Abstract
Inflammatory breast cancer (IBC) is rare and aggressive, with poor survival. While circulating tumor cells (CTCs) predict outcome in non-IBC patients, little data exists regarding their prognostic significance in IBC. This prospective study analyzed blood samples for CTCs from 63 stage III IBC patients to determine if CTCs present after primary systemic chemotherapy predicted relapse. CTC identification was not associated with tumor characteristics, lymph node positivity, or complete pathologic response to systemic therapy. At mean follow-up of 38 months, multivariable analysis demonstrated that detection of one or more CTCs predicted shortened relapse-free (log-rank P = 0.005, hazard ratio [HR] = 4.22, 95% confidence interval [CI] = 1.67 to 10.67, Cox P = 0.002) but not overall survival (log-rank P = 0.54, HR = 1.53, 95% CI = 0.41 to 5.79, Cox P = 0.53). All statistical tests were two-sided. In this study, CTCs after primary chemotherapy identified IBC patients at high risk for relapse.
Inflammatory breast cancer (IBC) is a rare (1%-6%), locally advanced breast carcinoma (LABC) with distinct features and poor prognosis (1,2). Compared with non-IBC LABC patients, IBC patients are more likely to be younger, with high-grade, estrogen receptor– (ER) and progesterone receptor (PR)–negative tumors (2–4). An IBC diagnosis is based on clinical criteria including an erythematous breast exhibiting “peau d’orange” (5,6). Pathologically, IBC is frequently characterized by tumor emboli within dermal lymphatics. IBC patients frequently have axillary node involvement and rapid progression (5).
IBC treatment guidelines were established in 2008 (7), including a multimodality approach of chemotherapy, surgery, and irradiation, improving five-year relapse-free survival (RFS) from 34% to 47% (8,9). Despite improvement, survival for IBC is clinically inferior to non-IBC LABC (10). Surveillance, Epidemiology, and End Results data report median survival for stage IIIB IBC at 2.9 vs 6.4 years for stage IIIB LABC patients (11). These statistics highlight the need for prognostic tools to identify IBC patients at high risk for relapse.
The poor outcome of IBC is likely due in part to dissemination of micrometastatic cancer cells. High-resolution imaging technologies are currently unable to detect micrometastases. Development of semi-automated systems, including CellSearch, has enabled clinicians and researchers to identify circulating tumor cells (CTCs) in peripheral blood and to demonstrate prognostic significance in metastatic (12,13) and nonmetastatic breast cancer patients (14–16). Detection of five or more CTCs per 7.5mL blood in metastatic (12,13) and one or more CTCs per 7.5mL blood in nonmetastatic patients (14–16) predicted worse relapse-free and overall survival (OS). Currently, little is known regarding the prognostic significance of CTCs in IBC patients.
This retrospective analysis of data collected in a prospective study analyzed blood for CTCs in 63 stage III IBC patients after primary systemic therapy (PST). Our goal was to determine if CTCs present after PST predicted relapse. All eligible patients with stage III IBC seen at The University of Texas MD Anderson Cancer Center from February 2005 through November 2014 were offered participation in this institutional review board–approved study (LAB-04-0698, PI: Lucci). Informed consent was obtained prior to blood collection. Patient characteristics and CTC results were blinded from investigators by use of a random numbering system. Bilateral breast cancer or other malignancy within five years of diagnosis of IBC rendered patients ineligible. All patients received PST, modified radical mastectomy, and postmastectomy radiation. Patients who developed distant metastatic disease or who became inoperable during their PST were excluded from the study. All patients with human epidermal growth factor receptor 2 (HER2)–positive tumors received trastuzumab or similar treatment.
Blood samples were collected after PST and before primary surgery. CTCs (per 7.5mL blood) were identified using the CellSearch System (Janssen), as described previously (14). CTCs were defined as nucleated cells lacking CD45 but expressing cytokeratins (CK) 8, 18, or 19. The presence of one or more CTCs meeting morphological criteria for malignancy was considered positive. Primary TNM staging and grade was designated according to the American Joint Commission on Cancer (17) and modified Black’s nuclear grading (18), respectively. Clinical stage was defined as TNM stage at primary diagnosis. Primary tumor estrogen and progesterone receptor status were evaluated with established immunostaining procedures (19). HER2-positive tumors had either strong (3+) immunohistochemical (IHC) staining or, when staining was moderate (2+), by fluorescence in situ hybridization result greater than 2. The endpoints were defined according to standardized definitions for efficacy end points (STEEP) criteria, with relapse-free survival (RFS) as the primary endpoint (20). Relapse-free survival was defined as the time elapsed between the date of diagnosis and the date of first evidence of regional invasive recurrence, distant metastasis, or death. Chi-square, Fisher’s exact, and log-rank tests were employed to detect differences between groups. P values of less than .05 were considered statistically significant, and all statistical tests were two-sided. The Monte Carlo permutation test was used to confirm log-rank and Cox regression statistical significance (21). Using the identity function for time variable, the test for proportionality of hazards was performed for multivariate Cox regression. Both the global test and the covariate-specific test for proportionality of hazards were insignificant.
Mean follow-up was 38 months (range = 1–92), average body mass index was 30.7kg/m2 (range = 19.7–48.4), and mean age was 52 years (range = 23–71). Twenty-two patients (34.9%) were premenopausal, 41 (65.0%) had grade 3 tumors, and 58 (92.1%) had lymph node metastases. Nineteen patients (30.2%) had ER-positive tumors, and 21 (33.3%) had HER2/neu overexpression/amplification. Sixteen of 62 patients (25.8%) had pathologic complete response (pCR, defined as absence of invasive tumor in breast and lymph nodes) following PST. One or more CTCs was identified in 27% of patients following four cycles of PST, which is higher than the PST (post-4 cycle) rates reported in the Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-1 and BEVERLY-2) trials. In the BEVERLY-1 trial, which included 92 nonmetastatic HER2/neu-negative IBC patients, the baseline (pretherapy) CTC positivity rate was 40%; this rate dropped to 6% before cycle 5 of PST. The BEVERLY-2 trial included 52 nonmetastatic, HER2/neu-positive IBC patients and reported a 35% baseline (pretherapy) positivity rate; this rate dropped to 13% before cycle 5 of PST (22). The lower positivity rates following the four cycles of PST reported in the BEVERLY trials might be a result of the therapy administered, which included fluorouracil, epirubicin, cyclophosphamide, and bevacizumab. CTC identification was not associated with menopausal status, high tumor grade, lymph node involvement, ER, PR or HER2/neu status, or pCR, which is in agreement with the BEVERLY-2 trial (22). In addition, CTC identification was not associated with high body mass index (>25kg/m2).
Twenty-three patients relapsed; nine patients had only regional invasive recurrences, nine had only distant metastases, and five experienced both regional invasive recurrences and distant metastases. Sites of distant metastases included brain (5), bone (3), and lung (1). Twelve patients died, and all deaths were breast cancer specific. Univariate analyses of relapse-free and overall survival associated with presence of CTCs are provided in Supplementary Table 1 (available online). On multivariable analysis (Table 1), the detection of one or more CTCs independently predicted shortened RFS (log-rank P = 0.005, hazard ratio [HR] = 4.22, 95% confidence interval [CI] = 1.67 to 10.67, Cox P = 0.002 (two sided Wald)) after adjusting for lymph node status, high grade, ER, PR, HER2/neu status, and high body mass index. Pathologic complete response was inversely associated with relapse (HR = 0.08, 95% CI = 0.01 to 0.66, P = 0.02). Ten of 17 (58.8%) CTC-positive patients relapsed compared with 13 of 46 (28.3%) who had no CTCs. The four-year RFS rate was lower (32.3%) in the CTC-positive group than in those with no CTCs (64.5%) (Figure 1A). As the number of CTCs increased, so did relapse hazard ratios. All (4/4) of the patients with two or more CTCs relapsed vs 19 of 59 (32.2%) patients with fewer than two CTCs. All patients with two or more CTCs exhibited progression at four years compared with 59.9% of patients who had no CTCs. Three patients had three or more CTCs and all of these patients relapsed, while 58.6% of patients with fewer than three CTCs demonstrated RFS at four years. Permutation testing at 104 repetitions confirmed statistical significance of the Cox regression and log-rank results.
Table 1.
Cox regression multivariable analyses of survival associated with presence of CTCs
| Outcome | RFS | OS | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P* |
P
permutation test† |
HR (95% CI) | P* |
P
permutation test† |
|
| Pathologic node negative vs | .60 | -- | .84 | -- | ||
| 1–3 lymph nodes | 1.28 (0.14 to 11.41) | -- | -- | 0.68 (0.06 to 7.88) | -- | -- |
| >3 lymph nodes | 1.76 (0.21 to 14.80) | -- | -- | 1.26 (0.13 to 12.18) | -- | -- |
| Histological high grade | 1.53 (0.42 to 5.50) | .52 | -- | 2.14 (0.37 to 12.45) | .40 | -- |
| ER-positive | 1.98 (0.49 to 7.98) | .34 | -- | 1.54 (0.27 to 8.94) | .63 | -- |
| PR-positive | 0.37 (0.08 to 1.75) | .21 | -- | 0.74 (0.12 to 4.56) | .74 | -- |
| HER2-positive | 0.82 (0.33 to 2.05) | .67 | -- | 0.38 (0.09 to 1.73) | .21 | -- |
| Pathological complete response | 0.08 (0.01 to 0.66) | .02 | -- | 0.29 (0.03 to 2.70) | .27 | -- |
| BMI > 25kg/m2 | 1.36 (0.51 to 3.61) | .53 | -- | 1.01 (0.29 to 3.48) | .98 | -- |
| One or more circulating tumor cells | 4.22 (1.67 to 10.67) | .002 | .004 | 1.53 (0.41 to 5.79) | .53 | .30 |
* Two-sided Wald’s test. BMI = body mass index; CI = confidence interval; CTC = circulating tumor cell; ER = estrogen receptor; HER2 = human epithelial growth factor receptor 2; HR = hazard ratio; OS = overall survival; PR = progesterone receptor; RFS = relapse-free survival.
† Two-sided Monte Carlo permutation test.
Figure 1.
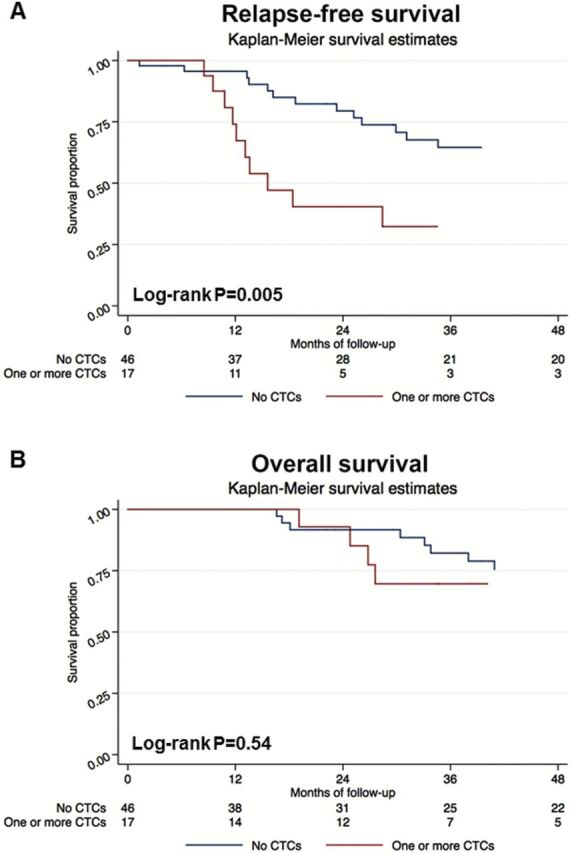
Kaplan-Meier survival estimates of probabilities of relapse-free survival (RFS) and overall survival (OS) according to circulating tumor cells in operable inflammatory breast cancer. A) The probability of RFS in patients with circulating tumor cell (CTC) count ≥ 1 is shown (hazard ratio [HR] = 4.22, 95% confidence interval [CI] = 1.67 to 10.67, log-rank P = 0.005). B) The probability of OS in patients with CTC count ≥ 1 is shown (HR = 1.53, 95% CI = 0.41 to 5.79, log-rank P = 0.54). All statistical tests were two-sided. CTC = circulating tumor cell.
One limitation of our data regarding increased relapse risk with increasing CTC numbers remains because of small numbers of patients who had multiple CTCs in this study. Confirmation of these results will require larger future studies. Using multivariable analysis, statistically significant differences in OS were not identified between patients who had at least one CTC compared with patients who had no CTCs (log-rank P = 0.54, HR = 1.53, 95% CI = 0.41 to 5.79, Cox P = 0.53) (Table 1). The four-year OS rate was 69.6% (95% CI = 48.7% to 99.6%) in this group vs 75.4% (95% CI = 61.9% to 91.9%) in patients with no CTCs (Figure 1B).
Studies investigating prognostic significance of CTCs in IBC are limited. A 2009 study reported that metastatic IBC patients had lower prevalence and fewer CTCs compared with metastatic non-IBC patients (23), and survival for patients with more than five CTCs was no different than those with five or fewer CTCs. In our study, the CTC identification rate (27.0%) was not substantially different than rates (21.5% to 31%) reported for non-IBC patients (14- 16,19). Outcomes data from the BEVERLY-1 and -2 trials will provide insight with respect to the prognostic significance of lower CTC positivity rates following PST regimens that include fluorouracil, epirubicin, cyclophosphamide, and bevacizumab in nonmetastatic IBC patients.
In this study, we found that only two factors present after PST, achievement of a pCR or presence of CTCs, predicted relapse. Because we observed no association between pCR and CTCs, the additional information provided by CTC status would be useful for designing clinical trials focused on patients at high risk for relapse who might benefit from additional adjuvant therapies. Our data illustrate the need for additional IBC studies to provide better understanding of mechanisms underlying the aggressive metastatic potential of IBC.
Funding
This work was supported by The University of Texas Morgan Welch Inflammatory Breast Cancer Research Program and Clinic.
Supplementary Material
The funder source had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
References
- 1. Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992;10 (6):1014–1024. [DOI] [PubMed] [Google Scholar]
- 2. Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol. 2003;21 (12):2254–2259. [DOI] [PubMed] [Google Scholar]
- 3. Parton M, Dowsett M, Ashley S, et al. High incidence of HER-2 positivity in inflammatory breast cancer. Breast. 2004;13 (2):97–103. [DOI] [PubMed] [Google Scholar]
- 4. Sawaki M, Ito Y, Akiyama F, et al. High prevalence of HER-2/neu and p53 overexpression in inflammatory breast cancer. Breast Cancer. 2006;13 (2):172–178. [DOI] [PubMed] [Google Scholar]
- 5. Cristofanilli M, Buzdar AU, Hortobagyi GN. Update on the management of inflammatory breast cancer. Oncologist. 2003;8 (2):141–148. [DOI] [PubMed] [Google Scholar]
- 6. Haagensen. Inflammatory carcinoma. Diseases of the breast. 2 ed Philadelphia (PA): Saunders; 1971. [Google Scholar]
- 7. Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22 (3):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. [DOI] [PubMed] [Google Scholar]
- 9. Masuda H, Brewer TM, Liu DD, et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann Oncol. 2014;25 (2):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Low JA, Berman AW, Steinberg SM, et al. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22 (20):4067–4074. [DOI] [PubMed] [Google Scholar]
- 11. Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97 (13):966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351 (8):781–791. [DOI] [PubMed] [Google Scholar]
- 13. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224. [DOI] [PubMed] [Google Scholar]
- 14. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13 (7):688–695. [DOI] [PubMed] [Google Scholar]
- 15. Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14 (21):7004–7010. [DOI] [PubMed] [Google Scholar]
- 16. Rack B, Schindlbeck C, Juckstock J, et al. Circulating Tumor Cells Predict Survival in Early Average-to-High Risk Breast Cancer Patients. J Natl Cancer Inst. 2014;106 (5):dju066 doi:10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. AJCC Cancer Staging Manual. 7 ed New York, NY: Springer; 2010. [Google Scholar]
- 18. Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105 (1):97–102. [PubMed] [Google Scholar]
- 19. Krishnamurthy S, Cristofanilli M, Singh B, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116 (14):3330–3337. [DOI] [PubMed] [Google Scholar]
- 20. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25 (15):2127–2132. [DOI] [PubMed] [Google Scholar]
- 21. Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Society. 1943;54(1–3):426–482. [Google Scholar]
- 22. Pierga JY, Petit T, Delozier T, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 2012;13 (4):375–384. [DOI] [PubMed] [Google Scholar]
- 23. Mego M, De Giorgi U, Hsu L, et al. Circulating tumor cells in metastatic inflammatory breast cancer. Ann Oncol. 2009;20 (11):1824–1828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


