0 | 1.62 (1.10 to 2.40) |
| 2 | 1.64 (1.05 to 2.56) | 2.33 (1.44 to 3.79) | |
| 1 | 1.83 (1.14 to 2.92) | 3.32 (2.02 to 5.47) | |
* Adjusted for age at diagnosis (years, continuous), cohort (Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative; also adjusts for sex), race/ethnicity (white, black, other, missing), stage at diagnosis (localized, locally advanced, metastatic, unknown), fasting time (<4, 4–8, 8–12, ≥12 hours, missing), and year of diagnosis (1984–1995, 1996–2005, 2006–2010). CI = confidence interval; HR = hazard ratio.
Three other TCA cycle intermediates were measured in our patients, but these circulating metabolites were not associated with survival, including fumarate (P trend = .18), malate (P trend = .03), and succinate (P trend = .07).
Discussion
Prior studies of altered metabolism and pancreatic cancer survival have demonstrated that obese patients (3,4) and those with diabetes (5–7) in the years before diagnosis have reduced survival. However, the association of circulating metabolites with survival has remained largely unexplored. In the current study, we identified associations of circulating intermediates of the TCA cycle and polymorphisms in Aconitase 1 with survival of patients with pancreatic cancer. The TCA cycle is a fundamental pathway of cellular metabolism comprised of eight enzyme-catalyzed reactions that provide reducing agents to drive mitochondrial ATP production and intermediates that can fuel anabolic pathways to produce lipids, nucleic acids, and proteins. Mutations in TCA cycle enzymes are known to promote cancer development and growth, including mutations in succinate dehydrogenase, fumarate hydratase, and isocitrate dehydrogenase 1 and 2 (10,35). Furthermore, these mutations have been associated with distinct cancer subsets with different patient prognoses (36,37) and measureable changes in levels of TCA cycle metabolites (38–40). Although mutations in genes encoding TCA cycle enzymes have not been a prominent feature of pancreatic adenocarcinoma, the importance of reprogrammed cellular metabolism has become increasingly apparent for this disease, including alterations that involve the TCA cycle and mutant KRAS-induced tumor cell dependencies for glucose, glutamine, and extracellular protein (11–15).
Aconitase functions as a TCA cycle enzyme, but it also plays a critical role in intracellular iron homeostasis and is alternatively known as iron regulatory protein-1 (IRP1). Depending on intracellular iron levels, it may function primarily as an aconitase enzyme or bind to iron-responsive elements within the untranslated regions of specific mRNA molecules, altering translation of proteins that regulate uptake, storage, and utilization of iron (41). Interestingly, these iron-regulatory functions of aconitase/IRP1 have also been linked to cancer development and progression (41,42). Additional studies will be required to determine whether the identified alterations in circulating TCA metabolites and ACO1 single-nucleotide variants reflect perturbations in one or both of these aconitase/IRP1 activities.
Median survival times were shorter by four to six months in patients with high levels of circulating aconitate and isocitrate. For context, two multi-agent chemotherapy programs have recently been adopted for treatment of patients with metastatic pancreatic cancer (43,44). These two chemotherapy regimens improved median overall survival by 1.8 months (gemcitabine plus nab-paclitaxel) and 4.3 months (FOLFIRNOX) in comparison with single-agent gemcitabine in patients with excellent functional status. Understanding the metabolic dependencies of cancer is a promising approach to identifying novel prognostic markers and therapeutic programs (45). The current study demonstrates that circulating metabolites may provide prognostic information in patients with pancreatic cancer, while focusing attention on enzymatic steps within the TCA cycle as potentially influencing disease progression.
Patients in this study were drawn from four prospective US cohorts. An important strength of a prospective cohort design is the ability to fully capture the spectrum of patients with pancreatic cancer in terms of disease aggressiveness and stage of disease, as individuals are enrolled prior to their diagnosis and are not identified at select tertiary care centers. Notably, survival times and stage distribution were highly similar to the 121 713 patients included in the National Cancer Data Base, which is thought to capture 76% of pancreatic cancer case patients diagnosed in the United States each year (41). Another important feature of a prospective cohort design is the collection of blood samples prior to cancer diagnosis, which limits metabolic changes induced by advanced disease and cancer-induced complications, such as an altered diet, biliary obstruction, and pancreatic insufficiency. Interestingly, the strongest associations of aconitate and isocitrate with survival were seen in patients with blood drawn within 10 years of cancer diagnosis, when subclinical invasive cancer is likely present (34). Whether these associations would be seen using blood samples collected at the time of cancer diagnosis requires further study. We rigorously piloted LC-MS methods in samples from our study subjects (21,22), removing metabolites unduly influenced by blood processing conditions or with poor reproducibility, which reduced the likelihood of false-negative results.
Limitations of the current study also require consideration. We did not collect systemic treatment information across the studies. Nevertheless, chemotherapy options are limited (2) and it is unlikely that chemotherapy differed by prediagnostic circulating metabolite levels, such that confounding by systemic treatment is not likely to have materially affected our results. We utilized overall mortality in our analyses, as opposed to pancreatic cancer–specific mortality. However, less than 5% of patients with pancreatic cancer are cured of their disease, such that almost all patients die from their cancer rather than other causes. We cannot rule out that our findings may be influenced by residual confounding by unmeasured factors. Nonetheless, we included relevant covariates in multivariable models and also noted survival associations with inherited variants within ACO1. We used strict Bonferroni corrections to define statistical significance. This approach to multiple-hypothesis testing correction reduces the likelihood of false-positive results but is likely to under-report statistically significant associations, particularly given that metabolite levels are correlated with one another and therefore not entirely independent tests. Our study participants were predominantly of European descent and further studies in other populations are warranted.
In nearly 500 patients with pancreatic cancer from four large US cohort studies, we found that elevated prediagnostic circulating levels of aconitate and isocitrate, two TCA cycle intermediates, were associated with an approximate two-fold increased hazard for death. Furthermore, survival was associated with tagging SNPs within ACO1, which encodes the enzyme that interconverts citrate, aconitate, and isocitrate. Taken together, these data identify circulating aconitate and isocitrate as novel prognostic markers in patients with pancreatic cancer and implicate altered metabolism related to the TCA cycle in pancreatic cancer progression.
Funding
HPFS is supported by National Institutes of Health (NIH) grant UM1 CA167552. NHS is supported by NIH grants UM1 CA186107, P01 CA87969, and R01 CA49449. PHS is supported by NIH grants CA 97193, CA 34944, CA 40360, HL 26490, and HL 34595. The Women’s Health Initiative (WHI) program is funded by the NIH through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221.
Additional support came from a Nestle Research Center award to the Broad Institute; from the Robert T. and Judith B. Hale Fund for Pancreatic Cancer, Perry S. Levy Fund for Gastrointestinal Cancer Research, Pappas Family Research Fund for Pancreatic Cancer, NIH R01 CA124908, and NIH P50 CA127003 to CSF; and from Department of Defense CA130288, Howard Hughes Medical Institute, Lustgarten Foundation, and Promises for Purple to BMW.
Supplementary Material
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors would like to thank the participants and staff of the HPFS, NHS, PHS, and WHI for their contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
References
- 1. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791):607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasenda B, Bass A, Koeberle D, et al. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer. 2014;14:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol. 2013;31(33):4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toriola AT, Stolzenberg-Solomon R, Dalidowitz L, et al. Diabetes and pancreatic cancer survival: a prospective cohort-based study. Br J Cancer. 2014;111(1):181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walter U, Kohlert T, Rahbari NN, et al. Impact of preoperative diabetes on long-term survival after curative resection of pancreatic adenocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21(4):1082–1089. [DOI] [PubMed] [Google Scholar]
- 7. Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morin A, Letouze E, Gimenez-Roqueplo AP, et al. Oncometabolites-driven tumorigenesis: From genetics to targeted therapy. Int J Cancer. 2014;135(10):2237–2248. [DOI] [PubMed] [Google Scholar]
- 11. Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baek G, Tse YF, Hu Z, et al. MCT4 Defines a Glycolytic Subtype of Pancreatic Cancer with Poor Prognosis and Unique Metabolic Dependencies. Cell Rep. 2014;9(6):2233–2249. [DOI] [PubMed] [Google Scholar]
- 13. Guillaumond F, Iovanna JL, Vasseur S. Pancreatic tumor cell metabolism: focus on glycolysis and its connected metabolic pathways. Arch Biochem Biophys. 2014;545:69–73. [DOI] [PubMed] [Google Scholar]
- 14. Sousa CM, Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35(7):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamphorst JJ, Nofal M, Commisso C, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–334. [DOI] [PubMed] [Google Scholar]
- 17. Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. [DOI] [PubMed] [Google Scholar]
- 18. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135. [DOI] [PubMed] [Google Scholar]
- 19. Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121. [DOI] [PubMed] [Google Scholar]
- 20. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. [DOI] [PubMed] [Google Scholar]
- 21. Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Townsend MK, Clish CB, Kraft P, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59(11):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang XR, Liang X, Pfeiffer RM, et al. Associations of 9p21 variants with cutaneous malignant melanoma, nevi, and pigmentation phenotypes in melanoma-prone families with and without CDKN2A mutations. Fam Cancer. 2010;9(4):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;46(9):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolpin BM, Ng K, Bao Y, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(6973):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286(12):1494–1497. [DOI] [PubMed] [Google Scholar]
- 28. Makuch RW. Adjusted survival curve estimation using covariates. J Chron Dis. 1982;35(6):437–443. [DOI] [PubMed] [Google Scholar]
- 29. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 30. Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grundberg E, Small KS, Hedman AK, et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet. 2012;44(10):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang TP, Beazley C, Montgomery SB, et al. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics. 2010;26(19):2474–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westra HJ, Peters MJ, Esko T, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Desideri E, Vegliante R, Ciriolo MR. Mitochondrial dysfunctions in cancer: genetic defects and oncogenic signaling impinging on TCA cycle activity. Cancer Lett. 2015;356(2 Pt A):217–223. [DOI] [PubMed] [Google Scholar]
- 36. Srinivasan R, Ricketts CJ, Sourbier C, et al. New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease. Clin Cancer Res. 2015;21(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borger DR, Goyal L, Yau T, et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin Cancer Res. 2014;20(7):1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janin M, Mylonas E, Saada V, et al. Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol. 2014;32(4):297–305. [DOI] [PubMed] [Google Scholar]
- 40. Richter S, Peitzsch M, Rapizzi E, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. 2014;99(10):3903–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkinson N, Pantopoulos K. The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol. 2014;5:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13(5):342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 44. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galluzzi L, Kepp O, Vander Heiden MG, et al. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.