Abstract
Background and aims
Cardiovascular (CV) disease is the leading cause of morbidity and mortality in hemodialysis (HD) patients. Kidney disease is associated with increased oxidative stress (OS), a nontraditional CV risk factor. Few studies evaluate the effect of OS markers on CV events (CVE) and survival in HD patients. The aim of this study is to examine potential determinants of OS markers and their predictive role on survival and CV morbidity and mortality in HD patients during a long-term follow-up (108 months).
Methods
We conducted an analytical cross-sectional prospective observational study, carried on a cohort of randomly selected HD patients. We registered in 44 HD patients baseline characteristics, OS markers, mortality and CVE over a period of 108 months and we used statistical analysis (descriptive, Kaplan-Meier, univariate and multivariate Cox model) for interpretation.
Results
Bound malondialdehyde (bMDA) was positively correlated with serum calcium, protein carbonyls (PC) were inversely correlated with diastolic blood pressure (DBP) and directly correlated with ferritin, NOx was directly correlated with ceruloplasmin) and serum albumin. Of the measured OS markers only bMDA was related to survival (HR=3.29 95% CI (1.28–8.44), p=0.01), and approached statistical significance in the effect on CV mortality (HR=2.85 95% CI (0.88–9.22), p=0.07). None of the measured OS markers was associated with CVE.
Conclusions
bMDA has a strong predictive value on survival in HD patients in a long-term follow-up (9 years). Its value is correlated with CV mortality but is not a predictor of CV events. Regular assessment of MDA in HD patients and the development of strategies aimed at reducing oxidative stress in these patients might be beneficial.
Keywords: oxidative stress, hemodialysis, cardiovascular disease, survival, malondialdehyde
Background and aims
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD). Cardiovascular mortality in patients with end-stage renal disease (ESRD) is 10 to 20 times higher than in the general population [1]. Classic risk factors (diabetes mellitus, smoking, dyslipidemia, high blood pressure, sedentary lifestyle, and obesity) do not entirely explain the high incidence of CV disease in these patients; other, non-traditional risk factors must play a role and amongst them oxidative stress (OS) and inflammation should be emphasized. OS and inflammation are inseparably linked, as they induce and amplify one another [2] and may cause cardiovascular damage. In addition, OS may be involved in the pathogenesis of other cardiovascular risk factors in CKD, such as anemia, amyloidosis and malnutrition [3,4].
HD patients are subjected to enhanced oxidative stress, as a result of increased pro-oxidant activity [5] and reduced anti-oxidant systems [3] related both to end stage renal disease (ESRD) and hemodialysis techniques [6,7]. Diabetes mellitus, advanced age, inflammation, uremia, bio-incompatibility of dialysis membranes and solutions (use of ultrapure dialysate results in less OS than standard dialysate) [8], intravenous iron therapy [9] are the main causes of increased pro-oxidant activity in HD patients [3]. Excessive reactive oxygen species (ROS) levels can produce cellular damage by interacting with biomolecules (proteins, lipids, and nucleic acids) and thus have negative effects on tissue function and structure.
ROS can react with polyunsaturated fatty acids producing lipid hydroperoxides. Malondialdehyde (MDA) is the breakdown product of the major chain reactions leading to definite oxidation of polyunsaturated fatty acids such as linolenic acid and thus is a useful indicator for assessing oxidative damage [10,11]. MDA can interact with DNA and proteins [7] and has been shown to have mutagenic and cytotoxic effects and possibly to be involved in the pathogenesis of several human diseases, including atherosclerosis [11]. MDA levels increase with the progression of kidney dysfunction and in HD patients with dialysis vintage [12]. In biological systems, MDA exists both free (fMDA) and bound (bMDA) to SH and/or NH2 groups of proteins, nucleic acids and lipoproteins. Chemically reactive fMDA is an index of recent and potential damage, while bMDA, excreted by the kidney, is a marker of an older injury [13]. MDA levels in HD patients can be reduced by L-carnitine [14] and N-acetylcysteine [15].
Proteins are susceptible to oxidative stress too, and recently some studies have demonstrated the deleterious effect of serum oxidized albumin on cardiovascular mortality and morbidity in HD patients [16,17]. The biomarker generally used to estimate protein oxidation are protein carbonyls (PC); increased levels have been described in HD patients [18].
Antioxidant systems including enzyme systems, water soluble, and fat-soluble free radical scavengers eliminate reactive oxygen species ideally before they inflict oxidative damage. Ceruloplasmin (CP) is a plasma protein that allows incorporation of iron into transferrin without the formation of toxic iron products. Under physiologic conditions, CP is also important in the control of membrane lipid oxidation, likely by direct oxidation of cations, thus preventing their catalysis of lipid peroxidation [19]. Nitric oxide (NO), part of the antioxidant system, is a gaseous lipophilic free radical cellular messenger synthesized by nitric oxide synthases and which plays an important role in the protection against CV disease. Few long-term follow-up studies that evaluate the impact of OS on morbidity and mortality in HD patients are available in the literature.
The aim of this study is to evaluate the impact of OS markers on CV morbidity and mortality and all-cause mortality in a long-term follow-up and to examine potential determinants of OS markers in HD patients.
Methods
Patients
We conducted an analytical longitudinal prospective observational study, carried on a cohort of HD patients, randomly selected, with the aim to investigate the possible predictive role of OS markers on survival and CV morbidity and mortality during a long term follow-up (108 months). All measurements were performed during a midweek non-dialysis day. Of the 90 patients on conventional HD treatment in Nefromed Dialysis Center Cluj-Napoca, 44 patients met the inclusion criteria and also agreed to participate in this study in 2005. Inclusion criteria were: prevalent HD patients, age>18 years, duration of maintenance hemodialysis at least 6 months (HD vintage), without residual renal function. We excluded patients with acute inflammation processes, terminal neoplasia, previous renal transplantation, immunosuppressive treatment, active hepatitis, antiviral treatment. All patients were on thrice weekly HD (4–5 h) regimen. Patients’ demographics data, duration of maintenance hemodialysis, and comorbidity conditions (diabetes, hypertension, virus hepatitis B or C infection, smoking status, statins treatment) at the time of enrolment were obtained from medical records. We also registered clinical data: age, weight, height, systolic blood pressure (SBP), diastolic blood pressure, (predialysis values). We registered previous cardiovascular disease (cardiac disease evaluated by: electrocardiogram with Q-wave infarction, or myocardial enzyme elevation, coronary revascularization, typical history of angina with abnormal coronarography, neurological disease with new onset focal neurological deficit, or carotid stenosis or lower extremity arterial disease with revascularization or amputation, new onset of intermittent claudication confirmed by Doppler or arteriography findings). We calculated body mass index (BMI) as BMI = (weight (kg)/height2 (m2) and pulse pressure (PP) (mmHg) with formula:PP=SBP-DBP. All patients remained in the study and were followed prospectively for 108 months or until death. During the follow-up period we registered every 6 months the general mortality, fatal CVE (myocardial infarction, congestive heart failure, stroke and sudden death) and non-fatal CVE with the same method as the registration of previous CV disease.
Laboratory parameters
All biochemical analyses were performed after an overnight fast between 7.00–9.00 a.m. always during a midweek non-dialysis day. Current measurements at the initiation of this study included serum electrolytes, albumin, creatinine, uric acid, iron profile (iron, transferrin and ferritin), lipid profile (total cholesterol, triglycerides (TG) and HDL-cholesterol), C-reactive protein (CRP), alkaline phosphatase, intact parathormone (iPTH) and transaminases. Pre-dialysis and post-dialysis urea levels were used to calculate Kt/V. Serum calcium was corrected (cCa) for albumin according to the formula: cCa (mg/dl)=serum calcium(mg/dl)+0.8x(4.0-serum albumin(g/dl), LDL-cholesterol was calculated with Friedewald formula: LDL-cholesterol=total cholesterol-(HDL-chol+TG/5). Hepatitis virus B and C detection was performed by electrochemiluminiscence for HBs antigen (HBs Ag) and hepatitis C virus antibodies (HCV Ab).
Free and bound MDA (nmol/ml) were measured using the thiobarbituric acid test [20] and Satoh [21] methods. Protein carbonyls (nmol/mg) were determined spectrophotometrically by Reznick method [22]. The antioxidant status was evaluated by measuring serum ceruloplasmin (mg%) colorimetrically by Ravin method [23] and nitric oxide was evaluated by measuring serum stable NO metabolites nitrate and nitrite (NOx) (μmol/l) by Griess reaction [24]. Measurements were performed in the Oxidative Stress Laboratory of the Physiology Department, University of Medicine and Pharmacy of Cluj.
Dialysis prescription
All patients were managed by nephrologists and were dialysed with bicarbonate based dialysate, volumetric ultrafiltration control, single use synthetic (polysulphone) dialyzers and heparin as standard anticoagulant. Dialysis prescription was guided by a goal of achieving a value of Kt/V≥1.2. Erythropoietin was prescribed via a standardized algorithm. Antihypertensive drugs were prescribed for patients having post-dialysis or inter-dialysis blood pressure persistently above 150/95 mmHg, at dry weight.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (25th–75th percentile) and categorical variables were expressed as percentages. For identifying correlations between two continuous variables, Pearson’s correlation coefficient or Spearman correlation coefficient were used. Cox proportional hazards regression analysis was used to examine the associations between variables and survival time. Calculation of survival rates were plotted by the Kaplan-Meier method and compared using log-rank test. Variables that were significant in the univariate analysis were included in a multivariate Cox proportional hazards regression model (Enter method). Hazard ratios (HR) and their 95% confidence intervals (CI) were calculated. The cut-off was obtained with ROC (receiver operating characteristic) curve analysis as the value which maximizes the Youden index. p≤0.05 was considered statistically significant. Statistical analyses were performed using Statistica 7.0.
Ethical issues
All patients signed an informed consent before entering the study. Their privacy was respected. The study protocol was in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and was approved by the University Ethics 24 Committee.
Results
Demographical and clinical characteristics of the patients (44 patients) are reported in Table I; 42 patients (95.4%) had an arterio-venous fistula, and 2 (4.6%) patients had a semi-permanent transcutaneous access. Comorbidity conditions included 45.4% hypertension, 52.6% had previous CV events, hepatitis virus infection 43.2%, (20 patients, 2 HBs Ag positive, 18 HCV Ab positive) and no diabetes mellitus. We registered only 4 patients with statin treatment (9.0%).
Table I.
Demographic, clinical and biochemical characteristics of patients.
| Parameter | Group (n=44) |
|---|---|
| Age (years) | 59.79±12.01 |
| Body mass index (kg/m2) | 24.18±4.49 |
| Gender (male) (%) | 28/44 (63.63) |
| Smoking (%) | 25/44 (56.81) |
| Hypertension (%) | 20/44 (45.4) |
| SBP (mmHg) | 129.19 (122.37–136) |
| DBP (mmHg) | 78.02 (74.31–81.73) |
| PP (mmHg) | 49.42 (44.99–53.85) |
| Previous CV events (%) | 23/44 (52.57) |
| HD vintage (months) | 101.10 (78.29–123.95) |
| Kt/V | 1.3 (1.2–1.6) |
| Total chol (mg/dl ) | 164.48±41.14 |
| LDL-chol (mg/dl) | 85.96±28.78 |
| HDL-chol (mg/dl) | 43.32±21.58 |
| Triglycerides (mg/dl) | 180.07±99.36 |
| Hemoglobin (g/l) | 11.08±1.53 |
| Serum albumin (g/l) | 4.21±0.31 |
| CRP (mg/dl) | 0.95±1.34 |
| cCa (mg/dl) | 8.50 (8.10–8.90) |
| P (mg/dl) | 6.50 (5.88–7.11) |
| Serum iPTH (pg/ml) | 719.9 (500.93–938.86) |
| HV positive (%) | 20/44 (45.45) |
| AST (UI/l) | 33.26±13.06 |
| ALT (UI/l) | 28.38±14.01 |
| bMDA (nmol/ml) | 2.40 (1.57–3.23) |
| fMDA (nmol/l) | 2.39±2.70 |
| PC (nmol/mg) | 1.37 (0.86–1.89) |
| CP (mg %) | 26.20 (23–37–29.03) |
| NOx (μmol/l) | 37.48 (25.58–49.39) |
| Epo Dose (U/Kg/week) | 94.41±71.62 |
| Statin treatment (%) | 4/44 (9) |
Data are presented as mean ± standard deviation or median (25th–75th percentile) or absolute or relative frequencies, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, CV: cardiovascular disease, HD: hemodialysis, chol: cholesterol, cCa: calcium corrected by serum albumin, P: phosphate, iPTH: intact parathyroid hormone, CRP: C-reactive protein, HV: hepatitis virus, AST: aspartat aminotransferase, ALT: alanine aminotransferase, bMDA: bound malondialdehyde, fMDA: free malondialdehyde, PC: protein carbonyls, CP: ceruloplasmin, NOx: nitrate/nitrite, EPO: erythropoietin.
The analysis of potential determinants of OS markers revealed a direct correlation of bMDA with cCa (r=0.30, p=0.05) of protein carbonyls with serum ferritin (r=0.33, p=0.04), of ceruloplasmin with NOx (r=0.35, p=0.02) and serum albumin with NOx (r=0.30, p=0.05). An inverse correlation was found between protein carbonyls and DBP (r=−0.31, p=0.02). We found no significant correlations between bMDA and cholesterol and between OS markers and erythropoietin dose.
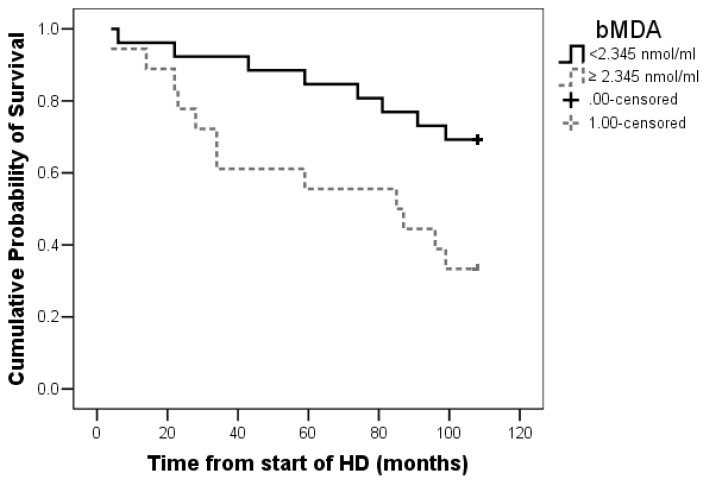
Total mortality rate was 45.4% (20 patients), 10 cardiovascular deaths (cardiovascular mortality rate 22.7%) and 10 deaths of other causes (infections, malignancies, hyperkalaemia and gastro-intestinal bleeding). Identified cut-off value for bMDA was 2.34 nmol/ml: bMDA lower than 2.34 nmol/ml is associated with better survival (p=0.01) (Figure 1).
Figure 1.
Kaplan-Meier survival curves stratified by bMDA (cut-off obtained with ROC curve): p=0.01 bMDA<2.345 nmol/l vs. bMDA≥2.345 nmol/l. bMDA: bound malondialdehyde, HD: hemodialysis
Univariate Cox’s proportional hazards regression analysis showed that PP (HR=1.04 CI 95% (1.00–1.08) p=0.029), cCa (HR=1.75 CI 95% (1.16–2.62) p=0.007), CRP (HR=1.47 CI 95% (1.03–2.11) p=0.03) and bMDA (HR=2.12 CI 95% (0.98–4.58) p=0.05) had a significant association with survival. These parameters were included in a multivariate Cox proportional hazards regression model which shows that bMDA has the strongest influence on survival (HR=3.29 CI 95% (1.28–8.44) p=0.01) (Table II).
Table II.
Cox regression univariate and multivariate analysis, hazard ratio and 95% confidence intervals for survival in HD patients.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| Parameter | Hazard ratio (95% CI) | p (value) | Hazard ratio (95% CI) | p (value) |
| Gender (male) | 1.17 (0.48–2.83) | 0.71 | ||
| Smoking (%) | 0.79 (0.23–2.71) | 0.71 | ||
| HD vintage (months) | 1.00 (1.00–1.00) | 0.07 | ||
| SBP (mmHg) | 1.00 (0.98–1.02) | 0.51 | ||
| DBP (mmHg) | 0.97 (0.94–1.01) | 0.23 | ||
| PP (mmHg) | 1.04 (1.00–1.08) | 0.029 | 1.05 (1.01–1.11) | 0.03 |
| BMI (kg/m2) | 0.94 (0.84–1.05) | 0.34 | ||
| Serum albumin (g/dl) | 0.31 (0.06–1.64) | 0.17 | ||
| Total chol (mg/dl) | 1.00 (0.99–1.01) | 0.11 | ||
| LDL-chol (mg/dl) | 1.01 (0.99–1.02) | 0.07 | ||
| TG (mg/dl) | 0.99 (0.99–1.00) | 0.38 | ||
| HDL-chol (mg/dl) | 1.01 (0.99–1.02) | 0.21 | ||
| Serum iPTH (pg/ml) | 1.00 (1.00–1.00) | 0.47 | ||
| P (mg/dl) | 1.02 (0.82–1.28) | 0.8 | ||
| cCa (mg/dl) | 1.75 (1.16–2.62) | 0.007 | 1.66 (1.02–2.71) | 0.04 |
| CRP (mg/dl) | 1.47 (1.03–2.11) | 0.03 | 1.27 (0.91–1.78) | 0.16 |
| bMDA (nmol/ml) | 2.12 (0.98–4.58) | 0.05 | 3.29 (1.28–8.44) | 0.01 |
| fMDA (nmol/ml) | 0.98 (0.83–1.15) | 0.83 | ||
| PC (nmol/mg) | 0.75 (0.34–1.68) | 0.49 | ||
| CP (mg %) | 1.03 (0.98–1.07) | 0.17 | ||
| NOx (μmol/l) | 1.00 (0.99–1.01) | 0.63 | ||
CI: confidence intervals, HD: hemodialysis, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, BMI: body mass index, chol: cholesterol, cCa: calcium corrected by serum albumin, P: phosphate, iPTH: intact parathyroid hormone, CRP: C-reactive protein, bMDA: bound malondialdehyde, fMDA: free malondialdehyde, PC: protein carbonyls, CP: ceruloplasmin, NOx: nitrate/nitrite.
Sixteen nonfatal CV events occurred within the follow-up period (36.3% of the patients). Univariate Cox regression analysis performed to evaluate the impact of OS markers on fatal and non-fatal CV events showed that only bMDA exerts an influence on fatal CV events that approaches statistical significance (HR=2.85 CI 95% (0.88–9.22) p=0.07). None of the OS markers showed any effect on non-fatal CV events (Table III).
Table III.
Cox regression univariate analysis, hazard ratio and 95% confidence intervals for CVE in HD patients.
| Parameter | non-fatal CVE (n=16) | fatal CVE (n=10) | ||
|---|---|---|---|---|
|
| ||||
| p | HR (95% CI) | p | HR (95% CI) | |
| bMDA (nmol/ml) | 0.10 | 2.01 (0.86–4.68) | 0.07 | 2.85 (0.88–9.22) |
| fMDA (nmol/ml) | 0.59 | 0.89 (0.58–1.35) | 0.79 | 0.96 (0.71–1.29) |
| PC (nmol/mg) | 0.68 | 0.95 (0.78–1.17) | 0.78 | 0.93 (0.56–1.56) |
| CP (mg %) | 0.90 | 0.99 (0.85–1.14) | 0.89 | 0.99 (0.92–1.07) |
| NOx (μmol/l) | 0.09 | 1.00 (0.99–1.01 ) | 0.90 | 1.00 (0.98–1.01) |
CI: confidence intervals, CVE: cardiovascular events, HD: hemodialysis, HR: hazard ratio, bMDA: bound malondialdehyde, fMDA: free malondialdehyde, PC: protein carbonyls, CP: ceruloplasmin, NOx: nitrate/nitrite.
Discussion
Our main finding is that bMDA is a strong predictor of survival in HD patients in a long-term (9 years) follow-up. Low levels of bMDA, suggesting lower oxidative stress, are associated with better survival.
In addition we identified that high bMDA tends to be associated with fatal CV events, but not with non-fatal CV events. Other studies in HD patients had partial similar results: MDA was the single strong predictor of prevalent CV disease in one transversal study, [25] and also MDA was associated with cardiovascular events in other studies [16,17]. Moreover, significantly lower levels of antioxidant markers were related with CV disease [4], but we have not found this association.
The results of our research, only partially similar to previous studies, might be influenced by the characteristics of the study design: absence of diabetic patients, long-term follow-up, high prevalence of hepatitis virus C positive patients. Although we found no correlations between virus status and OS markers Kato et al. observed that VHC infection can enhance oxidative stress in HD patients [26].
The role of OS in CV disease pathogenesis in CKD patients is well-known. Himmelfarb et al. were the first to propose the hypothesis that increased OS and its consequences is a major contributor to increased atherosclerosis and CV morbidity and mortality found in uremia [27,28]. Likewise, via formation of pro-inflammatory oxidized lipids and advanced protein oxidation and glycation end products, OS promotes inflammation. Conversely, by generating reactive oxygen, chlorine, and nitrogen species, activated leukocytes, macrophages and resident cells cause OS [29,11]. Oxidative stress and inflammation induce diminished endothelial function and impair vascular structural and functional parameters [30,31]
Our research is to our knowledge the longest follow-up (9 years) design in the literature to observe the predictive value of bMDA on survival and CV morbidity and mortality. We propose bMDA as a CV risk biomarker in HD patients.
OS markers are determinants of survival and cardiovascular events in HD patients, consequently identification of factors associated with levels of these OS markers is necessary for developing targeted therapies, in order to reduce OS. In our patients, many factors have been associated with OS markers. First, higher bMDA is associated with higher cCa, and indeed high cCa is as expected a predictor of high mortality. Hypercalcemia and increased OS might act synergistically in aggravating the severe vascular lesions found in HD patients.
Second the association of high values of protein carbonyls with high serum ferritin (an iron storage protein but also inflammation marker) might be either the effect of iron treatment or of the inflammation process which can increase OS [9]. Third, higher protein carbonyls were associated with low DBP and hence increased pulse pressure, which was also predictive for increased mortality.
Fourth, higher ceruloplasmin and serum albumin were associated with higher NOx which might reflect the vasodilation improvement in our patients with increased antioxidant capacity. Ceruloplasmin is an antioxidant but also an acute phase reactant [32]. In our study it seems to reflect the antioxidant capacity rather than inflammation because its levels do not correlate with any other inflammation markers. Moreover, NO, an inductor of vasodilation by regulation of vascular tone, inhibition of platelet aggregation and leukocyte adhesion, and prevention of smooth muscle cell proliferation, is affected in HD patients by many factors. NO may increase in some patients during HD by cytokine release [33] while in others it varies during dialysis, in correlation with BP variations [34].
In some studies in HD patients the OS markers (MDA values [29] and plasma advanced oxidation protein products) [35] were associated with lipid profile and erythropoietin treatment [13]. Erythropoietin has anti-oxidative effects [5] which depend on treatment duration and not EPO dose [36]. We did not observe correlations of measured OS markers with erythropoietin dose, the same as described above, and unlike De Vecchi et al. we found no correlation between OS and lipid profile.
Conclusions
bMDA has a strong predictive value on survival in HD patients and is related with CV mortality but is not a predictor of CV events in long-term follow-up (9 years). Regular assessment of MDA in HD patients and the development of strategies aimed at reducing oxidative stress in these patients might be beneficial.
The limit of this study is the relative low number of patients. Future studies might investigate whether MDA is a potential biomarker that can be used to guide strategies for the management of CV risk in HD.
Acknowledgement
This paper was published under the frame of European Social Fund, Human Resources Development Operational Program 2007–2013, project no POSDRU/159/1.5/S/138776.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–S23. [PubMed] [Google Scholar]
- 2.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 4.Hambali Z, Ahmad Z, Arab S, Khazaai H. Oxidative stress and its association with cardiovascular disease in chronic renal failure patients. Indian J Nephrol. 2011;21:21–25. doi: 10.4103/0971-4065.75218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inal M, Kanbak G, Sen S, Akyüz F, Sunal E. Antioxidant status and lipid peroxidation in hemodialysis patients undergoing erythropoietin and erythropoietin-vitamin E combined therapy. Free Radic Res. 1999;31:211–216. doi: 10.1080/10715769900300771. [DOI] [PubMed] [Google Scholar]
- 6.Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 7.El Mesallamy Fawzy Abdel fatah, Elhefnawy Khaled Abdel nabi, El Said Hanna Hosni. Plasma Retinol and Malondialdehyde Levels among Hemodialysis Patients. IJSR. 2015;4:193–200. [Google Scholar]
- 8.Elkabbaj D, Bahadi A, Cherrah Y, Errasfa M, Eljaoudi R. Impact of improving quality of dialysis fluid on oxidative stress and lipid profile in hemodialysis patients. ISRN Nephrol. 2013:717849. doi: 10.5402/2013/717849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishbane S, Mathew A, Vaziri ND. Iron toxicity: relevance for dialysis patients. Nephrol Dial Transplant. 2014;29:255–259. doi: 10.1093/ndt/gft269. [DOI] [PubMed] [Google Scholar]
- 10.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Sung CC, Hsu YC, Chen CC, Lin YF, Wu CC. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxid Med Cell Longev. 2013:301982. doi: 10.1155/2013/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peti A, Csiky B, Guth E, Kenyeres P, Mezosi E, Kovacs GL, Bajnok L. Effect of oxidative stress in hemodialysed patients. eJIFCC. 2011. Available from: www.ifcc.org/media/70145/eJIFCC_v22_02_02.pdf. [PMC free article] [PubMed]
- 13.De Vecchi AF, Bamonti F, Novembrino C, Ippolito S, Guerra L, Lonati S, et al. Free and total plasma malondialdehyde in chronic renal insufficiency and in dialysis patients. Nephrol Dial Transplant. 2009;24:2524–2529. doi: 10.1093/ndt/gfp102. [DOI] [PubMed] [Google Scholar]
- 14.Orasan R, Awon R, Racasan S, Patiu IM, Samasca G, Kacso IM, et al. Effects of L-Carnitine on Endothelial Dysfunction, Visfatin, Oxidative Stress, Inflammation and Anemia in Hemodialysis Patients. Acta Endocrinologica (Buc) 2011;7:219–228. [Google Scholar]
- 15.Trimarchi H, Mongitore MR, Baglioni P, Forrester M, Freixas EA, Schropp M, et al. N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients--a pilot study. Clin Nephrol. 2003;59:441–446. doi: 10.5414/cnp59441. [DOI] [PubMed] [Google Scholar]
- 16.Lim PS, Jeng Y, Wu MY, Pai MA, Wu TK, Liu CS, et al. Serum oxidized albumin and cardiovascular mortality in normoalbuminemic hemodialysis patients: a cohort study. PLoS One. 2013 Jul 29;8(7):e70822. doi: 10.1371/journal.pone.0070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terawaki H, Takada Y, Era S, Funakoshi Y, Nakayama K, Nakayama M, et al. The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther Apher Dial. 2010;14:465–471. doi: 10.1111/j.1744-9987.2010.00841.x. [DOI] [PubMed] [Google Scholar]
- 18.Tbahriti HF, Kaddous A, Bouchenak M, Mekki K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem Res Int. 2013:358985. doi: 10.1155/2013/358985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zengin S, AB, Karta S, Can B, Orkmez M, Taskin A, et al. An assessment of antioxidant status in patients with carbon monoxide poisoning. World J Emerg Med. 2014;5:91–95. doi: 10.5847/wjem.j.issn.1920-8642.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterbauer E, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 21.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 22.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonil assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 23.Ravin HA. An improved colorimetric enzymatic assay of ceruloplasmin. J Lab Clin Med. 1961;58:161–168. [PubMed] [Google Scholar]
- 24.Titheradge MA. The enzymatic measurement of nitrate and nitrite. In: Titheradge MA, editor. Methods in molecular biology. Vol. 100. New Jersey: Humana Press; 1998. pp. 83–91. [DOI] [PubMed] [Google Scholar]
- 25.Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, et al. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999;56:1078–1083. doi: 10.1046/j.1523-1755.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 26.Kato A, Odamaki M, Nakamura H, Yodoi J, Hishida A. Elevation of blood thioredoxin in hemodialysis patients with hepatitis C virus infection. Kidney Int. 2003;63:2262–2268. doi: 10.1046/j.1523-1755.2003.t01-3-00002.x. [DOI] [PubMed] [Google Scholar]
- 27.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 28.Himmelfarb J. Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg? Semin Dial. 2004;17:449–454. doi: 10.1111/j.0894-0959.2004.17605.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel ML, Rekha Sachan, Srivastava AN. Dyslipidemia and Oxidative Stress in Maintenance Hemodialysis Patient- An Emerging Threat to Patient. IJSRP. 2012;2:51–55. [Google Scholar]
- 30.Kaysen GA, Eiserich JP. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004;15:538–548. doi: 10.1097/01.asn.0000111744.00916.e6. [DOI] [PubMed] [Google Scholar]
- 31.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22:636–643. doi: 10.1111/j.1525-139X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 32.Panichi V, Taccola D, Rizza GM, Consani C, Migliori M, Filippi C, et al. Ceruloplasmin and acute phase protein levels are associated with cardiovascular disease in chronic dialysis patients. J Nephrol. 2004;17:715–720. [PubMed] [Google Scholar]
- 33.Sarkar SR, Kaitwatcharachai C, Levin NW. Nitric oxide and hemodialysis. Semin Dial. 2004;17:224–228. doi: 10.1111/j.0894-0959.2004.17310.x. [DOI] [PubMed] [Google Scholar]
- 34.Madore F, Prud’homme L, Austin JS, Blaise G, Francoeur M, Léveillé M, et al. Impact of nitric oxide on blood pressure in hemodialysis patients. Am J Kidney Dis. 1997;30:665–671. doi: 10.1016/s0272-6386(97)90491-1. [DOI] [PubMed] [Google Scholar]
- 35.Marques de Mattos A, Marino LV, Ovidio PP, Jordão AA, Almeida CC, Chiarello PG. Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial. 2012;16:68–74. doi: 10.1111/j.1744-9987.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrijevic ZM, Cvetkovic TP, Djordjevic VM, Pavlovic DD, Stefanovic NZ, Stojanovic IR, et al. How the duration period of erythropoietin treatment influences the oxidative status of hemodialysis patients. Int J Med Sci. 2012;9:808–815. doi: 10.7150/ijms.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]