Antibodies specific for PF4/heparin complexes (HIT antibodies) are a hallmark of heparin-induced thrombocytopenia (HIT) but only some antibody-positive patients experience thrombocytopenia and/or thrombotic complications (1). It is generally agreed that the serotonin-release assay (SRA), which detects heparin-dependent platelet-activating antibodies, is useful for identifying the subset of patients who are at risk for thrombocytopenia and/or thrombosis. The SRA is considered by some to be the “gold standard” assay for HIT diagnosis (2–5), but is technically demanding, involves use of radioactivity and is routinely available only through a small number of referral laboratories. We recently described a relatively simple method for detecting platelet-activating HIT antibodies based on expression of p-selectin (CD62p) by normal platelets treated with patient serum and exogenous platelet factor 4 (PF4) in the absence of heparin (6). Each of 57 patient samples studied were positive for HIT antibodies in a standard solid phase screening assay (IgG polyvinylsulfonate-PF4 ELISA, BloodCenter of Wisconsin, Milwaukee, WI) but only 33 were SRA-positive. Most, but not all of the SRA-positive samples, but none of the SRA-negative samples induced CD62p expression (6). These findings showed that while the PF4-augmented CD62p expression assay was highly specific for detection of platelet-activating HIT antibodies, its sensitivity was not sufficiently high to recommend it as a substitute for the SRA (6).
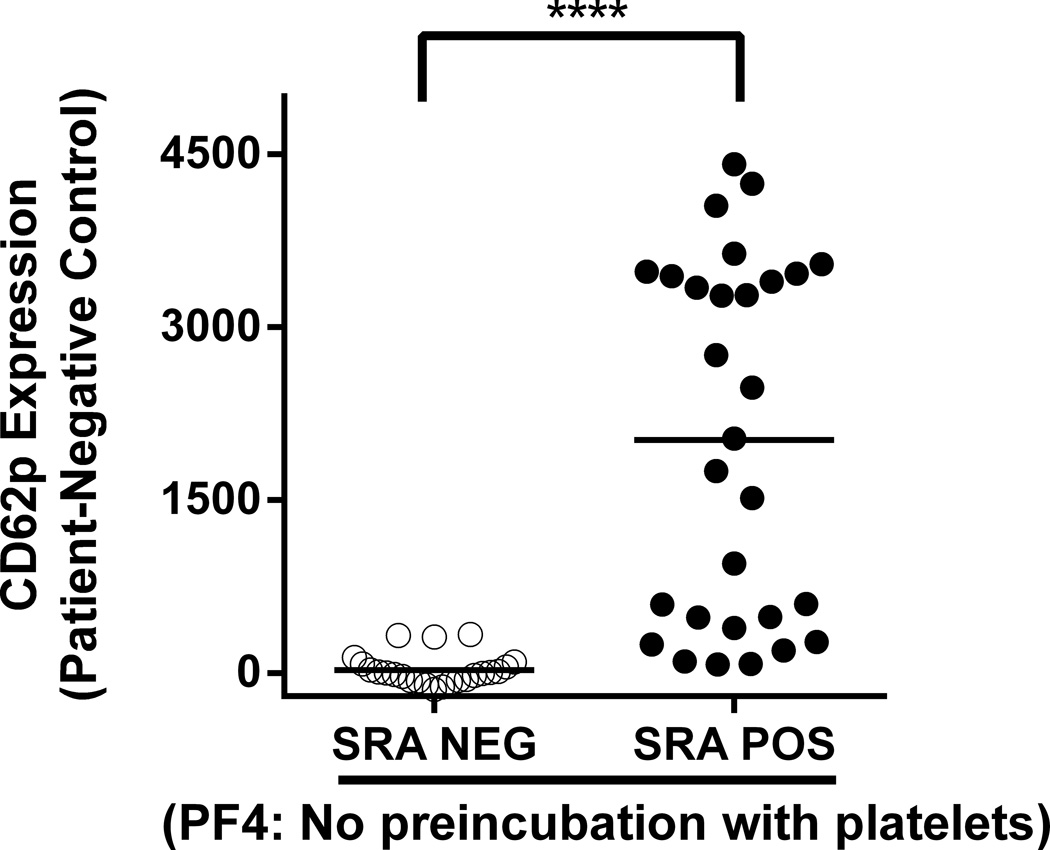
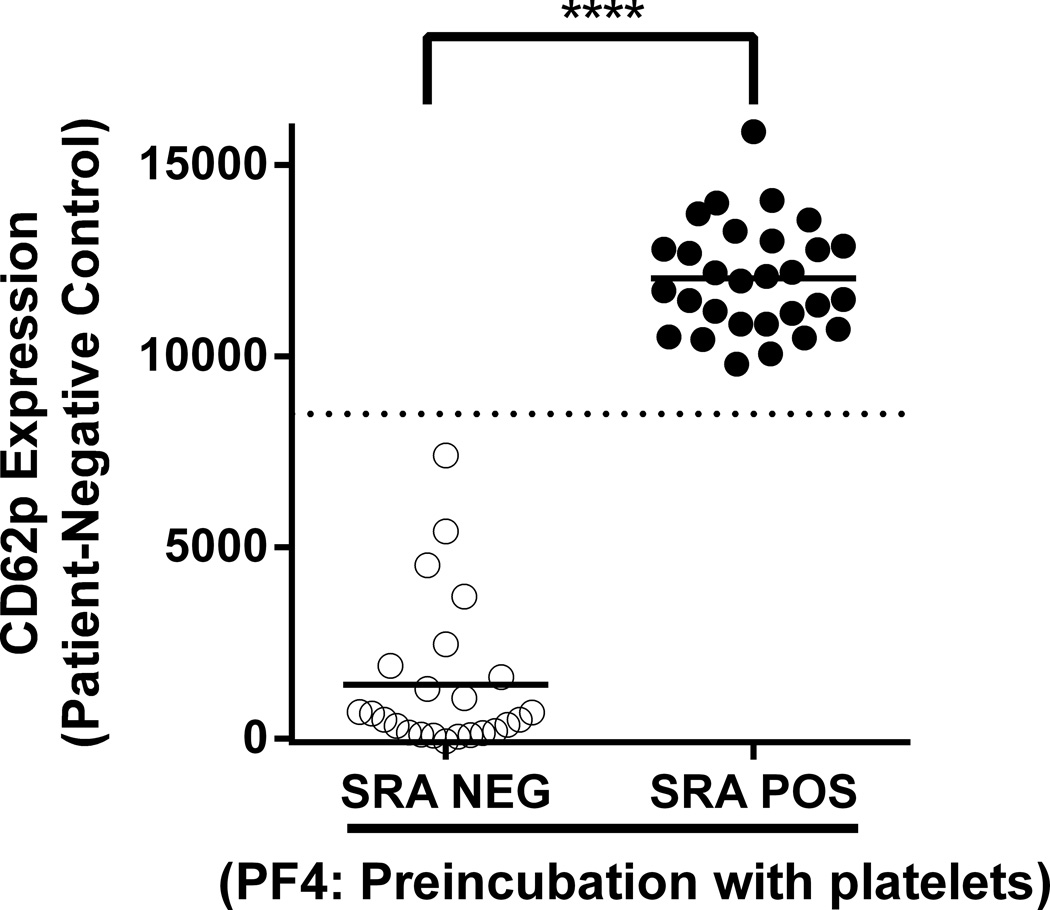
We have now defined new conditions that greatly improve the sensitivity of the CD62p expression assay for detecting “pathogenic” (SRA-positive) HIT antibodies. In the modified assay, platelets are pre-incubated with 62.5µg/mL human PF4 for 20 minutes at room temperature prior to addition of patient serum. In preliminary studies, this incubation time, temperature and concentration of PF4 were shown to be optimal for maximum induction of platelet CD62 expression (data not shown). The optimized assay was evaluated using 53 of the 57 HIT sera studied previously (6) after removing four samples that produced inconsistent results in the SRA. Testing was performed using platelet pools as targets, each pool comprising platelets from 2–3 healthy, randomly selected, group O blood donors. Known positive control (SRA+) and negative control samples were included in each run. All 53 samples were positive in the PF4/ELISA and 29 were also SRA-positive. Results obtained when PF4 and patient serum were added simultaneously to test platelets (6) are shown in Fig 1A where it can be seen that there is considerable overlap of CD62p expression values obtained with SRA-negative and SRA-positive samples. In contrast, when platelets pre-treated with PF4 were used as targets, strong positive reactions were obtained with each of the 29 SRA-positive but none of the SRA-negative sera (Fig 1B). It is apparent from Fig 1B that a cut-off defining a “positive” reaction can be selected (e.g. delta MFI of 8500) such that results obtained with the CD62p expression assay correlate perfectly with those obtained using the SRA. These findings cannot be explained simply on the basis of antibody strength (optical density in PF4/ELISA) because there was substantial overlap between the SRA-positive and SRA-negative groups in that assay (6).
Figure 1. SRA-positive, but not SRA-negative patient samples induce platelet CD62p expression in platelets pre-treated with PF4 in the absence of heparin.
Results shown were obtained with 53 samples from patients suspected of having HIT, all of which gave positive results in the IgG PF4 ELISA. Twenty-four samples were SRA-negative (open circles) and 29 were SRA-positive (closed circles). CD62p expression induced by SRA-positive samples was markedly enhanced with PF4-pretreated platelets (1B) relative to PF4 added to platelets at the time of serum incubation (1A). All positive reactions were significantly inhibited by high dose (100 U/mL) heparin (data not shown). Values on the ordinate depict median fluorescence intensity (MFI) obtained with patient sample minus MFI obtained with pooled normal serum. Horizontal bars depict average MFI obtained with each sample group. Horizontal dotted line in 1B depicts MFI of 8500 which completely differentiates SRA-negative from SRA-positive samples. Platelets isolated from a pool of 2–3 group O donors were incubated with human PF4 for 20 minutes at room temperature (RT) (1B) or not preincubated (1A) prior to addition of patient serum for 60 minutes. Platelet events were gated by GPIIb positivity and CD62p expression was measured by flow cytometry as previously described (6). Asterisks indicate significance of the difference between mean MFI values obtained with SRA-positive and SRA-negative samples [Mann Whitney; p<0.0001 (****)].
The key difference between the optimized CD62p expression assay (Fig1B) and its predecessor (6) is incubation of test platelets with PF4 prior to adding patient serum. A comparison of results shown in Fig 1B with those obtained when PF4 and patient serum were added simultaneously to platelets (Fig 1A) and the prior demonstration that activating HIT antibodies directly bind to PF4-treated platelets (6) suggests that increased sensitivity may be related to a structural modification induced in PF4 when it reacts with platelet glycosaminoglycans that is preferentially recognized by platelet-activating HIT antibodies. Studies are under way to characterize the molecular basis of this interaction.
In contrast to the SRA, the CD62p expression assay can be performed by any laboratory capable of isolating platelets from suitable donors and performing flow cytometric analysis and does not involve use of radioisotopes. Recently, the American Society of Hematology targeted inappropriate HIT testing as part of its “Choosing Wisely” campaign (7), a medical stewardship initiative, because of concern about “high false-positive rates” of commonly used HIT immunoassays and “substantial harm” that can result from incorrect diagnosis (7). Other published platelet activation assays have been unable to replace the cumbersome SRA due to suboptimal diagnostic accuracy (8, 9).
A limitation of our study is that we utilized non-consecutive archived samples that were available in sufficient quantities. Although we had no prior knowledge of how these samples might behave in the PF4-aumented CD62p expression assay, it is conceivable that the same close correlation between CD62p expression and SRA test results might not be found in a prospective study in which consecutive samples are evaluated. Another shortcoming is absence of detailed clinical information about patients tested, since a positive SRA assay is not a perfect surrogate for clinical HIT (10). A planned study will examine these issues.
If further studies show that the optimized CD62p expression assay can effectively be substituted for the SRA, a modified HIT diagnostic algorithm including clinical information and timely, easy-to-obtain CD62p expression test results could facilitate rapid and accurate identification of HIT among heparin-treated thrombocytopenic patients.
Acknowledgments
This study was supported in part by funds from the National Blood Foundation and the Clinical & Translational Science Institute of Southeastern Wisconsin (A.P) and by National Institutes of Health National Heart, Lung, and Blood Institute grant HL-13629 R.H.A).
Footnotes
Conflict of Interest
A patent application has been filed based partly on these findings (Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591
Human Research Approval
Studies involving human subjects were approved by the Institutional Review Board of the BloodCenter of Wisconsin.
Authorship Contributions
All authors were involved in study design. BRC and JGM provided the study materials and made helpful suggestions. AP and CGJ performed experiments and analyzed the data. RHA provided close oversight. AP wrote the manuscript and all authors edited the manuscript and approved the final version.
References
- 1.Shantsila E, Lip GY, Chong BH. Heparin-induced thrombocytopenia. A contemporary clinical approach to diagnosis and management. Chest. 2009 Jun;135(6):1651–1664. doi: 10.1378/chest.08-2830. [DOI] [PubMed] [Google Scholar]
- 2.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol. 2007 Dec;82(12):1037–1043. doi: 10.1002/ajh.21032. [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995 May 18;332(20):1330–1335. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143–149. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Morel-Kopp MC, Tan CW, Brighton TA, et al. Validation of whole blood impedance aggregometry as a new diagnostic tool for HIT: results of a large Australian study. Thromb Haemost. 2012 Mar;107(3):575–583. doi: 10.1160/TH11-09-0631. [DOI] [PubMed] [Google Scholar]
- 6.Padmanabhan A, Jones CG, Bougie DW, et al. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood. 2015 Jan 1;125(1):155–161. doi: 10.1182/blood-2014-06-580894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks LK, Bering H, Carson KR, et al. Five hematologic tests and treatments to question. Blood. 2014 Dec 4;124(24):3524–3528. doi: 10.1182/blood-2014-09-599399. [DOI] [PubMed] [Google Scholar]
- 8.Jy W, Mao WW, Horstman LL, et al. A flow cytometric assay of platelet activation marker P-selectin (CD62P) distinguishes heparin-induced thrombocytopenia (HIT) from HIT with thrombosis (HITT) Thromb Haemost. 1999 Oct;82(4):1255–1259. [PubMed] [Google Scholar]
- 9.Vitale M, Tazzari P, Ricci F, et al. Comparison between different laboratory tests for the detection and prevention of heparin-induced thrombocytopenia. Cytometry. 2001 Oct 15;46(5):290–295. doi: 10.1002/cyto.1170. [DOI] [PubMed] [Google Scholar]
- 10.Cuker A, Arepally G, Crowther MA, et al. The HIT Expert Probability (HEP) Score: a novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J Thromb Haemost. 2010 Dec;8(12):2642–2650. doi: 10.1111/j.1538-7836.2010.04059.x. [DOI] [PubMed] [Google Scholar]