Abstract
Introduction
Nitric oxide (NO) signaling can be mediated not only through classical cGMP, but also through S-nitrosylation. The impact of S-nitrosylation on erectile function and in NO regulation and oxidative stress in the penis, however, remains poorly understood.
Aims
To characterize the role of GSNOR, a major regulator of S-nitrosylation homeostasis, on erection physiology and on eNOS function and oxidative/nitrosative stress in the penis.
Materials and Methods
Adult GSNOR-deficient and WT mice were used. Erectile function was assessed in response to electrical stimulation of the cavernous nerve. Total NO in penile homogenates was measured by Griess reaction. Protein S-nitrosylation, endothelial NO synthase (eNOS) phosphorylation on Ser-1177 (positive regulatory site), eNOS uncoupling, and markers of oxidative stress (4-hydroxy-2-nonenal [4-HNE], malondialdehyde, and nitrotyrosine) in the penis were measured by Western blot.
Main outcome measures
Erectile function, eNOS function and oxidative stress in the penis of GSNOR-deficient mice.
Results
Erectile function was intact in GSNOR-deficient mice. Total S-nitrosylated proteins were increased (p<0.05) in the GSNOR−/− compared to WT mouse penis. While eNOS phosphorylation on Ser-1177 did not differ between the GSNOR−/− and WT mouse penis at baseline, electrical stimulation of the cavernous nerve increased (p<0.05) P-eNOS in the WT mouse penis, but failed to increase P-eNOS in the GSNOR−/− mouse penis. Total NO production was decreased (p<0.05), while eNOS uncoupling, 4-HNE, malondialdehyde, and nitrotyrosine were increased (p<0.05) in the GSNOR-deficient mouse penis compared to that of WT mice.
Conclusion
Transnitrosylation mechanisms play an important role in regulating NO bioactivity in the penis. Deficiency of GSNOR leads to eNOS dysfunction and increased oxidative damage, suggesting that homeostatic eNOS function in the penis is governed by transnitrosylation.
Keywords: S-nitrosylation, GSNOR, mouse, eNOS phosphorylation, eNOS uncoupling, oxidative/nitrosative stress, erectile function
Introduction
Penile erection is a complex process involving neurogenic, psychogenic and hormonal mechanisms with nitric oxide (NO) generally accepted to be the main mediator [1]. Traditional views of NO signaling in the penis suggest that NO is released by endothelium and neurons in penile tissue and binds to the heme group of soluble guanylyl cyclase to increase production of 3′,5′-cyclic guanosine monophosphate (cGMP), which in turn activates protein kinase G. cGMP/protein kinase G regulates intracellular signaling, which causes the relaxation of smooth muscle in the corpora cavernosa and penile erection [1].
Recent evidence demonstrates that many actions of NO can be mediated independently of cGMP signaling and are controlled enzymatically through S-nitrosylation. S-nitrosylation is a non-enzymatic reversible reaction consisting of the covalent attachment of a NO moiety to a reactive cysteine residue to form S-nitrosothiols (SNOs), which include S-nitrosylated proteins and low-molecular-weight S-nitrosoglutathione (GSNO). In addition, S-nitrosylation may occur through transnitrosylation involving GSNO and an acceptor thiol [2, 3]. GSNO, the most abundant endogenous SNO, serves as a stable intracellular reservoir of NO. Immunological detection of SNOs suggests that the NO synthase (NOS) isoforms, endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS), may be included among S-nitrosylatable proteins existing in diverse tissues ranging from endothelium to developing neurons and throughout blood vessel walls [4].
A key mechanism for regulating the action of S-nitrosothiols involves the enzyme alcohol dehydrogenase III, also known as S-nitrosoglutathione reductase (GSNOR) [5, 6]. GSNOR selectively metabolizes GSNO and thereby indirectly depletes the levels of S-nitrosylated proteins, which are in a dynamic equilibrium with GSNO [5]. GSNOR deficiency results in markedly increased levels of both GSNO and S-nitrosylated proteins [7, 8]. Thus, changes in GSNOR activity affect the entire S-nitrosothiol pool and thereby may modulate cellular signaling [5, 6].
We have previously shown GSNOR localizations in penile nerves as well as in vascular endothelium and smooth muscle cells of penile blood vessels [9]. However, the significance of transnitrosylation in erection physiology and in NO regulation and oxidative stress in the penis requires elucidation. In this study, we used GSNOR-deficient mice to characterize the role of GSNOR in erectile function and in eNOS function and oxidative/nitrosative stress in the penis.
Materials and Methods
Animals
Adult male (3-5 months old) homozygous GSNOR-deficient (GSNOR−/−) and age-matched wild-type (WT) mice (C57BL/6, The Jackson Laboratory, Bar Harbor, ME, USA) were used. Animals were cared for and housed under strict guidelines and all procedures were approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee.
In Vivo Erection Studies
Mice were anesthetized with 100 mg/kg Ketamine + 10 mg/kg Xylazine by intraperitoneal injection. To monitor mean arterial pressure (MAP), the right carotid artery was cannulated with polyethylene (PE) tubing filled with heparinized saline (100 U/ml). To monitor changes in intracavernosal pressure (ICP), the penis was denuded of skin and fascia and a 30-gauge needle connected via PE tubing to a pressure transducer was inserted into the right crus. The cavernous nerve was affixed with a bipolar electrode attached to a Grass Instruments S48 stimulator (Quincy, MA, USA) and stimulated at 1, 2, and 4 volts at 16 Hz with a 5 millisecond square-wave duration for 1 minute [10]. ICP was recorded using the DI-190 system (Dataq Instruments, Akron, OH, USA) from the start of electrical stimulation until 60 seconds after stimulation ended. Erectile function was represented by the normalized maximal ICP/MAP (max ICP) and total area under the curve/MAP (total ICP). Results were analyzed using the MATLAB program (Mathworks, Natick, MA, USA).
NO Assay
Griess reaction measuring total amount of nitrite and nitrate was performed with the commercially available kit (Oxford Biomedical Research, Rochester Hills, MI) on penile tissue collected at baseline, as previously described [11]. This kit employs metallic cadmium for quantitative conversion of nitrate to nitrite before quantitation of nitrite using the Griess assay, therefore providing for accurate determination of total NO (NOX) production. For Griess assays, absorbance was measured at 540 nm using a 680 microplate reader (Biorad, Hercules, CA).
Western Blot Analysis
For measurements of basal and stimulated levels of P-eNOS (Ser-1177), penes were collected at baseline and 1 min after a single electrical stimulation of the cavernous nerve at 4V for 60 sec, respectively. Penes were immediately snap frozen in liquid nitrogen and homogenized, as described previously [10]. NOS was partially purified and probed with polyclonal rabbit anti-P-eNOS (Ser-1177) antibody (Cell Signaling Technology, Beverly, MA, USA) at 1:450 dilution; the membrane was then stripped and probed with polyclonal rabbit anti-eNOS antibody (BD Transduction Laboratories, San Diego, CA) at 1:1,000 dilution [12]. In a separate group of mice, penes were removed from animals at baseline for measurements of eNOS uncoupling, protein S-nitrosylation, and markers of oxidative stress. For the analysis of dimeric and monomeric forms of eNOS, low-temperature sodium dodecyl sulfate (SDS)-gel electrophoresis was used with partially purified penile homogenates, as described previously [13]; membranes were then probed with polyclonal rabbit anti-eNOS antibody at 1:1,000 dilution. For the analysis of oxidative stress markers and S-nitrosylated proteins, membranes were probed with polyclonal rabbit anti-4-hydroxy-2-nonenal (4-HNE) antibody (Alpha Diagnostic International, San Antonio, TX) at 1:5,000 dilution, polyclonal rabbit anti-S-nitrocysteine, polyclonal rabbit anti-malondialdehyde (MDA), and monoclonal mouse anti-nitrotyrosine antibodies (Abcam Inc, Cambridge, MA) at 1:500, 1:500, and 1:2,000 dilutions, respectively [14]. Signals were standardized to β-actin (monoclonal mouse antibody at 1:7,000 dilution; Sigma Chemical, St. Louis, MO). Bands were detected by horseradish peroxidase conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare, Piscataway, NJ, USA), and analyzed using National Institutes of Health Image software. P-eNOS density was normalized relative to those of eNOS in partially purified samples. eNOS uncoupling was represented inversely as a ratio of active eNOS dimers to inactive eNOS monomers. The analysis of 4-HNE, MDA, nitrotyrosine, and protein-SNO was a densitometric composite of all proteins in each lane. All results were expressed relative to WT data.
Statistical Analyses
Statistical analyses were performed using two-way repeated measures ANOVA and Student t-test (SigmaStat Windows Version 3.00). In order to compare samples on western blots performed on different membranes, a modified t-test was used to compare GSNOR −/−mice to their control (% expression relative to WT mice). The data were expressed as the mean ± standard error of the mean (SEM). A value of p < 0.05 was considered to be statistically significant.
Results
Intact erectile function in GSNOR−/− mice
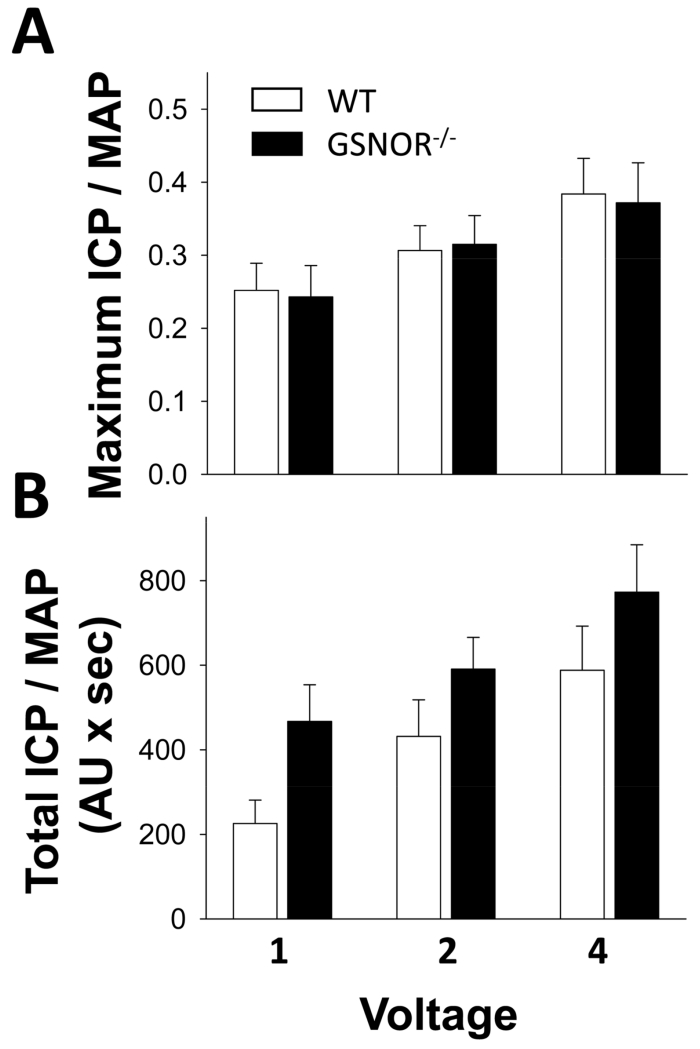
Erectile function, expressed as maximal ICP/MAP and total ICP/MAP (ICP area under the curve), was not changed at all voltages in GSNOR−/− mice compared to age-matched control WT mice (Figure 1).
Figure 1.
Erectile function is not impaired in GSNOR−/− mice compared to that in WT mice. Electrical stimulation of the cavernous nerve was performed at increasing voltages (1, 2, and 4 volts) at 16 Hz with square wave duration of 5 milliseconds for 1 minute. Erectile response to electrical stimulation of the cavernous nerve is indicated by maximal ICP/mean arterial pressure (MAP, A) and total ICP/MAP (B). Each bar represents the mean ± SEM. n = 9.
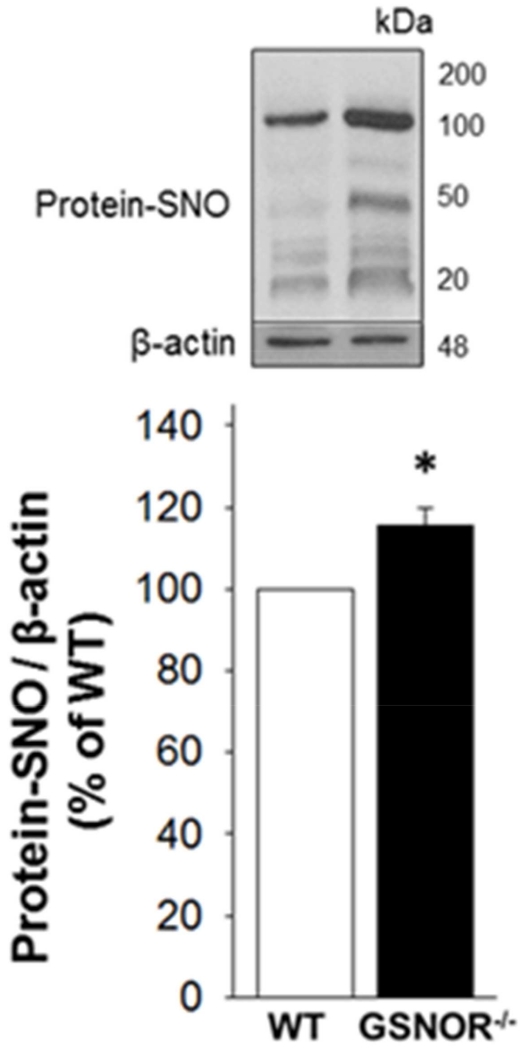
Increased protein S-nitrosylation in the GSNOR−/− mouse penis
Total S-nitrolysated proteins are increased (p<0.05) in the GSNOR−/− penile tissue (p<0.05) compared with that of WT mice (Figure 2).
Figure 2.
Protein S-nitrosylation is increased in GSNOR−/− compared to WT mouse penis. The upper panel displays representative Western immunoblots. The lower panel displays quantitative analysis of protein-SNO over β-actin. The analysis applies a densitometric composite of all proteins in each lane. Each bar represents the mean ± SEM. *p < 0.05 vs WT. n = 7.
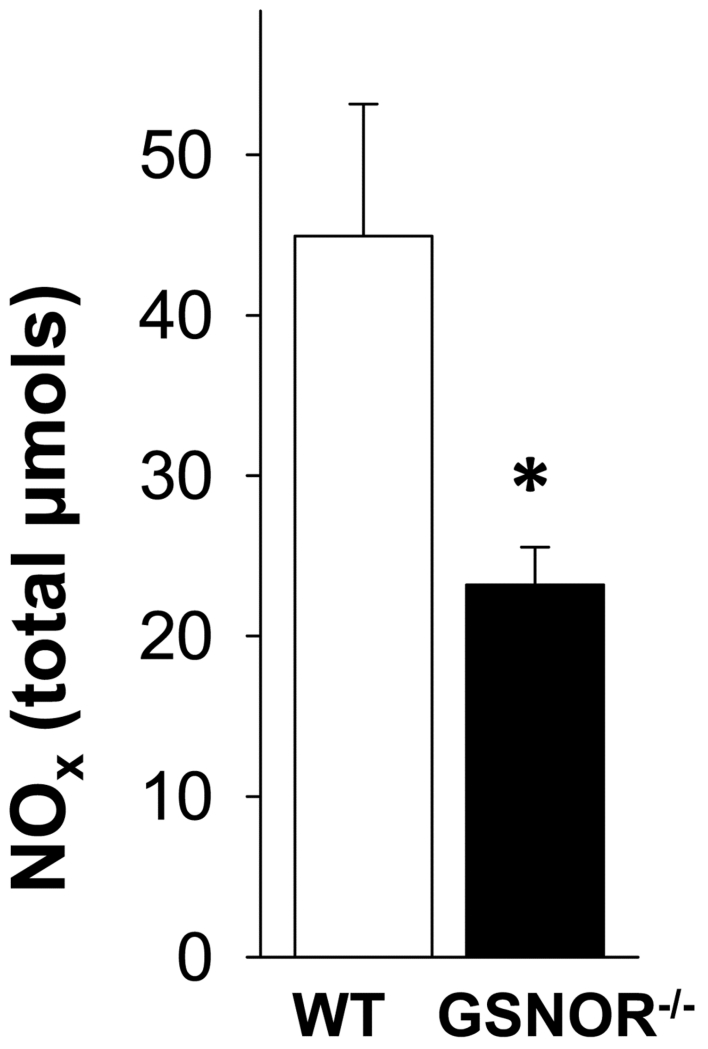
Decreased NO levels in the GSNOR−/− mouse penis
Total NOx formation was significantly (p<0.05) decreased in GSNOR−/− penile tissue compared with that of WT mice (Figure 3). This suggests the importance of GSNOR as a regulatory S-nitrosylation/denitrosylation mechanism of NO bioactivity in the penis.
Figure 3.
NO levels are decreased in GSNOR−/− compared to WT mouse penis. Each bar represents the mean ± SEM. *p < 0.05 vs WT. n=5.
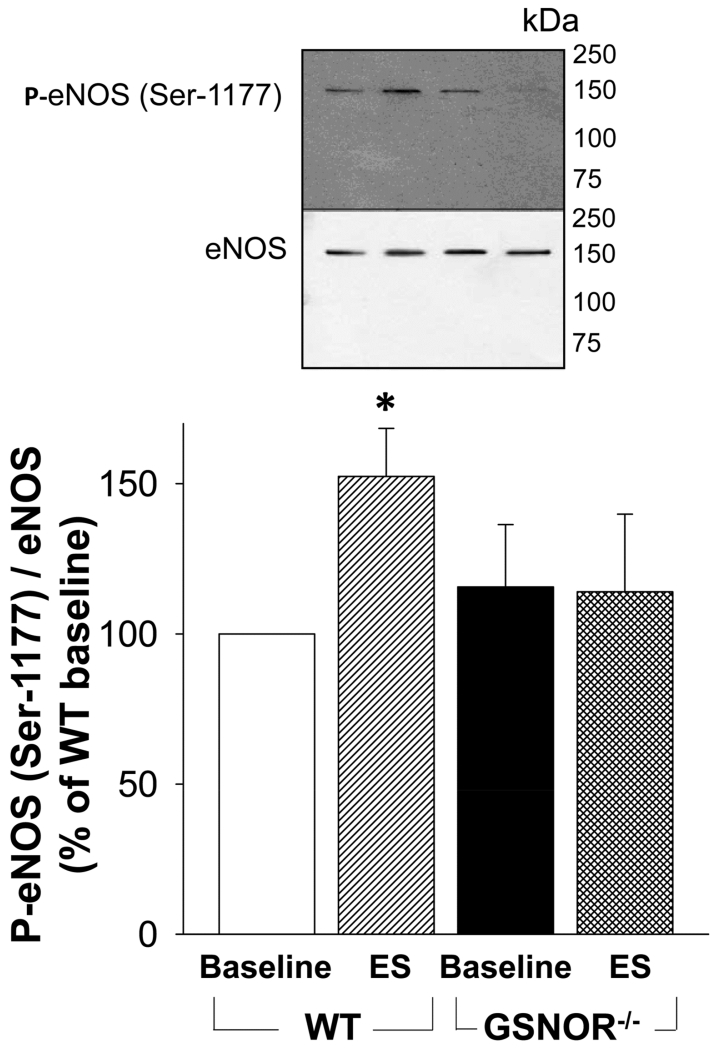
Decreased stimulated P-eNOS (Ser-1177) in the GSNOR−/− mouse penis
At baseline, eNOS phosphorylation on Ser-1177 did not differ between the GSNOR−/− and WT mouse penis. While electrical stimulation of the cavernous nerve increased (p<0.05) P-eNOS in the WT mouse penis, it failed to increase P-eNOS in GSNOR−/− mouse penis (Figure 4). These results suggest that GSNOR is involved in regulating agonist-stimulated eNOS phosphorylation on Ser-1177 in the penis.
Figure 4.
Stimulated levels of P-eNOS (Ser-1177) are decreased in GSNOR−/− compared to WT mouse penis. The upper panel displays representative Western immunoblots. The lower panel displays quantitative analysis of P-eNOS (Ser-1177) over eNOS in the same groups. Each bar represents the mean ± SEM. *p < 0.05 vs WT. n = 5.
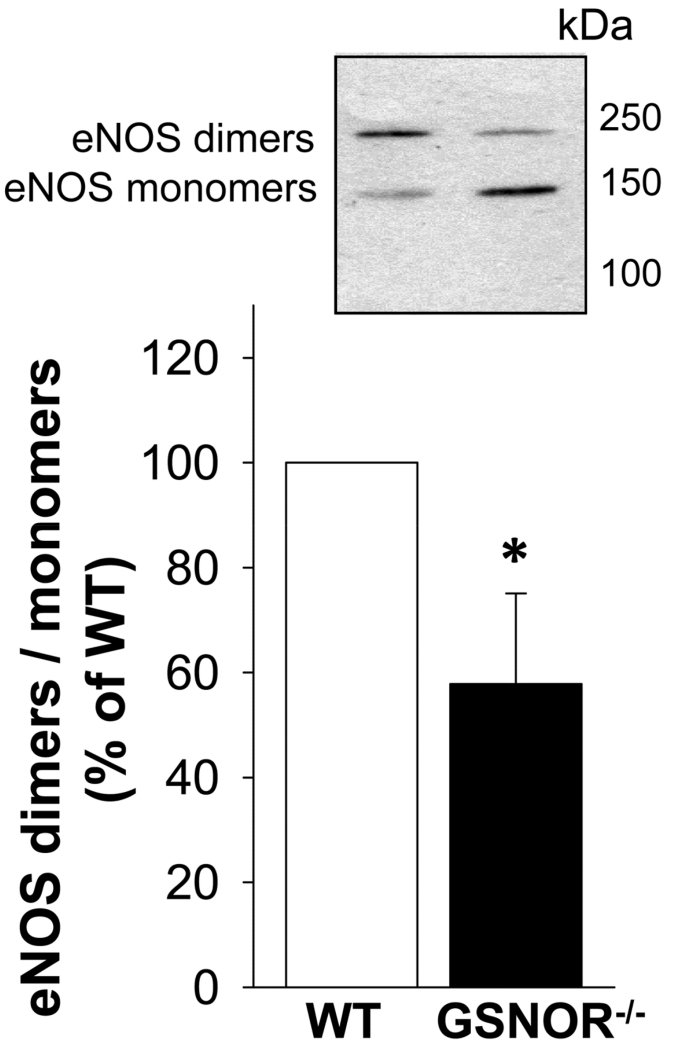
Increased eNOS uncoupling in the GSNOR−/− mouse penis
The ratio of functional eNOS dimers to nonfunctional eNOS monomers was significantly (p<0.05) decreased in the penis of GSNOR−/− compared to that of WT mice (Figure 5), indicating increased eNOS uncoupling.
Figure 5.
eNOS uncoupling is increased in GSNOR−/− compared to WT mouse penis. The upper panel displays a representative Western immunoblot. The lower panel displays quantitative analyses of eNOS dimers and monomers in the same groups. eNOS uncoupling is represented inversely as a ratio of active eNOS dimers to inactive eNOS monomers. Each bar represents the mean ± SEM. *p < 0.05 vs WT. n = 6.
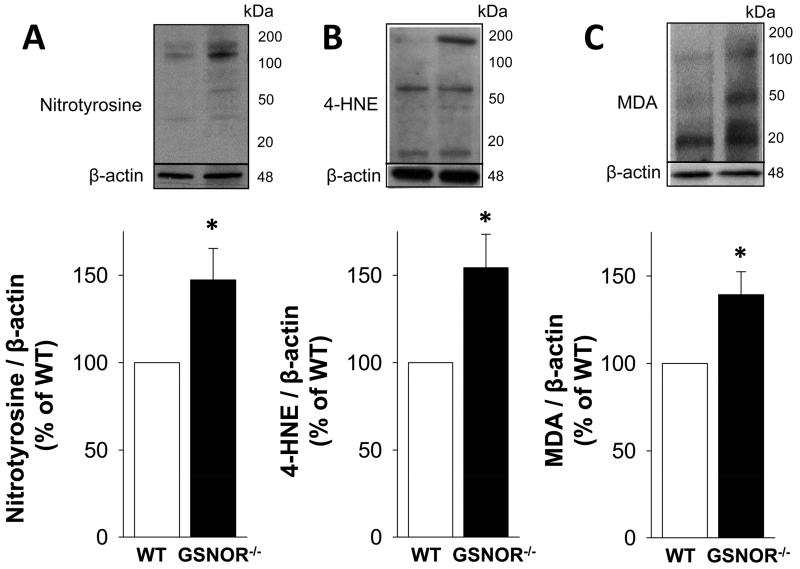
Increased oxidative stress in the GSNOR−/− mouse penis
Compared with that of the WT mouse penis, the amount of 4-HNE-modified proteins, MDA, and nitrotyrosine was significantly (p<0.05) increased in the GSNOR−/− mouse penis (Figure 6). These results suggest that GSNOR serves to control oxidative stress in the penis.
Figure 6.
Protein expressions of oxidative stress markers nitrotyrosine (A), 4-HNE (B), and MDA (C), are increased in GSNOR−/− compared to WT mouse penis. The upper panels display representative Western immunoblots. The lower panels display quantitative analyses of the proteins in the same groups. The analysis applies a densitometric composite of all proteins in each lane. Each bar represents the mean ± SEM. *p < 0.05 vs WT. n = 7.
Discussion
Our results show that transnitrosylation operates in the penis. GSNOR-deficient mice manifest increased levels of S-nitrosylated proteins, as well as altered NO functions in the penis by way of eNOS dysregulation, decreased NO production and increased markers of oxidative/nitrosative stress, despite apparently normal erectile function. These results demonstrate the importance of transnitrosylation mechanisms in the penis and indicate that uncontrolled S-nitrosylation leads to reduced physiologic NO signaling. Preserved erectile function in the face of eNOS dysfunction and increased oxidative stress in the penis suggests that NO reserves exist, sufficient to maintain penile erection.
GSNOR deficiency has been associated with increased nitrosative stress [5, 7], impaired cardiovascular function [15], tissue damage, and increased mortality in mouse models of sepsis [7]. In endothelial cells, S-nitrosylation inhibits eNOS function and NO/cGMP signaling at several steps: it inhibits soluble guanylate cyclase [16], cGMP phosphodiesterase [17], Akt [18], binding of eNOS to its positive regulatory protein heat shock protein 90 [19], and eNOS itself [20, 21]. In resting endothelial cells, eNOS is tonically inhibited by S-nitrosylation [21]. Upon agonist stimulation, eNOS is rapidly and transiently denitrosylated concomitant with the increase in eNOS phosphorylation at Ser-1177 and enzyme activation. As eNOS returns to resting activity levels, it is progressively renitrosylated, corresponding to the decline in eNOS Ser-1177 phosphorylation. We found unchanged levels of P-eNOS (Ser-1177) at baseline in the penis of GSNOR−/− mice, implying maintained penile vascular homeostasis in the face of un-opposed S-nitrosylation; however, insufficient denitrosylation in GSNOR-null mice resulted in the failure of eNOS to be phosphorylated in response to shear stress-associated penile blood flow generated by electrical stimulation of the cavernous nerve. These findings suggest that in the penis, denitrosylation is required for shear stress-induced eNOS activation by phosphorylation. Our results confirm in vitro data in isolated endothelial cells and further show in vivo the reciprocal regulation of eNOS by phosphorylation and S-nitrosylation.
S-nitrosylation of cysteine residues on proteins is dependent on intracellular redox state, and this posttranslational modification both occurs in the presence of elevated oxidative stress and induces oxidative stress [22]. Excessive S-nitrosylation of arginase has been implicated in eNOS uncoupling in arteriosclerotic vessels [23]. Furthermore, nitrosylation of 3 cysteines on eNOS (cysteines 93, 98, and 443), which comprise the eNOS dimer interface and are involved in the formation of eNOS functional dimer, has been associated with dimer collapse and eNOS uncoupling [20, 24, 25]. The loss of eNOS dimers in the penis of GSNOR−/− mice, observed in this study, implies that tight control of SNO levels is required for eNOS to be in its dimeric, functional state. Uncoupled eNOS conceivably contributes to increased oxidative/nitrosative stress. Increased oxidative/nitrosative stress in the penis of GSNOR−/− mice is evident by increased protein expression of 4-HNE, a product of lipid peroxidation [26], MDA, a product of ROS-induced degradation of polyunsaturated lipids [27], and nitrotyrosine, a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite [28]. These results indicate that in the penis, eNOS function is controlled by S-nitrosylation and that GSNOR protects against oxidative/nitrosative stress by keeping S-nitrosylation in check. Impaired agonist-stimulated eNOS phosphorylation and eNOS uncoupling, together with increased oxidative/nitrosative stress, apparently translate to decreased total NO production in the penis of GSNOR-deficient mice.
In spite of these molecular changes in the penis affecting eNOS function and NO production, erectile function was preserved in GSNOR-deficient mice. It is possible that neuronally-mediated penile erection is not affected by transnitrosylation, insofar as cavernous nerve-stimulated penile erection was intact. Alternatively, it is possible that an NO pool associated with available GSNO drives penile erection upon nerve stimulation despite dysfunctional eNOS. By virtue of GSNOR deficiency, these mice possibly store bioavailable NO in the form of GSNO, which can be donated to thiol groups forming vasoactive S-nitrosothiols [29]. This notion is consistent with the beneficial effect of GSNOR deficiency on cardiac repair [30] and ventricular systolic and diastolic function and tissue oxygenation [31] in a mouse model of myocardial injury. Similarly, pharmacological inhibition of GSNOR reduces vascular resistance and augments blood flow-mediated vasodilation in hypertensive rats [32], while targeted S-nitrosylation reduces neointimal hyperplasia and infarct size in animal models of carotid artery injury [33] and ischemia-reperfusion injury [34]. S-nitrosylation is thought to provide cardioprotection, in part, by modulating the activity of target proteins and by preventing the irreversible oxidation of critical cysteine residues that may occur with oxidative stress [35].
Although erectile function is preserved in GSNOR−/− mice, we hypothesize that S-nitrosylation-induced increased oxidative stress and deranged eNOS function in the penis predispose to erectile function loss upon further insult, such as additional oxidative/nitrosative stress. Consistent with this proposal, a nitrosylating agent S-nitrosothiol CysNO was found to impair mitochondrial function in bovine aortic endothelial cells, but complete loss of ATP and cell death occurred only after the cells were exposed to additional oxidative stress [36]. It is conceivable that heightened oxidative/nitrosative stress associated with vascular insults such as aging, diabetes, or hypercholesterolemia, may result in earlier vascular dysfunction in the penis and erectile dysfunction if transnitrosylation proceeds unchecked.
There are several potential limitations associated with our study. First, we did not directly measure eNOS S-nitrosylation. However, eNOS S-nitrosylation has been measured directly in human clitoral tissue [37], suggesting that eNOS may be nitrosylated similarly in penile tissue. Second, we measured total NO production, produced by all 3 NOS isoforms. Future studies are needed to distinguish NO production selectively by constitutive eNOS and nNOS versus inducible NOS. Third, as mentioned above, further studies are warranted to elucidate the presence and extent of S-nitrosylation in the penis in the context of pathological conditions, such as diabetes or hypercholesterolemia. Further research directions may serve to confirm these results in human corpus cavernosum and characterize nNOS nitrosylation in the penis.
In conclusion, unregulated S-nitrosylation in the penis resulted in reduced NO bioavailability, eNOS dysfunction, and increased oxidative/nitrosative stress, although erectile function was retained. Our results suggest that, in addition to traditional NO signaling involving cGMP, transnitrosylation plays an important role in regulating NO bioactivity in the penis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: “None” or details of Conflict
None
References
- 1.Burnett AL. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich) 2006;8(12 Suppl 4):53–62. doi: 10.1111/j.1524-6175.2006.06026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 4.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 6.Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochemical J. 1998;331(Pt 2):659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 8.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 9.Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, Burnett AL. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8:3325–3334. doi: 10.1111/j.1743-6109.2011.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, Burnett AL. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–3032. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 2014;28:76–84. doi: 10.1096/fj.13-228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt KJ, Musicki B, Palese MA, Crone JC, Becker RE, Moriaruty Jl, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric oxide synthase mediated penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musicki B, Liu T, Sezen SF, Burnett AL. Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis. J Sex Med. 2012;9:1980–1987. doi: 10.1111/j.1743-6109.2012.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musicki B, Bivalacqua TJ, Champion HC, Burnett AL. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J Sex Med. 2014;11:424–430. doi: 10.1111/jsm.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A. 2012;109:4314–4319. doi: 10.1073/pnas.1113319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Zhang P, Xu Z, Yue W, Zhuang Y, Chen Y, Lu Z. S-nitrosylation of PDE5 increases its ubiquitin-proteasomal degradation. Free Radic Biol Med. 2015;86:343–351. doi: 10.1016/j.freeradbiomed.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 22.Forrester MT, Foster MW, Stamler JS. Assessment and Application of the Biotin Switch Technique for Examining Protein S-Nitrosylation under Conditions of Pharmacologically Induced Oxidative Stress. J Biol Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummala M, Ryzhov V, Ravi K, Black SM. Identification of the cysteine nitrosylation sites in human endothelial nitric oxide synthase. DNA Cell Biol. 2008;27:25–33. doi: 10.1089/dna.2007.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altaany Z, Ju Y, Yang G2, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. 2014;7:ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 26.Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med. 2013;65:978–987. doi: 10.1016/j.freeradbiomed.2013.08.163. [DOI] [PubMed] [Google Scholar]
- 27.Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malondialdehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 28.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration: functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Stamler JS, Simon DI, Osborne JA, et al. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzistergos KE, Paulino EC, Dulce RA, Takeuchi LM, Bellio MA, Kulandavelu S, Cao Y, Balkan W, Kanashiro-Takeuchi RM, Hare JM. S-Nitrosoglutathione reductase deficiency enhances the proliferative expansion of adult heart progenitors and myocytes post myocardial infarction. J Am Heart Assoc. 2015;4:e001974. doi: 10.1161/JAHA.115.001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, Patton AK, Delany CS, Mutka SC, Blonder JP, Dubé GP, Rosenthal GJ, Springer ML. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. J Appl Physiol. 2013;114:752–760. doi: 10.1152/japplphysiol.01302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahnson ES, Kassam HA, Moyer TJ, Jiang W, Morgan CE, Vercammen JM, Jiang Q, Flynn ME, Stupp SI, Kibbe MR. Targeted Nitric Oxide Delivery by Supramolecular Nanofibers for the Prevention of Restenosis After Arterial Injury. Antioxid Redox Signal. 2016 Jan 21; doi: 10.1089/ars.2015.6363. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–25. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diers AR, Broniowska KA, Hogg N. Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol. 2013;1:1–7. doi: 10.1016/j.redox.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver JL, Kavoussi PK, Smith RP, Woodson RI, Corbett ST, Costabile RA, Palmer LA, Lysiak JJ. The role of regulatory proteins and S-nitrosylation of endothelial nitric oxide synthase in the human clitoris: implications for female sexual function. J Sex Med. 2014;11:1927–1935. doi: 10.1111/jsm.12576. [DOI] [PubMed] [Google Scholar]