Abstract
In most reviews addressing intracellular lipid trafficking, spontaneous diffusion of lipid monomers between the cellular organelles is considered biologically irrelevant because it is thought to be far too slow to significantly contribute to organelle biogenesis. This view is based on intervesicle transfer experiments carried out in vitro with few lipids as well as on the view that lipids are highly hydrophobic and thus cannot undergo spontaneous intermembrane diffusion at a significant rate. However, besides that single-chain lipids can translocate between vesicles in seconds, it has been demonstrated that the rate of spontaneous transfer of two-chain polar lipids can vary even 1000-fold, depending on the number of carbons and double bonds in the acyl chains. In addition, the rate of spontaneous lipid transfer can strongly depend on the experimental conditions such as vesicle composition and concentration. This review examines the studies suggesting that spontaneous lipid transfer is probably more relevant to intracellular trafficking of amphipathic lipids than commonly thought.
Keywords: lipid translocation, spontaneous diffusion, chemical activity, efflux, membrane contact site
Introduction
Why is interorganelle trafficking necessary in a eukaryotic cell? Nearly all cellular lipids are made in the endoplasmic reticulum (ER) yet are found in all other organelles, as well. Accordingly, mechanisms to transport these lipids from the ER to other organelles must exist. In principle, one or several of the following mechanisms could be involved: (i) transport in vesicles, (ii) protein-mediated transfer, (iii) monomer diffusion via the cytoplasm, and (iv) transient membrane (hemi) fusion. In most reviews on lipid trafficking, only the first two mechanisms are considered important for interorganellar lipid trafficking, while the last two are thought to be either too slow or rare to be biologically relevant. This view seems to be based on in vitro data showing that the half-time of glycerophospholipid (GPL) or sphingolipid diffusion between lipid vesicles is very slow, up to several days. However, it is very important to note that the rate of lipid intervesicle diffusion, measured in vitro, is highly dependent on the structure of the lipid as well as the experimental conditions.1 Another (possible) reason for downplaying spontaneous lipid transfer (SLT) is that this process is thought to be incompatible with lipid compositional gradients existing between organelles. However, SLT would not necessarily abolish such gradients since the other processes contributing to organelle lipid compositions, such as transbilayer movement and metabolic processes, are likely to be much faster than SLT. The purpose of this review is to briefly discuss the data that seem pertinent regarding the role of SLT in cellular physiology.
Definition of SLT
Traditionally, SLT has been defined as a process where a lipid molecule moves without the assistance of a protein carrier (a lipid transport protein) from one membrane to another. At low membrane concentrations, typically used in in vitro experiments, the rate-limiting step in this process is the efflux of a lipid molecule from a donor membrane, while at high membrane concentrations, membrane collisions can dominate SLT.1 Further mechanistic details can be found in previous publications.2–5 In this review, SLT is also considered to be the mechanism of transfer when proteins are indirectly involved in the transfer process, ie, when they tether two membranes, thereby increasing the probability of intermembrane lipid translocation.6 In addition, transient hemifusion (ie, only the outer membrane leaflets fuse) of two membranes is considered as an alternative mechanism of SLT as it allows for intermembrane lipid translocation.
Factors Influencing SLT
There are several factors influencing the rate of lipid monomer diffusion between lipid vesicles (SLT), such as the structure of the diffusing lipid, vesicle size (=bilayer curvature), vesicle concentration, and bilayer composition, as will be discussed below.
Lipid structure
It is well established that the number of alkyl chains, their length, and degree of unsaturation each have a remarkable effect on SLT. Single-chain lipids, such as fatty acids and lyso-GPLs, monoacylglycerol, sphingosine, and sphingosine 1-phosphate, have been shown or predicted to diffuse in seconds from a vesicle population to another,7–12 while this process takes hours or even days for typical two-chain GPLs and sphingolipids.13–17 Importantly, however, the rates of diffusion of two-chain GPLs differ remarkably, depending on the length and the degree of unsaturation of their alkyl chains. Thus, it has been shown that removing two methylene units from or adding a double bond to an acyl chain of a diacyl GPL increases the rate of diffusion 5- to 10-fold.18–20 Consequently, the rate of intervesicle diffusion of two-chain GPLs can differ by more than 1000-fold (ie, the half-time varies from minutes to days), depending on the number of alkyl chain carbons and double bonds. On the other hand, the polar head group structure has only a modest effect on SLT.21,22
The rate of spontaneous translocation of cholesterol is much faster than that of GPLs as expected from its structure containing fewer hydrophobic carbons. For instance, McLean and Phillips found that the half-time for cholesterol translocation between vesicles is only 2.4 hours,23 which is an order of magnitude less than the corresponding value for intact GPLs (see above). A similar value has been found by others,24,25 and even faster rates (~1 hour) have been reported.14 The rate of the spontaneous transfer of ergosterol (the cholesterol equivalent in yeast) has not been determined, but it is probably even faster than that of cholesterol due to the presence of two additional double bonds, albeit there is an extra methyl group in ergosterol. It is worthy to note here that the intermembrane translocation rate of the fluorescent cholesterol analog dehydroergosterol (and probably cholestatrienol as well) is significantly faster than that of cholesterol26 due to the additional double bonds that decrease the molecular hydrophobicity significantly. In contrast to GPLs and other polar lipids, triglycerides and cholesteryl esters are too hydrophobic to move spontaneously between membranes by the aqueous monomer diffusion mechanism. This applies to cardiolipin as well, which is intriguing as it may explain why this GPL remains fully confined to its site of synthesis, ie, mitochondria.19 Table 1 summarizes the measured or predicted rates of SLT for the main mammalian lipid classes.
Table 1.
Measured/predicted rates of spontaneous intervesicle translocation of different lipid classes in vitro.
| LIPID | TRANSLOCATION (t1/2) | REFERENCE |
|---|---|---|
| Triacylglycerols | Days-weeks | Predicted |
| Diacylglycerols | Hours –> days | Predicted |
| Monoacylglycerols | Seconds –> minutes | Predicted |
| Cholesterol/ergosterol | 1–3 h | 14,23,24,25 |
| Cholesterol esters | Days-weeks | Predicted |
| Fatty acids | Seconds | 7,10,11 |
| Fatty acid-CoAs | Seconds | Predicted |
| Di-acyl-GPLs | Hours –> days | 1, 9,14,19, 20, 23 |
| Lyso-gpLs | Seconds –> minutes | 7 |
| Cardiolipin | Weeks –> months | Pred. in ref 15 |
| Shingomyelin | Hours –> days | 15 |
| Glycosphingolipids | Days | 13,16,17 |
| Ceramide | Days | Predicted |
| Shingosine | Seconds-minutes | Predicted |
| Shingosine-1-P | Seconds | Predicted |
Bilayer curvature and vesicle concentration
It has been demonstrated that SLT is markedly dependent on the donor surface curvature in vitro, ie, the rate of transfer (efflux) increases markedly with increasing curvature of the donor surface. Thus, transfer of phosphatidylcholine (PC) or cholesterol is significantly faster from small vesicles with a high bilayer curvature vs. large vesicles with low bilayer curvature.23,24 The effect of interphase curvature is due to the lipid–lipid interactions that become weaker with increasing interphase curvature, which increases the chemical activity of the lipids and thus their efflux propensity. PC and cholesterol transfer from lipoprotein particles also increases markedly with increasing particle curvature,27,28 albeit the apoproteins could also contribute to the faster transfer (see below). Vesicle concentration can also have marked effect on SLT as was demonstrated by Jones and Thompson1 who found that intervesicle transfer of PC increased six fold when the vesicle concentration was increased from 0.1 to 40 mM, which is similar to the estimated concentration on membranes in cells. Kinetic data analyses indicated that the increase of SLT at high vesicle concentrations is due to enhancement of lipid translocation by intervesicle collisions.2
Lipid composition
Also the lipid composition of the (donor) bilayer can have a marked effect on SLT as anticipated based on the fact that the lipid composition can influence bilayer packing remarkably. Thus, cholesterol efflux is much faster from bilayers consisting of unsaturated vs. saturated lipids29 and, strikingly, Wimley and Thompson found that inclusion of 30 mol% of PE in PC vesicles enhanced PC translocation by 100-fold at high vesicle concentrations.30 Other studies have shown that fatty acids and other single-chain amphiphiles can markedly increase the efflux of cholesterol from membranes.31,32 It has also been reported that the composition of the acceptor vesicles can influence SLT.33 However, in this study, the transfer of an unnatural, short-chain fluorescent PC was studied, and thus, this result needs to be verified by using probe-free, long-chain lipids.
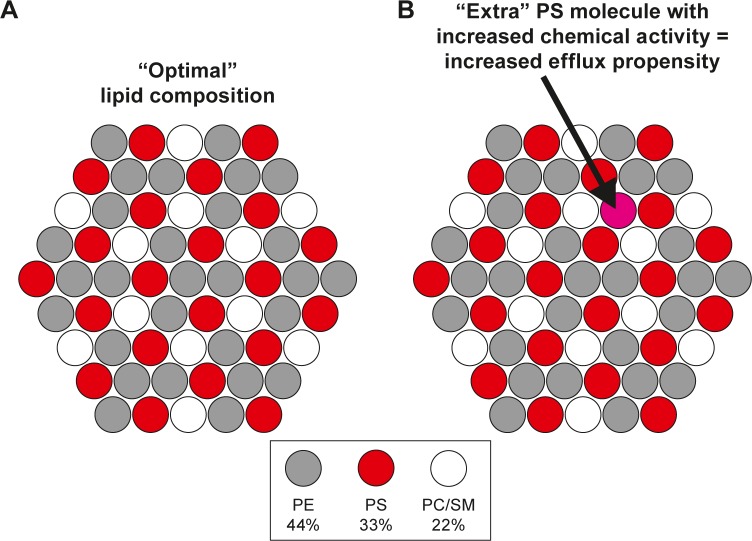
Lange et al have found that the amount of cholesterol relative to phospholipids can be critical for cholesterol efflux and transfer.34,35 It was proposed that when the cholesterol to phospholipid ratio exceeds a critical value, the cholesterol molecules in excess (ie, not complexed with the phospholipids) will have an increased chemical activity, and that the active cholesterol molecules have an increased tendency to efflux from the membrane.34–37 However, there is yet no direct experimental evidence for such cholesterol–phospholipid complexes, first proposed by Radhakrishnan and McConnell.38 In fact, the several critical cholesterol/phospholipid stoichiometric ratios observed in the experiments are more readily explained by the superlattice (regular distribution) model, which does not involve complex formation but proposes that (i) each critical ratio corresponds to a specific, regular lateral arrangement of cholesterol and phospholipids and (ii) and those specific arrangements (or cholesterol to phospholipid ratios) are more stable than random arrangements because they correspond to local minima in the membrane-free energy.39–41 Notably, the superlattice model also predicts several critical phospholipid/phospholipid ratios for multicomponent membranes.41 Thus, an excess of a particular phospholipid can exist when the composition deviates from a critical ratio (Fig. 1). Similar to the case of cholesterol, the phospholipid molecules in excess should have an increased chemical activity and thus an increased efflux propensity.42
Figure 1.
Lateral arrangement of the different GPLs in the inner leaflet on the human erythrocyte membrane as predicted by the superlattice model. (A) The membrane is viewed from above so that only the phospholipid head groups are visible. The superlattice model considers three distinct groups of phospholipids, ie, PE (a small head group), PS (a negatively charged head group), and PC + SM (a big head group). The arrangement shown here is consistent with the experimentally observed ratio of the three lipid groups (ie, 4:3:2, mole/mole).75 Notably, the model does not propose any long-range lateral order in the erythrocyte membrane (unlike implied by the model image shown). Rather, optimization of short-range lipid–lipid interactions is thought to drive the lipids toward the regular arrangement. Note also that in the proposed arrangement, no PS molecule (red) is proximal to another PS. (B) In case that a PE or PC/SM would be replaced by a PS molecule, this extra PS (pointed by an arrow) would necessarily be in contact with other PS molecules, which is energetically unfavorable due to a Coulombic repulsion between the negatively charged PS molecules. Thus, this extra PS would have an increased chemical activity and thus increased efflux propensity.
Membrane proteins
Membrane proteins are highly abundant in biological membranes and, consequently, a major fraction of membrane lipids must reside next to a membrane protein molecule. The intrabilayer sequences of these proteins contain multiple protruding aminoacyl chains, which make the protein surface rough. Because of this, the lipid molecules in the protein boundary cannot pack as well as those outside the boundary, and thus, they would have an increased chemical activity. Such protein-induced packing perturbations are probably responsible for some transmembrane peptides that act as nonspecific lipid scramblases in vitro, ie, they greatly enhance transbilayer movement of phospholipids.43 Also some cellular membrane proteins, such as cytochrome b544 and opsins, members of the ubiquitous family of G-protein-coupled receptors,45,46 act as highly efficient phospholipid scramblases in vitro. Besides such passive protein-induced lipid scrambling, active lipid scrambling also seems to exist as in the case of the ABCA1 transporter.5
The anticipated increased chemical activity of the lipids in protein boundaries probably translates to their increased propensity of efflux from the membrane. Since efflux is the rate-limiting step in SLT (see above), membrane proteins could thus significantly enhance SLT. In line with this prediction, the rate of cholesterol transfer between liposomes was considerably increased upon addition of proteins to lipid vesicles.25 Also, the fact that GPL transfer from small reconstituted lipoproteins is an order of magnitude faster than from neat lipid vesicles25 is consistent with this model. However, the smaller size of the lipoproteins may also contribute to the faster rate of transfer from these particles (see above). Besides proteins, small amphipathic molecules, such as fatty acids and sphingosine, can also increase the chemical potential of a membrane lipid and thus its efflux, as shown for cholesterol by Lange et al.32
SLT in Cells
Membrane contact sites
There are yet no data demonstrating that SLT contributes significantly to interorganelle lipid translocation. However, there is little evidence against this either. We will now discuss where in a cell could SLT be particularly relevant. The most obvious sites are the inter-membrane contacts thought to be present between the ER (the site of lipid synthesis) and several other organelles such as the mitochondria, Golgi apparatus, plasma membrane (PM), peroxisomes, and endosomes.47–52 Such membrane contacts should increase SLT significantly because the closeness of the acceptor and donor membranes increases the likelihood that a lipid molecule, after its efflux from the donor membrane, inserts into the acceptor membrane.6
The best studied membrane contact sites are those between the mitochondria-associated ER membranes (MAMs) and the outer mitochondrial membranes (OMMs).49,53–55 Several studies have proposed that these contact sites are involved in lipid transfer between the ER and mitochondria and that specific proteins play a role there. However, despite that this issue has been studied for more than two decades,56 no protein directly involved in lipid transfer between these membranes has been identified.48,49,53 This makes one wonder whether such proteins indeed exist? Another reason underlying this, perhaps provocative question is that the half-time of phosphatidylserine (PS) translocation from MAM (where it is synthetized) to mitochondria is slow, ie, the half-time of translocation was 7–15 hours for typical PS species.57,58 Notably, such long half-times are similar to those predicted for spontaneous transfer of PS between membranes, and thus do not support the involvement of lipid-transfer protein(s).
Besides SLT, lipids might move between MAMs and the OMM upon a transient hemifusion of the respective membranes. Thus, Ca2+ could cross-link PS molecules via their head groups,59 thereby creating PS-rich domains in MAM. Such domains could act as nucleation sites for a transient hemifusion of MAM with the outer leaflet of the OMM, which then would allow translocation of PS and other lipids between the two organelles (Fig. 2). Consistent with this speculative model, (i) PS synthesis requires a relatively high Ca2+ concentration in vitro,60 (ii) Ca2+ is released from MAM lumen into the intermembrane space between the MAM and the OMMs,61,62 and (iii) Ca2+ can induce (hemi)fusion of PS-rich membranes in vitro.63 An alternative model suggests that the localized synthesis of PS in MAM49 increases the concentration of this GPL beyond its optimal concentration (Fig. 1), thus creating a pool of active PS molecules prone to efflux from MAM to the OMM. The latter, but not the former, model is consistent with the slower translocation of the more hydrophobic PS molecules from MAM to mitochondria.57,58,64 However, in both models, tethering proteins would enhance (indirectly) PS translocation from MAM to mitochondria by keeping these organelles close to each other.
Figure 2.
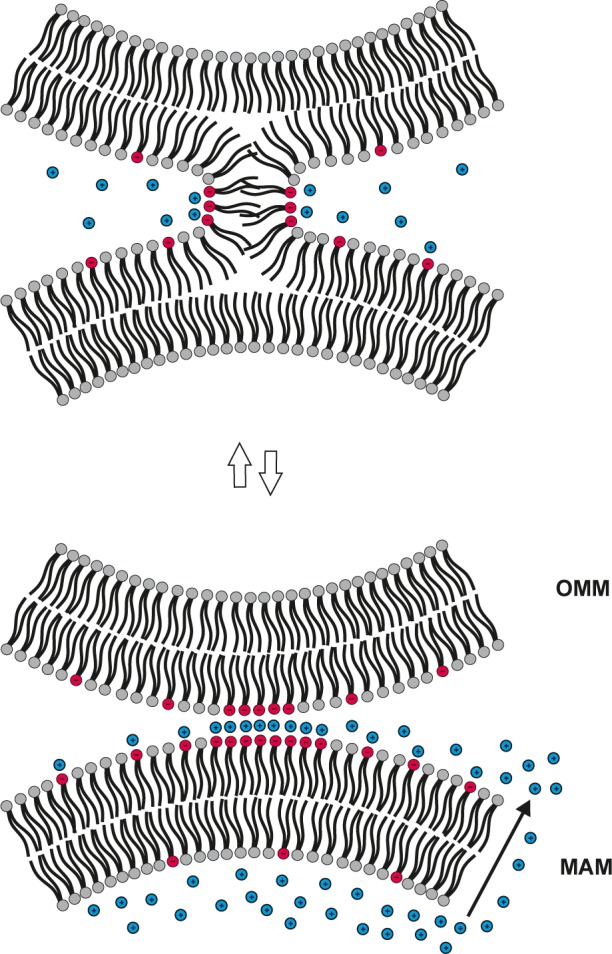
Hypothetical model for Ca2+-induced hemifusion of MAM and the OMM. The concentration of Ca2+ in the lumen of the MAM is of orders of magnitude higher than that in the cytoplasm (see text). When Ca2+ is released (via a protein channel, not shown) from the lumen of MAM (arrow) to the space separating MAM and OMM, this should strongly stimulate the synthesis of PS (red), which is Ca2+-dependent. The PS molecules in the cytosolic surface of MAM attract Ca2+ ions (blue), which leads to the formation of PS-rich domains and subsequent bridging of the PS domains with negatively charged lipids in OMM by Ca2+. This destabilizes the contacting membranes, thus promoting their hemifusion that allows the lipids move from MAM to OMM and vice versa by lateral diffusion. The double arrows indicate that the process could be reversible, ie, when the concentration of Ca2+ decreases, the membranes detach from each other. An alternative model suggests that the rapid Ca2+-induced synthesis of PS creates an excess of this lipid in MAM (Fig. 1). The PS molecules in excess have an increased chemical activity, which makes them prone to efflux from MAM to OMM.
Since contact sites also seem to exist between the ER and the PM, it is feasible that aqueous diffusion or membrane hemifusion could be involved in lipid translocation between these membranes. Regarding possible hemifusion, it is notable that the concentration of PS in the PM inner leaflet is very high, ie, 10–20 mol%.65 Such a high concentration of PS could drive hemifusion of the ER and PM membranes, thus promoting lipid transfer via the contact sites. However, we have shown that the rate of transfer of fluorescent PS molecules from the PM to mitochondria decreases exponentially with increasing chain length (hydrophobicity) of the lipid.66 This result is inconsistent with the hemifusion mechanism (which should be insensitive to lipid hydrophobicity), but consistent with spontaneous diffusion via the cytoplasm being the rate-limiting step in the transport of PS from the PM to mitochondria.
A recent study addressed the relevance of SLT between PM/ER contact sites in vitro by employing a rather involved approach based on various fluorescent probes.67 It was concluded that neither PC nor cholesterol translocates spontaneously through contact sites between PM and ER membranes. In particular, the lack of cholesterol transfer is puzzling as cholesterol translocates quite rapidly between lipid vesicles in vitro (see above). Also, it has been shown that intracellular cholesterol in glutaraldehyde-fixed, nonleaky cells could be rapidly and completely oxidized by extracellular cholesterol oxidase.68 Since fixing should stop both vesicle- and protein-mediated cholesterol transfer to the PM,68 these data imply that spontaneous translocation of cholesterol, possibly via membrane contact sites, could be involved.
Is the deacylation/reacylation cycle involved in lipid translocation?
In mammalian cells, GPLs are rapidly remodeled after their synthesis, ie, their acyl chains are exchanged for others.69 This so-called Lands pathway70 involves removal of an acyl chain by an A-type phospholipase followed by acylation of the lyso-GPL with another fatty acid. Since the spontaneous diffusion of lyso-GPLs is very fast (see above), it should rapidly distribute to other membranes where it could be reacylated to an intact GPL. If so, spontaneous intermembrane transfer of a GPL would have been achieved in effect.71 However, there is as yet no experimental proof for this putative mechanism of intermembrane lipid translocation.
Future Perspectives
There are very few experimental studies addressing the importance of SLT in vivo. Besides the presumption that SLT is irrelevant for organelle biogenesis, there is a lack of methods suitable to investigate the importance of this mechanism. First of all, there is, or will be, no specific method to inhibit or eliminate SLT, such as knockdown of gene products, which has been highly useful in studies on the other translocation mechanisms. Thus, the evidence for or against the importance of SLT is likely to be indirect, ie, will derive from experiments where the other mechanisms of translocation have been eliminated. Also studies where the hydrophobicity of a lipid under study is varied in a systematic manner would be useful since the different transport modes respond differently to varying lipid hydrophobicity.57 Of note, there is substantial evidence that particular lipid-binding/-transfer proteins are enriched at membrane contact sites, where they appear to facilitate the interorganelle transport of specific lipids.72–74 This observation is not discordant with the SLT mechanisms discussed here since one can envision that transfer of protein-dependent and -independent mechanisms complement each other in the transfer of distinct lipid species with particular acyl chain length and degree of saturation.
Acknowledgments
I am grateful to Dr. Vesa Olkkonen and the members of my group for critical reading of the manuscript.
Abbreviations
- ER
endoplasmic reticulum
- GPL
glycerophospholipid
- PM
plasma membrane
- PC
phosphatidylcholine
- PS
phosphatidylserine
- SLT
spontaneous lipid transfer
Footnotes
ACADEMIC EDITOR: Tim Levine, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1695 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the Academy of Finland (grant 259818) and the Sigrid Juselius Foundation. The author confirms that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: PS. Wrote the first draft of the manuscript: PS. Developed the structure and arguments for the paper: PS. Made critical revisions: PS. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Jones JD, Thompson TE. Spontaneous phosphatidylcholine transfer by collision between vesicles at high lipid concentration. Biochemistry. 1989;28(1):129–134. doi: 10.1021/bi00427a019. [DOI] [PubMed] [Google Scholar]
- 2.Brown RE. Spontaneous lipid transfer between organized lipid assemblies. Biochim Biophys Acta. 1992;1113(3–4):375–389. doi: 10.1016/0304-4157(92)90007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pownall HJ, Bick DL, Massey JB. Spontaneous phospholipid transfer: development of a quantitative model. Biochemistry. 1991;30(23):5696–5700. doi: 10.1021/bi00237a009. [DOI] [PubMed] [Google Scholar]
- 4.Nichols JW. Thermodynamics and kinetics of phospholipid monomer-vesicle interaction. Biochemistry. 1985;24(23):6390–6398. doi: 10.1021/bi00344a011. [DOI] [PubMed] [Google Scholar]
- 5.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289(35):24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wustner D, Solanko K. How cholesterol interacts with proteins and lipids during its intracellular transport. Biochim Biophys Acta. 2015;1848(9):1908–1926. doi: 10.1016/j.bbamem.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Doody MC, Pownall HJ, Kao YJ, Smith LC. Mechanism and kinetics of transfer of a fluorescent fatty acid between single-walled phosphatidylcholine vesicles. Biochemistry. 1980;19(1):108–116. doi: 10.1021/bi00542a017. [DOI] [PubMed] [Google Scholar]
- 8.McLean LR Phillips MC. Kinetics of phosphatidylcholine and lysophosphatidylcholine exchange between unilamellar vesicles. Biochemistry. 1984;23(20):4624–4630. doi: 10.1021/bi00315a017. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell JE, Jr, Lee KJ, Huestis WH. Lipid transfer between phosphatidylcholine vesicles and human erythrocytes: exponential decrease in rate with increasing acyl chain length. Biochemistry. 1985;24(12):2857–2864. doi: 10.1021/bi00333a007. [DOI] [PubMed] [Google Scholar]
- 10.Peeters RA, Veerkamp JH, Demel RA. Are fatty acid-binding proteins involved in fatty acid transfer? Biochim Biophys Acta. 1989;1002(1):8–13. doi: 10.1016/0005-2760(89)90057-x. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16(2–3):99–108. doi: 10.1385/JMN:16:2-3:99. [DOI] [PubMed] [Google Scholar]
- 12.Lagakos WS, Guan X, Ho SY, et al. Liver fatty acid-binding protein binds monoacylglycerol in vitro and in mouse liver cytosol. J Biol Chem. 2013;288(27):19805–19815. doi: 10.1074/jbc.M113.473579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa-Freire MC, Barenholz Y, Thompson TE. Glucocerebroside transfer between phosphatidylcholine bilayers. Biochemistry. 1982;21(6):1244–1248. doi: 10.1021/bi00535a021. [DOI] [PubMed] [Google Scholar]
- 14.Jones JD, Thompson TE. Mechanism of spontaneous, concentration-dependent phospholipid transfer between bilayers. Biochemistry. 1990;29(6):1593–1600. doi: 10.1021/bi00458a034. [DOI] [PubMed] [Google Scholar]
- 15.Frank A, Barenholz Y, Lichtenberg D, Thompson TE. Spontaneous transfer of sphingomyelin between phospholipid bilayers. Biochemistry. 1983;22(17):5647–5651. [Google Scholar]
- 16.Brown RE, Thompson TE. Spontaneous transfer of ganglioside GM1 between phospholipid vesicles. Biochemistry. 1987;26(17):5454–5460. doi: 10.1021/bi00391a036. [DOI] [PubMed] [Google Scholar]
- 17.Jones JD, Almeida PF, Thompson TE. Spontaneous interbilayer transfer of hexosylceramides between phospholipid bilayers. Biochemistry. 1990;29(16):3892–3897. doi: 10.1021/bi00468a015. [DOI] [PubMed] [Google Scholar]
- 18.Massey JB, Gotto AM, Jr, Pownall HJ. Kinetics and mechanism of the spontaneous transfer of fluorescent phosphatidylcholines between apolipoprotein-phospholipid recombinants. Biochemistry. 1982;21(15):3630–3636. doi: 10.1021/bi00258a016. [DOI] [PubMed] [Google Scholar]
- 19.Silvius JR, Leventis R. Spontaneous interbilayer transfer of phospholipids: dependence on acyl chain composition. Biochemistry. 1993;32(48):13318–13326. doi: 10.1021/bi00211a045. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Li M, Charubin K, et al. Effects of nanoparticle morphology and acyl chain length on spontaneous lipid transfer rates. Langmuir. 2015;31(47):12920–12928. doi: 10.1021/acs.langmuir.5b03291. [DOI] [PubMed] [Google Scholar]
- 21.Slater SJ, Ho C, Taddeo FJ, Kelly MB, Stubbs CD. Contribution of hydrogen bonding to lipid-lipid interactions in membranes and the role of lipid order: effects of cholesterol, increased phospholipid unsaturation, and ethanol. Biochemistry. 1993;32(14):3714–3721. doi: 10.1021/bi00065a025. [DOI] [PubMed] [Google Scholar]
- 22.Massey JB, Gotto AM, Jr, Pownall HJ. Kinetics and mechanism of the spontaneous transfer of fluorescent phospholipids between apolipoprotein-phospholipid recombinants. Effect of the polar headgroup. J Biol Chem. 1982;257(10):5444–5448. [PubMed] [Google Scholar]
- 23.McLean LR, Phillips MC. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry. 1981;20(10):2893–2900. doi: 10.1021/bi00513a028. [DOI] [PubMed] [Google Scholar]
- 24.Thomas PD, Poznansky MJ. Effect of surface curvature on the rate of cholesterol transfer between lipid vesicles. Biochem J. 1988;254(1):155–160. doi: 10.1042/bj2540155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letizia JY, Phillips MC. Effects of apolipoproteins on the kinetics of cholesterol exchange. Biochemistry. 1991;30(3):866–873. doi: 10.1021/bi00217a041. [DOI] [PubMed] [Google Scholar]
- 26.Bar LK, Chong PL, Barenholz Y, Thompson TE. Spontaneous transfer between phospholipid bilayers of dehydroergosterol, a fluorescent cholesterol analog. Biochim Biophys Acta. 1989;983(1):109–112. doi: 10.1016/0005-2736(89)90386-6. [DOI] [PubMed] [Google Scholar]
- 27.Massey JB, Hickson D, She HS, et al. Measurement and prediction of the rates of spontaneous transfer of phospholipids between plasma lipoproteins. Biochim Biophys Acta. 1984;794(2):274–280. doi: 10.1016/0005-2760(84)90156-5. [DOI] [PubMed] [Google Scholar]
- 28.Lund-Katz S, Hammerschlag B, Phillips MC. Kinetics and mechanism of free cholesterol exchange between human serum high- and low-density lipoproteins. Biochemistry. 1982;21(12):2964–2969. doi: 10.1021/bi00541a025. [DOI] [PubMed] [Google Scholar]
- 29.Lopez CA, de Vries AH, Marrink SJ. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci Rep. 2013;3:2071. doi: 10.1038/srep02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wimley WC, Thompson TE. Phosphatidylethanolamine enhances the concentration-dependent exchange of phospholipids between bilayers. Biochemistry. 1991;30(17):4200–4204. doi: 10.1021/bi00231a014. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RA, Hamilton JA, Worgall TS, Deckelbaum RJ. Free fatty acids modulate intermembrane trafficking of cholesterol by increasing lipid mobilities: novel 13C NMR analyses of free cholesterol partitioning. Biochemistry. 2003;42(6):1637–1645. doi: 10.1021/bi0264465. [DOI] [PubMed] [Google Scholar]
- 32.Lange Y, Ye J, Duban ME, Steck TL. Activation of membrane cholesterol by 63 amphipaths. Biochemistry. 2009;48(36):8505–8515. doi: 10.1021/bi900951r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pankov R, Markovska T, Antonov P, Ivanova L, Momchilova A. Influence of membrane phospholipid composition and structural organization on spontaneous lipid transfer between membranes. Gen Physiol Biophys. 2006;25(3):313–324. [PubMed] [Google Scholar]
- 34.Steck TL, Lange Y. Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 2010;20(11):680–687. doi: 10.1016/j.tcb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange Y, Tabei SM, Ye J, Steck TL. Stability and stoichiometry of bilayer phospholipid-cholesterol complexes: relationship to cellular sterol distribution and homeostasis. Biochemistry. 2013;52(40):6950–6959. doi: 10.1021/bi400862q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange Y, Ye J, Steck TL. Essentially all excess fibroblast cholesterol moves from plasma membranes to intracellular compartments. PLoS One. 2014;9(7):e98482. doi: 10.1371/journal.pone.0098482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radhakrishnan A, McConnell HM. Chemical activity of cholesterol in membranes. Biochemistry. 2000;39(28):8119–8124. doi: 10.1021/bi0005097. [DOI] [PubMed] [Google Scholar]
- 38.Radhakrishnan A, McConnell HM. Condensed complexes of cholesterol and phospholipids. Biophys J. 1999;77(3):1507–1517. doi: 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virtanen JA, Ruonala M, Vauhkonen M, Somerharju P. Lateral organization of liquid-crystalline cholesterol-dimyristoylphosphatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry. 1995;34(36):11568–11581. doi: 10.1021/bi00036a033. [DOI] [PubMed] [Google Scholar]
- 40.Tang D, Chong PL. E/M dips. evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somerharju P, Virtanen JA, Cheng KH, Hermansson M. The superlattice model of lateral organization of membranes and its implications on membrane lipid homeostasis. Biochim Biophys Acta. 2009;1788(1):12–23. doi: 10.1016/j.bbamem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Batchu KC, Hokynar K, Jeltsch M, Mattonet K, Somerharju P. Substrate efflux propensity is the key determinant of Ca2+-independent phospholipase A-beta (iPLAbeta)-mediated glycerophospholipid hydrolysis. J Biol Chem. 2015;290(16):10093–10103. doi: 10.1074/jbc.M115.642835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kol MA, van Dalen A, de Kroon AI, de Kruijff B. Translocation of phospholipids is facilitated by a subset of membrane-spanning proteins of the bacterial cytoplasmic membrane. J Biol Chem. 2003;278(27):24586–24593. doi: 10.1074/jbc.M301875200. [DOI] [PubMed] [Google Scholar]
- 44.Greenhut SF, Roseman MA. Cytochrome b5 induced flip-flop of phospholipids in sonicated vesicles. Biochemistry. 1985;24(5):1252–1260. doi: 10.1021/bi00326a030. [DOI] [PubMed] [Google Scholar]
- 45.Menon I, Huber T, Sanyal S, et al. Opsin is a phospholipid flippase. Curr Biol. 2011;21(2):149–153. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goren MA, Morizumi T, Menon I, et al. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat Commun. 2014;5:5115. doi: 10.1038/ncomms6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833(11):2526–2541. doi: 10.1016/j.bbamcr.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol. 2010;11(10):739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- 49.Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841(4):595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Flis VV, Daum G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb Perspect Biol. 2013;5(6):10. doi: 10.1101/cshperspect.a013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.English AR, Voeltz GK. Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol. 2013;5(4):a013227. doi: 10.1101/cshperspect.a013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olkkonen VM. OSBP-related protein family in lipid transport over membrane contact sites. Lipid Insights. 2015;8(suppl 1):1–9. doi: 10.4137/LPI.S31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16(1):1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 54.Lahiri S, Toulmay A, Prinz WA. Membrane contact sites, gateways for lipid homeostasis. Curr Opin Cell Biol. 2015;33:82–87. doi: 10.1016/j.ceb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol. 2016;17(2):69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voelker DR. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J Biol Chem. 1985;260(27):14671–14676. [PubMed] [Google Scholar]
- 57.Heikinheimo L, Somerharju P. Preferential decarboxylation of hydrophilic phosphatidylserine species in cultured cells. Implications on the mechanism of transport to mitochondria and cellular aminophospholipid species compositions. J Biol Chem. 1998;273(6):3327–3335. doi: 10.1074/jbc.273.6.3327. [DOI] [PubMed] [Google Scholar]
- 58.Kainu V, Hermansson M, Hanninen S, Hokynar K, Somerharju P. Import of phosphatidylserine to and export of phosphatidylethanolamine molecular species from mitochondria. Biochim Biophys Acta. 2013;1831(2):429–437. doi: 10.1016/j.bbalip.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka Y, Schroit AJ. Calcium/phosphate-induced immobilization of fluorescent phosphatidylserine in synthetic bilayer membranes: inhibition of lipid transfer between vesicles. Biochemistry. 1986;25(8):2141–2148. doi: 10.1021/bi00356a044. [DOI] [PubMed] [Google Scholar]
- 60.Kanfer JN. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- 61.Giacomello M, Drago I, Bortolozzi M, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38(2):280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13(10):607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarafdar PK, Chakraborty H, Dennison SM, Lentz BR. Phosphatidylserine inhibits and calcium promotes model membrane fusion. Biophys J. 2012;103(9):1880–1889. doi: 10.1016/j.bpj.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heikinheimo L, Somerharju P. Translocation of phosphatidylthreonine and - serine to mitochondria diminishes exponentially with increasing molecular hydrophobicity. Traffic. 2002;3(5):367–377. doi: 10.1034/j.1600-0854.2002.30506.x. [DOI] [PubMed] [Google Scholar]
- 65.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 66.Heikinheimo L, Somerharju P. Translocation of pyrene-labeled phosphatidylserine from the plasma membrane to mitochondria diminishes systematically with molecular hydrophobicity: implications on the maintenance of high phosphatidylserine content in the inner leaflet of the plasma membrane. Biochim Biophys Acta. 2002;1591(1–3):75–85. doi: 10.1016/s0167-4889(02)00253-7. [DOI] [PubMed] [Google Scholar]
- 67.Merklinger E, Schloetel JG, Spitta L, Thiele C, Lang T. No evidence for spontaneous lipid transfer at ER-PM membrane contact sites. J Membr Biol. 2015 doi: 10.1007/s00232-015-9845-2. [DOI] [PubMed] [Google Scholar]
- 68.Lange Y, Ye J, Steck TL. Activation mobilizes the cholesterol in the late endosomes-lysosomes of niemann pick type C cells. PLoS One. 2012;7(1):e30051. doi: 10.1371/journal.pone.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kainu V, Hermansson M, Somerharju P. Electrospray ionization mass spectrometry and exogenous heavy isotope-labeled lipid species provide detailed information on aminophospholipid acyl chain remodeling. J Biol Chem. 2008;283(6):3676–3687. doi: 10.1074/jbc.M709176200. [DOI] [PubMed] [Google Scholar]
- 70.Lands WE. Stories about acyl chains. Biochim Biophys Acta. 2000;1483(1):1–14. doi: 10.1016/s1388-1981(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 71.Hermansson M, Hokynar K, Somerharju P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog Lipid Res. 2011;50(3):240–257. doi: 10.1016/j.plipres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Yamaji T, Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic. 2015;16(2):101–122. doi: 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- 73.Mesmin B, Antonny B, Drin G. Insights into the mechanisms of sterol transport between organelles. Cell Mol Life Sci. 2013;70(18):3405–3421. doi: 10.1007/s00018-012-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung J, Torta F, Masai K, et al. Intracellular Transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349(6246):428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Virtanen JA, Cheng KH, Somerharju P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc Natl Acad Sci U S A. 1998;95(9):4964–4969. doi: 10.1073/pnas.95.9.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]