Abstract
Background and aim
Obesity is a major risk factor for the onset of insulin resistance (IR), hyperinsulinemia and type 2 diabetes mellitus (T2DM) Evidence data has proven that beyond important weight loss bariatric surgery especially Roux-en-Y gastric bypass (RYGB) and bilio-pancreatic diversion (BPD) leads to significant early reduction of insulinemia and of IR calculated through the homeostatic model assessment (HOMA-IR), independently of fat mass decrease. Sleeve gastrectomy (SG) is now used as a sole weight loss operation with good results. Therefore, the aim of the present study was to investigate the early changes of fasting blood glucose, insulin and HOMA-IR in a group of morbidly obese (MO) patients i.e. at 7, 30 and 90 days after SG.
Methods
The study included 20 MO patients (7 male and 13 female) submitted to SG. Anthropometrical (weight, body mass index –BMI, percent excess BMI loss -%EBMIL) and biochemical (plasma glucose, insulin and calculated HOMA-IR ) evaluation were performed before and at 7, 30 and 90 days after SG. In addition, a second group of 10 normal weight healthy subjects with a BMI ranging form 19 kg/m2 to 23.14 kg/m2, matched for age and gender was investigated.
Results
Plasma glucose (p=0.018), insulin (p=0.004) and HOMA-IR (p=0.006) values were statistically different between the studied groups. After surgery, at every follow-up point, there were statistically different weight and BMI mean values relative to the operation day (p<0.003). BMI, decreased at 7 days (estimated reduction=2.79; 95% CI:[2.12;3.45]), at 30 days (estimated reduction=5.65; 95% CI:[3.57;7.73]) and at 90 days (estimated reduction=10.88; 95% CI:[7.35;14.41]) respectively after SG. We noted a tendency toward statistical significant change of mean insulin values at 7 days after surgery (corrected p=0.075), no statistical change at 30 days (corrected p=0.327) and a significant change at 90 days (corrected p=0.027) after SG as compared to baseline. There was a significant change in mean values of HOMA-IR at 30 days (corrected p=0.009) and at 90 days (corrected p=0.021) after the operation day.
Conclusions
The present study showed important early changes consisting in reductions of mean values of plasma insulin and HOMA-IR after SG.
Keywords: morbid obesity, insulin resistance, sleeve gastrectomy
Introduction
Obesity is a major risk factor for the onset of insulin resistance (IR), hyperinsulinemia and type 2 diabetes mellitus (T2DM) [1], a “combination” of conditions that are associated with significant morbidity and mortality [2]. It is well known that weight loss is associated with decrease of IR, and therefore, it delays or prevents the onset of diabetes and can restore blood glucose control in patients with T2DM [3,4]. However, management of weight loss based on diet, exercise and behavioral therapy are associated with poor long term results [5].
Consistent data has proven that bariatric surgery offers an important and sustained weight loss along with significant amelioration/remission of T2DM [6–8]. Moreover, rapid improvement of IR and early resolution of T2DM have been observed to occur after Roux-en-Y gastric bypass (RYGB) [9,10,12] and bilio-pancreatic diversion with duodenal switch (BPD-DS) [11], implying that these changes are unrelated to weight loss, and more likely induced by some hormonal effects of the bariatric procedures through the entero-insular axis [13]. Furthermore, studies have shown that after bariatric surgery, patients who are still obese have insulin sensitivity that is similar to non-obese patients, which, again, indicate that additional factors may be involved in decreasing IR [14]. However, the fact that bariatric surgery reduces IR before significant weight loss remains poorly explained [14,15].
Sleeve gastrectomy (SG) is a purely restrictive operation and has been initially described as a modification of BPD and as part of a staged surgical approach for high-risk morbidly obese (MO) patients. More recently it has been used as a sole weight loss operation [16–19]. SG is regarded as a surgical intervention which in addition to significant weight loss has an early high rate of T2DM resolution [20–22], independent of loss of fat mass, suggesting that it is more than a simple restrictive procedure [18]. Yet, the precise mechanisms involved in the early control of blood glucose after SG are still under research.
This study was designed to test the hypothesis that IR changes rapidly in MO patients submitted to SG. Therefore, the aim of the present study was to investigate the changes of fasting blood glucose, insulin and HOMA-IR in a group of MO patients at 7, 30 and 90 days after SG.
Materials and methods
Study subjects and ethics statement
Twenty MO patients (7 male and 13 female) form the Second Surgical Clinic of Cluj-Napoca were initially enrolled in this study in a prospective and consecutive manner. To be mentioned that in some cases the selected patients missed the follow-up appointments. Patients who met the 1991 bariatric surgery criteria of the National Institutes of Health [23] and those who agreed to participate to the study were included into the study. The exclusion criteria were- acute and chronic inflammatory diseases, infectious diseases, severe endocrine, cardiovascular, hepatic, renal diseases and cancer. Out of our sample of patients 7 had T2DM (treated with oral antidiabetics and with a good blood glucose control), 4 were with impaired fasting glucose (IFG) and 8 of them were within normal range of fasting blood glucose. Because insulinemia is included in the equation for HOMA index calculation, T2DM patients on insulin treatment were excluded. In addition, a second group of 10 normal weight healthy subjects with a BMI ranging form 19 kg/m2 to 23.14 kg/m2, matched for age and gender was investigated.
The present study was carried out in accordance with the ethical principles of the Helsinki Declaration. The research protocol was analyzed and approved by the Ethics Committee of Iuliu Haţieganu University of Medicine and Pharmacy (NO.341/2.06.2015) Cluj-Napoca and written informed consent was obtained from all participants included in the study.
Study design
This was a prospective interventional clinical study, in which MO patients were evaluated anthropometrically and biochemically before and at 7, 30 and 90 days after SG. Protocol appointments included measurements of weight and height based on which BMI and percent excess BMI loss (%EBMIL) were calculated [24]. Also, at the same points, tests were run for the measurement of fasting blood glucose and insulin. Before surgery, diagnosis of T2DM was based on a positive history of T2DM or fasting plasma glucose (FPG) according to criteria established by the American Diabetes Association [25].
After overnight fast blood samples were collected and plasma was obtained through centrifugation. The plasma samples were stored at -80 °C until analysis. The measurements of fasting blood glucose and insulin, were conducted at the Second Surgical Clinic Laboratory and at the Oxidative Stress Laboratory form the Physiology Department of Iuliu Haţieganu University of Medicine and Pharmacy form Cluj-Napoca.
Assays
FPG levels were determined through the biochemical method using the Medist kit. For fasting plasma insulin levels we used the commercially ELISA kit INS-EASIA KAP1251 (DIAsource) according to the specific protocol of the manufacturer. Homeostasis model assessment of insulin resistance (HOMA-IR) was used to evaluate insulin resistance using the following formula HOMA-IR= (fasting insulin μU/ml x fasting glucose mg/dl/)/22.5x18 [26].
Surgical procedure
The greater curvature including the complete fundus was resected from the distal antrum (2–3 cm proximal to the pylorus) to the angle of His (1 cm). We used a laparoscopic stapler, EndoGIA (Autosuture, Norwalk, CT, USA) with a 60-mm cartridge in order to divide the stomach parallel to and alongside a 34 CH bougie. The resected portion of the stomach was extracted from the right upper abdominal 15-mm port site. A running absorbable suture was applied to the staple-line to prevent hemorrhage.
Statistical analysis
The univariate Gaussian distribution for continuous variable was verified separately for control and MO group, by normality tests (Shapiro-Wilk test, D’Agostino skewness test Anscombe-Glynn kurtosis test) and quantile-quantile (Q-Q) plot. The descriptive statistics were expressed as mean ± SD (standard deviation) for all variables as they had a Gaussian distribution. We used the Student’s (t) test for independent samples to assess the significant differences of IR characteristics in control and MO patients and paired Student test to analyze the changes in IR variables at every time-point after surgery as compared to baseline. The bivariate correlations between quantitative variables were performed by Pearson coefficient. The estimated level of statistical significance for all two-sided tests, except for paired t-tests, was set at p<0.05. In the case of multiple comparisons of repeated measures (comparison of IR characteristics before and after surgery follow-up points), we used the Bonferroni’s correction in order to keep the error rate (α) to the specified level of 0.05. In this case, the estimated significance was corrected by multiplying with the number of performed t tests. Because of the missing data, the number of subjects at every follow-up point being different, we used pairwise deletion in all the tests.
The statistical analysis was performed with the IBM SPSS v.19 (Armonk, NY: IBM Corp) and Statistica, version 7.
Results
The subjects’ physical and laboratory characteristics are presented in table 1. Mean values of plasma glucose (p=0.018), insulin (p=0.004) and HOMA-IR (p=0.006) values were statistically different between the studied groups. We noted higher levels of plasma glucose, insulin and HOMA-IR in the MO group. None of the patients presented perioperative complications.
Table 1.
Anthropometric and metabolic characteristics of the subjects at baseline.
| Control group | MO group | Differences in means values | |
|---|---|---|---|
| Mean±SD* | Mean±SD | p-values** | |
| Weight (kg) | 64.60±9.67 | 131±28.48 | 0.015 |
| BMI (kg/m2) | 21.72±1.58 | 46.45±6.94 | 0.01 |
| Fasting glucose (mg/dl) | 91.30±5.23 | 101.90±22.28 | 0.018 |
| Fasting insulin (μUI/ml) | 15.77±1.63 | 28.04±11.35 | 0.004 |
| HOMA-IR | 3.50±0.39 | 7.09±3.15 | 0.006 |
BMI body mass index, HOMA-IR homeostasis model assessment of insulin resistance
SD=standard deviation for each group;
Student’s t test for independent groups
After surgery, there were statistically different mean weight and BMI values, at every follow-up point, relative to the operation day (paired Student’s t test, corrected p<0.003). The analysis of weight changes revealed an estimated mean reduction of 7.31 kg (95% CI:[6;9]) at 7 days, of 15.6 kg (95% CI: [9;22]) at 30 days and of 32 kg (95% CI: [21;42]) at 90 days after SG. Regarding BMI, we observed decreases at 7 days (estimated reduction=2.79; 95% CI:[2.12;3.45]), at 30 days (estimated reduction=5.65; 95% CI:[3.57;7.73]) and at 90 days (estimated reduction=10.88; 95% CI:[7.35;14.41]). Mean BMI showed that by the end of the study the patients were still obese (BMI=37.80±6.72). The %EBMIL was 12.88±5.77% at 7 days, 25.63±13.77% at 30 days and 48.15±12.22 % at 90 days after SG.
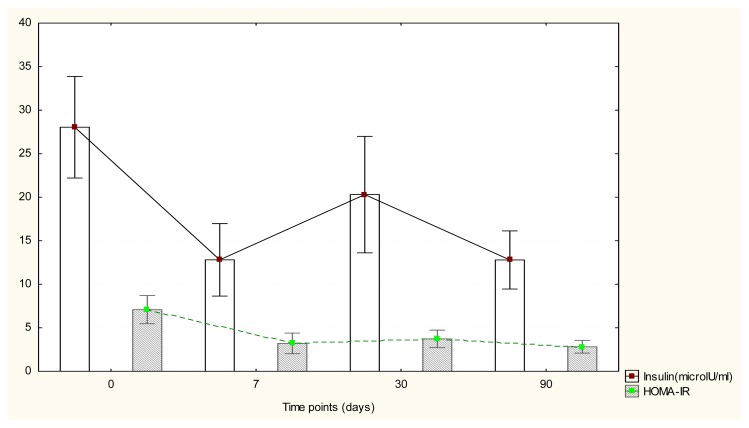
Regarding insulin values, we noted a tendency toward statistical significant change at 7 days after surgery (corrected p=0.075), no statistical change at 30 days (corrected p=0.327) and a significant change of mean values (corrected p=0.027) at 90 days after SG as compared to baseline (Figure.1). For analyzed cases, we obtained an estimated mean reduction of insulin of 12.73 microlU/ml at 7 days (95% CI: [2;23.5], of 8.10 microlU/ml at 30 days (95% CI:[−2.09;18.31]) and of 21.99 microlU/ml at 90 days (95% CI:[9.33;34.64]).
Figure 1.
Insulin and HOMA-IR changes after surgery.
As for HOMA-IR values, there was a significant change in mean values at 30 days after the operation day (corrected p=0.009) and at 90 days (corrected p=0.021) (Figure 1). At 30 days we noted a HOMA-IR reduction level of 3.08 points (95% CI: [1.30;4.85]) while at 90 days a drop of 6.78 points (95% CI:[3.10;10.45]).
No changes were observed as far as blood glucose was concerned (corrected p>0.05).
Because of the small sample size, when analyzing the correlations at baseline and at each follow-up point, we did not identify any statistically significant relationship (p>0.05).
Discussion
The present study aimed at analyzing the early changes of HOMA-IR, fasting plasma insulin and glucose in a group of MO patients who underwent SG. Fasting plasma insulin and HOMA-IR changed early after surgery and reached values comparable with those of the control group by the end of the study. Also, we observed that at each follow-up point BMI dropped, but, the patients were still obese (BMI=37.80 kg/m2) at 3 months after SG.
Significant decrease of IR during the first 3 months following bariatric surgery (laparoscopic adjustable gastric banding LAGB and RYGB) has been reported previously [10]. LAGB is a purely restrictive technique, therefore the findings showed that the caloric restriction imposed by LAGB plays an important role in reducing IR. However, the authors observed that IR was lower after RYGB as compared to the LAGB patients. RYGB implies the rearrangement of the gastrointestinal tract and therefore the study suggested that beyond caloric restriction other mechanisms may also be involved in the greater drop of IR following this type of intervention [10]. Furthermore, some studies showed early improvement in IR within days of surgery [9,11,12] clearly unrelated to weight loss, which occurred much more slowly. Taken together, mounting evidence showed significant improvement of glucose homeostasis beyond food restriction and weight loss, e.g. by favorable changes in gastrointestinal hormones, such as incretins that enhance insulin sensitivity, at least after some kinds of bariatric procedures [13,27,28].
SG has been initially proposed as a first step operation in high risk patients submitted to laparoscopic BPD-DS in order to shorten the duration of the intervention, with the duodeno-ileostomy and ileo-ileostomy as a second stage a few months later [29]. Patients achieved remarkable weight loss after the first stage of this approach, and therefore SG has started to be used as an independent antiobesity operation [28]. Although SG was considered at first a purely restrictive operation, which does not involve rearrangement of the gastrointestinal tract, studies have shown rapid improvements of HOMA-IR and plasma glucose suggesting the hypothesis that SG might be more than a simple gastric restriction [30, 31]
Herein, the mean values of fasting plasma insulin and HOMA-IR were increased at baseline. Early after surgery, at each follow-up point we observed important changes of these parameters and all in all these changes consisted in reductions of the plasma levels. Moreover, the values reached by the end of the study were comparable with those of the control group, even though the patients were still obese. However, as we can see in the figure, although 7 days after surgery there is a tendency towards decrease of both parameters, an increase can be observed 30 days after SG. An interesting issue to be addressed is whether the decrease of IR is influenced by the presence/absence of T2DM. If we look at the literature we see that some show a lesser increase in insulin sensitivity in T2DM patients when compared with those with IFG, while others show no significant difference between normo and hyperglycaemic patients after bariatric surgery. Taken together, there is no consensus on insulin sensitivity change in non-diabetic patients when compared with diabetic patients [15].
An important drop in HOMA-IR to near normal values was reported also by Eickhoff et al. [32] as early as 3 months after SG in a group of obese patients with IFG or type 2 diabetes. Rizello et al.[30] showed in a group of MO patients with T2DM submitted to SG a sharp (5 days) and significant reduction of insulin concentration (from 41.8±13.2μg/l to 14.8±7.92μg/l) and HOMA IR (from 17.1±9.4 to 4.3±3.4). Furthermore, they noted that at the 15th postoperative day serum insulin concentration and HOMA-IR remained significantly lower in the absence of significant weight modifications. However 30 and 60 days after SG these values remained substantially unchanged in spite of a greater weight loss [30]. A recent study showed that HOMA-IR and BMI decreased significantly in the first week after SG, but, however percent weight loss was not correlated with percent changes in all of the glycemic parameters at this follow-up schedule [31]. Other studies however, did not reveal a significant decrease of HOMA-IR at one week or one month after SG, but only by the 3rd month [33,34]. In this respect, Rao et al.[15] showed in an elegant meta-analysis that the change in HOMA-IR at 1–2 weeks was not statistically significant, although it was strongly trending and that an increasing follow-up time was found to produce an increasing effect size.
Taken together these data suggest that SG displays an additional mechanism (other than caloric restriction and weight loss) that contributes to changes in T2DM and HOMA-IR and confirms that LSG is more than a restrictive procedure. A hormonal mechanism has been suggested. Firstly, ghrelin which is a hormone produced mainly by the gastric fundus may play a role in short- and long-term energy balance as it seems to suppress the insulin-sensitizing hormone adiponectin, block hepatic insulin signaling, and inhibit insulin secretion. Thus, the removal of the gastric fundus through SG may lead to reduced ghrelin secretion and therefore to improvement of the glucose metabolism [18]. Secondly, YY peptide (PYY) and glucagon-like peptide 1 (GLP-1) seem to be increased after SG even though this procedure does not involve rerouting of the food. A potential explanation for PYY and GLP-1 increases following LSG could be accelerated gastric emptying and earlier contact of chyme with the L cells of the hindgut [35]. Last but not least, the mechanisms that are involved in SG’s beneficial effects on glucose metabolism are complex and merit further investigation.
Our study has its limitations. First, the small sample of patients and lack of continuity in presenting at all the follow-up points. Secondly, our patients belonged to different categories in regard to the glycaemic state.
In conclusion, the present study has confirmed the hypothesis that there is an early change of IR after SG. Furthermore, this change is based on reductions of HOMA-IR and fasting plasma insulin values.
Acknowledgments
This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143(4):897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip S, Plank LD, Murphy R. Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta-analysis of outcomes. Obes Surg. 2013;23(12):1994–2003. doi: 10.1007/s11695-013-1030-z. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson J, Lindström J, Tuomilehto J. Potential for the prevention of type 2 diabetes. Br Med Bull. 2001;60:183–199. doi: 10.1093/bmb/60.1.183. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and andmeta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Heneghan HM, Nissen S, Schauer PR. Gastrointestinal surgery for obesity and diabetes: weight loss and control of hyperglycemia. Curr Atheroscler Rep. 2012;14(6):579–587. doi: 10.1007/s11883-012-0285-5. [DOI] [PubMed] [Google Scholar]
- 9.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 10.Ballantyne GH, Farkas D, Laker S, Wasielewski A. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16(9):1189–1197. doi: 10.1381/096089206778392158. [DOI] [PubMed] [Google Scholar]
- 11.Adami GF, Cordera R, Camerini G, Marinari GM, Scopinaro N. Recovery of insulin sensitivity in obese patients at short term after biliopancreatic diversion. J Surg Res. 2003;113(2):217–221. doi: 10.1016/s0022-4804(03)00189-6. [DOI] [PubMed] [Google Scholar]
- 12.Faria G, Preto J, da Costa EL, Guimarães JT, Calhau C, Taveira-Gomes A. Acute improvement in insulin resistance after laparoscopic Roux-en-Y gastric bypass: is 3 days enough to correct insulin metabolism? Obes Surg. 2013;23(1):103–110. doi: 10.1007/s11695-012-0803-0. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, Overduin J, Shannon MH, Foster-Schubert KE 2004 ABS Consensus Conference. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1(3):358–368. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Fuentes E, Garcia-Almeida JM, Garcia-Arnes J, Rivas-Marín J, Gallego-Perales JL, González-Jiménez B, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16(9):1179–1188. doi: 10.1381/096089206778392383. [DOI] [PubMed] [Google Scholar]
- 15.Rao RS, Yanagisawa R, Kini S. Insulin resistance and bariatric surgery. Obes Rev. 2012;13(4):316–328. doi: 10.1111/j.1467-789X.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery. Sleeve gastrectomy as a bariatric procedure. Surg Obes Rel Dis. 2007;3(6):573–576. doi: 10.1016/j.soard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25–27, 2007. Obes Surg. 2008;18(5):487–496. doi: 10.1007/s11695-008-9471-5. [DOI] [PubMed] [Google Scholar]
- 18.Papailiou J, Albanopoulos K, Toutouzas KG, Tsigris C, Nikiteas N, Zografos G. Morbid obesity and sleeve gastrectomy: how does it work? Obes Surg. 2010;20(10):1448–1455. doi: 10.1007/s11695-010-0148-5. [DOI] [PubMed] [Google Scholar]
- 19.Slater BJ, Bellatorre N, Eisenberg D. Early postoperative outcomes and medication cost savings after laparoscopic sleeve gastrectomy in morbidly obese patients with type 2 diabetes. J Obes. 2011;2011:350523. doi: 10.1155/2011/350523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6(6):707–713. doi: 10.1016/j.soard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Vidal J, Ibarzabal A, Romero F, Delgado S, Momblán D, Flores L, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. 2008;18(9):1077–1082. doi: 10.1007/s11695-008-9547-2. [DOI] [PubMed] [Google Scholar]
- 22.Bayham BE, Greenway FL, Bellanger DE, O’Neil CE. Early resolution of type 2 diabetes seen after Roux-en-Y gastric bypass and vertical sleeve gastrectomy. Diabetes Technol Ther. 2012;14(1):30–34. doi: 10.1089/dia.2011.0151. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard VS, Hall WH. Gastrointestinal surgery for severe obesity. Obes Surg. 1991;1(3):257–265. doi: 10.1381/096089291765560962. [DOI] [PubMed] [Google Scholar]
- 24.Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606. doi: 10.1007/s11695-015-1645-3. [DOI] [PubMed] [Google Scholar]
- 25.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 26.Mattheus DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessement: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 28.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 29.Gagner M, Inabnet WB, Pomp A. Laparoscopic gastrectomy with second stage biliopancreatic diversion and duodenal switch in the super-obese. In: Inabnet WB, DeMaria EJ, Ikramuddin S, editors. Laparoscopic Bariatric Surgery. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 143–149. [Google Scholar]
- 30.Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20(1):50–55. doi: 10.1007/s11695-009-0017-2. [DOI] [PubMed] [Google Scholar]
- 31.Meydan C, Goldstein N, Weiss-Shwartz E, Lederfine D, Goitein D, Rubin M, et al. Immediate Metabolic Response Following Sleeve Gastrectomy in Obese Diabetics. Obes Surg. 2015;25(11):2023–2029. doi: 10.1007/s11695-015-1669-8. [DOI] [PubMed] [Google Scholar]
- 32.Eickhoff H, Guimarães A, Louro TM, Seiça RM, Castro E, Sousa F. Insulin resistance and beta cell function before and after sleeve gastrectomy in obese patients with impaired fasting glucose or type 2 diabetes. Surg Endosc. 2015;29(2):438–443. doi: 10.1007/s00464-014-3675-7. [DOI] [PubMed] [Google Scholar]
- 33.Mallipedhi A, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):860–869. doi: 10.1016/j.soard.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbély Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7(5):561–568. doi: 10.1016/j.soard.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]