Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and, to a lesser extent, in the noradrenergic neurons of the locus coeruleus (LC). Most cases of PD are idiopathic and sporadic and are believed to be the result of both environmental and genetic factors. Here, to the best of our knowledge, we report the first evidence that chronic restraint stress (8 h/day, 5 days/week) substantially reduces nigral DA and LC noradrenergic neuronal cell numbers in rats. Loss of DA neurons in the SNpc was evident after 2 weeks of stress and progressed in a time-dependent manner, reaching up to 61% at 16 weeks. This reduction was accompanied by robust microglial activation and oxidative stress and was marked by nitrotyrosine in the SNpc and LC of the midbrain. These results indicate that chronic stress triggers DA and noradrenergic neurodegeneration by increasing oxidative stress, and that activated microglia in the substantia nigra and LC may play an important role in modulating the neurotoxic effects of oxidative stress. Taken together, these data suggest that exposure to chronic stress triggers DA and noradrenergic neurodegeneration, which is a cause of PD.
1. Introduction
Parkinson’s disease (PD) is a late-onset neurodegenerative disease that is characterized by slow movements, tremor, stiffness, and postural instability (Thomas and Beal, 2007) and generally occurs in 1% of the population over the age of 60 years (de Lau et al., 2006). The neuropathological hallmarks of PD include progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Causes of PD include viral infection, head trauma, herbicides, pesticides, heavy metal exposure, and genetic mutations (Davie-CA, 2008). In particular, mutations in specific genes, such as α-synuclein (SNCA), parkin (PRKN), PTEN-induced putative kinase 1 (PINK1), DJ-1, and ATP13A2, are known to cause PD. However, genetic PD is estimated to account for approximately 5% of the PD cases, and at least 90% of the PD cases are sporadic and have unknown etiology.
Chronic stress has previously been shown to contribute to neuronal loss in the hippocampus (Uno et al., 1989), and experimental evidence suggests that it also contributes to the damage of catecholaminergic neurons. In particular, clinical studies demonstrated that stressful life events may induce PD development. Among these studies, a study involving the prisoners of war reportedly had considerably higher incidence rates of PD development after 35 years of their release (Gibberd and Simmonds, 1980). This finding is consistent with the experimental data indicating that chronic stress decreases dopamine levels in the striatum (STR), nucleus accumbens, and the frontal cortex (Rasheed et al., 2010) as well as reduced the level of dopamine metabolites in the prefrontal cortex (Mizoguchi et al., 2000) and accelerated 6-hydroxy dopamine (OHDA)- or lipopolysaccharide (LPS)-induced DA neurodegeneration in the animal models of PD (Smith et al., 2008; de Pablos et al., 2014). These experimental data suggest that chronic stress preferentially exerts neurotoxic effects on the DA system and accelerates DA cell damage in the brain. However, in those studies, chronic stress is considered to promote the loss of DA neurons, which is the primarily triggered by extrinsic neurotoxins; it remains unknown whether prolonged intrinsic stress exposures trigger the loss of DA neurons.
To the best of our knowledge, our study is the first to generate evidence using an in vivo model that acute stress can induce the activation of microglial cells in the brain (Sugama et al., 2007). Moreover, similar observations have been made following chronic stress (Tynan et al., 2010; Hinwood et al., 2012) and sub-chronic stress (Wohleb et al., 2012). Microglia participate in neuroinflammation and can produce reactive oxygen species (ROS) that may contribute to neurodegeneration in PD (McGeer et al., 1988). Thus, in this study, we tested whether chronic stress contributes to the degeneration of catecholaminergic neurons by activating microglial cells. In the present experiments, we examined the effects of prolonged chronic stress on the number of tyrosine hydroxylase (TH) neurons, microglial activation, and oxidative stress in the SNpc and locus coeruleus (LC) of rats.
2. Methods
2.1. Animals and treatments
All procedures were approved by the Institutional Animal Care and Use Committee of Nippon Medical School and were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals; animal suffering and sacrifice were minimized. Male Wistar rats (280–320 g, 8 weeks old) were purchased from Japan Laboratory Animals, Inc. (Tokyo, Japan). All animals were housed in a room maintained at 20–22°C with a 12-h light/12-h dark cycle and food and water were provided ad libitum. Repeated restraint stress (RS) experiments were performed as previously described (Watanabe et al., 1992) with minor modifications. Briefly, RS was performed by tightly wrapping rats in the wire net without restricting respiration for 8 h per day (from 8 am to 4 pm) for 5 days per week. The procedure was repeated according to the experimental schedules of 1 day and of 2, 4, 8, and 16 weeks of RS. Control rats were not subjected to restrictions of food and water, and their weights increased by 29.8 ± 1.8 g per week, whereas RS-treated rat weights only increased by 9.0 ± 2.0 g per week. However, the weights of unstressed (RS-untreated) rats, which were deprived of food and water for 8 h per day, did not differ from those of the control rats. Tissues were immediately collected after the last session of stress; unstressed control animals were age matched.
2.2. Immunohistochemistry and immunofluorescence
Immunohistochemistry analyses were performed as previously described with minor modifications (Sugama et al., 2013) using anti-TH rabbit (1:10,000; Calbiochem, SD, USA) and anti-OX-42 mouse (1:500; Serotec, Oxford, UK) antibodies. Immunofluorescent primary antibodies included anti-nitrotyrosine (NT) and polyclonal rabbit antibody (1:200; Millipore, Temecula, USA) and OX-42 (1:200) and were observed using laser scanning confocal microscopy (LSM 710; Carl Zeiss, Germany). To observe Nissl bodies, 40-μm brain sections were mounted on glass slides and were stained with 0.75% cresyl violet and dehydrated using graded alcohols; coverslips were applied using Multi Mount (Matsunami Glass Ind., Ltd., Japan).
2.3. Fluoro-Jade B staining
Fluoro-Jade B (FJB; Millipore) staining was performed according to the manufacturer’s instructions. After staining reactions, sections were mounted on gelatin-coated slides and were dehydrated; coverslips were applied using Vectashield D (Vector Lab, Inc., Burlingame, USA) prior to observation using laser scanning confocal microscopy (LSM 710; Carl Zeiss, Germany).
2.4. In situ hybridization
In situ hybridization was performed as previously described with minor modifications (Sugama et al., 2000). Tissues were pre-hybridized in hybridization solution containing 50% formamide, 5% dextran sulfate, 1 × Denhardt’s solution, 0.25% SDS, 200-μg/ml Escherichia coli transfer RNA, 600-mM NaCl, and 1-mM EDTA. Digoxigenin (DIG)-labeled sense and anti-sense RNA probes were synthesized from the template, and mouse TH cDNA was sub-cloned into pGEM T-Easy vectors (Promega, Madison, USA) using DIG RNA labeling kits (Roche Diagnostics, Manheim, Germany). SP6 and T7 RNA polymerases were used for anti-sense and sense probe labeling with the restriction enzyme-digested plasmids SphI and SpeI, respectively.
2.5. Cell counting of substantia nigra
The total number of TH-immunoreactive (ir) cell bodies in SNpc neurons was counted as previously described (Sugama et al., 2003). Briefly, digital images of TH-ir SNpc neurons were obtained at a magnification of 50× on an Olympus microscope fitted with a video camera (Olympus, Tokyo, Japan). Counting frames were generated using MCID image analysis software (Imaging Research Inc., Ontario, Canada) and were systemically scanned over the outlined SNpc using a motorized stage. Neurons were counted as they appeared within the counting frame. Average neuron densities were calculated by dividing the number of neuron profiles by the calculated volume. The total number of neurons was estimated as the product of neuron densities and SNpc volumes as previously described (Coggeshall, 1992; Gundersen, 1992; Sugama et al., 2003).
2.6. Quantification of immunoreactivity and in situ hybridization
To quantify immunohistochemical and in situ hybridization data, mean optical densities in the target area were measured from each section using image analysis software (WinROOF; Sugama et al., 2013).
2.7. Western blot analysis
Western blot analysis was performed as described in our previous report (Sugama et al., 2000) using anti-TH rabbit polyclonal (1:20,000; Calbiochem, SD, USA), anti-inducible nitric oxide synthase (iNOS) rabbit polyclonal (1:2000; Chemicon, CA, USA), and anti-GAPDH rabbit polyclonal (1:4000; Santa Cruz Biotechnology, Inc., Santa Cruz, USA) primary antibodies. Autoradiograms were scanned using the Imaging Densitometer GS-800 (Bio-Rad, Hercules, USA) and were analyzed using Quantity One 4.0 software (Bio-Rad), which calculates relative quantities associated with the background signals of the corresponding lane.
2.8. High-performance liquid chromatography (HPLC)
Rat STR samples were dissected on ice; they were homogenized in ice-cold 5% perchloroacetic acid and centrifuged. Soluble fractions were neutralized with 1 M potassium carbonate, and norepinephrine (NE), dopamine (DA), and 5-hydroxytryptophan (5-HT) concentrations were determined using high-performance liquid chromatography with electrochemical detection (EDT-300; Eicom, Kyoto, Japan), and were quantified using a PowerChrom (AD Instruments, Australia) with external standards (Sigma).
2.9. Footprint analysis
Footprint analyses were performed to assess the motor function as previously described (Li et al., 2010). Briefly, front and hind limbs of the rats were dipped into non-toxic paint, and these rats were placed on a runway leading to its home cage. The floor of the runway was lined with white paper and footprints were recorded; distances between front and hind limbs were analyzed. Data from rats that did not smoothly transverse the runway were excluded from analyses.
2.10. Statistical Analysis
Data are presented as means ± standard errors of the mean (SEM; four rats per group). Differences were identified using Student’s t-test and one-way ANOVA followed by Bonferroni analysis and were considered to be statistically significant at a p value of <0.05.
3. Results
3.1. Tyrosine hydroxylase neuron loss in the substantia nigra
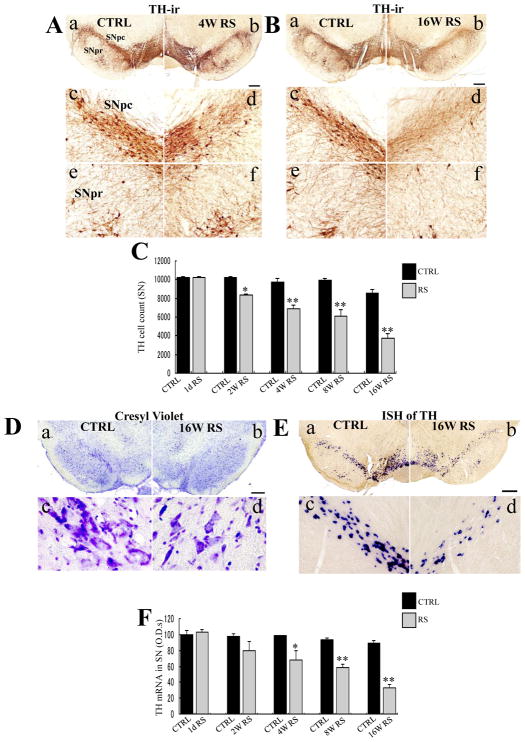
On comparing TH-ir neurons at the substantia nigra (SN), chronic RS significantly affected the loss of TH-ir cells [F (9,30) = 37.272; p < 0.0001; Fig. 1C). Moreover, decreases in TH-ir neuronal cell numbers were significant at 2 weeks in RS rats as compared with those in non-stressed age-matched rats (n = 4, p < 0.05). Cell numbers were further decreased after 4 weeks of RS (Fig. 1A); neuron loss was marked in the intermediate part of the SNpc (Fig. 1A-d) as compared with age-matched controls (Fig. 1A-c).
Fig. 1.
TH immunoreactivity in the substantia nigra pars compacta (SNpc) following chronic restraint stress after 4 (A) and 16 weeks of restraint stress (RS; B). a, b: age-matched control substantia nigra (SN) (a), stressed SN (b) in low-power photomicrograph; c, d: enlargements of (a) and (b) focusing on the SNpc; e, f: enlargements of (a) and (b) focusing on the SNpr; Scale bar, 500 μm under low-power- and 50 μm under high-power magnifications. C: Histogram of total tyrosine hydroxylase (TH)-ir cell counts in the SN of age-matched control (CTRL, black) and stressed rats (gray) at each time point; D, E: Cresyl violet staining (D) and TH mRNA expression (E) in the SNpc following 16 weeks of RS; a, b: age-matched control SN (a), stressed SN (b). c, d: enlargements of (a) and (b), respectively; Scale bar, 500 μm at low-power- and 20 μm at high-power magnifications in D and 50 μm at high-power magnifications in E. F: Histogram demonstrating TH mRNA optic densities (ODs) in the SN of age-matched control (CTRL, black) and stressed rats (gray) at each time point. Asterisks (C, F) indicate *p < 0.05 and **p < 0.01 as well as n = 4; Data are presented as means ± standard errors of the mean (SEM).
Greater durations of stress regimens accelerated neuron loss; in the RS rats, notable neuron loss was detected in the entire SNpc at 16 weeks (Fig. 1B-b), including in the medial and lateral parts (Fig. 1B-b, d), compared with that detected in the control rats (Fig. 1B-a, c). Dendrite densities in the SNpr were also remarkably decreased in RS rats (Fig. 1B-f) compared with control rats (Fig. 1B-e).
The number of Nissl bodies in the cytoplasm and nuclei of cells from the intermediate part of the SN was also substantially reduced after 16 weeks of RS (Fig. 1D-d) as compared with that in the age-matched control rats (Fig. 1D-c).
Subsequent in situ hybridization experiments revealed significant decreases in TH mRNA expression in the SN (Fig. 1E; F (9,30) = 14.712; p < 0.0001). Moreover, decreases in TH mRNA expression were significant from the 4th week of RS (n = 4, p < 0.05; Fig. 1F) and were greater after the 8th (n = 4, p < 0.01) and 16th week of RS (n = 4, p < 0.01; Fig. 1F). Finally, the number of TH-ir cells in the ventral tegmental area (VTA) was significantly decreased by chronic RS (data not shown).
3.2. Tyrosine hydroxylase neuronal loss in the locus coeruleus
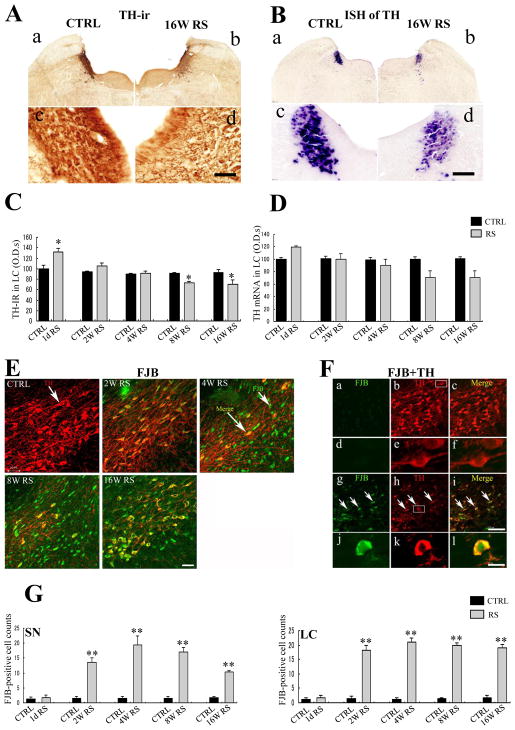
To investigate the effects of chronic RS in the LC, TH protein and mRNA expression were determined in the LC of rats subjected to chronic stress. Among control rats, TH-ir cells had relatively round morphology (Fig. 2A-c), whereas some TH cells had shrunk following 16 weeks of RS (Fig. 2A-d). Chronic RS also significantly affected TH-immunoreactivity in the LC (F (9,30) = 11.498; p < 0.0001; Fig. 2C), with 24% decreases at 8 weeks (n = 4, p < 0.05; Fig. 2C), and further decreases after 16 weeks of RS (n = 4, p < 0.05; Fig. 2C).
Fig. 2.
A, B TH-immunoreactivity (A) and TH mRNA expression (B) in the locus coeruleus (LC) in CTRL rats (a, c) and after 16 weeks of RS (b, d); Scale bar, 500 μm at low-power- and 50 μm at high-power magnifications. C, D: Histogram of total TH-immunoreactivity (C) and TH mRNA (D) in the LC of age-matched control (CTRL; black) and stressed rats (gray) at each time point. E: Merged images of TH (red) and Fluoro Jade B (FJB; green) in the SN of CTRL rats and of the rats treated with RS for 2, 4, 8, and 16 weeks; Scale bar, 50 μm. F: Double immunofluorescence staining of TH (red) and FJB (green) in the SN of control rats (a, b, c, d, e, f) and 16 week RS-treated rats (g, h, i, j, k, l); Merged images at 16 weeks of RS (i, l) sharply differ from those of the CTRL (c, f) and demonstrate chronic stress-induced neurodegeneration in the SN; Scale bar, 120 μm at lower magnification and 20 μm at higher magnification. G: Histogram of FJB-positive cell numbers in the SNpc and LC of age-matched control rats (black) and stressed rats (gray) at each time point; asterisks (C, D, and G) indicate *p < 0.05 and **p < 0.01 as well as n = 4; Data are presented as means ± SEM.
Subsequent in situ hybridization of TH mRNA revealed significant decreases in expression in the LC following 4 weeks of chronic RS (F (9,30) = 5.052; p < 0.0001; Fig. 2D). However, decreases in TH mRNA were not significant at 8 (n=4, p = 0.117) and 16 weeks (n = 4, p = 0.152; Fig. 2B-d), as compared with that in non-stressed age-matched control rats (Fig. 2B-c).
3.3. Fluoro-Jade-B immunoreactive cells
Fluoro-Jade B (FJB)-positive cells were detected as the markers of degeneration in the SN and LC. Under control conditions, FJB-positive cells were scarcely detected in the SN and LC. In contrast, chronic RS significantly increased FJB-positive cell numbers (F (9, 30) = 33.196; p < 0.0001; Fig. 2G). In these experiments, FJB-positive cells appeared in the SN as early as 2 weeks following the initiation of RS (Fig. 2E); their numbers were significantly increased at 4 (n = 4, p < 0.01), 8 (n = 4, p < 0.01), and 16 weeks of RS (n = 4, p < 0.01) as compared with those in control rats (Fig. 2E, G). Similar observations were made in the LC (F (9, 30) = 89.974; p < 0.0001; Fig. 2G).
To identify FJB-positive cell types, double immunofluorescence experiments were performed using sections from control versus the 16-week RS-treated rats (Fig. 2F). Under control conditions, healthy TH-ir cells (Fig. 2F-b, e) did not show FJB immunoreactivity (Fig. 2F-a, d). In contrast, TH-ir cells were smaller in the 16-week RS rats (Fig. 2F-h, k) and were co-localized with FJB (Fig. 2F-g, j), as shown in the merged image (Fig. 2F-i, l).
3.4. Western blot analysis of tyrosine hydroxylase protein in the brain
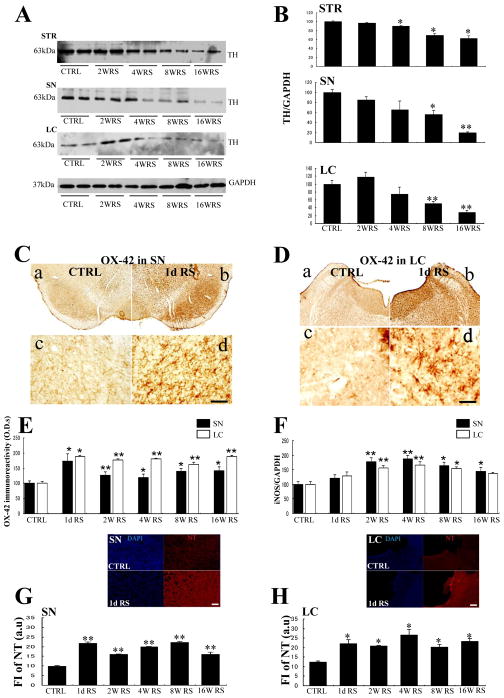
Western blot analysis were performed to confirm TH protein levels in the mid-brain (SN, LC) and the STR. In these experiments, TH signals were significantly reduced by 35% in the SN after 8 weeks of RS (n = 4, p < 0.05; Fig. 3A, B). Moreover, TH protein expression was significantly reduced by 49% in the LC at the same time point (n = 4, p < 0.01; Fig. 3A, B) and by 11% in the STR following 4 weeks of RS (n = 4, p < 0.05; Fig. 3A, B).
Fig. 3.
A: Western blot analysis showing changes of TH protein expression in the striatum (STR), SN, and LC at each time point. B: Quantification of western blot analyses showing that TH protein expression is elevated in the STR, SN, and LC. C, D: OX-42 immunoreactive cells in the SNpc (C) and LC (D) of the control rats (a) and 1-day (8 h) RS-treated rats (b); c, d: enlargements of (a) and (b), respectively; Scale bar, 500 μm at low-power- and 50 μm at high-power magnification; E. Histogram showing ODs of OX-42 immunoreactive microglia in the SN (black) and LC (white) after acute (1 day) and chronic RS; F: Histogram showing the quantification of Western blot analysis of iNOS in the SN and LC; G, H: Histograms showing NT fluorescence [arbitrary unit (au)] in the SN (G) and LC (H) at each time point; Asterisks (B, E, F, G, H) indicate *p < 0.05 and **p < 0.01 as well as n = 4; Data are presented as means ± SEM. Representative immunofluorescence images of DAPI (blue) and NT (red) in CTRL (upper) and of 1-day RS (lower) in SN and LC are shown at the top of each histogram; Scale bar, 50 μm.
3.5. HPLC
To further investigate neurotransmitter responses to chronic stress in the brain, we measured DA, NE, and 5-HT levels in the STR using HPLC. In these experiments, DA levels were significantly decreased after 4 and 8 weeks of RS (n = 4, p < 0.05; Table 1) and significant decreases in 5-HT levels were observed after 8 weeks (n = 4, p < 0.05; Table 1). However, NE levels were unaffected by chronic stress.
Table 1.
Neurotransmitters in the STR of CTRL and chronic RS rat (HPLC) Expressed as mean ± S.E.M. (ng/100 mg tissue)
| CTRL | 4W RS | 8W RS | |
|---|---|---|---|
| DA | 731.99 ± 45.20 | 448.22 ± 6.83* | 454.77 ± 13.60* |
| NE | 7.69 ± 0.55 | 13.88 ± 3.41 | 10.01 ± 1.29 |
| 5-HT | 23.08 ± 2.31 | 17.13 ± 2.35 | 19.62 ± 0.58* |
3.6. Microglial activation
In a previous study, we demonstrated that acute stress can lead to the morphological activation of microglia (Sugama et al., 2007) but did not determine changes over the course of chronic stress. In the present study, microglial responses were investigated following acute and chronic RS using the complement receptor 3 marker OX-42, which is commonly used to evaluate microglial activation in the SN and LC.
Under control conditions, microglial cells showed typical resting phenotypes with mild OX-42 immunoreactivity and small cell bodies in the SN (Fig. 3C-c) and LC (Fig. 3D-c). In contrast, single 8-h sessions of acute RS for substantially transformed microglial morphology into more intensive and larger cell bodies, indicating typical microglial activation. Stress-dependent microglial activation was observed in the SN (Fig. 3C-d) and in the LC (Fig. 3D-d) and persisted for the entire 16-week duration of RS (Fig. 3E).
To investigate inflammation, we quantified iNOS protein expression using Western blot analysis and showed significant increases of iNOS protein in the SN and LC from 2–16 weeks of RS (Fig. 3F).
3.7. Comparison of oxidative stress levels following acute and chronic restraint stress
In subsequent experiments, we investigated whether chronic stress induces morphological activation and increases oxidative stress in microglia by measuring NT levels as a marker of oxidation in the SN and LC of acute- and chronic-stressed rats and non-stressed controls.
Under control conditions, NT immunoreactivity was not detected in the SN or in the LC. However, following a single RS session, NT immunoreactivity was immediately increased in the SN (Fig. 3G) and in the LC (Fig. 3H) and was maintained for 2, 4, 8, and 16 weeks of RS. Finally, the source of NT was indicated by colocalization with OX-42-ir microglia (data not shown).
3.8. Footprint analyses of spatial gait
Footprint analyses were performed to evaluate the motor function on the final day of RS in the rats treated with RS for 4 and 8 weeks. No significant differences in motor function were observed between the control and stressed rats after 4 weeks. However, significant differences in the stride lengths of both front and hind limbs were observed after 8 weeks of RS (Table 2).
Table 2.
Stride Length (cm) of CTRL and chronic RS rats. Expressed as mean ± S.E.M.
| CTRL | 4W RS | 8W RS | |
|---|---|---|---|
| Front left | 15.35 ± 0.18 | 14.50 ± 0.35 | 10.07 ± 0.63 * |
| Front right | 15.37 ± 0.43 | 13.97 ± 0.74 | 11.17 ± 0.55* |
| Back left | 15.60 ± 0.14 | 14.07 ± 0.56 | 9.95 ± 0.70 * |
| Back right | 15.15 ± 0.69 | 13.75 ± 0.59 | 10.65 ± 0.55* |
4. Discussion
In the present study, exposure to chronic RS significantly reduced the number of DA neurons in the SNpc and that of noradrenergic neurons in the LC. In addition, neuronal loss was accompanied by robust microglial activation; the oxidative stress marker NT was significantly increased in the SN and LC. These observations demonstrate significant DA neuron loss in rat SN after only 2 weeks of RS and further intensification following persistent exposure to chronic stress for up to 16 weeks. Chronic stress also reduced the levels of TH mRNA and protein as well as the levels of DA neurotransmitter measured using in situ hybridization, Western blot analysis, and HPLC assays, respectively. To ensure that the observed loss of TH immunoreactivity reflected neuronal loss rather than altered TH expression, cell numbers were evaluated using cresyl violet staining of Nissl bodies and by FJB immunofluorescence labeling of degenerating cells, regardless of whether they were apoptotic or necrotic. Accordingly, the number of Nissl-stained cells was similar to that of TH-ir cells. Importantly, FJB-positive cells initially appeared in the SN after only 2 weeks of chronic RS, indicating early degeneration with TH-ir loss. Moreover, FJB-staining was specific for cells that showed shrinking morphology, indicating that the induction of DA neurodegeneration was caused by chronic stress in the SN.
Noradrenergic neurodegeneration reportedly occurs in conjunction with DA neuron loss in PD (Zarow et al., 2003). Accordingly, the present data show neurodegenerative changes in the LC after only 8 weeks of RS, suggesting that chronic stress alone induces both DA and noradrenergic neurodegeneration.
Because previous studies have demonstrated that acute stress induced robust microglial activation in the brain (Sugama et al., 2007, 2013, Walker et al., 2013), we investigated microglial changes following chronic RS and showed persistent microglial activation in the whole brain as well as in the SN and LC. In addition, the magnitude of microglial activation, estimated according to morphological changes, was remarkable after only 1 day of RS followed by gradual waning after 4 weeks of RS. These data are consistent with data in the recent reports of chronic stress-induced microglial activation in the brain (Tynan et al., 2010, Hinwood et al., 2012, Wohleb et al., 2012).
Microglia are regarded as the major source of ROS such as NO, H2O2, O2−, and peroxynitrite (ONOO−/ONOOH; Block and Hong, 2007). Because ONOO− is generated from nitric oxide (NO) and superoxide (O2−) and results in the nitration of free tyrosine and tyrosine residues in polypeptides (Ischiropoulos, 2003), NT concentrations directly reflect ONOO− activity. Consistent with an earlier report showing that long-term exposure to stress increases NT concentrations in the cerebral cortex (Olivenza et al., 2000), NT accumulated in the SN and LC in the present RS-treated rats. We also observed the colocalization of NT with activated microglia (data not shown), suggesting that these cells may be the predominant source of NT. Injection of 3-NT into the brain reportedly causes striatal neurodegeneration (Mihm et al., 2001), and activated microglia that are engaged in neuroinflammation have been shown to be neurotoxic (Qian et al., 2010). Therefore, the exposure of microglial cells to neurotoxic agents results in the production of proinflammatory mediators such as TNFα, IL-1β, IL-6, and ROS. In particular, activated microglia generate abundant superoxide through NADPH oxidase and directly damage DA cells (Gao et al., 2003; Wang et al., 2014). Taken together with the present data, these observations suggest that microglia are activated during chronic stress and may contribute to neurodegenerative changes by increasing oxidative stress.
As a limitation, the stress regimen employed here can be considered severe and prolonged. The aim of this study was to mimic a succession of stressful working weeks. It is not yet verified to what extent this model can be compared to humans. In addition, because the present rats were exposed to the same stress for the entire duration of the study, adaptations may have limited the subsequent inflammatory reactions. Hence, further studies are required to compare the neurodegenerative effects of varied stress regimens.
In conclusion, the present data are the first in vivo evidence that exposure to chronic stress triggers DA and noradrenergic neurodegeneration, potentially contributing to the etiology of PD through increased oxidative stresses and microglial activation. These observations indicate that severe and prolonged stress may trigger the onset of sporadic PD, thereby contributing to its progression.
Research Highlights.
The present study investigated the effects of chronic stress on dopaminergic neurodegeneration.
The chronic stress significantly triggered the dopaminergic neuron loss in the substantia nigra.
The chronic stress also reduced noradrenergic neuron in the locus coeruleus.
The activated microglia induced by stress exposures is detected in the substantia nigra and locus coeruleus.
Nitrotyrosine as a marker of oxidative stress is also significantly increased in the substantia nigra and locus coeruleus.
Acknowledgments
This study was supported in part by a Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors thank Sheila Silverstein for proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Smith AD, Castro SL, Zigmond MJ. Stress-induced Parkinson’s disease: a working hypothesis. Physiology & Behavior. 2002;77:527–531. doi: 10.1016/s0031-9384(02)00939-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neurons systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EH. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 1998;24:76–84. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Benskey M, Lee KY, Parikh K, Lookingland KJ, Goudreau JL. Sustained resistance to acute MPTP toxicity by hypothalamic dopamine neurons following chronic neurotoxicant exposure is associated with sustained up-regulation of parkin protein. NeuroToxicology. 2013;37:144–153. doi: 10.1016/j.neuro.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hedreen JC, Price DL. Parkinson’s disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–8. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Munoz MF, Machado A, Venero JL. Chronic stress enhances microglial activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation. 2014;11:34. doi: 10.1186/1742-2094-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M, Conti B, Kakinuma Y. Chronic restraint stress triggers the dopaminergic and noradrenergic neurodegeneration: possible role of chronic stress for the onset of Parkinson’s disease. Brain, Behav, Immun. doi: 10.1016/j.bbi.2015.08.015. under revise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Sugama S, Cho BP, DeGiorgio LA, Shimizu Y, Kim SS, Kim YS, Shin DH, Volpe BT, Reis DJ, Cho S, Joh TH. Temporal and sequential analysis of microglia in the substantia nigra following medial forebrain bundle axotomy in rat. Neuroscience. 2003;116:925–933. doi: 10.1016/s0306-4522(02)00572-9. [DOI] [PubMed] [Google Scholar]
- Gibberd FB, Simmonds JP. Neurological disease in ex-Rar-East prisoners of war. Lancet. 1980;2:135–137. doi: 10.1016/s0140-6736(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed N, Ahmad A, Pandey CP, Chaturvedi RK, Lohani M, Palit G. Differential responose of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem Res. 2010;35:22–32. doi: 10.1007/s11064-009-0026-5. [DOI] [PubMed] [Google Scholar]
- Smith LK, Jadavji NM, Colwell KL, Perehudoff K, Metz G. Stress accelerates neuronal degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur J Neurosci. 2008;27:2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: Involvement of Interleukin-18. Neuroscience. 2007;146:1388–99. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Current Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Inflammation. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain:role of microglia. J Neurosci. 2000;20:6309–16. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substanita nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Hwang O. Role of oxidative stress in Parkinson’s disease. Experimental Neurobiology. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Meth Enzymol. 1996;269:185–194. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Cohen G. 6-Hydroxydopamine: evidence for superoxide radical as an oxidative intermediate. Science. 1973;181:456–457. doi: 10.1126/science.181.4098.456. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Kapur SA. dopaminergic hypothesis of major depression. Clin Neuropharmacol. 1995;18:S57–65. [Google Scholar]