Introduction
Genetic factors may explain the differences in individual sensitivity to the psychosis-inducing effects of cannabis.1,2 In view of the converging data from candidate gene and genome-wide association studies that the D2-AKT1 signaling pathway is relevant for the pathophysiology and outcome of schizophrenia,3 and on the basis of previous association between cannabis-related psychosis and both DRD2 (rs1076560)1 and AKT1 (rs2494732),2 we hypothesized that these polymorphisms interact in increasing the risk of psychosis in cannabis users. We expected the genetic pathway × cannabis use interaction model to better predict the individual’s odds of psychotic disorder than the single candidate gene×cannabis use interaction model.
Materials and Methods
Participants were recruited as part of the Genetic and Psychosis project (GAP),2 a case–control study, carried out at the Institute of Psychiatry, London. All patients presenting to the Adult services (18–65 years) of the South London and Maudsley Mental Health NHS Foundation Trust, between December 2005 and October 2010, with their first episode of psychosis, were recruited into the study. Over the same time frame, from the area served by the mental health units, we recruited a sample of control subjects, aged 18–65 years, representing the local population in terms of ethnicity and other main sociodemographics according to the appropriate census data (www.statistics.gov.uk/census). Using the same methodology as in ref. 2, only patients with a diagnosis of nonorganic psychosis (International Classification of Diseases, 10: F20–F29 and F30–F33) were included, and control subjects were excluded if they met criteria for a psychotic disorder or if they reported a previous diagnosis of psychotic illness. For a more detailed description of the GAP study methods, (see refs 1, 2).
The data presented here are based on the subset of the whole GAP sample (450 participants (222 first-episode psychosis patients and 228 healthy individuals) with complete information on the following: (i) sociodemographics (age, gender, and self-reported ethnicity); (ii) lifetime use of cannabis, stimulants, tobacco, and alcohol; and (iii) DRD2 rs1076560 and AKT1 rs2494732 genotypes. To confirm self-report of ethnicity, genetic ancestry was derived using a panel of 57 ancestry-informative genetic markers, as performed previously.2 These were genotyped using iPLEX technology developed for the MassArray platform (Sequenom, San Diego, CA, USA). Ancestry scores were derived using the program Structure to implement a model-based (Markov Chain Monte Carlo) clustering algorithm. Having determined the best solution for K (the probable true number of underlying genetic groups) in initial analyses, individuals who scored between 96 and 100% for genetic cluster membership were used to create a three-way ancestral axis on the basis of Black African (N=81), European Caucasian (N=118), and Asian (N=16) ancestry. These reference groups were used to index genetic ancestry for the remaining sample. Further information on the makeup of the marker panel as well as a figure reporting plots of three-way ancestral axis on the basis of Black African, European Caucasian, and Other are available on request. Ninety percent (N=407) of participants had information on both self-reported ethnicity and ancestry markers. The level of overall agreement between self-reported and genetic ethnicities (96%) was reassuringly high.
Multivariable logistic regressions were used to evaluate the main and interaction effects between two measures of cannabis exposure (lifetime use and frequency of use) and DRD2 rs1076560/AKT1 rs2494732 (individuals carrying one or more of each of the two ‘risk’ alleles (DRD2 T and AKT1 C); individuals carrying one or more ‘risk’ alleles of only one of the genes (DRD2 T or AKT1 C); individuals carrying no ‘risk’ alleles) on presence of a psychotic disorder, after adjusting for potential confounders (sociodemographics and other drug use). The interaction model examined the probability of having a psychotic disorder among cannabis users carrying ‘risk’ allele(s) from both (DRD2 T carriers/AKT1 C carriers) or only one of the genes (DRD2 T carriers/AKT1 TT and DRD2 GG/AKT1 C carriers) compared with DRD2 GG/AKT1 TT subjects. Odds ratio (OR) of psychosis among cannabis-naive subjects carrying ‘risk’ allele(s) from both or only one of the genes were also calculated from the estimates provided by the model.
The study was granted ethical approval by the South London and Maudsley and Institute of Psychiatry Local Research Ethics Committee. All cases and control subjects who were included gave informed written consent, signing the consent document, to the publication of data originating from the study.
Results
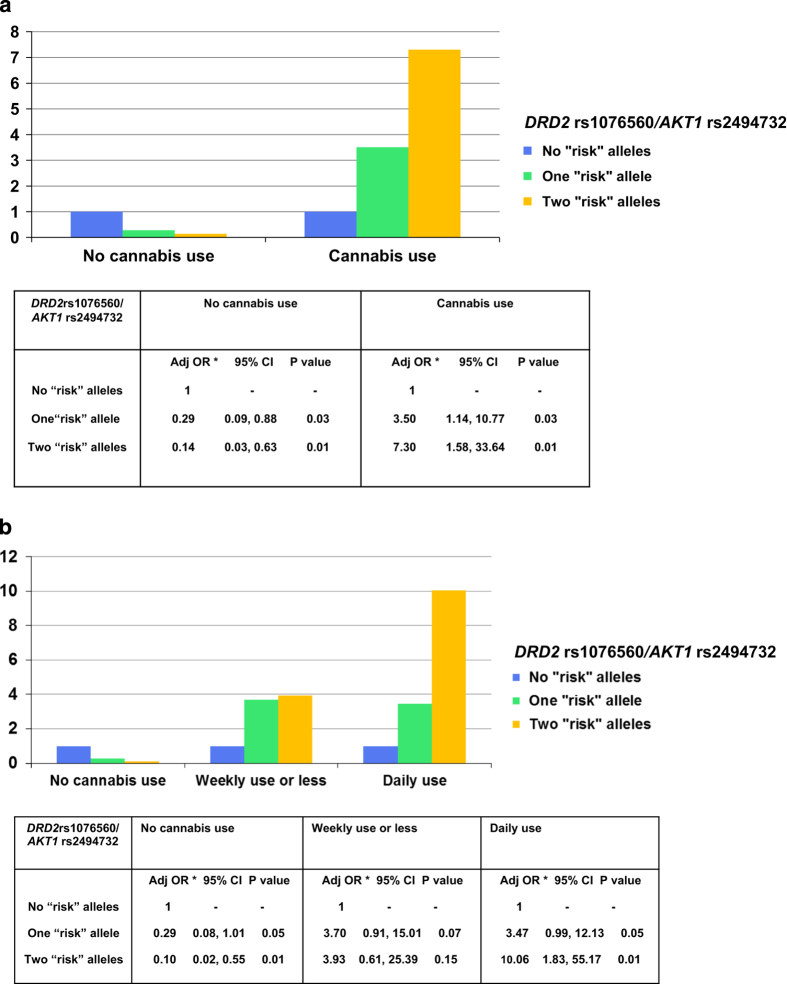
First-episode psychosis and control subjects differed significantly for some demographic characteristics and in their patterns of drug use. However, the DRD2/AKT1 genotype was not associated with any sociodemographic variable or cannabis use (all P>0.1; Table 1). A multivariable logistic regression adjusting for the modeled potential confounders showed a significant interaction between DRD2 rs1076560/AKT1 rs2494732 genotypes and lifetime cannabis use on probability of suffering from a psychotic disorder. The analysis showed an increasing probability of suffering from a psychotic disorder in cannabis users depending on DRD2/AKT1 (N=450, likelihood ratio test=7.66; P=0.022). When compared with the no ‘risk’ allele group, cannabis users carrying ‘risk’ allele(s) from only one (OR=3.50; 95% confidence interval: 1.14, 10.77) or both the genes (OR=7.30; 95% confidence interval: 1.58, 33.64) showed increased odds of having psychotic disorder. On the contrary, among those who had never used cannabis, carrying ‘risk’ allele(s) from only one (OR=0.29; 95% confidence interval: 0.09, 0.88) or both the genes (OR=0.14; 95% confidence interval: 0.03, 0.63) was associated with lower odds of suffering a psychotic disorder compared with the DRD2 GG/AKT1 TT genotype (Figure 1a). A second multivariable logistic regression adjusting for the potential confounders showed a significant interaction between DRD2/AKT1 and lifetime frequency of cannabis use on risk of psychosis (N=402, likelihood ratio test =11.91; P=0.042). Among both occasional and daily cannabis users, subjects carrying ‘risk’ allele(s) from one or both genes showed increased odds of having psychotic disorder when compared with the no ‘risk’ allele group; however, only among daily cannabis users did the increased odds of psychosis reach significance. In particular, there was a weak association between daily use and psychosis risk in subjects carrying ‘risk’ allele(s) from only one gene (OR=3.47; 95% confidence interval: 0.99, 12.13) but a strong association between daily use and psychosis risk in subjects carrying ‘risk’ allele(s) from both the genes (OR=10.06; 95% confidence interval: 1.83, 55.17). On the contrary, among those who had never used cannabis, carrying ‘risk’ allele(s) from only one (OR=0.29; 95% confidence interval: 0.08, 1.01) or both the genes (OR=0.10; 95% confidence interval: 0.02, 0.55) was associated with lower odds of suffering a psychotic disorder compared with the DRD2 GG/AKT1 TT genotype (Figure 1b).
Table 1. Demographic measures and patterns of drug use in FEP patients and control subjects.
| FEP patients, n=222 | Control subjects, n=228 | Statistical comparisons | |
|---|---|---|---|
| M±s.d. | M±s.d. | ||
| Age of psychosis onset | 26.07±6.19 | ||
| Age at assessment | 26.96±6.46 | 28.17±7.12 | F=3.54, P=0.06 (ANOVA) |
| Range | 18–44 | 18–44 | |
| Distribution | χ 2 =5.23; P=0.07 | ||
| 18–29 | 151 (68.0) | 145 (63.6) | |
| 30–39 | 59 (26.6) | 57 (25) | |
| 40–49 | 12 (5.4) | 26 (11.4) | |
| Gender | χ 2, P>0.1 | ||
| Male | 149 (67.1) | 141 (61.8) | |
| Female | 73 (32.9) | 87 (38.2) | |
| Self-reported ethnicity | χ 2 =35.44, P<0.001 | ||
| White Caucasian | 72 (32.4) | 134 (58.8) | |
| Black Caribbean | 68 (30.6) | 37 (16.2) | |
| Black African | 57 (25.7) | 31 (13.6) | |
| Asian/other | 25 (11.3) | 26 (11.4) | |
| DRD2 rs1076560/AKT1 rs2494732 | P>0.1 | ||
| No ‘risk’ alleles | 54 (24.3) | 57 (25) | |
| One ‘risk’ allele | 124 (55.9) | 133 (58.3) | |
| Two ‘risk’ alleles | 44 (19.8) | 38 (16.7) | |
| Tobacco use | χ 2 =27.19, P<0.001 | ||
| Nicotine dependence | 157 (70.7) | 106 (46.5) | |
| Not nicotine dependence | 65 (29.3) | 122 (53.5) | |
| Stimulant use | P>0.1 | ||
| Yes | 93 (41.9) | 81 (35.5) | |
| Never | 129 (58.1) | 147 (64.5) | |
| Alcohol use | χ 2 =23.58, P<0.001 | ||
| Harmful drinking behavior | 157 (70.7) | 203 (89) | |
| Not harmful drinking behavior | 65 (29.3) | 25 (11) | |
| Cannabis use | χ 2 =4.00, P=0.05 | ||
| Yes | 158 (71.2) | 142 (62.3) | |
| No ‘risk’ alleles | 40 (25.3) | 36 (25.4) | |
| One ‘risk’ allele | 89 (56.3) | 82 (57.7) | |
| Two ‘risk’ alleles | 29 (18.3) | 24 (23.2) | |
| Never | 64 (28.8) | 86 (37.7) | |
| No ‘risk’ alleles | 14 (21.9) | 21 (24.4) | |
| One ‘risk’ allele | 35 (54.7) | 51 (59.3) | |
| Two ‘risk’ alleles | 15 (23.4) | 14 (16.3) | |
| Frequency of use | χ 2 =14.38, P<0.001 | ||
| Dailya | 92 (61.1) | 43 (39.8) | |
| No ‘risk’ alleles | 24 (26.1) | 11 (25.6) | |
| One ‘risk’ allele | 51 (55.4) | 22 (51.2) | |
| Two ‘risk’ alleles | 17 (18.5) | 10 (23.2) | |
| Weekly or lessa | 52 (38.9) | 65 (60.2) | |
| No ‘risk’ alleles | 12 (23.1) | 18 (27.7) | |
| One ‘risk’ allele | 29 (55.7) | 37 (56.9) | |
| Two ‘risk’ alleles | 11 (21.2) | 10 (15.4) | |
| No detailsa | 14 | 34 | |
| Age of first usea | 16.51±4.99 | 16.51±5.81 | P>0.1 (ANOVA) |
| No ‘risk’ alleles | 16.35±4.63 | 16.20±7.46 | |
| One ‘risk’ allele | 16.51±5.03 | 16.64±5.10 | |
| Two ‘risk’ alleles | 16.66±5.35 | 16.6±4.83 | |
| Age of onset minus age of first useb | 9.56±7.00 |
Abbreviations: ANOVA, analysis of variance; FEP, first-episode psychosis; no ‘risk’ alleles, DRD2 GG/AKT1 TT; one ‘risk’ allele, DRD2 T carriers/AKT1 TT+DRD2 GG/AKT1 C carriers; two ‘risk’ alleles, DRD2 T carriers/AKT1 C carriers.
In those who had ever used cannabis.
No patient started to use cannabis for the first time after the psychosis onset.
Data are presented as M±s.d. or n (%).
Figure 1.
(a) Interaction between DRD2 rs1076560/AKT1 rs2494732 and lifetime cannabis use on psychosis risk. (b) Interaction between DRD2 rs1076560/AKT1 rs2494732 and lifetime frequency of cannabis use on psychosis risk. *Adjusted for gender, age, ethnicity, nicotine dependence, stimulants use, and harmful drinking behavior.
Discussion
The present results suggest an interaction between DRD2 rs1076560 and AKT1 rs2494732 genotypes on psychosis risk among cannabis users. Individuals carrying the DRD2 T allele or the AKT1 C allele have an increased psychosis risk in the context of cannabis use; however, the risk is especially increased in subjects who carry ‘risk’ alleles from both genes. In line with previous findings,1,2 the psychosis risk in cannabis users depends on the frequency of use, with the highest probability of psychotic disorder among daily users carrying both the risk variants. Our results indicate a model of interaction known as ‘qualitative G×E interaction’ with a crossover pattern: carriers of risk allele(s) for one of the two genes (DRD2 rs1076560 T or AKT1 rs2494732 C allele), compared with individuals carrying no ‘risk’ alleles (DRD2 rs1076560 GG/AKT1 rs2494732 TT), have a lower probability of psychotic disorder if they never used cannabis but a higher probability if they have a history of cannabis use, especially of daily use. Similarly, carriers of both the ‘risk’ alleles (DRD2 rs1076560 T allele and AKT1 rs2494732 C allele), compared with the other groups, have the lowest probability of psychotic disorder if they never used cannabis but the highest probability if they have a history of cannabis use, especially of daily use. Our findings are in line with previous results in the field1,2,4,5 and indicate that specific minor alleles may prevent or promote the risk for psychosis depending on the presence and degree of cannabis use. Such findings require validation in experimental designs and animal studies where both changes in the exposure and in the genotype can be modeled.
Striatal dopamine is altered in both schizophrenia patients and cannabis users,6 and cannabis-induced psychosis is related to the effects of cannabis on the striatum.7 The DRD2 T allele has been associated with both greater levels of striatal dopamine8 and cannabis-related psychosis,2 and is linked with AKT1 expression and psychosis-related endophenotypes by interaction with AKT1 rs1130233.3 Rs1130233 is in high linkage disequilibrium with rs2494732 (r 2=0.45, D′=0.94) and both single-nucleotide polymorphisms have been implicated in cannabis-related psychosis,1,7 likely altering striatal function.7 Cannabinoids activate AKT1 signaling downstream of D2,1,7 and schizophrenia patients with comorbid substance dependence have been reported to have abnormal post-synaptic D2 function.9 Consistent with this, our results suggest vulnerability to the psychotogenic effects of cannabis use involving genes that control dopamine signaling, particularly postsynaptically.
Acknowledgments
This study was supported by The Genetic and Psychotic Disorders (GAP) Study, Institute of Psychiatry, Psychology & Neuroscience, King’s College London and The South London and Maudsley (SLaM) Mental Health NHS Foundation Trust.
Funding
This work was supported by the UK National Institute of Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health, South London and Maudsley (SLAM) and the Institute of Psychiatry at King’s College London, The Psychiatry Research Trust, the Maudsley Charity Research Fund, and by the European Community’s Seventh Framework Program (grant agreement no. HEALTHF2-2009-241909; Project EU-GEI).
Footnotes
AB is a full-time employee of Hoffman-La Roche. The other authors declare no conflict of interest.
References
- Di Forti M , Iyegbe C , Sallis H , Kolliakou A , Falcone MA , Paparelli A et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry 2012; 72 (10): 811–816. [DOI] [PubMed] [Google Scholar]
- Colizzi M , Iyegbe C , Powell J , Ursini G , Porcelli A , Bonvino A et al. Interaction between functional genetic variation of DRD2 and cannabis use on risk of psychosis. Schizophr Bull, e-pub ahead of print 2015. 10.1093/schbul/sbv032. [DOI] [PMC free article] [PubMed]
- Blasi G , Napolitano F , Ursini G , Taurisano P , Romano R , Caforio G et al. DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing, and response to olanzapine treatment in schizophrenia. Proc Natl Acad Sci USA 2011; 108 (3): 1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkel R . Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry 2011; 68: 148–157. [DOI] [PubMed] [Google Scholar]
- Colizzi M , Fazio L , Ferranti L , Porcelli A , Masellis R , Marvulli D et al. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related behavior. Neuropsychopharmacology 2015; 40 (3): 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM , Mehta M , Di Forti M . Different dopaminergic abnormalities underlie cannabis dependence and cannabis-induced psychosis. Biol Psychiatry 2014; 75 (6): 430–431. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S , Atakan Z , Martin-Santos R , Crippa JA , Kambeitz J , Prata D et al. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry 2012; 17: 1152–1155. [DOI] [PubMed] [Google Scholar]
- Bertolino A , Taurisano P , Pisciotta NM , Blasi G , Fazio L , Romano R et al. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS ONE 2010; 5 (2): e9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL , Urban N , Slifstein M , Xu X , Kegeles LS , Girgis RR et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry 2013; 18 (8): 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]