CME/CNE Information
Activity Available Online: To access the article, post-test, and evaluation online, go to http://www.cmscscholar.org.
Target Audience: The target audience for this activity is physicians, physician assistants, nursing professionals, and other health-care providers involved in the management of patients with multiple sclerosis (MS).
Learning Objectives:
1) Be able to discuss the risk of new onset of fatigue and comorbidities associated with increased fatigue with MS patients and their families
2) Be able to identify and address comorbidities that are most associated with fatigue in MS patients
Accreditation Statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Consortium of Multiple Sclerosis Centers (CMSC), Nurse Practitioner Alternatives (NPA), and Delaware Media Group. The CMSC is accredited by the ACCME to provide continuing medical education for physicians.
The CMSC designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurse Practitioner Alternatives (NPA) is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center's Commission on Accreditation.
NPA designates this enduring material for a maximum of 1.0 Continuing Nursing Education credits.
Disclosures: Francois Bethoux, MD, Editor in Chief of the International Journal of MS Care (IJMSC), has served as Physician Planner for this activity. He has disclosed no relevant financial relationships.
Laurie Scudder, DNP, NP, has served as Nurse Planner for this activity. She has disclosed no relevant financial relationships.
The authors (listed in the article's author byline) and the anonymous peer reviewers for the IJMSC have disclosed no relevant financial relationships.
Method of Participation:
Release Date: April 1, 2016
Valid for Credit Through: April 1, 2017
In order to receive CME/CNE credit, participants must:
1) Review the CME/CNE information, including learning objectives and author disclosures.
2) Study the educational content.
3) Complete the post-test and evaluation, which are available at http://www.cmscscholar.org.
Statements of Credit are awarded upon successful completion of the post-test with a passing score of >70% and the evaluation.
There is no fee to participate in this activity.
Disclosure of Unlabeled Use: This CME/CNE activity may contain discussion of published and/or investigational uses of agents that are not approved by the FDA. CMSC, NPA, and Delaware Media Group do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the authors and do not necessarily represent the views of CMSC, NPA, or Delaware Media Group.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any medications, diagnostic procedures, or treatments discussed in this publication should not be used by clinicians or other health-care professionals without first evaluating their patients' conditions, considering possible contraindications or risks, reviewing any applicable manufacturer's product information, and comparing any therapeutic approach with the recommendations of other authorities.
Abstract
Background: Fatigue is commonly reported by people with multiple sclerosis (MS). Comorbidity is also common in MS, but its association with the presence of fatigue or fatigue changes over time is poorly understood.
Methods: Nine hundred forty-nine people with definite MS were recruited from four Canadian centers. The Fatigue Impact Scale for Daily Use and a validated comorbidity questionnaire were completed at three visits over 2 years. Participants were classified into groups with no fatigue versus any fatigue. Logistic regression was used to determine the relationship between fatigue and each comorbidity at baseline, year 1, year 2, and overall.
Results: The incidence of fatigue during the study was 38.8%. The prevalence of fatigue was greater in those who were older (P = .0004), had a longer time since symptom onset (P = .005), and had greater disability (P < .0001). After adjustment, depression (odds ratio [OR], 2.58; 95% confidence interval [CI], 2.03–3.27), irritable bowel syndrome (OR, 1.71; 95% CI, 1.18–2.48), migraine (OR, 1.69; 95% CI, 1.27–2.27), and anxiety (OR, 1.57; 95% CI, 1.15–2.16) were independently associated with fatigue that persisted during the study. There was also an individual-level effect of depression on worsening fatigue (OR, 1.49; 95% CI, 1.08–2.07).
Conclusions: Comorbidity is associated with fatigue in MS. Depression is associated with fatigue and with increased risk of worsening fatigue over 2 years. However, other comorbid conditions commonly associated with MS are also associated with persistent fatigue, even after accounting for depression. Further investigation is required to understand the mechanisms by which comorbidities influence fatigue.
Fatigue is the most common symptom of multiple sclerosis (MS),1 and many people with MS consider fatigue to be among their worst symptoms.2 The prevalence of fatigue in MS ranges from 50% to 83%1–3; this reported variability may be explained by differences in sample characteristics and by how fatigue is operationalized and measured. Fatigue in MS has been conceptualized as “a significant lack of physical and/or mental energy that is perceived by the individual or caretaker to interfere with usual or desired activity.”4(p2) Fatigue is associated with reduced employment and lower quality of life.5 Despite its importance, however, few studies have examined fatigue in MS over time,6–10 and only one incidence estimate of 25% over 1 year has been reported.10
Fatigue is a prominent and chronic symptom of many neurologic conditions and can be characterized as peripheral (ie, arising at the neuromuscular junction) or central, with the latter reflecting its effect on cognitive and motor functioning and arising in the context of central nervous system dysfunction, as in MS.11 In MS, fatigue has also been conceptualized as either primary (ie, a direct consequence of MS pathology) or secondary to other factors, which may or may not be related in some way to MS.12 A few studies have explored determinants of secondary fatigue in MS, but they are limited to sociodemographics,3 pain, and psychiatric comorbidities, which are more common in individuals with fatigue.6–9,13,14
The impact of nonpsychiatric comorbidities on fatigue in MS has received limited attention, although preliminary evidence suggests that they can affect fatigue management.15 Such medical comorbidities are common in MS and have deleterious effects on outcomes, including mortality.16 Still, it remains poorly understood how comorbidities affect the risk and pattern of fatigue in MS. We aimed to 1) determine the incidence of fatigue and changes in fatigue over time in MS and 2) identify physical and mental comorbid conditions that are associated with fatigue and with changes in fatigue over time in MS.
Materials and Methods
Study Population
We recruited consecutive patients attending routine visits between July 1, 2010, and March 31, 2011, at four MS clinics across Canada: British Columbia, Alberta, Manitoba, and Nova Scotia. A trained research coordinator approached potential participants using a standardized recruitment script. The inclusion criteria were as follows: a confirmed diagnosis of MS according to the prevailing diagnostic criteria at the time the participant was diagnosed,17–20 age 18 years or older at enrollment, sufficient knowledge of English to be able to complete the questionnaires, and ability to provide informed consent. Ethics approval was obtained at all of the sites.
Clinical Information
A trained reviewer used a standard form to extract demographic and clinical information from medical records: sex, date of birth, race, clinical course,21 age at MS symptom onset, Expanded Disability Status Scale (EDSS) score22 logged by the treating neurologist on the day of recruitment and at follow-up visits, history of relapses (location, resolution, and treatment) since the last visit, and disease-modifying therapy use.
Questionnaires
Participants completed questionnaires at each of three assessments over 2 years (baseline, year 1, and year 2). All self-report measures were administered to participants at their routine clinic visits. Because we aimed to obtain a representative consecutive series of patients as they presented to the clinics, we attempted to minimize the response burden for participants. Twenty-one physical and psychiatric comorbidities (such as diabetes and depression), including those that could be considered secondary to MS (such as osteoporosis) were captured via a questionnaire that asked, “Has a doctor ever told you that you have any of the following conditions?” to which a yes or no answer was required.23 The comorbidities selected had been reported to affect outcomes in MS, were potentially modifiable with treatment, and affected a sufficient number of individuals with MS to be relevant at the population level, and the questionnaire had been validated previously against medical record review.23 We also included an alcohol dependence measure (assessed by the four-item CAGE [Cutting down, Annoyance by criticism, Guilty feeling, Eye-openers] questionnaire)24 given the high prevalence of alcohol dependence in the MS population25 and its associations with anxiety and depression.26
We measured current symptoms of depression using the depression subscale of the Hospital Anxiety and Depression Scale, which has been validated for use in MS, and we used the recommended cutoff score of 8 or greater to define clinically significant depressive symptoms.27,28 Lifetime history of depression was measured using the aforementioned comorbidity questionnaire. To account for current and past depression in the analyses, four mutually exclusive groups were defined: 1) no history of depression and no current depressive symptoms (reference group); 2) no history of depression and current depressive symptoms; 3) a history of depression (diagnosed before the study visit when questionnaires were administered) and no current depressive symptoms; and 4) a history of depression and current depressive symptoms. If depression was diagnosed before completion of the Hospital Anxiety and Depression Scale (and not at the same visit), a person was considered to have a history of depression.
Multiple self-report questionnaires exist for the evaluation of fatigue, all of which have limitations.29 We selected a brief scale that minimized participant burden and measured fatigue using the Fatigue Impact Scale for Daily Use (D-FIS).30 This unidimensional scale with item content derived from the Fatigue Impact Scale2 examines perceived effect on cognitive and physical functioning. It has been validated in patients with MS previously27 and has data available regarding clinically important change. Scores range from 0 to 36, with higher scores indicating worse fatigue. Based on the responsiveness of this scale in its original validation study to flu-related fatigue30 and based on the clinically meaningful difference values suggested from its subsequent validation study in people with MS (ie, standard error of the mean = 3.18, and ½ standard deviation = 3.65),31 a change of at least 4 points on the D-FIS between visits was considered clinically important.30 Fatigue was operationalized in two ways: 1) no versus any fatigue (D-FIS scores <5.0 vs. ≥5.0) and 2) no versus mild, moderate, or severe fatigue (D-FIS scores grouped as <5.0, ≥5 to <12.0, ≥12.0 to <19.0, and ≥19.0, respectively). These cut-points were chosen to be clinically meaningful based on findings in the initial validation of the D-FIS and its subsequent validation in people with MS. In individuals with the flu, the mean D-FIS score 10 days or more after flu (ie, recovered) was less than 5, and the mean D-FIS score on day 1 of the flu was 19, and scores greater than 19 were associated with missed work due to illness. In the MS population, 19 was the median D-FIS score for the fatigued MS sample. For longitudinal analyses, worsening fatigue (increase of ≥4 points between visits) was contrasted with fatigue that stayed the same or improved.
Data Analyses
Descriptive statistics (means, medians) were calculated with the accompanying measure of variability (standard deviation [SD], interquartile range). Those with and without any fatigue were compared using t tests, Wilcoxon rank sum tests, or tests of proportions. For all logistic models, odds ratios (ORs) with 95% confidence intervals (CIs) are reported. The Box-Tidwell model transformation was used to test the assumption of linearity of the logit model.32 To assess potential differential effects of age (continuous) and sex on the relationship between comorbidities and fatigue, baseline logistic regression analyses were adjusted using an interaction term for age or sex. Covariates used in all other logistic models include time since symptom onset (continuous, in years), disability status (EDSS score, categorized as 0.0–3.0, 3.5–5.5, and 6.0–9.0), educational level (high school or less, greater than high school, other), age (continuous, in years), and sex. The level of statistical significance was set at α= 0.05 to minimize type 2 error when assessing potentially novel associations.33 The statistical software programs STATA version 13.0 (StataCorp LP, College Station, TX) and SAS version 9.3 (SAS Institute Inc, Cary, NC) were used for the analyses.
Fatigue Prevalence
Clinical and demographic characteristics, including disease course, age, sex, disability status (EDSS score), educational level, and time since symptom onset, were compared between those with and without any fatigue at baseline (ie, D-FIS score ≥5 vs. <5). Disease courses were examined as distinct entities to characterize the population (ie, primary progressive, relapsing-remitting, and secondary progressive MS). The full range of the EDSS was used.
Fatigue Incidence
We calculated the incidence of any fatigue at years 1 and 2 as the number of people who developed fatigue at that visit divided by the number of people at risk for incident fatigue (ie, those in the no fatigue group at any previous visit). Incident comorbidities were calculated similarly. The cumulative incidence of fatigue, and of comorbidity, over 2 years was also calculated. Risk factors for incident fatigue at any point during the study, including sex, age, educational level, alcohol dependence, disease-modifying therapy use, and disease course, were assessed using univariate logistic regression.
Comorbidities
For multivariable analyses we included specific comorbidities with a prevalence of at least 5% in the sample (ie, depression, hypertension, migraine, hypercholesterolemia, anxiety, chronic obstructive pulmonary disease [COPD], irritable bowel syndrome [IBS], autoimmune thyroid disease, and osteoporosis) because these conditions were considered to be sufficiently frequent to be relevant at the population level, and each had a sufficient number of individuals affected to provide stable estimates. For the longitudinal analyses (see later herein) we also examined the presence of any comorbidity (including only those with a prevalence of ≥5% based on the sample size because there would be a small number of other incident comorbidities).
Fatigue and Comorbidities over Time
Using an unadjusted ratio, we compared the frequency of worsening fatigue among participants with each specific incident comorbidity with those who had not newly developed that comorbidity (but could already have the comorbidity and be in the comparison group). To determine the effect of specific comorbidities on fatigue (no, mild, moderate, or severe) over time, we used ordinal generalized estimating equation (GEE) models with an independence correlation structure to account for repeated measures within individuals. We used the four fatigue categorizations for these analyses. We included time × comorbidity interaction terms to determine the effect of the development of each comorbidity on fatigue. The GEE models generate ORs that are population averages of within-subject and between-subject effects, but they tend to be dominated by between-subject effects.
To better determine within-person effects over time, we modeled change in fatigue with logistic GEE using the method recommended by Twisk.34 Change scores were calculated as the change in fatigue between visits, and then they were categorized into two groups based on whether an increase of 4 points or more (worsening) was reached. Time-varying covariates were also modeled in the logistic GEE of worsening fatigue as change scores (eg, difference in EDSS scores between visits; incident comorbidity).
Results
Study Participants
As previously reported,35 of 1632 patients screened for eligibility and scheduled for an MS clinic visit during the study period, 1144 met the inclusion criteria. Of these, 949 consented to participate (83.0%), of whom 94.6% completed the study. Twenty-nine participants were lost to follow-up at year 1, and 22 were lost to follow-up at year 2. No baseline differences were found in age, proportion reporting fatigue, median EDSS score, or prevalence of comorbidity between those who completed the study and those who were lost to follow-up (data not shown). Most participants were female and white, with a mean (SD) age of 48.6 (11.4) years (Table 1). The mean (SD) time since symptom onset was 15.4 (10.2) years, and 72.4% had relapsing-remitting MS (Table 1).
Table 1.
Demographic characteristics of the multiple sclerosis cohort at baseline
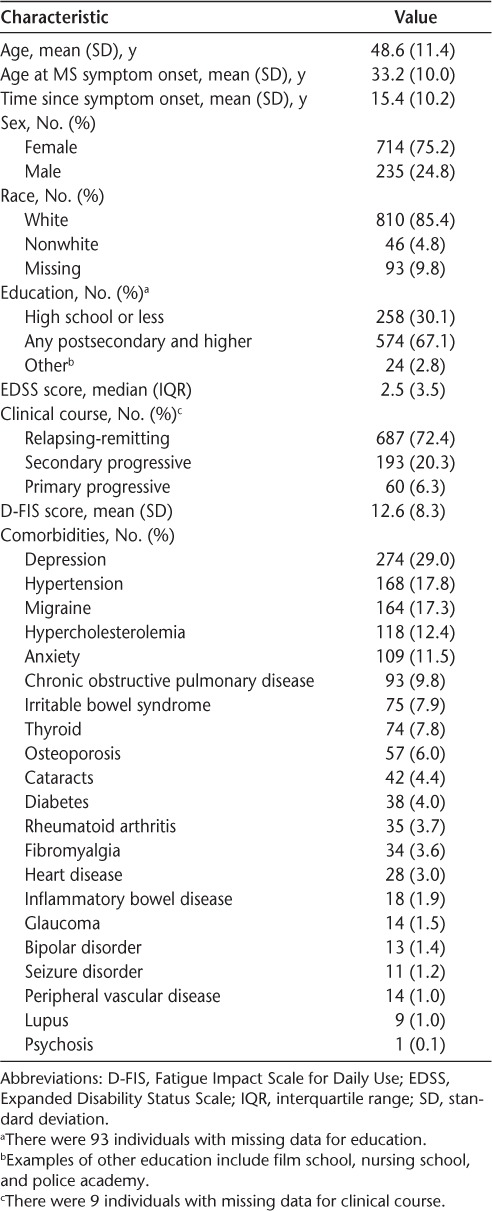
At baseline, 41.5% of the participants had at least one comorbidity; most common was depression, followed by hypertension, migraine, hypercholesterolemia, and anxiety (Table 1). The incidence of any comorbidity per 100 persons was 18.3 at year 1, 21.2 at year 2, and 28.3 over the entire study period. The most common incident comorbidities over the entire study period were depression (5.5 of 100 persons), anxiety (4.6 of 100 persons), and hypercholesterolemia (3.7 of 100 persons).
Prevalence and Severity of Fatigue at Baseline
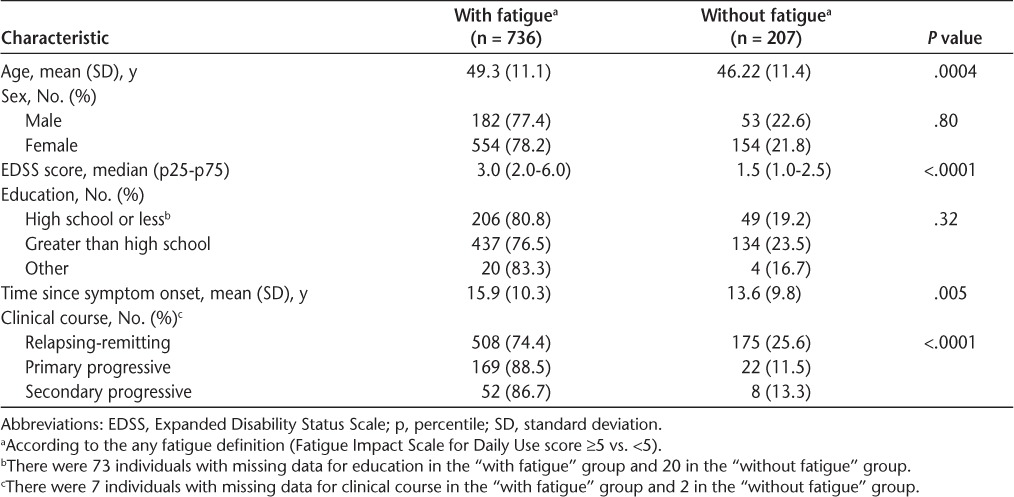
The prevalence of no fatigue was 21.8% (95% CI, 19.3%–24.6%); mild fatigue, 24.1% (95% CI, 21.5%–27.0%); moderate fatigue, 27.3% (95% CI, 24.5%–30.2%); and severe fatigue, 26.1% (95% CI, 23.4%–29.0%), with a median D-FIS score of 12.0 (interquartile range = 13.0). The highest prevalence of any fatigue was reported by those with primary progressive MS, followed by secondary progressive and relapsing-remitting MS (Table 2). The prevalence of any fatigue was greater in those who were older (P = .0004), who had a longer time since symptom onset (P = .005), and who had greater disability (P < .0001). There was an association between any fatigue and disability; for every unit increase on the EDSS, the odds of any fatigue increased by 1.64 (95% CI, 1.39–1.93). There was also a relationship between any fatigue and a history of depression/current depressive symptoms. Compared with patients with no current symptoms and no history of depression, those with no current depressive symptoms but a history of depression had an adjusted OR of any fatigue of 3.28 (95% CI, 2.10–5.13), whereas it was 9.94 (95% CI, 5.11–19.30) in those with current symptoms and no history of depression and 10.82 (95% CI, 5.12–22.87) in those with current symptoms and a history of depression.
Table 2.
Clinical and demographic characteristics comparing participants with and without fatigue at baseline
Fatigue and Comorbidities at Baseline
In individuals reporting at least one comorbidity, 84.5% reported experiencing any fatigue compared with 66.8% of those without a comorbidity (OR, 2.96; 95% CI, 2.16–4.07). The highest median fatigue scores at baseline were found in those with glaucoma, fibromyalgia, cataracts, or anxiety. Participants with lupus, fibromyalgia, and inflammatory bowel disease at baseline were more likely to report also having any fatigue (Supplementary Table 1, which is published in the online version of this article at ijmsc.org). Age was an effect modifier in the relationship between fatigue and osteoporosis at baseline (P = .015), where younger individuals with osteoporosis were more likely to report fatigue than were older individuals. Sex was not an effect modifier between fatigue and any comorbidity.
Fatigue Incidence
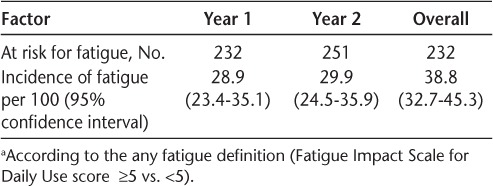
The incidence of any fatigue at years 1 and 2 and the cumulative incidence overall are presented in Table 3. Of the characteristics (covariates) explored, time since symptom onset was associated with an increased risk of any incident fatigue at any point in the study (OR, 1.04; 95% CI, 1.01–1.07), but age, sex, educational level, clinical course, disability status, alcohol abuse, and the use of disease-modifying therapy were not.
Table 3.
Incidence of fatiguea at two time points
Fatigue and Comorbidities over Time
For each specific comorbidity explored (depression, hypertension, migraine, hypercholesterolemia, anxiety, COPD, IBS, autoimmune thyroid disease, and osteoporosis), we compared the proportion of those with worsening fatigue in those with each comorbidity with those without. At time 1, compared with those without depression, those with depression were more likely to report worsening fatigue (ratio of proportions, 1.59; 95% CI, 1.04–2.43). At time 2, patients with COPD were more likely to report worsening fatigue than those without (ratio of proportions, 1.84; 95% CI, 1.04–3.26).
From the ordinal GEE models, several comorbidities were associated with fatigue (Supplementary Table 1). Even after adjusting for disease duration, disability status, age, sex, educational level, and all other comorbidities, the specific comorbidities anxiety, IBS, depression, and migraine were each independently associated with increased odds of fatigue that persisted over time (all P < .006) (Supplementary Table 2). Also, the presence of any comorbidity was associated with increased odds of fatigue that persisted over time. However, there was no association between developing a comorbidity and subsequent incident fatigue for any of the specific comorbidities examined (data not shown).
When we modeled worsening fatigue using logistic GEE and adjusted for disease duration, disability status, age, sex, and the presence of other comorbidities (as indicated previously herein), there was an individual-level effect of depression (OR, 1.49; 95% CI, 1.08–2.07) on the presence of worsening fatigue (Supplementary Table 3). This effect was maintained after adjusting for the presence of current depressive symptoms (Supplementary Table 4).
Discussion
We examined the relationship between comorbidities and the presence, onset, and worsening of fatigue over 2 years in a large, consecutive sample of people with MS. The incidence of fatigue over 2 years was 38.8%. The presence of any comorbidity was associated with persistent fatigue over the 2-year study period; this effect was largely driven by the presence of IBS, depression, and migraine. Depression was also associated with fatigue that worsened over time.
The 1-year incidence of fatigue in the present study (28.9%) is similar to that reported in a previous study of fatigue in MS (25%) that used a different fatigue scale but conceptualized fatigue in a similar manner and divided participants into those with and without any fatigue.10 Although other longitudinal studies of fatigue in MS have not reported incidence estimates, factors associated with fatigue have been identified, including mood, age, neurologic disability, and MS course.6–9 The present study confirmed these associations.
Several previous studies have extensively examined the relationship between depression and fatigue in MS using self-report questionnaires of current depressive symptoms, as did we.6–9,13,14 Overlap in the concepts examined by self-report scales makes it challenging to distinguish fatigue and depression in MS.36 However, we also examined history of depression and found this to be an important determinant of fatigue as well. Notably, we found a differential relationship between fatigue and depression based on the presence of depressive symptoms and a reported history of depression. Individuals with current clinically significant depressive symptoms and a history of depression had the highest odds of fatigue, followed by those with current symptoms but without a history and those with no current symptoms but a history of depression. This suggests a relationship between depression and fatigue that persists over time in people with MS. Anxiety was also associated with increased odds of fatigue over time, but this effect was attenuated once depression was accounted for. Symptoms of anxiety, depression, and fatigue overlap and often cluster together, although exact biological mechanisms have yet to be elucidated.37 From the standpoint of clinical management, it is important to note that those without clinically significant depressive symptoms but a history of depression still had more than three times the odds of being fatigued relative to those with no symptoms or history. Thus, although fatigue is a common symptom of depression, the presence of current clinically significant depressive symptoms does not seem necessary for an association between depression and fatigue to be seen. This may reflect subthreshold depression, which in the general population is associated with an increased risk of depression relapse,38 greater disability,39 and functional impairment.40 In MS specifically, subthreshold depression is related to an increase in generalized psychological distress and morbidity.41 In MS, it is also possible that the association of depression and fatigue reflects shared underlying pathologic changes.42
Aside from depression, the other comorbidities that were independently associated with fatigue in this MS sample were IBS and migraine. Fatigue is common in people with migraine, with an estimated prevalence between 40% and 85%,43–45 and there is strong evidence that it is associated with both headache frequency and pain.46 Mechanisms of fatigue in migraine are not well described, although as a neurologic disorder with central fatigue, structural and functional neurologic changes are implicated,11 including cortical thickness47 and other structural abnormalities in gray matter.48 Fatigue has been more extensively described in IBS, where it is a somatic concern affecting up to 60% of individuals.49–51 Psychological theories have been posited explaining this relationship that include cognitive and behavioral models.50 Specifically, in IBS it has been suggested that those who engage in more pleasurable activities may be less fatigued as these pleasant activities are reinforced, whereas those who focus greater mental attention on their disease may become more fatigued.50
Comorbidities contribute to the overall disease burden of MS, and the pathologic processes inherent to each comorbid condition may cause secondary fatigue and exacerbate existing MS fatigue. Persons with secondary fatigue report greater levels of fatigue than those with isolated primary fatigue,52 and a broader understanding of secondary fatigue in MS is needed. Although the existing literature on secondary fatigue in MS has focused on the presence of sleep disorders, depression, disability, and mobility as contributing factors,12,52 the present findings demonstrate that other physical comorbidities can also contribute. Moreover, such comorbidities may present a novel target for intervention that could improve fatigue and quality of life. Intervention studies that specifically evaluate this hypothesis are warranted, including promotion of health behaviors such as quitting smoking, physical activity, and healthy eating that may prevent some comorbidities. Furthermore, the presence of comorbidities should be considered when establishing treatment regimens for fatigue in MS. Notably, in a study of a fatigue self-management education program in MS, those with diabetes were slower to show improvement in fatigue after the intervention, and although those with arthritis had dramatic initial gains, these were not maintained over time,15 implying that different rehabilitation targets or timelines may be needed in the presence of comorbidity.
This study has strengths that include the enrollment of a large, representative sample and the description of previously unreported relationships between fatigue and comorbidity in MS. Most participants completed the entire study, and the cohort was recruited consecutively from multiple MS centers across Canada, two of which serve as the only MS centers in their jurisdictions. Although generalization of these findings to other settings is needed, the publicly funded and regional nature of our participating centers suggests that this sample was representative of the Canadian MS population. It is possible that the clinic-attending sample underrepresented patients with progressive MS with severe disability, which suggests that our estimates of fatigue may be underestimates. Nonetheless, compared with previous studies, the incidence of fatigue was assessed over a longer period in a larger cohort of people with MS. We used a brief validated measure of fatigue that, similar to other self-report scales, did not distinguish between primary and secondary fatigue, as this requires clinical judgment. Although fatigue may be considered an inherently continuous construct, we categorized the D-FIS; this may lead to loss of power, and the categories chosen may not reflect ideal clinically meaningful thresholds. Although the comorbidity questionnaire had previously been validated against medical record review, use of such self-reported data could lead to misclassification. However, reporting errors would be unlikely to differ between those with and without fatigue and would tend to attenuate any observed associations. Some comorbidities associated with fatigue may not have been captured. For example, we did not examine sleep disorders, which are already known to contribute to secondary fatigue in MS. Given the number of analyses, some of these findings could reflect a type I error and need to be replicated. However, these limitations do not negate the clinical relevance of these findings.
Fatigue is common in MS. We confirmed that depression is associated with fatigue, but we also showed that there is a differential effect of a history of depression and current depressive symptom severity on fatigue, as well as that depression increases the risk of worsening fatigue over a 2-year period. In addition to depression, IBS, migraine, and anxiety were also found to be associated with persistent fatigue even after accounting for depression. Although fatigue remains one of the most common and disabling symptoms affecting people with MS, addressing these comorbidities may offer some means of reducing fatigue in MS.
PracticePoints.
The incidence of fatigue in MS is 38.8 per 100 persons over 2 years.
Depression, anxiety, irritable bowel syndrome, and migraine are associated with an increased likelihood of developing fatigue.
Depression at baseline is associated with worsening fatigue over time.
Supplementary Material
Footnotes
Note: Supplementary material for this article is available on IJMSC Online at ijmsc.org.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This work was supported by CIHR (CIBG 101829), the Rx & D Health Research Foundation, and the MS Society of Canada (through a Don Paty Career Development Award to Dr. Marrie). The funding sources had no involvement in the study design; the collection, analysis, and interpretation of data; writing the article; or the decision to submit the article for publication.
References
- 1.Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12:24–38. doi: 10.1191/135248506ms1262oa. [DOI] [PubMed] [Google Scholar]
- 2.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 3.Lerdal A, Celius EG, Moum T. Fatigue and its association with sociodemographic variables among multiple sclerosis patients. Mult Scler. 2003;9:509–514. doi: 10.1191/1352458503ms943oa. [DOI] [PubMed] [Google Scholar]
- 4.Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998. [Google Scholar]
- 5.Flensner G, Landtblom AM, Soderhamn O, Ek AC. Work capacity and health-related quality of life among individuals with multiple sclerosis reduced by fatigue: a cross-sectional study. BMC Public Health. 2013;13:224. doi: 10.1186/1471-2458-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerdal A, Celius EG, Krupp L, Dahl AA. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol. 2007;14:1338–1343. doi: 10.1111/j.1468-1331.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 7.Patrick E, Christodoulou C, Krupp LB. Longitudinal correlates of fatigue in multiple sclerosis. Mult Scler. 2009;15:258–261. doi: 10.1177/1352458508097466. [DOI] [PubMed] [Google Scholar]
- 8.Brown RF, Valpiani EM, Tennant CC et al. Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother. 2009;82(pt 1):41–56. doi: 10.1348/147608308X345614. [DOI] [PubMed] [Google Scholar]
- 9.Johansson S, Ytterberg C, Hillert J, Widen Holmqvist L, von Koch L. A longitudinal study of variations in and predictors of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:454–457. doi: 10.1136/jnnp.2007.121129. [DOI] [PubMed] [Google Scholar]
- 10.Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Fatigue in multiple sclerosis persists over time: a longitudinal study. J Neurol. 2006;253:1466–1470. doi: 10.1007/s00415-006-0247-3. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 12.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33:1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakshi R, Shaikh ZA, Miletich RS et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler. 2000;6:181–185. doi: 10.1177/135245850000600308. [DOI] [PubMed] [Google Scholar]
- 14.Kroencke DC, Lynch SG, Denney DR. Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler. 2000;6:131–136. doi: 10.1177/135245850000600213. [DOI] [PubMed] [Google Scholar]
- 15.Finlayson M, Preissner K, Cho C. Impact of comorbidity on fatigue management intervention outcomes among people with multiple sclerosis: an exploratory investigation. Int J MS Care. 2013;15:21–26. doi: 10.7224/1537-2073.2012-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krokki O, Bloigu R, Ansakorpi H, Reunanen M, Remes A. Neurological comorbidity and survival in multiple sclerosis. Mult Scler Relat Disord. 2014;3:72–77. doi: 10.1016/j.msard.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 17.McDonald WI, Compston A, Edan G et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Bertolotto A, Deisenhammer F et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 2010;9:740–750. doi: 10.1016/S1474-4422(10)70103-4. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Edan G et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 20.Poser CM, Paty DW, Scheinberg L et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 21.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey: National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 23.Horton M, Rudick RA, Hara-Cleaver C, Marrie RA. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology. 2010;35:83–90. doi: 10.1159/000311013. [DOI] [PubMed] [Google Scholar]
- 24.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 25.Marrie RA, Reingold S, Cohen J et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21:305–317. doi: 10.1177/1352458514564487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay KA, Tremlett H, Fisk JD et al. Adverse health behaviours are associated with depression and anxiety in multiple sclerosis: a prospective multisite study. Mult Scler. 2015 doi: 10.1177/1352458515599073. Aug 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler. 2009;15:1518–1524. doi: 10.1177/1352458509347150. [DOI] [PubMed] [Google Scholar]
- 28.Patten SB, Burton JM, Fiest KM et al. Validity of four screening scales for major depression in MS. Mult Scler. 2015;21:1064–1071. doi: 10.1177/1352458514559297. [DOI] [PubMed] [Google Scholar]
- 29.Elbers RG, Rietberg MB, van Wegen EE et al. Self-report fatigue questionnaires in multiple sclerosis, Parkinson's disease and stroke: a systematic review of measurement properties. Qual Life Res. 2012;21:925–944. doi: 10.1007/s11136-011-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisk JD, Doble SE. Construction and validation of a fatigue impact scale for daily administration (D-FIS) Qual Life Res. 2002;11:263–272. doi: 10.1023/a:1015295106602. [DOI] [PubMed] [Google Scholar]
- 31.Benito-Leon J, Martinez-Martin P, Frades B et al. Impact of fatigue in multiple sclerosis: the Fatigue Impact Scale for Daily Use (D-FIS) Mult Scler. 2007;13:645–651. doi: 10.1177/1352458506073528. [DOI] [PubMed] [Google Scholar]
- 32.Box G, Tidwell P. Transformation of the independent variables. Technometrics. 1962;4:531–550. [Google Scholar]
- 33.Rothman K. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 34.Twisk J. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. New York, NY: Cambridge University Press; 2003. Other possibilities for modelling longitudinal data. [Google Scholar]
- 35.Fiest KM, Fisk JD, Patten SB et al. Comorbidity is associated with pain-related activity limitations in multiple sclerosis. Mult Scler Relat Disord. 2015;4:470–476. doi: 10.1016/j.msard.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Gunzier D, Perzynski A, Morris N, Bermel R, Lewis S, Miller D. Disentangling multiple sclerosis and depression: an adjusted depression screening score for patient-centered care. J Behav Med. 2015;38:237–250. doi: 10.1007/s10865-014-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood B, van der Mei IA, Ponsonby AL et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19:217–224. doi: 10.1177/1352458512450351. [DOI] [PubMed] [Google Scholar]
- 38.Judd LL, Akiskal HS, Maser JD et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 39.Preisig M, Merikangas KR, Angst J. Clinical significance and comorbidity of subthreshold depression and anxiety in the community. Acta Psychiatr Scand. 2001;104:96–103. doi: 10.1034/j.1600-0447.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 40.Rapaport MH, Judd LL. Minor depressive disorder and subsyndromal depressive symptoms: functional impairment and response to treatment. J Affect Disord. 1998;48:227–232. doi: 10.1016/s0165-0327(97)00196-1. [DOI] [PubMed] [Google Scholar]
- 41.Feinstein A, Feinstein K. Depression associated with multiple sclerosis: looking beyond diagnosis to symptom expression. J Affect Disord. 2001;66:193–198. doi: 10.1016/s0165-0327(00)00298-6. [DOI] [PubMed] [Google Scholar]
- 42.Gobbi C, Rocca MA, Riccitelli G et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler. 2014;20:192–201. doi: 10.1177/1352458513493684. [DOI] [PubMed] [Google Scholar]
- 43.Seidel S, Hartl T, Weber M et al. Quality of sleep, fatigue and daytime sleepiness in migraine: a controlled study. Cephalalgia. 2009;29:662–669. doi: 10.1111/j.1468-2982.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 44.Takeshima T, Ishizaki K, Fukuhara Y et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache. 2004;44:8–19. doi: 10.1111/j.1526-4610.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 45.Peres MF, Zukerman E, Young WB, Silberstein SD. Fatigue in chronic migraine patients. Cephalalgia. 2002;22:720–724. doi: 10.1046/j.1468-2982.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 46.Raggi A, Giovannetti AM, Quintas R et al. A systematic review of the psychosocial difficulties relevant to patients with migraine. J Headache Pain. 2012;13:595–606. doi: 10.1007/s10194-012-0482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messina R, Rocca MA, Colombo B et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013;268:170–180. doi: 10.1148/radiol.13122004. [DOI] [PubMed] [Google Scholar]
- 48.Rocca MA, Ceccarelli A, Falini A et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 49.Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024–6030. doi: 10.3748/wjg.v20.i20.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lackner JM, Gudleski GD, Dimuro J, Keefer L, Brenner DM. Psychosocial predictors of self-reported fatigue in patients with moderate to severe irritable bowel syndrome. Behav Res Ther. 2013;51:323–331. doi: 10.1016/j.brat.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 52.Forwell S, Brunham S, Tremlett H, Morrison W, Oger J. Primary and nonprimary fatigue in multiple sclerosis. Int J MS Care. 2008;10:14–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


