Abstract
Bladder cancer is one of the most commonly diagnosed malignancies worldwide, derived from the urothelium of the urinary bladder and defined by long asymptomatic and atypical clinical picture. Its complex etiopathogenesis is dependent on numerous risk factors that can be divided into three distinct categories: genetic and molecular abnormalities, chemical or environmental exposure and previous genitourinary disorders and family history of different malignancies. Various genetic polymorphisms and microRNA might represent useful diagnostic or prognostic biomarkers. Genetic and molecular abnormalities - risk factors are represented by miRNA or genetic polymorphisms proved to be part of bladder carcinogenesis such as: genetic mutations of oncogenes TP53, Ras, Rb1 or p21 oncoproteins, cyclin D or genetic polymorhisms of XPD,ERCC1, CYP1B1, NQO1C609T, MDM2SNP309, CHEK2, ERCC6, NRF2, NQO1Pro187Ser polymorphism and microRNA (miR-143, −145, −222, −210, −10b, 576-3p). The aim of our article is to highlight the most recent acquisitions via molecular biomarkers (miRNAs and genetic polymorphisms) involved in bladder cancer in order to provide early diagnosis, precise therapy according to the molecular profile of bladder tumors, as well as to improve clinical outcome, survival rates and life quality of oncological patients. These molecular biomarkers play a key role in bladder carcinogenesis, clinical evolution, prognosis and therapeutic response and explain the molecular mechanisms involved in bladder carcinogenesis; they can also be selected as therapeutic targets in developing novel therapeutic strategies in bladder malignancies. Moreover, the purpose in defining these molecular non invasive biomarkers is also to develop non invasive screening programs in bladder malignancies with the result of decreasing bladder cancer incidence in risk population.
Keywords: bladder cancer, molecular diagnostic biomarkers, miRNA, genetic polymorphism, early diagnosis
Introduction
Bladder cancer has a complex etiopathogenesis dependent on various factors: chemical carcinogens (smoking, professional exposure: industry carcinogens), diet (artificial sweeteners, coffee consumption and meat consumption, total fluid intake), previous treatments (pelvic radiation, drug abuse, chronic treatments with analgesics and anti-inflammatory drugs, hormone therapy) or genetic factors (genetic polymorphisms, microRNAs) [1,2]. These risk factors are involved in bladder cancer etiopathogenesis, clinical evolution, prognosis, response to specific therapy or survival rates [3]. Dietary factors are mentioned also as part of bladder carcinogenesis (meat consumption, total fluid intake, vegetables, artificial sweeteners) and various disorders in the patient’s medical history (chronic infections and inflammations of urinary bladder, Schistosomiasis, HPV, chronic litiasis, long term catheterization) [4,5,6,]. We can also mention as important etiological risk factors: the patient’s clinical and family history - previous disorders (chronic inflammations and fibrosis, uro-genital malignancies, congenital abnormalities of urinary tract) [7] and various malignancies [8]. The genetic component is very important in bladder carcinogenesis (CCND1, CHEK2, CYP1B1, XPC, ERCC2 and ERCC5, MDM2SNP309 genetic variants, NRF2 and NRF2 target genes, p53 and Rab oncogene) [9,10,11]. It will be important to identify and define various miRNA and genetic polymorphisms involved in bladder carcinogenesis and use these genetic variants as diagnostic or prognostic biomarkers or as useful non invasive parameters in patients surveillance or screening programs, but also as important parameters in the improvement of the EORTC scale (European Organization for Research and Treatment of Cancer) in predicting progression and recurrences in bladder cancer [12,13].
The aim of recent research studies in bladder cancer pathology is to identify novel diagnostic and prognostic biomarkers in bladder cancer using medical genetics and functional genomics technology and provide early diagnosis, precise therapy and improve clinical outcome, life quality and survival rates of patients with bladder cancer [14].
Novel genetic biomarkers
Bladder cancer is a highly heterogenous malignancy derived from the urothelium of the urinary bladder with profound genetic valences (genetic polymorphisms, miRNA) that could characterize etiopathogenesis, evolution, prognosis, response to specific therapy or survival rates.
Much interest has been given to urine tests in developing non invasive diagnosis biomarkers for bladder cancer [8]. The usual diagnostic tools, such as cystoscopy, require experience, while histopathological examination represents the golden standard nowadays in bladder cancer positive diagnosis. Urine cytology has a high specificity but lack of sensitivity in low-grade urothelial carcinomas. Studying non invasive molecular biomarkers (miRNA or genetic mutations) in bladder carcinogenesis and harvesting biomarkers from the blood of bladder cancer patients and compare with their tumors and normal tissue profiling could lead to a relatively non-invasive, cost-effective test with equivalent or improved sensitivity and specificity [12,13]. Identifying miRNA and genetic polymorphisms involved in bladder cancer ethiopathogenesis using functional genomic technologies, recent research studies aim at: developing new diagnostic and prognostic strategies based on non invasive tumoral biomarkers, novel therapeutic/chemoprevention strategies, improvement of the clinical evolution of these patients and also improved EORTC scale for bladder cancerGenetic polymorphisms. Intra-familial clusters have been reported in bladder cancer and recent studies documented the influence of genetic polymorphisms in bladder carcinogenesis. This hypothesis opens new research ways in finding novel diagnostic biomarker in bladder cancer, but also in predicting bladder cancer evolution, prognosis and develop novel therapeutic strategies according to molecular profile. Family history of bladder cancer is a well-known risk factor in bladder cancer ethiopathogenesis and susceptibility, new data emerging under the influence of family history tumors rather than bladder cancer. Recent research studies identified tumors in whose case family history influenced survival rates, prognosis and clinical evolution. Many genetic polymorphisms are identified in bladder cancer etiopathogenesis which influence tumor susceptibility, prognosis or therapy response [13,14].
Studying genetic polymorphisms involved in bladder cancer has proved that NQO1Pro187Ser is an important part of bladder carcinogenesis involved in the etiopathogenesis of urinary system malignancies: bladder cancer, prostate cancer or renal cell carcinomas[15,16], but also a risk factor for other malignancies like: breast cancer, colorectal cancer or esophageal carcinoma [17,18,19].
Relevant clinical research studies have shown that NQO1C609T is involved in bladder cancer and after clinical validation, this genetic polymorphism might represent an important diagnostic or prognostic biomarker in clinical oncology [19,20,21,22].
MDM2SNP309 promotes various genetic mutations of p53 involved in bladder carcinogenesis and is correlated with poor prognosis and fast evolution for T1 stage bladder malignancies. MDM2SNP309 also represents an important risk factor in developing bladder cancer and might be seriously considered in the oncological evaluation. It may help to develop precise therapy in bladder malignancies according to the molecular profile of the tumour [22,23].
P53Arg72Pro genetic polymorphism might be considered an important prognostic tool for invasive tumors versus superficial types of bladder cancer and could represent a useful parameter in bladder cancer surveillance or EORTC scale of prediction progression and recurrences of bladder cancer [24,25].
P53 is well-known as a supressor oncogene responsible for apoptotic processes, cell senescence, proliferation and control [26]. Its genetic mutation is frequently involved in various carcinogenetic processes including bladder malignancies [27].
miRNA
MiRNAs are non coding small molecules made of 19–22 nucleotides involved in gene regulation and part of various malignancies ethiopathogenesis. miRNAs might correlate malignancies with clinical evolution, prognosis or survival rates [28].
Studying bladder cancer etiopathogenesis has proven that miRNA-222 is correlated with the presence of in situ carcinoma, progression rate, fast evolution and poor survival rate [29].
miRNA -143 is also prognostic biomarker correlated with survival rates in bladder malignancies [30].
MiRNA-145 is involved in bladder cancer and might represent an important diagnostic biomarker in low grade, non muscle invasive bladder cancers [30]. MiRNA-21 is up-regulated in high grade bladder cancer and might represent a trustable diagnosis biomarker which can differentiate high grade bladder cancer from low grade [30,31,32,33].
MiRNA-137 might represent an important prognostic biomarker in bladder malignancies, which is correlated with cell proliferation, progression, tumor invasion and metastasis. Like
miRNA-137, miRNA-10b is a biomarker associated with tumor progression, metastatic processes but also important target in defining precise therapy in bladder cancers [34]. MiRNA-29c,10b and miRNA-210 might also represent diagnostic biomarkers in bladder malignancies and after clinical validation might provide early diagnosis in bladder cancer, improve clinical outcome and survival rates of these patients [34,35,36,37]. Being aware of the diagnostic difficulties in bladder cancer due to its long atypical asymptomatic clinical evolution (common genitourinary symptoms: painless hematuria, dysuria, urgency, frequency) and limited values of medical imaging techniques (cystoscopy, CT urography, ultrasonograpy) especially in superficial bladder malignancies, research studies try to define novel diagnostic biomarkers which might provide early diagnosis, precise therapy in bladder cancer, improve clinical outcome, quality of quality and survival rates [38,39,40,41,42,43].
Nowadays bladder cancer diagnosis is based on: cystoscopic examination mainly, urine cytology and histological examination [43]. Except for medical imaging tests and clinical examination, we mention complementary tests used in the positive diagnostic of bladder malignancies: UroVysion test (FISH), ImmunoCyt, BTA ( bladder tumor antigen) and NMP22 [43,44,45,46].
BTA (bladder tumor antigen) is a qualitative, non invasive, easy to perform diagnostic test in bladder cancer which measures complement factor H related protein and might be used along with urine cytology, cystoscopic and histopathological examination in bladder cancer diagnostic evaluation [46,47,48,49].
NMP22 (nuclear matrix protein 22) is an important biomarker and together with UroVysion test (fluorescence in situ hybridization) might represent useful non invasive surveillance instrument in bladder malignancies [50,51].
ImunoCyt is another non invasive diagnostic tools in bladder cancer which combines immuno- fluorescence technique with urine cytology and its characterized by a high rate of false positive results induced by genitourinary benign disorders [52,53,54].
Conclusions
Bladder cancer is a real health problem worldwide because of its incidence, prevalence, high recurrence rate, with a long silent clinical evolution, which is diagnosed using medical imaging techniques and histopathological approach. A wide range of non invasive genetic biomarkers have been evaluated that can provide early diagnosis and, above all, may estimate and characterize bladder malignancies evolution, prognosis, survival rate, response to therapy, and can be also included as useful parameters in non invasive screening programs. Blood profiling for bladder cancers pathology might represent an interesting non-invasive test, showing accurate information about the tumor grade, therapy response and patients prognosis. Using-these molecular biomarkers in clinical practice might provide early diagnosis in bladder malignancies, precise therapy and improve clinical outcome patients.
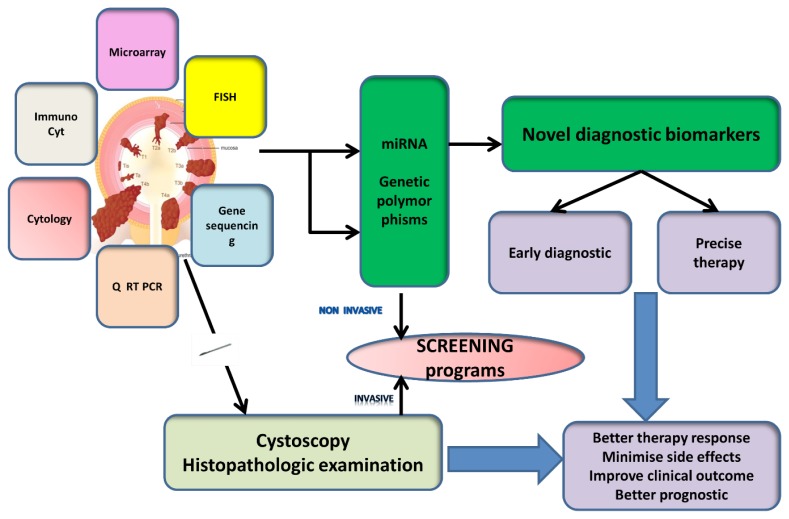
Figure 1.
Bladder cancer diagnostic techniques-personal synthetic approach.
Table I.
| MiRNA | Diagnostic value in bladder cancer | Specifications |
|---|---|---|
| MiRNA-145 | + | Diagnostic biomarker in muscle invasive bladder cancers |
| MiRNA-21 | + | Diagnostic biomarker in high grade bladder cancers |
Table II.
| miRNA | Prognostic value in bladder cancer | Specifications |
|---|---|---|
| MiRNA-137 | ++ | Poor prognostic biomarker for metastatic processes and fast clinical evolution |
| MiRNA-143 | ++ | Poor prognostic biomarker |
| MiRNA-222 | ++ | Poor prognostic biomarker |
| MiRNA-10b | ++ | Poor prognostic biomarkers for invasion and metastatic processes |
| MiRNA-29c | + | Prognostic biomarker Suppresses cell growth in bladder cancer |
Acknowledgments
Dr. Truta Anamaria acknowledges financial support from an POSDRU grant no.159/1.5/S/138776 with title:”Model colaborativ institutional pentru translatarea cercetarii biomedicale in practica clinica –TRANSCENT “[Institutional collaborative model for the translation of biomedical research into clinical practice].
References
- 1.Banaszkiewicz M, Constantinou M, Pietrusiński M, Kępczyński Ł, Jędrzejczyk A, Rożniecki M, et al. Concomitance of oncogenic HPV types, CHEK2 gene mutations, and CYP1B1 gene polymorphism as an increased risk factor for malignancy. Cent European J Urol. 2013;66:23–29. doi: 10.5173/ceju.2013.01.art7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daugherty SE, Lacey JV, Jr, Pfeiffer RM, Park Y, Hoover RN, Silverman DT. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2013;133(2):462–472. doi: 10.1002/ijc.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Yang L, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and bladder cancer risk: a meta-analysis. J Infect Dis. 2011;204(2):217–223. doi: 10.1093/infdis/jir248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali AM, Nelvigi GG, Keshavaiah VG, Ratkal CS. Extensive xanthogranulomatous cystitis mimicking bladder cancer. Urol Ann. 2014;6(4):373–375. doi: 10.4103/0974-7796.141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudziec E, Gogol-Döring A, Cookson V, Chen W, Catto J. Integrated epigenome profiling of repressive histone modifications, DNA methylation and gene expression in normal and malignant urothelial cells. PLoS One. 2012;7(3):e32750. doi: 10.1371/journal.pone.0032750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu CL, Odegaard JI, Herbert DR, Hsieh MH. A novel mouse model of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 2012;8(3):e1002605. doi: 10.1371/journal.ppat.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Z, Li S, Xu X, Xu X, Wang X, Wu J, et al. MicroRNA-576-3p inhibits proliferation in bladder cancer cells by targeting cyclin D1. Mol Cells. 2015;38(2):130–137. doi: 10.14348/molcells.2015.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouissi K, Bahria IB, Bougatef K, Marrakchi R, Stambouli N, Hamdi K, et al. The effect of tobacco, XPC, ERCC2 and ERCC5 genetic variants in bladder cancer development. BMC Cancer. 2011;11:101. doi: 10.1186/1471-2407-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reszka E, Jablonowski Z, Wieczorek E, Jablonska E, Krol MB, Gromadzinska J, et al. Polymorphisms of NRF2 and NRF2 target genes in urinary bladder cancer patients. J Cancer Res Clin Oncol. 2014;140(10):1723–1731. doi: 10.1007/s00432-014-1733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y, Wu W, Yin Z, Guan P, Zhou B. MDM2 SNP309, gene-gene interaction, and tumor susceptibility: an updated meta-analysis. BMC Cancer. 2011;11:208. doi: 10.1186/1471-2407-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho JR, Chapeaublanc E, Kirkwood L, Nicolle R, Benhamou S, Lebret T, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One. 2012;7(6):e39469. doi: 10.1371/journal.pone.0039469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walczak R, Bar K, Walczak J. The value of EORTC risk tables in evaluating recurrent non–muscle–invasive bladder cancer in everyday practice. Cent European J Urol. 2014;66(04):418–422. doi: 10.5173/ceju.2013.04.art6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemminki K, Bermejo JL, Ji J, Kumar R. Familial bladder cancer and the related genes. Curr Opin Urol. 2011;21(5):386–392. doi: 10.1097/MOU.0b013e32834958ff. [DOI] [PubMed] [Google Scholar]
- 14.Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11(11):4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang D, Zhu JH, Chen MB, Shen WX, He J. The association between NQO1 Pro187Ser polymorphism and urinary system cancer susceptibility: a meta-analysis of 22 studies. Cancer Invest. 2015;33(2):39–40. doi: 10.3109/07357907.2014.998836. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Jin T, Su HX, Zhu JH, Wang DW, Zhu SJ, et al. The association between NQO1 Pro187Ser polymorphism and bladder cancer susceptibility: a meta-analysis of 15 studies. PLoS One. 2015;10(1):e0116500. doi: 10.1371/journal.pone.0116500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Tian H, Yu KZ, Shen WY, Mao ZC, Jin CH, et al. Association between NQO1 Pro187Ser polymorphism and esophageal cancer: a meta-analysis. Tumour Biol. 2014;35(3):2063–2068. doi: 10.1007/s13277-013-1273-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Zhu F, Sun J, Meng X. Meta-analysis of the association between NQO1 Pro187Ser polymorphism and colorectal cancer in Asians. Tumour Biol. 2014;35(3):2111–2116. doi: 10.1007/s13277-013-1280-3. [DOI] [PubMed] [Google Scholar]
- 19.Peng Q, Lu Y, Lao X, Chen Z, Li R, Sui J, et al. The NQO1 Pro187Ser polymorphism and breast cancer susceptibility: evidence from an updated meta-analysis. Diagn Pathol. 2014;9:100. doi: 10.1186/1746-1596-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lajin B, Alachkar A. The NQO1 polymorphism C609T (Pro187Ser) and cancer susceptibility: a comprehensive meta-analysis. Br J Cancer. 2013;109(5):1325–1337. doi: 10.1038/bjc.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong M, Yi Q, Wang W. Association between NQO1 C609T polymorphism and bladder cancer susceptibility: a systemic review and meta-analysis. Tumour Biol. 2013;34(5):2551–2556. doi: 10.1007/s13277-013-0799-7. [DOI] [PubMed] [Google Scholar]
- 22.Zheng B, Wang Z, Chai R. NQO1 C609T polymorphism and colorectal cancer susceptibility: a meta-analysis. Arch Med Sci. 2014;10(4):651–660. doi: 10.5114/aoms.2014.44856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson H, Hultman P, Rosell J, Söderkvist P, Jahnson S. MDM2 SNP309 promoter polymorphism and p53 mutations in urinary bladder carcinoma stage T1. BMC Urol. 2013;13:5. doi: 10.1186/1471-2490-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikawa Y, Nadaoka J, Saito M, Kumazawa T, Inoue T, Yuasa T, et al. Clinical implications of the MDM2 SNP309 and p53 Arg72Pro polymorphisms in transitional cell carcinoma of the bladder. Oncol Rep. 2008;20(1):49–55. [PubMed] [Google Scholar]
- 25.Toffoli G, Biason P, Russo A, De Mattia E, Cecchin E, Hattinger CM, et al. Effect of TP53 Arg72Pro and MDM2 SNP309 polymorphisms on the risk of high-grade osteosarcoma development and survival. Clin Cancer Res. 2009;15(10):3550–3556. doi: 10.1158/1078-0432.CCR-08-2249. [DOI] [PubMed] [Google Scholar]
- 26.He F, Mo L, Zheng XY, Hu C, Lepor H, Lee EY, et al. Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer Res. 2009;69(24):9413–9421. doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (Phila) 2008;1(6):439–451. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veerla S, Lindgren D, Kvist A, Frigyesi A, Staaf J, Persson H, et al. MiRNA expression in urothelial carcinomas: important roles of miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and metastasis, and frequent homozygous losses of miR-31. Int J Cancer. 2009;124(9):2236–2242. doi: 10.1002/ijc.24183. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DQ, Zhou CK, Jiang XW, Chen J, Shi BK. Increased expression of miR-222 is associated with poor prognosis in bladder cancer. World J Surg Oncol. 2014;12:241. doi: 10.1186/1477-7819-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dip N, Reis ST, Srougi M, Dall’Oglio MF, Leite KR. Expression profile of microrna-145 in urothelial bladder cancer. Int Braz J Urol. 2013;39(1):95–101. doi: 10.1590/S1677-5538.IBJU.2013.01.12. [DOI] [PubMed] [Google Scholar]
- 31.Guancial EA, Bellmunt J, Yeh S, Rosenberg JE, Berman DM. The evolving understanding of microRNA in bladder cancer. Urol Oncol. 2014;32(1):41.e31–41.e40. doi: 10.1016/j.urolonc.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monfared H, Ziaee SA, Hashemitabar M, Khayatzadeh H, Kheyrollahi V, Tavallaei M, et al. Co-regulated expression of TGF-β Variants and miR-21 in bladder cancer. Urol J. 2013;10(3):981–987. [PubMed] [Google Scholar]
- 33.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10(7):396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 34.Xiu Y, Liu Z, Xia S, Jin C, Yin H, Zhao W, et al. MicroRNA-137 upregulation increases bladder cancer cell proliferation and invasion by targeting PAQR3. PLoS One. 2014;9(10):e109734. doi: 10.1371/journal.pone.0109734. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K, et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol Rep. 2014;31(4):1832–1838. doi: 10.3892/or.2014.3048. [DOI] [PubMed] [Google Scholar]
- 36.Biagioni F, Bossel Ben-Moshe N, Fontemaggi G, Yarden Y, Domany E, Blandino G. The locus of microRNA-10b: a critical target for breast cancer insurgence and dissemination. Cell Cycle. 2013;12(15):2371–2375. doi: 10.4161/cc.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa P, et al. Synthetic miRNA-mowers targeting miR-183-96-182 cluster or miR-210 inhibit growth and migration and induce apoptosis in bladder cancer cells. PLoS One. 2012;7(12):e52280. doi: 10.1371/journal.pone.0052280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 39.Seiler R, Thalmann GN, Rotzer D, Perren A, Fleischmann A. CCND1/CyclinD1 status in metastasizing bladder cancer: a prognosticator and predictor of chemotherapeutic response. Mod Pathol. 2014;27(1):87–95. doi: 10.1038/modpathol.2013.125. [DOI] [PubMed] [Google Scholar]
- 40.Stamatiou K, Papadoliopoulos I, Dahanis S, Zafiropoulos G, Polizois K. The accuracy of ultrasonography in the diagnosis of superficial bladder tumors in patients presenting with hematuria. Ann Saudi Med. 2009;29(2):134–137. doi: 10.4103/0256-4947.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francica G, Bellini SA, Scarano F, Miragliuolo A, De Marino FA, Maniscalco M. Correlation of transabdominal sonographic and cystoscopic findings in the diagnosis of focal abnormalities of the urinary bladder wall: a prospective study. J Ultrasound Med. 2008;27(6):887–894. doi: 10.7863/jum.2008.27.6.887. [DOI] [PubMed] [Google Scholar]
- 42.Helenius M, Dahlman P, Lonnemark M, Brekkan E, Wernroth L, Magnusson A. Comparison of post contrast CT urography phases in bladder cancer detection. Eur Radiol. 2016;26(2):585–591. doi: 10.1007/s00330-015-3844-7. [DOI] [PubMed] [Google Scholar]
- 43.Seideman C, Canter D, Kim P, Cordon B, Weizer A, Oliva I, et al. Multicenter evaluation of the role of UroVysion FISH assay in surveillance of patients with bladder cancer: does FISH positivity anticipate recurrence? World J Urol. 2015;33(9):1309–1313. doi: 10.1007/s00345-014-1452-9. [DOI] [PubMed] [Google Scholar]
- 44.Dimashkieh H, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121(10):591–597. doi: 10.1002/cncy.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coskuner E, Cevik I, Ozkan A, Dillioglugil O, Akdas A. In the cystoscopic follow-up of non-muscle-invasive transitional cell carcinoma, NMP-22 works for high grades, but unreliable in low grades and upper urinary tract tumors. Int Urol Nephrol. 2012;44(3):793–798. doi: 10.1007/s11255-012-0144-x. [DOI] [PubMed] [Google Scholar]
- 46.Mansoor I, Calam RR, Al-Khafaji B. Role of urinary NMP-22 combined with urine cytology in follow-up surveillance of recurring superficial bladder urothelial carcinoma. Anal Quant Cytol Histol. 2008;30(1):25–32. [PubMed] [Google Scholar]
- 47.Guo A, Wang X, Gao L, Shi J, Sun C, Wan Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can Urol Assoc J. 2014;8(5–6):E347–E352. doi: 10.5489/cuaj.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raitanen MP, Marttila T, Kaasinen E, Rintala E, Aine R, Tammela TL. Sensitivity of human complement factor H related protein (BTA stat) test and voided urine cytology in the diagnosis of bladder cancer. J Urol. 2000;163(6):1689–1692. [PubMed] [Google Scholar]
- 49.O’Brien T, Ray E, Singh R, Coker B, Beard R British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial) Eur Urol. 2011;60(4):703–710. doi: 10.1016/j.eururo.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 50.Ahn JS, Kim HS, Chang SG, Jeon SH. The clinical usefulness of nuclear matrix protein-22 in patients with atypical urine cytology. Korean J Urol. 2011;52(9):603–606. doi: 10.4111/kju.2011.52.9.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balci M, Tuncel A, Guzel O, Aslan Y, Sezgin T, Bilgin O, et al. Use of the nuclear matrix protein 22 Bladder Chek test™ in the diagnosis of residual urothelial cancer before a second transurethral resection of bladder cancer. Int Urol Nephrol. 2015;47(3):473–477. doi: 10.1007/s11255-015-0921-4. [DOI] [PubMed] [Google Scholar]
- 52.Odisho AY, Berry AB, Ahmad AE, Cooperberg MR, Carroll PR, Konety BR. Reflex ImmunoCyt testing for the diagnosis of bladder cancer in patients with atypical urine cytology. Eur Urol. 2013;63(5):936–940. doi: 10.1016/j.eururo.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soyuer I, Sofikerim M, Tokat F, Soyuer S, Ozturk F. Which urine marker test provides more diagnostic value in conjunction with standard cytology-ImmunoCyt/uCyt+ or Cytokeratin 20 expression. Diagn Pathol. 2009;4:20. doi: 10.1186/1746-1596-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vergara-Lluri ME, Hu E, Rao JY, Levin M, Apple SK, Moatamed NA. Comparative evaluation of ProEx C and immunoCyt/uCyt assays in atypical urine cytology. Arch Pathol Lab Med. 2014;138(9):1215–1222. doi: 10.5858/arpa.2013-0433-OA. [DOI] [PubMed] [Google Scholar]