Abstract
Background
One of the pathogenic mechanisms of the nonalcoholic fatty liver disease (NAFLD) is the accumulation of reactive oxygen species, which in turn aggravates the disease progress. We have investigated novel cerium dioxide nanoparticles (nCeO2) due to their promising antioxidant auto-regenerative ability and low toxicity.
Methods
30 white male Wistar rats were divided into 3 groups: control, monosodium glutamate (MSG)-induced obesity and MSG treated with nCeO2 (MSG+nCeO2) groups. Newborn rats of control group were injected with saline (control). MSG− and MSG+nCeO2 groups were injected with MSG (4 mg/g concentration, 8 μl/g volume) between the 2nd and the 10th days of life subcutaneously [13]. At the age of 1 month, rats of group II were administered water 2.9 ml/kg orally, MSG+nCeO2 group received 1 mM solution of nCeO2 1 mg/kg orally. 4-months rats were sacrificed and the liver was harvested for histological and biochemical analysis. To assess the morphological changes in the liver we used NAS (NAFLD activity score). The content of lipid peroxidation products and enzymatic activity of superoxide dismutase (SOD) and catalase in the liver were studied by standard biochemical methods [Refs].
Results
In 4-month rats we found significantly lower total score (1.3±0.26 vs 3.6±0.34, p<0.001), degree of steatosis (1.1±0.18 vs 2.1±0.18, p<0.001), manifestation of lobular inflammation (0.2±0.13 vs 1.2±0.2, p<0.001) and ballooning degeneration (0.0±0.0 vs 0.3±0.15, p=0.034) due to NAS in the nCeO2 group compared to the MSG-group. nCeO2 significantly decreased lipid peroxidation in the liver tissue, namely it reduced the conjugated dienes content by 27% (p<0.05), TBA-products – by 43% (p<0.05) and Schiff bases – by 21% (p<0.05).
Conclusions
Due to its antioxidant properties nCeO2 significantly reduces the incidence of NASH and improves the main NAFLD histological features.
Keywords: non-alcoholic fatty liver disease, reactive oxygen species, Wistar rats, lipid peroxidation, nanoparticles
Background
The attention to the worldwide epidemic of obesity is heightened as it is the fifth leading risk factor for global deaths [1]. Mortality rate from all causes in obese population is at least 20% higher compared to normal-weight [2]. Incidence of obesity strongly correlates with a wide range of the related diseases, such as cardiovascular diseases (except congestive heart failure) [3], type 2 diabetes, all types of cancers (except esophageal cancer in females), asthma, gallbladder disease, osteoarthritis and chronic back pain [4]. In obese individuals lipid metabolism is impaired, which leads to excessive fat accumulation in the body. One of the main sites of potential fat deposition is the liver that is a prime cause of the development of nonalcoholic fatty liver disease (NAFLD).
NAFLD is the most common cause of liver dysfunction in the western world because of its close association with obesity, insulin resistance and dyslipidaemia [5]. NAFLD is defined as the accumulation of lipids within the hepatocytes exceeding 5% of liver weight in the absence of excessive ethanol intake (conventionally defined as an intake of ethanol 20 g/day) and without other causes of liver diseases [6]. The mechanisms involved in the development of fatty liver and in the progression of the disease are unclear, but these may be related to a metabolic profile in the context of a genetic predisposition [7]. Insulin resistance, oxidative stress, cytokines and obesity are identified as the major risk factors involved in NAFLD/NASH pathogenesis. These factors can promote intra-hepatic fat accumulation and lipotoxicity, development of an inflammatory status, oxidative stress, apoptosis and fibrogenesis that determine the progression of the disease [8].
Recently, antioxidant effects of cerium dioxide nanoparticles (nCeO2), a type of engineered nanomaterials, have been reported. These are currently investigated for their possible therapeutic significance. nCeO2 possess catalytic activity, which arises from the presence of two valence states (Ce3+ and Ce4+). Due to the oxygen vacancies present in these nanoparticles, they are able to react with the surrounding reactive oxygen species, thus introducing nCeO2 as a potential in vivo mimetic for endogenous anti-oxidants like superoxide dismutase [9]. However, there are conflicting results about the efficiency of nCeO2 anti-oxidant activity.
One of the pathogenic mechanisms of the NAFLD is the accumulation of reactive oxygen species, which in turn aggravate the disease progress [10]. Consequently, antioxidant therapy is necessary for the successful treatment of the liver injury. Thus, we aimed at investigating the novel cerium dioxide nanoparticles (nCeO2), which have shown promising antioxidant auto-regenerative ability and low toxicity [11,12]
The aim of this study was to investigate the influence of nCeO2 on lipid peroxidation, and antioxidant enzymes activity in rats with experimental induced NAFLD.
Methods
Study design
In order to develop the experimental NAFLD in rats the model of monosodium glutamate (MSG)-induced obesity was used [13–15]. White male Wistar rats (n=30) were used in the study and divided into 3 groups: control, MSG− and MSG+ nCeO2 groups. Newborn rats of control group were injected with saline (control). MSG− and MSG+nCeO2 groups were injected with MSG (4 mg/g, 8 μl/g volume) at 2nd-10th day of life subcutaneously [13]. At the age of 1 month, rats of group II were administered water in a volume of 2.9 ml/kg orally, MSG+nCeO2 group was treated with 1 mM solution of nCeO2 (1 mg/kg orally). The treatments were given intermittently in two-week courses alternated with two-week breaks for 3 months. During the experiment, rats aged between one and four months were fed with standard laboratory chow and tap water ad libitum. 4-month rats were sacrificed and the liver was removed for histological and biochemical analysis.
Histological study
Liver tissue was fixed in 10% formalin, dehydrated and imbedded in paraffin wax. Paraffin sections of 5 μm were cut and stained with hematoxylin and eosin. The specimen were examined under a XS-4130 MICROmed microscope. To assess morphological changes in the liver we used NAS (NAFLD activity score), which included histological features and has been defined as unweighted sum of scores for steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2). According to NAS scores ≥5 are considered as non-alcoholic steatohepatitis (NASH), while those with a NAS <3 are considered as not NASH [16].
Biochemical measurement of lipid peroxidation and antioxidant systems activity
Liver tissue was homogenated for subsequent biochemical analysis. In the liver tissue the content of diene conjugates (DC), TBA-active products and Schiff bases were determined by standard biochemical methods. For evaluation of diene conjugates [17] and Schiff bases [18] 5 ml of mixture of heptane and isopropyl alcohol (1:1) was added to the sample containing 100 μg of protein, homogenized (10 min) and centrifugated (1000 g, 15 min). Water (0.5 ml) was added and two phases were separated. Ethanol (1.5 ml) was added to an upper heptane phase (0.3 ml) and this mixture was subjected to spectrophotometric analysis at 233 nm for the detection of diene conjugates. The DC level was expressed as nmol/mg of protein. Schiff bases were detected in heptane phase with fluorophotometer RF-510, Shimadzu (Japan) at l=360 nm (excitation) and l=420 nm (emission) and expressed as absorbance units (a.u.)/mg of protein.
The TBA-active products were detected by formation of the colored complex with TBA. Briefly, sample with 0.5 mg of protein in TRIS was centrifuged with 0.2 ml of 17% trichloroacetic acid. 0.25 ml of 0.8% TBA was added to 0.5 ml of supernatant and the obtained solution was heated in a boiling water bath for 10 min. The optical density was evaluated by spectrophotometry at 532 nm. The concentration of TBA-active products was presented as nmol/mg of protein.
Antioxidant systems activity was assessed by detection of the activity of superoxide dismutase (SOD) and catalase. SOD activity was determined by Chevari’s method [19] based on the ability of SOD to compete with nitroblue tetrazolium for superoxide anions, which are formed during the interaction of NADH with phenazine methosulfate. In the presence of SOD percentage of superoxide anions recovery by nitroblue tetrazolium is decreased. Catalase activity was detected by the reaction of H2O2 cleavage [20]. H2O2 forms a colored complex with molybdenum salts. The sample containing 100 μg of protein was added to 2 ml of 0.03% H2O2. After 10 min the reaction was stopped by adding of 1 ml of 4% ammonium molybdate. The optical density was detected spectrophotometrically at 410 nm against the blank sample.
Statistical analysis
Statistical analysis was performed by using SPSS-20 software. All data in this study were expressed as means ± standard error (M±SE) or %. Data distribution was analyzed using the Kolmogorov-Smirnov normality test. Continuous variables with parametric distribution were analyzed using Analysis of Variance (ANOVA) and if the differences were significant, a post-hoc Tukeys test was performed. For data with non-parametric distribution Kruskall-Wallis and post-hoc Dunn’s test were conducted for multiple comparisons. For comparisons of categorical variables we conducted χ2 test. The difference between groups was defined to be statistically significant when a p-value was less than 0.05.
Results
Neonatal subcutaneous injection of MSG led to the development of visceral obesity and NAFLD as consequence of metabolic disorder in 4-month rats. Histological analysis confirmed the development of NAFLD in rats. There was evidence of steatosis, lobular inflammation and ballooning degeneration in liver tissue in 4-month treated with MSG neonatally.
In contrast, there was significantly lower total score (1.3±0.26 vs. 3.6±0.34, p<0.001), degree of steatosis (1.1±0.18 vs. 2.1±0.18, p<0.001), manifestation of lobular inflammation (0.2±0.13 vs. 1.2±0.2, p<0.001) and ballooning degeneration (0.0±0.0 vs. 0.3±0.15, p=0.034) due to NAS in the nCeO2 group as compared to MSG-group (Table I, Figures 1, 2). NASH was confirmed only in 30% of the MSG-group of rats (p=0.036).
Table I.
Morphological changes of the liver tissue in the experimental groups.
| Control group (n=20) | MSG-obesity (n=10) | MSG+nCeO2 (n=10) | p | |
|---|---|---|---|---|
| Steatosis (0–3) | 0.10±0.1a | 2.1±0.18c | 1.10±0.18b | <0.001 |
| Lobular inflammation (0–2) | 0.0±0.0a | 1.2±0.20b | 0.2±0.13a | <0.001 |
| Ballooning degeneration (0–2) | 0.0±0.0a | 0.3±0.15a | 0.0±0.0a | 0.034 |
| Total NAS (0–8) | 0.10±0.1a | 3.6±0.34c | 1.3±0.26b | <0.001 |
| Prevalence of NASH, % | - | 30 | - | 0.036 |
Data are presented as the M ± SEM. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis. a, b, c, Values at the same row with different superscript letters shows significant differences with p < 0.05.
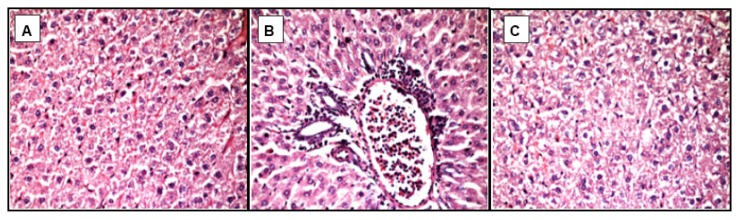
Figure 1.
Light microscopic micrographs of the liver tissue of MSG-group rats stained with hematoxylin and eosin, ×400.
A - pronounced total microvesicular steatosis;
B - microvesicular steatosis with perivascular leukocyte infiltration in zone 3 (mild lobular inflammation);
C - focal necrosis as a result of hepatocytes ballooning degeneration – lack of nuclei (center).
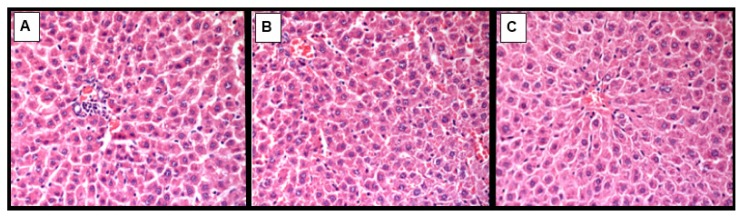
Figure 2.
Light microscopic micrographs of the liver tissue of MSG+nCeO2 rats stained with hematoxylin and eosin, ×400.
A - single perivascular lobular lymphocytes infiltration;
B - focal mild microvesicular steatosis;
C - mainly normal histological structure of hepatocytes.
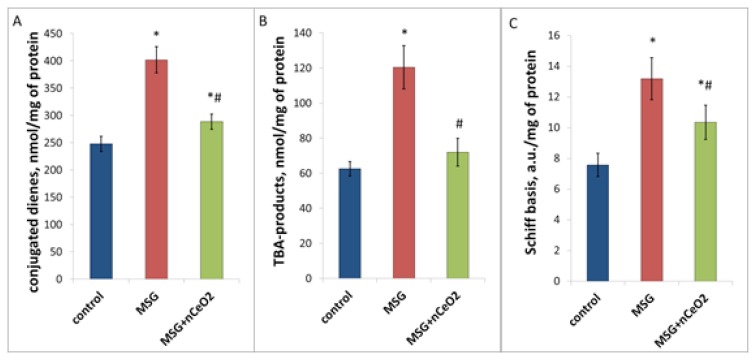
Biochemical analysis showed the increase of lipid peroxidation in the liver tissue under conditions of NAFLD development. This was confirmed by a significant elevation of the lipid peroxidation products. DC in MSG-group was higher by 62.2% (p<0.05), TBA-products – by 92.3% (p<0.05) and Schiff bases – by 72.3% (p<0.05) compared to control values (Figure 3). Short-term periodic oral administration of nCeO2 significantly decreased the lipid peroxidation in liver tissue, namely reduced the DC content by 27% (p<0.05), TBA-products – by 43% (p<0.05) and Schiff bases – by 21% (p<0.05) (Figure 4).
Figure 3.
Lipid peroxidation in the rat liver under conditions of MSG-obesity and nCeO2 (1 mg/kg) treatment. A, B, C – content of conjugated dienes, TBA-products and Schiff basis in the liver, correspondingly. * – p<0.05 compared to control rats without obesity, # – p<0.05 compared to rats with MSG-obesity. Data are presented as the M ± SEM. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis.
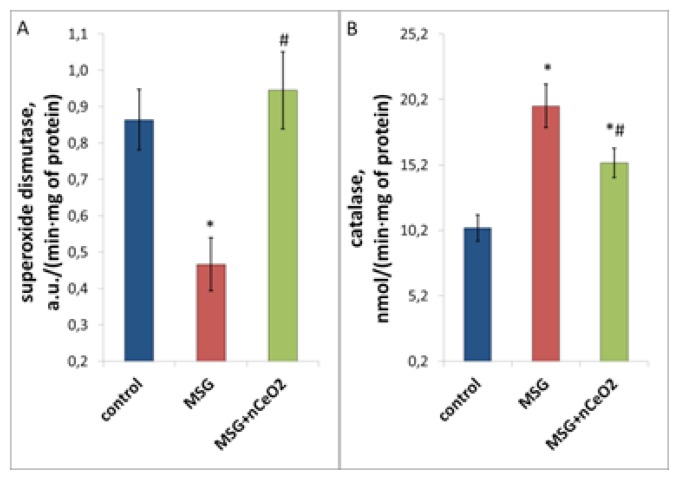
Figure 4.
Antioxidant enzymes activity in the rat liver under conditions of MSG-obesity and nCeO2 (1 mg/kg) treatment. A, B – enzymatic activity of superoxide dismutase and catalase in the liver, correspondingly. * – p<0.05 compared to control rats without obesity, # – p<0.05 compared to rats with MSG-obesity. Data are presented as the M ± SEM. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis.
Worsening of pro/antioxidant balance in the rat liver in case of NAFLD is also caused by alteration of antioxidant enzyme activity. SOD activity in obese rats was decreased by 46.1% (p<0.05), while catalase activity was increased by 90.0% (p<0.05) compared to control (Figure 4). Treatment with nCeO2 led to the restoration of SOD activity to the control values and decrease of excessive catalase activity by 22.1% (p<0.05) compared to the MSG-group (Figure 4). Thus, the studied nanoparticles improved the impaired pro/antioxidant state in the liver under conditions of MSG-induced NAFLD.
Discussion
Oxidative stress is one of the main fundamental phenomena during the development of obesity and NAFLD [20]. nCeO2 mimic SOD and/or catalase activity and have shown promise as a therapeutic application due to their antioxidant auto-regenerative ability and low toxicity [22,23]. The electronic structure of CNPs at the nanoscale underlines their antioxidant activity. Both large surface-to-volume ratio with the reduction in particle size [24,25] and the ability to reversibly switch between Ce3+and Ce4 +present on the surface [26,27] result in the formation of oxygen defects in the crystal lattice that act as “reactive sites” or “hot spots” for reactive oxygen species (ROS) scavenging [28].
Physiochemical properties (size, shape, agglomeration status in liquid and coating) of nCeO2 also could influence their biological response and catalytic activity. Several nanocomposites of CNPs which are used for coating biocompatible polymers (polyethylene glycol, dextran, polyacrylic acid, citric and oleic acid) have been described [29]. In our study, we used near shaped, citrate coated nCeO2 with particle size of 2–5 nm. Sodium citrate in nanocomposites serves not only as a stabilizer, which determines the compositional stability of nCeO2 in water and biologically relevant media, but also plays an important role in the pharmacokinetics of nCeO2 in the cells. Citrate as a component of Krebs cycle provides nCeO2 to the mitochondria, where ROS form under the pathological conditions, leading to oxidative stress.
In recent years, there has been a substantial interest in nCeO2 as a therapeutic agent, and many examples can be found where these nanoparticles have been tested for the treatment of several pathologies, where an imbalance of the redox state occurs [30]. In the present study, we first investigated the influence of nCeO2 on liver lipid peroxidation and antioxidant enzymes activity in rats with experimental NAFLD.
Several previous studies clearly demonstrated the biodistribution of nCeO2 mainly in the spleen and liver, also with trace amounts found in the lungs and kidneys, and virtually none in the heart or brain. Despite the strong accumulation in the spleen and liver for a period of at least 30 days, histological analysis does not reveal any alteration of the organ cytology and show the typical morphology of the tissues without difference between treated and control rats [31]. Only in one study granulomatous formations were found after 30 days in the liver of rats exposed to a single dose of nCeO2 [32]. However, this hepatic damage was probably due to the high dose of administered nCeO2 (85 mg/kg), which resulted in an impaired nanoparticle clearance.
Hirst et al. reported that weekly administration of nCeO2 for 2 or 5 weeks with 0.5 mg/kg to mice with induced liver toxicity (by CCl4) showed similar findings to mice treated with N-acetyl cystine (NAC), a common therapeutic to reduce oxidative stress [31]. These data are in agreement with our findings, where we demonstrate that attenuation of pathological lipid peroxidation after administration of nCeO2 reduces damaging influence of ROS on the liver tissue in MSG-induced animal model of NAFLD.
There is an interesting study reported by Rocca et al, in which nCeO2 were tested both in vitro and in vivo as novel anti-obesity pharmaceutical formulation. They mentioned that nCeO2 interfere with the adipogenic pathway by reducing the mRNA transcription of genes involved in adipogenesis, and by hindering the triglycerides accumulation in 3T3-L1 pre-adipocytes. Intraperitonal administration of 0.5 mg/kg nCeO2 to Wistar rats, did not have toxic effects, but caused significant reduction of weight gain and decrease of plasma levels of insulin, leptin, glucose and triglycerides as compared to control group [33].
Pourkhalili et al. demonstrated that the beneficial antioxidant properties are inherent only to nanoparticles of CeO2 and can be strengthened by additional administration of sodium selenite. The improvement of antioxidant enzymes activity and decrease of cholesterol, triglyceride and low density lipoprotein levels have been demonstrated in streptozotocin-induced diabetic rats after 2 week intraperitoneal injection of nCeO2 with sodium selenite alone or in combination, but the metal form of CeO2 showed no significant improvement [34].
Conclusion
The administration of nCeO2 under conditions of oxidative stress can reduce/prevent its pathological effects. Due to the ability of Ce3+ conversion into Ce4+ nCeO2 effectively neutralizes hydrogen peroxide and hydroxyl radical without generating ROS. Such attenuation of pathological lipid peroxidation reduces the pathological influence of ROS on the liver tissue, which is confirmed by significant improvement of the main NAFLD histological features and restoration of antioxidant enzymes activity. In summary, nCeo2 may have the potential to be used as a treatment for NAFLD, but it should be tested using other animal models of obesity.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing death. Am J Public Health. 2014;104:512–519. doi: 10.2105/AJPH.2013.301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Flanders WD, Ward EM, Jemal A. Body mass index in young adulthood and premature death: analyses of the US National Health Interview Survey linked mortality files. Am J Epidemiol. 2011;174:934–944. doi: 10.1093/aje/kwr169. [DOI] [PubMed] [Google Scholar]
- 4.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126–133. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- 6.Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol. 2014;20:16831–16840. doi: 10.3748/wjg.v20.i45.16831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abenavoli L, Greco M, Nazionale I, Peta V, Milic N, Accattato F, et al. Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:519–527. doi: 10.1586/17474124.2015.1004312. [DOI] [PubMed] [Google Scholar]
- 9.Minarchick VC, Stapleton PA, Sabolsky EM, Nurkiewicz TR. Cerium Dioxide Nanoparticle Exposure Improves Microvascular Dysfunction and Reduces Oxidative Stress in Spontaneously Hypertensive Rats. Front Physiol. 2015;6:339. doi: 10.3389/fphys.2015.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yefimenko OY, Savchenko YO, Falalyeyeva TM, Beregova TV, Zholobak NM, Spivak MY, et al. Nanocrystalline cerium dioxide efficacy for gastrointestinal motility: potential for prokinetic treatment and prevention in elderly. EPMA J. 2015;6(1):6. doi: 10.1186/s13167-015-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobyliak NM, Falalyeyeva TM, Kuryk OG, Beregova TV, Bodnar PM, Zholobak NM, et al. Antioxidative effects of cerium dioxide nanoparticles ameliorate age-related male infertility: optimistic results in rats and the review of clinical clues for integrative concept of men health and fertility. EPMA J. 2015;6(1):12. doi: 10.1186/s13167-015-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savcheniuk OA, Virchenko OV, Falalyeyeva TM, Beregova TV, Babenko LP, Lazarenko LM, et al. The efficacy of probiotics for monosodium glutamate-induced obesity: dietology concerns and opportunities for prevention. EPMA J. 2014;5:2. doi: 10.1186/1878-5085-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savcheniuk O, Kobyliak N, Kondro M, Virchenko O, Falalyeyeva T, Beregova T. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement Altern Med. 2014;14:247. doi: 10.1186/1472-6882-14-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondro M, Mykhalchyshyn G, Bodnar P, Kobyliak N, Falalyeyeva T. Metabolic profile and morpho-functional state of the liver in rats with glutamate-induced obesity. Curr Issues Pharm Med Sci. 2013;26:379–381. [Google Scholar]
- 16.Kobyliak N, Abenavoli L. The role of liver biopsy to assess non-alcoholic fatty liver disease. Rev Recent Clin Trials. 2014;9:159–169. doi: 10.2174/1574887109666141216102231. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilov VB, Gavrilova AR, Khmara NF. Measurement of diene conjugates in blood plasma using the UV absorption of heptane and isopropanol extracts. Lab Delo. 1988;(2):60–64. [PubMed] [Google Scholar]
- 18.Kolesova OE, Markin AA, Fedorova TN. Lipid peroxidation and methods of determining its products in biological media. Lab Delo. 1984;(9):540–546. [PubMed] [Google Scholar]
- 19.Chevari S, Chaba I, Sekeĭ I. Role of superoxide dismutase in cellular oxidative processes and method of its determination in biological materials. Lab Delo. 1985;(11):678–681. [PubMed] [Google Scholar]
- 20.Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE. A method of determining catalase activity. Lab Delo. 1988;(1):16–19. [PubMed] [Google Scholar]
- 21.Shrivastava A, Chaturvedi U, Singh SV, Saxena JK, Bhatia G. Lipid lowering and antioxidant effect of miglitol in triton treated hyperlipidemic and high fat diet induced obese rats. Lipids. 2013;48:597–607. doi: 10.1007/s11745-012-3753-3. [DOI] [PubMed] [Google Scholar]
- 22.Shcherbakov AB, Zholobak NM, Ivanov K, Tretyakov YuD, Spivak NYa. Nanomaterials based on the nanocrystalline ceria: properties and use perspectives in biology and medicine. Biotechnologia Acta. 2011;4:9–28. [Google Scholar]
- 23.Shcherbakov AB, Ivanov VK, Zholobak NM, Ivanova OS, Krysanov EIu, Baranchikov AE, et al. Nanocrystaline ceria based materials--perspectives for biomedical application. Biofizika. 2011;56:995–1015. [PubMed] [Google Scholar]
- 24.Nolan M, Parker SC, Watson GW. Reduction of NO2 on ceria surfaces. J Phys Chem B. 2006;110:2256–2262. doi: 10.1021/jp055624b. [DOI] [PubMed] [Google Scholar]
- 25.Hochella MF, Jr, Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, et al. Nanominerals, mineral nanoparticles, and earth systems. Science. 2008;319:1631–1635. doi: 10.1126/science.1141134. [DOI] [PubMed] [Google Scholar]
- 26.Naganuma T, Traversa E. Stability of the Ce3+ valence state in cerium oxide nanoparticle layers. Nanoscale. 2012;4:4950–4953. doi: 10.1039/c2nr30406f. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande S, Patil S, Kuchibhatla SVNT, Seal S. Size Dependency Variation in Lattice Parameter and Valency States in Nanocrystalline Cerium Oxide. Appl Phys Lett. 2005;87:133113–133116. [Google Scholar]
- 28.Celardo I, Traversa E, Ghibelli L. Cerium oxide nanoparticles: a promise for applications in therapy. J Exp Ther Oncol. 2011;9:47–51. [PubMed] [Google Scholar]
- 29.Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine (Lond) 2013;8:1483–1508. doi: 10.2217/nnm.13.133. [DOI] [PubMed] [Google Scholar]
- 30.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 31.Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, et al. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol. 2013;28:107–118. doi: 10.1002/tox.20704. [DOI] [PubMed] [Google Scholar]
- 32.Tseng MT, Lu X, Duan X, Hardas SS, Sultana R, Wu P, et al. Alteration of hepatic structure and oxidative stress induced by intravenous nanoceria. Toxicol Appl Pharmacol. 2012;260:173–182. doi: 10.1016/j.taap.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Rocca A, Moscato S, Ronca F, Nitti S, Mattoli V, Giorgi M, et al. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine. 2015;11:1725–1734. doi: 10.1016/j.nano.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, Hassani S, Pakzad M, Baeeri M, et al. Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes. 2011;2:204–210. doi: 10.4239/wjd.v2.i11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]