Abstract
Background:
Electric power morcellation during laparoscopic hysterectomy allows some women to undergo minimally invasive surgery but may disrupt underlying occult malignancies and increase the risk of tumor dissemination.
Methods:
We developed a state transition Markov cohort simulation model of the risks and benefits of hysterectomy (abdominal, laparoscopic, and laparoscopic with electric power morcellation) for women with presumed benign gynecologic disease. The model considered perioperative morbidity, mortality, risk of cancer and dissemination, and outcomes in women with an underlying malignancy. We explored the effectiveness from a societal perspective stratified by age (<40, 40–49, 50–59, and ≥60 years).
Results:
Under all scenarios, modeled laparoscopic hysterectomy without morcellation was the most beneficial strategy. Laparoscopic hysterectomy with morcellation was associated with 80.83 more intraoperative complications, 199.64 fewer perioperative complications, and 241.80 fewer readmissions than abdominal hysterectomy per 10 000 women. Per 10 000 women younger than age 40 years, laparoscopic hysterectomy with morcellation was associated with 1.57 more cases of disseminated cancer and 0.97 fewer deaths than abdominal hysterectomy. The excess cases of disseminated cancer per 10 000 women with morcellation compared with abdominal hysterectomy increased with age to 47.54 per 10 000 in women age 60 years and older. Compared with abdominal hysterectomy, this resulted in 0.30 (age 40–49 years), 5.07 (age 50–59 years), and 18.14 (age 60 years and older) excess deaths per 10 000 women in the respective age groups.
Conclusion:
Laparoscopic hysterectomy without morcellation is the most beneficial approach of the three methods of hysterectomy studied. In older women, the risks of electric power morcellation may outweigh the benefits of minimally invasive hysterectomy.
Minimally invasive hysterectomy, removal of the uterus either through laparoscopic or robotic assistance, is now commonly performed in women. Minimally invasive hysterectomy is performed using several small abdominal incisions as opposed to the larger incision required when laparotomy, or open, abdominal hysterectomy is undertaken. One challenge of minimally invasive hysterectomy, however, is that the uterus cannot be removed through the small abdominal incisions that are utilized. Most commonly, the uterus is removed intact through the vagina. Alternatively, the uterus can be fragmented into smaller pieces and removed through the small incisions used for minimally invasive hysterectomy, a procedure known as morcellation.
Use of electromechanical devices, known as electric power morcellators, is a frequently used approach for morcellation. The use of electric power morcellation has come under scrutiny since the recognition that occult uterine malignancies may be present in nine to 100 per 10 000 women who undergo power morcellation (1–4). Inadvertent morcellation of uterine malignancies may increase the risk of tumor dissemination and compromise survival (5–8).
Despite the potential risks of electric power morcellation, use of power morcellators may allow for removal of the uterus through a minimally invasive surgical approach in women who would otherwise require laparotomy (9,10). Compared with laparotomy, minimally invasive hysterectomy is associated with a number of benefits, including decreased perioperative morbidity, less pain, and an earlier return to normal activities (11,12). From a societal perspective, eliminating the use of electric power morcellation may increase the rate of laparotomy for hysterectomy and thus also be associated with adverse health consequences for women who require hysterectomy.
Given the uncertainty surrounding the use of electric power morcellation, we developed a computer simulation model of the risks and benefits of the procedure. Specifically, we estimated the morbidity, mortality, quality of life, and cost implications associated with three modalities of hysterectomy in women undergoing uterine removal for presumed benign gynecologic diseases.
Methods
Analytic Framework and Model Structure
Our model is a computer-simulation state-transition Markov model of the risks and benefits of hysterectomy in women age 18 to 65 years (Supplemental Materials, available online). The base-case analysis represents women requiring hysterectomy without a preoperative diagnosis of cancer and compares three modalities of hysterectomy: 1) total abdominal, 2) laparoscopic (either total laparoscopic or laparoscopic-assisted vaginal), or 3) laparoscopic with use of electric power morcellation to facilitate removal of the uterus. As the prevalence of cancer is highly dependent on age, all models were stratified into the following age groups: younger than 40, 40 to 49, 50 to 59, and 60 years and older (4).
The model structure consisted of three components: perioperative morbidity and mortality, risk of cancer and dissemination, and outcomes of cancer in women with an underlying malignancy. The perioperative phase of the model included complications, resource utilization, readmission, and death within six weeks of surgery. Patients were assumed to have recovered from surgery by six weeks postoperatively.
Although women underwent hysterectomy for presumed benign gynecologic disease, there is an underlying risk of occult malignancy (1,2,4,5,7). Our base-case analysis was predicated on a woman who could have undergone any one of three types of hysterectomy, so we therefore assumed that the underlying risk of cancer was similar for all modalities of hysterectomy. For women with underlying invasive cancer, we assumed that the neoplasms were a mix of epithelial endometrial tumors and uterine sarcomas (4). For women who underwent either a total abdominal or laparoscopic hysterectomy, we assumed that the uterus was removed intact without tumor disruption. In contrast, among women who underwent laparoscopic hysterectomy with electric power morcellation, patients with an underlying malignancy were at risk for dissemination (7). Separate estimates were used for the risk of dissemination for endometrial tumors and uterine sarcomas.
The third component of the model included care of patients diagnosed with an invasive cancer. Further therapy, cost, and outcome were based on age and stage at the time of diagnosis (13). Patients with tumor dissemination due to electric power morcellation were classified as stage IV (metastatic) tumors. Separate estimates were developed for epithelial endometrial tumors and uterine sarcomas. Women with smooth muscle tumors of indeterminate behavior, neoplasms with worrisome histologic features but that do not meet the criteria for classification as sarcomas, are also at risk for recurrence (14). At the time of recurrence, most women require repeat surgical exploration and resection. These tumors may recur as either smooth muscle tumors of uncertain malignant potential or sarcomas. Women with tumors that recur as a sarcoma were assumed to have metastatic disease and classified as stage IV sarcomas (13).
Modeling Approach and Parameter Estimates
Clinical, epidemiologic, and oncologic data were drawn from available literature and cancer registry data. Complications analyzed included intraoperative injuries, perioperative complications, and resource utilization (transfusion, readmission, length of stay, and return to work), as defined in a recent Cochrane review of hysterectomy for benign gynecologic diseases (11,12,15–34). As studies specifically evaluating the morbidity associated with laparoscopic hysterectomy with electric power morcellation are lacking, we assumed that morbidity was similar to those of women who underwent laparoscopic hysterectomy. We utilized data from large observational studies and registries to estimate the risk of perioperative death for each modality of hysterectomy (35–41).
Estimates of cancer incidence, age-specific stage distributions, and stage and age-specific survival are derived from the Surveillance, Epidemiology, and End Results (SEER) database (13). For women who underwent laparoscopic hysterectomy with electric power morcellation, we assumed a risk of tumor dissemination for patients with occult stage I-III neoplasms. The risk of clinically significant tumor dissemination was estimated as 20.0% for patients with epithelial endometrial tumors and 28.6% for women with uterine sarcomas (7). Survival was calculated at yearly intervals until year 5 after diagnosis.
For women with smooth muscle tumors of indeterminate potential, we estimated a recurrence rate of 7.3%, with 33.3% recurring as sarcomas and 66.7% as tumors of uncertain malignant potential (14). Age- and stage-specific survival estimates for women with sarcomas were derived as described above. All patients who recurred with tumors of indeterminate potential were assumed to have survived.
Cost-Effectiveness Analysis
We estimated costs, changes in life-years, and changes in quality-adjusted life-years (QALYs). Quality-of-life weights (utilities) were applied to postsurgery health states and cancer outcomes based upon the available literature (42–51). The base cost of each type of hysterectomy and the cost of perioperative complications were estimated from published reports (Supplementary Tables, available online). Stage-specific costs of cancer care are estimated as incremental costs compared with age-matched subjects without cancer. The cost of cancer care was analyzed based on the phases-of-care model (first year after diagnosis, continuing care, and last year of life), as previously reported (52–54). All costs and effectiveness were discounted by 3% annually and reported in 2013 US dollars (55).
Compared with abdominal hysterectomy, we projected the difference in cost, change in life-years, change in QALYs, and incremental cost-effectiveness ratios (ICERs) for laparoscopic hysterectomy with electric power morcellation. We report outcomes per 10 000 women. All analyses were from a societal perspective that incorporates healthcare costs and loss of labor costs.
Sensitivity Analyses
Probabilistic sensitivity analyses were used to simultaneously sample from multiple parameter estimates in order to assess the uncertainty around our base case assumptions. We performed 1000 simulations and reported the mean and 95% confidence intervals and interquartile ranges for ICERs.
In addition, we conducted a series of scenario analyses in which underlying assumptions of the risk and behavior of the occult malignancy were varied. First, we developed a simulation in which the risk of malignancy was reduced by 25%, and, alternatively, increased by 25% for each age group. A second scenario analysis varied the risk of tumor dissemination. We estimated outcomes if the risk of dissemination was increased to 40% in women with endometrial tumors and 60% for uterine sarcomas. We then changed the risk of dissemination to 10% for both tumor types. A third analysis was undertaken, in which we assumed that 75% of the tumors were sarcomas and 25% were epithelial endometrial tumors.
Results
Across all scenarios modeled, laparoscopic hysterectomy was the least costly and most effective modality of hysterectomy (Table 1). Per 10 000 women, compared with laparoscopic hysterectomy, laparoscopic hysterectomy with morcellation was more costly, associated with decreased quality of life, and lower overall life-years. Similarly, laparoscopic hysterectomy was more favorable than total abdominal hysterectomy for all parameters.
Table 1.
Cost and effectiveness of laparoscopic hysterectomy with morcellation compared with laparoscopic hysterectomy and total abdominal hysterectomy compared to laparoscopic hysterectomy*
| Age group, y | Rate per 10 000 patients | ||||
|---|---|---|---|---|---|
| Incremental cost, millions USD Estimate (95% CI) |
Change in QALYs No. (95% CI) |
QALY ICER Median (IQR) |
Change in life-years No. (95% CI) |
Life-years ICER Median (IQR) |
|
| Laparoscopic hysterectomy with morcellation vs laparoscopic hysterectomy | |||||
| <40 | 2.61 (-31.95 to 34.70) | -1.76 (-87.58 to 83.41) | -626.47 (-386528.87–399518.89) | -3.67 (-9.35 to 2.08) | -537720.25 (-3929056.58–2590720.86) |
| 40–49 | 3.20 (-27.96 to 36.67) | -6.82 (-89.27 to 80.06) | -25037.43 (-408760.17–349304.56) | -8.63 (-14.93 to -2.08) | -389632.54 (-1680275.23–853283.30) |
| 50–59 | 4.36 (-28.02 to 38.53) | -25.52 (-114.06 to 67.91) | -57142.86 (-382461.94–273648.88) | -28.79 (-43.07 to -17.85) | -152135.62 (-546337.12–272979.01) |
| ≥60 | 10.31 (-24.83 to 44.32) | -73.50 (-183.38 to 34.47) | -108762.86 (-304964.60–33435.88) | -99.28 (-136.51 to -67.60) | -101865.72 (-223600.75–9610.66) |
| Total abdominal hysterectomy vs laparoscopic hysterectomy | |||||
| <40 | 16.41 (-25.19 to 55.91) | -16.62 (-100.20 to 78.06) | -126147.64 (-596032.94–417811.52) | -8.16 (-11.75 to -3.78) | -2002608.19 (-3675576.93– -360600.93) |
| 40–49 | 17.59 (-17.74 to 55.92) | -18.04 (-109.50 to 74.26) | -119801.95 (-614346.21–368115.51) | -8.16 (-11.75 to -3.78) | -2069758.12 (-3777693.91– -561055.35) |
| 50–59 | 17.74 (-17.79 to 55.75) | -18.38 (-108.59 to 76.41) | -154333.50 (-624948.07–368908.40) | -8.15 (-11.73 to -3.77) | -2053237.71 (-3846839.11– -566830.81) |
| ≥60 | 17.14 (-21.17 to 56.69) | -15.05 (-119.05 to 97.64) | -106121.92 (-524029.50–346190.69) | -8.09 (-11.67 to -3.75) | -2042317.71 (-3793868.91– -514003.25) |
* CI = confidence interval; ICER = incremental cost-effectiveness ratio; IQR = interquartile range; QALY = quality-adjusted life-year.
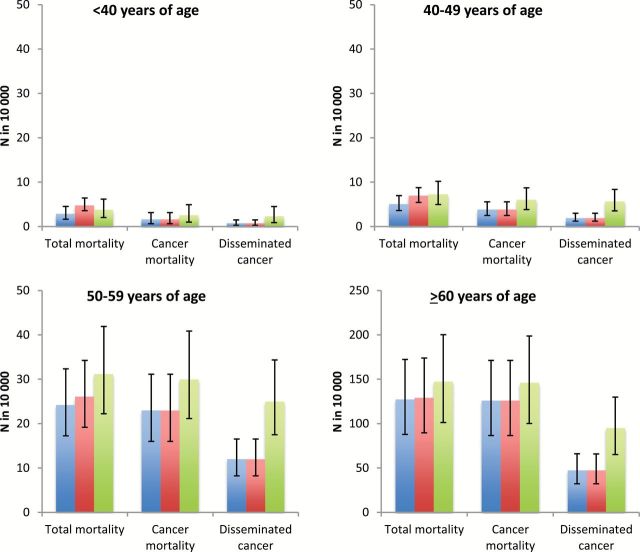
As laparoscopic hysterectomy is often not technically feasible, further comparisons were made between abdominal hysterectomy and laparoscopic hysterectomy with morcellation. Per 10 000 women, laparoscopic hysterectomy with morcellation was associated with 80.83 (95% confidence interval [CI] = 21.35 to 138.03) more intraoperative complications, 199.64 (95% CI = -301.70 to -107.52) fewer perioperative complications, and 241.80 (95% CI = -257.50 to -227.25) fewer readmissions than abdominal hysterectomy. Figure 1 displays estimates of mortality and the risk of disseminated cancer for each type of hysterectomy, stratified by age.
Figure 1.
Risk of cancer dissemination and mortality stratified by modality of hysterectomy and age at the time of surgery. Blue line represents laparoscopic hysterectomy, red line abdominal hysterectomy, and green line laparoscopic hysterectomy with morcellation.
Per 10 000 women younger than age 40 years, laparoscopic hysterectomy with morcellation was associated with 1.57 more cases of disseminated cancer and 0.94 more cancer-associated deaths, but 0.97 fewer overall deaths than abdominal hysterectomy (Table 2). The excess cases of disseminated cancer per 10 000 women with morcellation increased to 3.75 in women age 40 to 49 years, 12.97 in those age 50 to 59 years, and 47.54 in women age 60 years and older. Compared with abdominal hysterectomy, this translated into 0.30, 5.07, and 18.14 excess deaths per 10 000 women in the respective age groups.
Table 2.
Projected estimates of cancer dissemination and perioperative mortality in women who undergo laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy
| Age group, y | Rate per 10 000 patients | ||
|---|---|---|---|
| Total mortality Estimate (95% CI) |
Cancer-associated mortality Estimate (95% CI) |
Disseminated cancer Estimate (95% CI) |
|
| Main simulation | |||
| <40 | -0.97 (-2.01 to 0.30) | 0.94 (0.36 to 1.85) | 1.57 (0.61 to 2.99) |
| 40–49 | 0.30 (-1.02 to 1.97) | 2.21 (1.20 to 3.74) | 3.75 (2.30 to 5.75) |
| 50–59 | 5.07 (2.45 to 8.71) | 6.99 (4.48 to 10.51) | 12.97 (8.87 to 18.20) |
| ≥60 | 18.14 (11.92 to 25.43) | 20.05 (13.56 to 27.59) | 47.54 (32.89 to 64.91) |
| Sensitivity analyses | |||
| Higher prevalence of cancer* | |||
| <40 | -0.77 (-1.87 to 0.59) | 1.14 (0.44 to 2.21) | 1.92 (0.74 to 3.65) |
| 40–49 | 0.65 (-0.73 to 2.42) | 2.56 (1.47 to 4.17) | 4.44 (2.78 to 6.62) |
| 50–59 | 6.46 (3.45 to 10.37) | 8.37 (5.51 to 12.19) | 15.82 (10.95 to 22.01) |
| ≥60 | 23.01 (15.26 to 32.21) | 24.93 (16.87 to 34.17) | 59.27 (41.03 to 80.88) |
| Lower prevalence of cancer† | |||
| <40 | -1.17 (-2.14 to -0.05) | 0.74 (0.27 to 1.46) | 1.22 (0.48 to 2.36) |
| 40–49 | -0.05 (-1.32 to 1.55) | 1.86 (0.94 to 3.30) | 3.05 (1.79 to 4.86) |
| 50–59 | 3.69 (1.41 to 7.06) | 5.60 (3.43 to 8.90) | 10.12 (6.74 to 14.54) |
| ≥60 | 13.27 (8.44 to 18.96) | 15.18 (10.25 to 20.94) | 35.81 (24.80 to 49.16) |
| Higher rate of tumor dissemination‡ | |||
| <40 | -0.14 (-1.52 to 1.69) | 1.77 (0.69 to 3.47) | 3.04 (1.16 to 5.79) |
| 40–49 | 1.77 (0.18 to 3.87) | 3.69 (2.27 to 5.55) | 6.68 (4.32 to 9.56) |
| 50–59 | 10.84 (6.72 to 16.16) | 12.75 (8.71 to 17.89) | 24.92 (17.58 to 33.90) |
| ≥60 | 38.15 (25.66 to 52.80) | 40.06 (27.20 to 54.67) | 96.78 (67.08 to 131.96) |
| Lower rate of tumor dissemination§ | |||
| <40 | -1.43 (-2.33 to -0.37) | 0.48 (0.17 to 1.06) | 0.75 (0.28 to 1.51) |
| 40–49 | -0.51 (-1.73 to 1.07) | 1.40 (0.58 to 2.76) | 2.13 (1.07 to 3.77) |
| 50–59 | 1.96 (0.06 to 5.02) | 3.87 (2.17 to 6.72) | 6.42 (4.05 to 10.01) |
| ≥60 | 7.58 (4.34 to 11.71) | 9.49 (6.38 to 13.54) | 20.52 (14.06 to 28.24) |
| Higher proportion of sarcomas‖ | |||
| <40 | -0.93 (-1.98 to 0.34) | 0.98 (0.39 to 1.94) | 1.64 (0.64 to 3.12) |
| 40–49 | 0.32 (-1.02 to 2.04) | 2.23 (1.22 to 3.77) | 3.87 (2.39 to 5.92) |
| 50–59 | 4.59 (2.02 to 8.17) | 6.50 (4.09 to 9.80) | 12.91 (8.81 to 18.12) |
| ≥60 | 13.91 (8.66 to 20.27) | 15.83 (10.50 to 22.04) | 47.78 (32.96 to 65.65) |
* Prevalence of cancer increased by 25%.
† Prevalence of cancer reduced by 25%.
‡ Risk of dissemination assumed to be 40% in women with endometrial tumors and 60% in women with sarcomas.
§ Risk of dissemination assumed to be 10% in women with either endometrial tumor or uterine sarcomas.
‖ Risk of underlying tumors assumed to be sarcomas in 75% and endometrial tumors in 25%.
Sensitivity Analyses
If the prevalence of cancer was increased by 25% for each age group, the mortality trends remained similar to the base case model. However, if the prevalence of cancer was reduced by 25%, in the age 40 to 49 years cohort, laparoscopic hysterectomy with morcellation became the more favorable modality with 0.05 fewer deaths per 10 000 women. Similarly, if the rate of tumor dissemination was increased, the mortality trends were similar to the base case analysis; however, if the risk of tumor dissemination was only 10% in those that underwent morcellation, laparoscopic hysterectomy with morcellation becomes a more favorable strategy in women age 40 to 49 years (0.51 fewer deaths per 10 000 women than abdominal hysterectomy). In a scenario analysis in which it was assumed that 75% of occult tumors were sarcomas, laparoscopic hysterectomy with morcellation remained more favorable than abdominal hysterectomy in women younger than age 40 years (-0.93 deaths per 10 000 compared with abdominal hysterectomy), while abdominal hysterectomy was associated with fewer deaths in the other three age strata.
Cost-Effectiveness
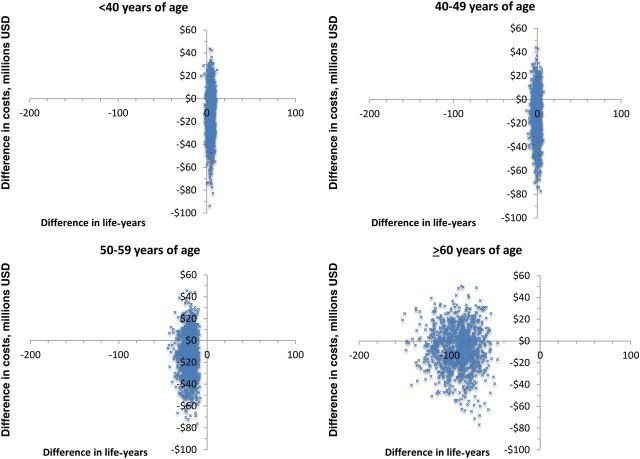
For the base case scenarios, use of laparoscopic hysterectomy with morcellation was less costly than abdominal hysterectomy for all age strata (Table 3). For every 10 000 women, use of laparoscopic hysterectomy with morcellation would result in an increase of 4.49 life-years for women younger than age 40 years, but was associated with 0.47, 20.64, and 91.19 fewer life years for women age 40 to 49, 50 to 59, and older than 60 years, respectively. Figure 2 displays the cost-effectiveness plane (cost vs life-years) for laparoscopic hysterectomy with morcellation from 1000 Monte Carlo simulations.
Table 3.
Cost and effectiveness of laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy
| Age group, y | Rate per 10 000 patients | ||||
|---|---|---|---|---|---|
| Incremental cost, millions USD Estimate (95% CI) |
Change in QALYs No. (95% CI) |
QALY ICER Median (IQR) |
Change in life-years No. (95% CI) |
Life-years ICER Median (IQR) |
|
| Main simulation | |||||
| <40 | -13.81 (-54.84 to 25.29) | 14.87 (-78.96 to 109.72) | -82748.18 (-569039.71–412563.56) | 4.49 (-0.63 to 8.77) | -2850553.66 (-6260810.00–185168.53) |
| 40–49 | -14.39 (-54.29 to 22.90) | 11.22 (-89.04 to 107.54) | -67233.20 (-532589.74–463735.82) | -0.47 (-7.01 to 4.73) | 823366.28 (-7767664.48–7652659.38) |
| 50–59 | -13.38 (-52.57 to 24.71) | -7.14 (-107.71 to 88.56) | 79398.87 (-385996.03–591027.87) | -20.64 (-34.55 to -10.19) | 588818.19 (-14350.23–1389005.93) |
| ≥60 | -6.83 (-47.46 to 30.76) | -58.45 (-172.87 to 55.30) | 68314.39 (-157555.05–299516.64) | -91.19 (-127.76 to -59.77) | 74207.99 (-72433.38–215128.86) |
| Sensitivity analyses | |||||
| Higher prevalence of cancer* | |||||
| <40 | -13.75 (-54.81 to 25.32) | 14.19 (-79.38 to 109.16) | -82562.82 (-581409.69–420264.87) | 3.67 (-1.90 to 8.26) | -2829468.49 (-7019694.32–890487.25) |
| 40–49 | -14.29 (-54.18 to 23.02) | 9.95 (-86.84 to 110.55) | -37083.31 (-505988.90–498183.10) | -1.94 (-8.71 to 3.50) | 2352347.64 (-3962810.46–8300772.62) |
| 50–59 | -12.95 (-52.24 to 25.09) | -11.95 (-113.36 to 83.31) | 103252.20 (-303805.40–616188.80) | -26.58 (-41.97 to -14.35) | 443104.59 (-27302.45–1049457.88) |
| ≥60 | -4.98 (-45.47 to 32.32) | -76.81 (-203.75 to 42.79) | 44307.16 (-133431.56–219951.68) | -115.50 (-159.75 to -76.88) | 43285.16 (-74120.85–153448.02) |
| Lower prevalence of cancer† | |||||
| <40 | -13.87 (-54.87 to 25.26) | 15.54 (-78.42 to 110.27) | -87048.59 (-569755.10–412626.91) | 5.30 (0.70 to 9.37) | -2597937.65 (-5537859.78–23256.98) |
| 40–49 | -14.49 (-54.40 to 22.79) | 12.49 (-87.63 to 109.88) | -78557.68 (-528267.40–439677.64) | 1.00 (-5.20 to 5.96) | -1460618.65 (-8857400.97–5546840.92) |
| 50–59 | -13.81 (-52.98 to 24.17) | -2.32 (-103.64 to 92.60) | 47204.70 (-427764.42–575159.75) | -14.71 (-27.35 to -5.64) | 855307.38 (10498.37–2040596.51) |
| ≥60 | -8.67 (-49.22 to 29.15) | -40.09 (-142.27 to 65.08) | 71040.34 (-221417.03–368525.86) | -66.87 (-95.94 to -42.76) | 129265.27 (-70717.96–322922.87) |
| Higher rate of tumor dissemination‡ | |||||
| <40 | -13.57 (-54.72 to 25.58) | 12.06 (-80.76 to 106.02) | -71616.25 (-569703.21–445454.68) | 1.06 (-6.50 to 6.73) | -1136348.51 (-8427112.67–4427816.09) |
| 40–49 | -13.95 (-53.81 to 23.39) | 5.90 (-93.17 to 102.23) | -2809.41 (-501401.00–542348.48) | -6.66 (-14.80 to -0.32) | 1833214.75 (-112437.41–4717430.36) |
| 50–59 | -11.56 (-50.81 to 26.37) | -27.28 (-128.05 to 71.74) | 114736.13 (-209787.67–559681.62) | -45.43 (-66.22 to -28.16) | 228689.23 (-45318.81–576862.79) |
| ≥60 | 0.92 (-39.08 to 38.25) | -132.23 (-271.98 to 2.95) | -13170.67 (-118205.81–83671.58) | -191.98 (-264.08 to -129.65) | -5729.99 (-76072.49–59788.31) |
| Lower rate of tumor dissemination§ | |||||
| <40 | -13.94 (-54.91 to 25.22) | 16.42 (-77.33 to 111.30) | -88217.60 (-553802.84–419107.41) | 6.39 (2.00 to 10.17) | -2178020.87 (-4516861.22– -40097.39) |
| 40–49 | -14.63 (-54.56 to 22.67) | 14.16 (-86.84 to 110.55) | -95969.66 (-542571.33–413740.34) | 2.96 (-2.80 to 7.80) | -3151372.36 (-9068072.53–1235814.25) |
| 50–59 | -14.37 (-53.54 to 23.60) | 3.83 (-97.69 to 97.66) | -10489.60 (-526375.93–529430.80) | -7.16 (-18.08 to 0.18) | 1778319.72 (3063.72–4980259.31) |
| ≥60 | -11.05 (-51.96 to 26.66) | -18.54 (-124.52 to 89.89) | 51496.04 (-322886.06–417451.79) | -37.05 (-56.38 to -21.98) | 293802.07 (-63058.64–655149.40) |
| Higher proportion of sarcomas‖ | |||||
| <40 | -13.79 (-54.83 to 25.29) | 14.67 (-78.74 to 109.52) | -82807.73 (-571963.79–408106.57) | 4.23 (-1.01 to 8.64) | -2759212.78 (-6502502.97–312751.74) |
| 40–49 | -14.36 (-54.26 to 22.95) | 10.98 (-88.77 to 107.28) | -63600.33 (-524275.95–454578.73) | -0.79 (-7.39 to 4.53) | 1320971.24 (-7027970.35–8398248.30) |
| 50–59 | -13.36 (-52.56 to 24.76) | -6.38 (-107.26 to 88.37) | 82007.15 (-373331.13–596563.92) | -19.49 (-33.28 to -8.97) | 619047.17 (-16502.39–1480717.68) |
| ≥60 | -7.08 (-47.73 to 30.60) | -52.79 (-159.85 to 56.42) | 69804.20 (-168037.50–317138.98) | -79.50 (-114.16 to -50.74) | 89723.28 (-80583.31–250252.92) |
* Prevalence of cancer increased by 25%. CI = confidence interval; ICER = incremental cost-effectiveness ratio; IQR = interquartile range; QALY = quality-adjusted life-year.
† Prevalence of cancer reduced by 25%.
‡ Risk of dissemination assumed to be 40% in women with endometrial tumors and 60% in women with sarcomas.
§ Risk of dissemination assumed to be 10% in women with either endometrial tumor or uterine sarcomas.
‖ Risk of underlying tumors assumed to be sarcomas in 75% and endometrial tumors in 25%.
Figure 2.
Probabilistic sensitivity analysis of cost vs life-years for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy stratified by age.
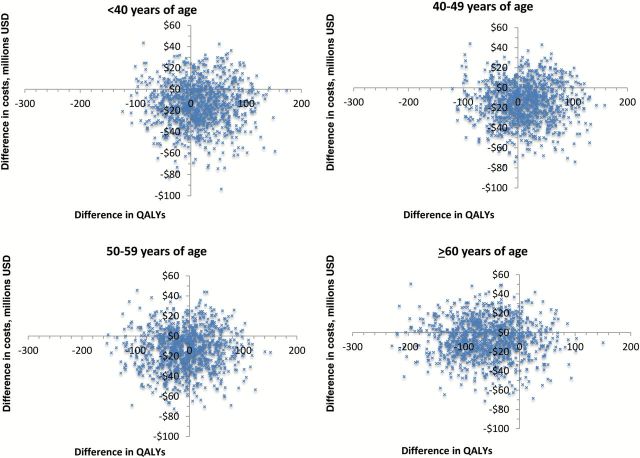
When quality of life is included, laparoscopic hysterectomy with morcellation became a more favorable strategy for women age 40 to 49 years. For 10 000 women age 40 to 49 years, laparoscopic hysterectomy with morcellation was associated with an improvement of 11.22 quality-adjusted life-years compared with abdominal hysterectomy. Figure 3 displays the cost-effectiveness plane (cost vs quality-adjusted life-years) for laparoscopic hysterectomy with morcellation from 1000 Monte Carlo simulations.
Figure 3.
Probabilistic sensitivity analysis of cost vs quality-adjusted life-years for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy stratified by age. QALY = quality-adjusted life-year.
Cost-Effectiveness Sensitivity Analyses
In a series of sensitivity analyses, the findings were largely unchanged. A higher prevalence of cancer resulted in similar rankings. However, if the prevalence of cancer was reduced by 25%, morcellation was associated with an incremental increase of 1.00 life-year per 10 000 women and became a favorable strategy.
If the rate of tumor dissemination was higher, laparoscopic hysterectomy with morcellation remained the most effective strategy for women younger than age 40 years. In this scenario, morcellation was favored from a QALY perspective, but was associated with an incremental decrease of 6.66 life-years per 10 000 women in those age 40 to 49 years (Table 3). If the rate of tumor dissemination was lower, laparoscopic hysterectomy with morcellation in women age 50 to 59 years was associated with 3.83 greater QALYs but 7.16 fewer overall life-years per 10 000 women (Table 3). This was the only potential scenario in which the benefits of morcellation outweighed the risk for women older than age 50 years. If a higher percentage of neoplasms were sarcomas, the rankings mirrored the base case scenario.
Discussion
Laparoscopic hysterectomy without morcellation is the most beneficial approach for hysterectomy among the three modalities studied. For those women who cannot undergo laparoscopic hysterectomy without morcellation, our findings suggest that the risks and benefits of electric power morcellation for hysterectomy are highly dependent upon age. In women younger than age 40 years, electric power morcellation is associated with greater quality-adjusted life-years and overall life-years compared with abdominal hysterectomy. However, the magnitude of benefit in this age group was relatively small. In contrast, for older women, the risk of electric power morcellation greatly outweighs the benefits of the procedure.
The prevalence of underlying malignancy in women who undergo hysterectomy with electric power morcellation has been the subject of controversy (1–4). A quantitative assessment by the US Food and Drug Administration (FDA) of women who underwent hysterectomy for fibroids suggested that the prevalence of unsuspected uterine sarcomas was one in 352, while population-based data reported underlying malignancies in one in 368 women after electric power morcellation (3,4). In contrast, other studies have claimed that the prevalence of uterine sarcomas is much lower (56). The controversy stems from a multitude of factors, including differences in the populations studied, the difficulty of identifying use of electric power morcellators from medical records, and imprecise coding of pathologic outcomes. We utilized an overall baseline risk of cancer of 0.27% and report age-specific outcomes as well as results from a number of simulations based on higher and lower rates of malignancy.
While much of the controversy surrounding electric power morcellation has focused on uterine sarcomas, women are also at risk for unsuspected endometrial cancers, which are much more common than uterine sarcomas (5). In one report, endometrial hyperplasia, a precursor to epithelial endometrial cancer, was common (1 in 99), perhaps suggesting that many of the morcellated cancers are in fact endometrial cancers and not uterine sarcomas (4). Compared with uterine sarcomas, the prognosis for these endometrial tumors is more favorable. While we modeled a range of histologic distributions, our findings remained largely unchanged when the distributions of sarcomas and endometrial cancers were varied.
The most important risk of morcellation is tumor dissemination of an occult malignancy. In a series of women with presumed stage I leiomyosarcoma who underwent immediate re-exploration after morcellation, 29% had disseminated intraperitoneal disease (7). A second series noted dissemination of leiomyosarcoma in 57% of patients (2). Importantly, even women diagnosed with smooth muscle tumors of unknown significance are also at risk for tumor dissemination and recurrence (2,7). To date, the risk of dissemination of morcellated endometrial tumors remains poorly defined (5). Our assumptions of the risk of dissemination are based on reported case series, and we also performed a variety of sensitivity analyses modeling varying risks of dissemination.
In our models, laparoscopic hysterectomy without morcellation was the most beneficial approach to hysterectomy under a wide variety of scenarios. While electric power morcellation was initially introduced to facilitate minimally invasive hysterectomy in women who would otherwise require laparotomy, concern has been raised that power morcellation is being utilized in women who could undergo a minimally invasive procedure without the devices. These data reinforce that electric power morcellation should not be performed in women who could undergo minimally invasive surgery without use of the devices.
We recognize a number of limitations in our projections. First, the risk of unrecognized cancer is relatively low and prospective trials to define cancer risks, as well as the risk of perioperative complications in women who undergo morcellation, are lacking. Our risk estimates are based on population-level prevalence estimates in women who underwent electric power morcellation. While we performed a number of sensitivity analyses incorporating other reported estimates, our estimates may underestimate the true risk of cancer in some subgroups. Second, data describing the outcomes of women with endometrial tumors who undergo morcellation are limited and we thus report a range of sensitivity analyses. Because data describing the characteristics of women with cancers who have undergone morcellation are limited, we are unable to further stratify our models for other important factors such as race and uterine size. Similarly, it is difficult to adjust for surgeon preferences and unmeasured factors that may have impacted route of surgery. Lastly, many women who undergo minimally invasive hysterectomy now undergo a robotic-assisted procedure. Our estimates are based on the performance of laparoscopic hysterectomy. Prior studies have consistently shown that robotic hysterectomy is substantially more costly than laparoscopic hysterectomy, which would likely have altered our cost estimates (57).
The controversy surrounding the use of electric power morcellators for gynecologic surgery demonstrates the challenges associated with surgical innovation. Electric power morcellators were developed more than two decades ago and diffused into clinical practice with minimal data supporting either safety or efficacy. A highly publicized case of one patient with a uterine sarcoma who underwent electric power morcellation led to intense public scrutiny (58). This heightened awareness stimulated position statements from professional societies and changes in hospital policies, as well as reports describing widely varying estimates of the risk of cancer associated with morcellation (56). The FDA’s process for device regulation, 510(k), has also been criticized for lack of oversight for new surgical devices (59). The controversy around electric power morcellation clearly demonstrates the need for better data and heightened regulation before new surgical devices are allowed to diffuse into widespread practice.
Despite these potential limitations of our study, these findings provide important data to inform policy and clinical decision-making. Notably, our findings are in accord with a recent FDA advisory statement that warned against use of electric power morcellators in peri- and postmenopausal women (60). Regardless of the surgical approach chosen, efforts to detect occult malignancy should precede intervention. Surgical technique should be individualized, and all patients should be thoroughly counseled regarding the risk of electric power morcellation. As women age, the risk of underlying occult malignancy rises, increasing the risks associated with electric power morcellation. For many women, this risk will outweigh the benefits of minimally invasive surgery and the procedure should be used with caution.
Funding
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Supplementary Material
The authors have no conflicts of interest or disclosures.
References
- 1. Hagemann IS, Hagemann AR, LiVolsi VA, Montone KT, Chu CS. Risk of occult malignancy in morcellated hysterectomy: a case series. Int J Gynecol Pathol. 2011;30 (5):476–483. [DOI] [PubMed] [Google Scholar]
- 2. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7 (11):e50058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Accessed September 29, 2014.
- 4. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312 (12):1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18 (5):1065–1070. [DOI] [PubMed] [Google Scholar]
- 6. George S, Barysauskas C, Serrano C, et al. Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer. 2014;120 (20):3154–3158. [DOI] [PubMed] [Google Scholar]
- 7. Oduyebo T, Rauh-Hain AJ, Meserve EE, et al. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma. Gynecol Oncol. 2014;132 (2):360–365. [DOI] [PubMed] [Google Scholar]
- 8. Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122 (2):255–259. [DOI] [PubMed] [Google Scholar]
- 9. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311 (9):905–906. [DOI] [PubMed] [Google Scholar]
- 10. Society of Gynecologic Oncology. Morcellation. 2013. https://http://www.sgo.org/newsroom/position-statements-2/morcellation/. Accessed February 15, 2014.
- 11. Johnson N, Barlow D, Lethaby A, Tavender E, Curr L, Garry R. Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;330 (7506):1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009; (3):CD003677. [DOI] [PubMed] [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results Database. http://healthservices.cancer.gov/seermedicare/overview/. Accessed July 15, 2014
- 14. Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009;113 (3):324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kluivers KB, Johnson NP, Chien P, Vierhout ME, Bongers M, Mol BW. Comparison of laparoscopic and abdominal hysterectomy in terms of quality of life: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2008;136 (1):3–8. [DOI] [PubMed] [Google Scholar]
- 16. Ellstrom M, Olsen MF, Olsson JH, Nordberg G, Bengtsson A, Hahlin M. Pain and pulmonary function following laparoscopic and abdominal hysterectomy: a randomized study. Acta Obstet Gynecol Scand. 1998;77 (9):923–928. [PubMed] [Google Scholar]
- 17. Falcone T, Paraiso MF, Mascha E. Prospective randomized clinical trial of laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy. Am J Obstet Gynecol. 1999;180 (4):955–962. [DOI] [PubMed] [Google Scholar]
- 18. Ferrari MM, Berlanda N, Mezzopane R, Ragusa G, Cavallo M, Pardi G. Identifying the indications for laparoscopically assisted vaginal hysterectomy: a prospective, randomised comparison with abdominal hysterectomy in patients with symptomatic uterine fibroids. BJOG. 2000;107 (5):620–625. [DOI] [PubMed] [Google Scholar]
- 19. Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328 (7432):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harkki-Siren P, Sjoberg J, Toivonen J, Tiitinen A. Clinical outcome and tissue trauma after laparoscopic and abdominal hysterectomy: a randomized controlled study. Acta Obstet Gynecol Scand. 2000;79 (10):866–871. [PubMed] [Google Scholar]
- 21. Hwang JL, Seow KM, Tsai YL, Huang LW, Hsieh BC, Lee C. Comparative study of vaginal, laparoscopically assisted vaginal and abdominal hysterectomies for uterine myoma larger than 6cm in diameter or uterus weighing at least 450 g: a prospective randomized study. Acta Obstet Gynecol Scand. 2002;81 (12):1132–1138. [DOI] [PubMed] [Google Scholar]
- 22. Kluivers KB, Hendriks JC, Mol BW, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: a randomized, controlled trial. J Minim Invasive Gynecol. 2007;14 (2):145–152. [DOI] [PubMed] [Google Scholar]
- 23. Kongwattanakul K, Khampitak K. Comparison of laparoscopically assisted vaginal hysterectomy and abdominal hysterectomy: a randomized controlled trial. J Minim Invasive Gynecol. 2012;19 (1):89–94. [DOI] [PubMed] [Google Scholar]
- 24. Langebrekke A, Eraker R, Nesheim BI, Urnes A, Busund B, Sponland G. Abdominal hysterectomy should not be considered as a primary method for uterine removal. A prospective randomised study of 100 patients referred to hysterectomy. Acta Obstet Gynecol Scand. 1996;75 (4):404–407. [DOI] [PubMed] [Google Scholar]
- 25. Lumsden MA, Twaddle S, Hawthorn R, et al. A randomised comparison and economic evaluation of laparoscopic-assisted hysterectomy and abdominal hysterectomy. BJOG. 2000;107 (11):1386–1391. [DOI] [PubMed] [Google Scholar]
- 26. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multicenter study. Am J Obstet Gynecol. 1999;180 (2, pt 1): 270–275. [DOI] [PubMed] [Google Scholar]
- 27. Olsson JH, Ellstrom M, Hahlin M. A randomised prospective trial comparing laparoscopic and abdominal hysterectomy. Br J Obstet Gynaecol. 1996;103 (4):345–350. [DOI] [PubMed] [Google Scholar]
- 28. Perino A, Cucinella G, Venezia R, Castelli A, Cittadini E. Total laparoscopic hysterectomy versus total abdominal hysterectomy: an assessment of the learning curve in a prospective randomized study. Hum Reprod. 1999;14 (12):2996–2999. [DOI] [PubMed] [Google Scholar]
- 29. Raju KS, Auld BJ. A randomised prospective study of laparoscopic vaginal hysterectomy versus abdominal hysterectomy each with bilateral salpingo-oophorectomy. Br J Obstet Gynaecol. 1994;101 (12):1068–1071. [DOI] [PubMed] [Google Scholar]
- 30. Ribeiro SC, Ribeiro RM, Santos NC, Pinotti JA. A randomized study of total abdominal, vaginal and laparoscopic hysterectomy. Int J Gynaecol Obstet. 2003;83 (1):37–43. [DOI] [PubMed] [Google Scholar]
- 31. Schutz K, Possover M, Merker A, Michels W, Schneider A. Prospective randomized comparison of laparoscopic-assisted vaginal hysterectomy (LAVH) with abdominal hysterectomy (AH) for the treatment of the uterus weighing >200g. Surg Endosc. 2002;16 (1):121–125. [DOI] [PubMed] [Google Scholar]
- 32. Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9 (3):333–338. [DOI] [PubMed] [Google Scholar]
- 33. Summitt RL, Jr, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopically assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92 (3):321–326. [PubMed] [Google Scholar]
- 34. Yuen PM, Mak TW, Yim SF, et al. Metabolic and inflammatory responses after laparoscopic and abdominal hysterectomy. Am J Obstet Gynecol. 1998;179 (1):1–5. [DOI] [PubMed] [Google Scholar]
- 35. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116 (4):909–915. [DOI] [PubMed] [Google Scholar]
- 36. Brummer TH, Jalkanen J, Fraser J, et al. FINHYST, a prospective study of 5279 hysterectomies: complications and their risk factors. Hum Reprod. 2011;26 (7):1741–1751. [DOI] [PubMed] [Google Scholar]
- 37. Hansen CT, Moller C, Daugbjerg S, Utzon J, Kehlet H, Ottesen B. Establishment of a national Danish hysterectomy database: preliminary report on the first 13,425 hysterectomies. Acta Obstet Gynecol Scand. 2008;87 (5):546–557. [DOI] [PubMed] [Google Scholar]
- 38. Kafy S, Huang JY, Al-Sunaidi M, Wiener D, Tulandi T. Audit of morbidity and mortality rates of 1792 hysterectomies. J Minim Invasive Gynecol. 2006;13 (1):55–59. [DOI] [PubMed] [Google Scholar]
- 39. Makinen J, Johansson J, Tomas C, et al. Morbidity of 10 110 hysterectomies by type of approach. Hum Reprod. 2001;16 (7):1473–1478. [DOI] [PubMed] [Google Scholar]
- 40. Varol N, Healey M, Tang P, Sheehan P, Maher P, Hill D. Ten-year review of hysterectomy morbidity and mortality: can we change direction? Aust N Z J Obstet Gynaecol. 2001;41 (3):295–302. [DOI] [PubMed] [Google Scholar]
- 41. Wiser A, Holcroft CA, Tulandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomy for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10 (2):117–122. [Google Scholar]
- 42. Fennessy FM, Kong CY, Tempany CM, Swan JS. Quality-of-life assessment of fibroid treatment options and outcomes. Radiology. 2011;259 (3):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gorlero F, Lijoi D, Biamonti M, et al. Hysterectomy and women satisfaction: total versus subtotal technique. Arch Gynecol Obstet. 2008;278 (5):405–410. [DOI] [PubMed] [Google Scholar]
- 44. Grann VR, Jacobson JS, Sundararajan V, Albert SM, Troxel AB, Neugut AI. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am. 1999;5 (5):283–292. [PubMed] [Google Scholar]
- 45. Grann VR, Jacobson JS, Thomason D, Hershman D, Heitjan DF, Neugut AI. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: an updated decision analysis. J Clin Oncol. 2002;20 (10):2520–2529. [DOI] [PubMed] [Google Scholar]
- 46. Guest JF, Sladkevicius E, Gough N, Linch M, Grimer R. Utility values for advanced soft tissue sarcoma health States from the general public in the United kingdom. Sarcoma. 2013;2013:863056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113 (2):326–335. [DOI] [PubMed] [Google Scholar]
- 48. Sculpher M, Manca A, Abbott J, Fountain J, Mason S, Garry R. Cost effectiveness analysis of laparoscopic hysterectomy compared with standard hysterectomy: results from a randomised trial. BMJ. 2004;328 (7432):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soini EJ, Garcia San Andres B, Joensuu T. Trabectedin in the treatment of metastatic soft tissue sarcoma: cost-effectiveness, cost-utility and value of information. Ann Oncol. 2011;22 (1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38 (6):583–637. [DOI] [PubMed] [Google Scholar]
- 51. Yang KY, Caughey AB, Little SE, Cheung MK, Chen LM. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Familial Cancer. 2011;10 (3):535–543. [DOI] [PubMed] [Google Scholar]
- 52. Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40 (8 suppl): IV-104–17. [DOI] [PubMed] [Google Scholar]
- 53. Warren JL, Brown ML, Fay MP, Schussler N, Potosky AL, Riley GF. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20 (1):307–316. [DOI] [PubMed] [Google Scholar]
- 54. Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100 (9):630–641. [DOI] [PubMed] [Google Scholar]
- 55. United States Department of Labor. Bureau of Labor Statistics. http://www.bls.gov/cps/cpsaat37.htm. Accessed October 3, 2014.
- 56. AAGL Statement to the FDA on Power Morcellation. http://www.aagl.org/aaglnews/aagl-statement-to-the-fda-on-power-morcellation/. Accessed September 29, 2014.
- 57. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309 (7):689–698. [DOI] [PubMed] [Google Scholar]
- 58. Levitz J, Kamp J. Debate grows over possible dangers from a type of hysterectomy. The Wall Streeet Journal. 2014. [Google Scholar]
- 59. Curfman GD, Redberg RF. Medical devices--balancing regulation and innovation. N Engl J Med. 2011;365 (11):975–977. [DOI] [PubMed] [Google Scholar]
- 60. Updated Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed December 3, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.