Abstract
Schizophrenia is a severe psychiatric disorder with multi-factorial characteristics. A number of findings have shown disrupted synaptic connectivity in schizophrenia patients and emerging evidence suggests that this results from dysfunctional oligodendrocytes, the cells responsible for myelinating axons in white matter to promote neuronal conduction. The exact cause of this is not known, although recent imaging and molecular profiling studies of schizophrenia patients have identified changes in white matter tracts connecting multiple brain regions with effects on protein signaling networks involved in the myelination process. Further understanding of oligodendrocyte dysfunction in schizophrenia could lead to identification of novel drug targets for this devastating disease.
Introduction
Schizophrenia (SCZ) is a group of severe psychiatric disorders with lifelong disability occurring in >50% of the sufferers, making it one of the 10 most costly illnesses worldwide.1 The course of the disease is heterogeneous and characterized variously by the well-known positive symptoms such as psychosis, hallucinations, and delusions, as well as negative symptoms and cognitive deficits.2 Despite recent advances leading to new scientific insights into this disorder, consistent neurobiological markers for SCZ are lacking and diagnosis still relies on subjective assessment of a cluster of signs and symptoms, based on psychiatric rating systems such as the International Statistical Classification of Diseases and Related Health Problems 10th Revision and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.3 Treatment with antipsychotics helps to relieve some of the positive symptoms, although this has little or no effect on the negative symptoms or cognitive deficits, and most patients continue to suffer from these throughout their lifetimes.4,5
Considerable efforts are now underway using imaging and biomarker studies, which have marginally increased our understanding of the neurobiological basis of the disease. It is anticipated that further efforts in this area will lead to improved diagnosis or evaluation of the course of the disease and may also lay the groundwork for the development of new innovative treatment strategies. The main findings of these studies have led to the concept that the neurological deficits arise from an interaction between genetic6 and environmental factors.7 This result in SCZ symptoms that emerge during early adulthood and associated structural alterations in specific brain regions, leading to dysfunctional neuronal circuits and impaired connectivity through effects on white matter in complex brain networks.8–10
This review details the latest findings concerning the role of oligodendrocytes in the neuronal disconnectivity in SCZ from studies that have used imaging and biomarker profiling approaches. Most importantly, it will highlight how further studies along these avenues will result in increased understanding of the pathways affected in this devastating disease as well as the identification of much-needed novel drug targets for improved patient outcomes.
Schizophrenia—a result of brain disconnectivity?
One of the most recurrent findings has implicated disrupted intra- and inter-region connectivity as being the cause of many hallmark symptoms of SCZ. This is because normal brain function requires coordinated function of multiple brain regions in tasks such as perception and cognition, as well as for emotions and mood responses. Disconnectivity has been demonstrated in fronto-temporal regions,11 cortico-thalamo-cerebellar loops,12 and inter-hemispheric fibers crossing in the corpus callosum.13 A meta-analysis of 15 voxel-based diffusion tensor imaging studies revealed reduced fractional anisotropy as a measure of fiber density, myelination, and intra-tract coherence in left frontal and temporal lobe white matter in SCZ patients. These findings point towards disconnectivity in two distinct white matter tracts, one linking the frontal cortex, thalamus, and cingulate gyrus and the other forming a connection between the frontal cortex, insula, hippocampus, and temporal cortex.14 However, as chronic patients were used in these studies, it is possible that antipsychotic treatment was a confounding factor. Nevertheless, a recent meta-analysis of first episode patients with only marginal treatment also showed a reduction in fractional anisotropy, this time in the fronto-limbic circuitry involving the left inferior longitudinal fasciculus, left inferior fronto-occipital fasciculus, and inter-hemispheric fibers of the corpus callosum.15 Such effects have been associated with deficits in white matter integrity and one study showed that the myelin-associated water fraction was decreased in the genu of the corpus callosum of first episode patients, whereas chronic patients showed reductions in the same region along with additional changes in the frontal cortex.16 Thus, the chronic form of the disease may show changes, which affect a greater number of brain regions.
These studies have led to the hypothesis that brain disconnectivity and the consequential effects on cognitive function are likely to be owing to disruption of axon mylelination by oligodendrocytes. This is likely to be reflected by alterations in the patterns of oligodendrocyte messenger RNA (mRNA) transcripts and proteins. Myelination of axon fibers by oligodendrocytes is essential for rapid conduction of action potentials. This process continues through development into young adulthood and this could be important as this timing coincides with the average age of onset of SCZ.17 It is well known that oligodendrocyte dysfunction can lead to disturbances in myelination and consequently to deficient propagation of nerve impulses, compromising cognitive, neural, and glial functions as observed in SCZ.18 This is supported by the findings of microscopic stereology and immunohistochemical studies that showed reduced oligodendrocyte density in gray matter of the prefrontal cortex, anterior thalamic nucleus, and the cornuammonis four region of the hippocampus in SCZ patients.19–23 Furthermore, electron microscopy studies have revealed dystrophic and degenerative changes of pericapillary oligodendrocytes in the SCZ prefrontal cortex.24 However, other studies found no reductions of oligodendrocyte density in white matter,25,26 although the two-dimensional assessment of cell density used in some of these cases may have been confounded by volume differences owing to tissue shrinkage that is sometimes associated with formalin fixation.27 Although the primary reason for myelin and oligodendrocyte abnormalities in SCZ is not known, similar effects can be found in found in other neurological diseases with signs of inflammatory infiltration and microglial activation.
The impact of environmental factors leading to oligodendrocyte damage
Along with genetic factors, an array of environmental disturbances can contribute to the development of SCZ.28 Epidemiological studies have shown that obstetric complications such as bleeding during pregnancy, abnormal fetal growth, premature labor, or delivery problems are associated with an increased risk of SCZ later in life.29,30 All of these scenarios can lead to the development of fetal hypoxia and inflammation,31,32 which can become pathological and have a harmful impact on tissue growth. The influence of fetal or perinatal hypoxia on SCZ-like symptoms has been shown in several reports on animal models.33,34 Likewise, the potential involvement of inflammation has been supported by a meta-analysis, which found that more than half of the SCZ candidate genes are associated with hypoxia regulation or vascular function.35 On the other hand, there are reports showing that hypoxia at low levels is needed for blood vessel formation during embryogenesis36,37 and inflammation can protect the immature brain against viral or bacterial infection during pregnancy and may resolve itself without noxious effects.31
Hypoxia and inflammation induce changes in gene expression and signaling pathways associated with both physiological and pathological responses throughout the brain, although this occurs predominantly in the microglia. Hypoxia has been shown to activate microglia in the corpus callosum, thereby leading to deficits in myelination and consequently impaired axon functions.38 Microglia react rapidly to pathological changes in the brain by producing and releasing various pro-inflammatory cytokines and by generation of free radicals. Other studies have shown that an appropriate interaction between microglia and neurons is required to balance the processes of synaptogenesis and neuronal death during neurodevelopment and brain injury response.39
Microglia are mainly activated through inflammation by damage-associated molecular pattern molecules, including ATP high-mobility group box 1 and S100 molecules, as well as pathogen-associated molecular pattern molecules, such as lipopolysaccharide.40 Once activated, the microglia themselves can produce both cytokines and growth factors, as well as carry out antigen presentation and phagocytosis.41 Thus, microglia can affect other cell types in the area such as the myelinating oligodendrocytes through regulation of inflammation and growth pathways. This is important as some studies have reported increased density of microglia in post-mortem samples from patients with SCZ42–46 and patients who had committed suicide during acute psychosis had elevated microglial cell numbers in the anterior cingulate cortex, mediodorsal thalamus, dorsolateral prefrontal cortex, and hippocampus.44,45 Also, in vivo positron emission tomography imaging analyses demonstrated microglial activation in the hippocampus of patients with SCZ using the benzodiazepine-like ligand [11C] (R)-PK11195,47 although treatment of patients with antipsychotics in this study may have been a confounding factor as these drugs are known to decrease cytokine levels.48
Taken together, the above findings provide a plausible link between the changes identified by imaging studies regarding white matter density of brains from SCZ patients to alterations in oligodendrocyte functions, such axonal myelination. Studies have shown that cytokines released from activated microglia through hypoxia and inflammation are capable of damaging oligodendrocytes during neurodevelopment stages.32 For example, embryonic or neonatal animals treated with specific growth factors or cytokines such as epidermal growth factor, neuregulin 1 (NRG1), interleukin-1, or interleukin-6 exhibit disturbed oligodendrocyte function, abnormal neurotransmission, synaptic loss, and SCZ-like behavioral abnormalities after puberty.49 Furthermore, activation of microglia by lipopolysaccharide led to reduced survival of oligodendrocyte precursor cells in oligodendrocyte/microglia co-cultures. Thus, such disturbances could result in impaired connectivity of the developing brain up to the time of adulthood, leading to increased vulnerability of SCZ development. An elegant review has also suggested involvement of microglial or astroglial activation in white matter pathologies in SCZ.50 Another study showed that the anti-inflamamtory agent minocycline could be used to inhibit cytokine release and increase survival and proliferation of oligodendrocyte precursor cells in an animal model of hypoxia,51 The same study also found that long-term impairment of white matter diffusivity in these animals was attenuated by minocycline, as shown by magnetic resonance imaging/diffusion tensor imaging analysis. Taken together, these findings provide strong evidence that neuroinflammation is associated with oligodendrocyte dysfunction in SCZ, which is likely to lead to alterations in myelination and the white matter tract disturbances associated with disrupted brain connectivity and impaired cognition.
Differential expression of myelination markers in schizophrenia
In line with the hypothesis of oligodendrocyte dysfunction in SCZ patients and the findings described above, several molecular profiling studies of brain tissues from SCZ patients have now revealed changes in a number of proteins related to myelination. For example, a transcriptomic study carried out in 2001 found altered expression levels of myelination-related mRNAs in post-mortem dorsolateral prefrontal cortex samples, consistent with the oligodendrocyte hypothesis (Table 1).52 These findings have been confirmed by other researchers who also investigated myelination-related mRNA levels from post-mortem brains using microarray analyses.53–55 In addition to these findings, decreased expression in myelin- and oligodendrocyte-related mRNAs was found in other studies by quantitative PCR and in situ hybridization in post-mortem hippocampus and cortical/sub-cortical brain regions from patients with SCZ.56–59 More recently, a study employed RNA sequencing in a transcriptomic analysis of post-mortem superior temporal gyrus, which revealed significant alterations of cortical promoter usage and splicing in SCZ patients.60 Changes in claudin-11 (CLDN11) mRNA have also been detected in several studies.55,61–64 CLDN11 is expressed predominantly by oligodendrocytes and contributes to ~7% of the total myelin mass.65 Gow and collaborators reported that claudin-11 is essential for the formation of tight junctions in central nervous system (CNS) myelin through the observation that claudin-11 knockout mice exhibited neurological deficits, such as slowed CNS nerve conduction.66
Table 1. Classical myelin proteins and most oligendrocyte-related proteins differential expressed in schizophrenia.
| Proteins | UniProt ID, human | Cellular location | Gene expression reference | Proteome reference |
|---|---|---|---|---|
| 2,3-Cyclic-nucleotide 3-phosphodiesterase (CNP) | P09543.2 | Plasma membrane | 52, 56, 60–62, 64 | 16, 54, 70, 71 |
| Claudin-11 (CLDN11) | O75508.2 | Tight junction | 55, 61–63 | — |
| Myelin basic protein (MBP) | P02686 | Plasma membrane | 55, 60, 88 | 68, 69, 71, 74 |
| Myelin proteolipid protein (PLP1) | P60201.2 | Plasma membrane | 55, 67 | 72 |
| Myelin-associated glycoprotein (MAG) | P20916.1 | Plasma membrane | 52, 53, 55, 56, 60–62, 67 | 73 |
| Myelin oligodendrocyte glycoprotein (MOG) | Q16653.1 | Plasma membrane | 55, 56, 60, 61, 75 | 70, 71, 74 |
| Hyaluronan and proteoglycan link hyaluprotein 2 (HAPLN2) | Q9GZV7 | Secreted | — | 71 |
| Ermin (ERMN) | Q8TAM6 | Cytoskeleton | — | 71 |
| Gelsolin (GSN) | P06396 | Cytoskeleton | — | 54 |
| Transferrin (TRF) | P02787 | Cytoplasm | 52, 55, 60, 61, 64, 67, 75 | 54, 69, 106, 107 |
In addition, proteomic studies of post-mortem brain tissues from SCZ patients have been carried out, which identified changes in proteins associated with oligodendrocytes and the process of myelination. In 2004, Prabakaran and collaborators analyzed frontal cortex samples from SCZ patients and controls and found differential expression of transferrin and 2ʹ,3ʹ-cyclic-nucleotide 3ʹ-phosphodiesterase (CNP).54 Other studies supported these results through identification of changes in other myelination-related proteins in several different brain regions, such as myelin basic protein (MBP), myelin proteolipid protein (PLP1),67 myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG).16,54,68–75 The functions of each of these oligodendrocyte proteins are described in more detail in the following sections with a focus on their roles in myelination. It should be noted that there are some discrepancies in the literature regarding the effects on some of these molecules in post-mortem tissues, which could be due to differences in factors such as the methods applied, duration of drug treatment or post-mortem factors. However, another intriguing possibility is that the differences could relate to variations in the brain regions involved and potentially to different studies analyzing different subtypes of the disorder. For example, a recent T1 structural imaging and resting-state functional magnetic resonance imaging scanning study by Chang and co-workers found aberrant bilateral connectivity of default mode network, inferior frontal gyrus, and cerebellum only in patients with auditory verbal hallucinations, whereas disturbances in superior temporal gyrus and precentral gyrus were specific to non-auditory verbal hallucination patients.51
CNP
CNP is an enzyme that catalyses the hydrolysis of a phosphodiester bond in 2ʹ,3ʹ-cyclic phosphate to generate 2ʹ-phosphate.76,77 This protein comprises ~4% of the total CNS myelin protein mass and is found in the inner and outer margins of myelin paranodal loops and oligodendrocyte cytoplasm, although it is absent from compact myelin.78 CNP undergoes post-translational modification by the addition of isoprenyl and palmitoyllipidic radicals, facilitating its binding to the plasma membranes.79,80 In addition to its phosphodiesterase activity, CNP links tubulin to cellular membranes, regulating the microtubule distribution in the cytoplasm. Consequently, it can regulate cellular morphology.81,82 Consistent with a potential role in SCZ, two genetic association studies have implicated CNP in SCZ pathogenesis.83,84 Likewise, changes in the levels of the CNP protein have been associated with behavioral deficits in mice. Edgar et al. 85 that CNP knockout mice show decreased emotionality and fear compared with control mice. In addition, we found that it is a potential protein biomarker for SCZ, with altered levels found in some brain regions.74
MBP
MBP has several isoforms, although an 18.5 kDa polypeptide is the major form in adult humans and this is highly conserved in mammals. MBP isolated from brain tissues contains numerous post-translational modifications including deimination, phosphorylation, deamidation, methylation, and N-terminal acylation.86 This protein is also attached to the plasma membrane and is responsible for maintaining adhesion of the cytoplasmic surfaces of multi-lamellar compact myelin.87 In carrying out this role, MBP interacts with a variety of proteins such as calmodulin, actin, tubulin, and SH3 domain-containing proteins. Thus, it may be involved as a signaling hub in the processes of myelin development and remodeling.86 Besides the detection of altered MBP expression in SCZ using transcriptomic88 and proteomic analyses, imaging approaches have also found abnormal MBP staining in samples of SCZ brain tissues.89 However, another study, which used western blot analysis, found no changes in MBP protein levels in post-mortem brain tissues from patients with SCZ and other psychiatric disorders.90 Also, transcriptomic studies found no changes in MBP in post-mortem hippocampus, anterior cingulate cortex, and putamen of patients with SCZ.62 In the case of the latter, the discrepancy could be owing to differences in drug treatments and/or potential confounding factors associated with post-mortem tissues such as differences in post-mortem intervals or agonal periods. In addition, differences across studies could be due to the established fact that mRNA and protein levels are not necessarily correlated and deduction from mRNA levels alone is insufficient.91 This is why many researchers are turning to protein-based methods as these macromolecules can offer a real-time readout of physiological and biological change. Therefore, further work is essential using proteomic-based methods to establish whether MBP can be a useful biomarker of perturbed myelination in SCZ.
PLP1
PLP1 is the major protein constituent of compact myelin in the CNS comprising ~45% of the total content.92 It has four transmembrane-spanning domains with cytoplasmic N and C termini,93,94 and it is highly hydrophobic and contains several covalently bound fatty acid moieties essential for its function. The extracellular loops of PLP1 include four cysteine residues connected through intramolecular disulfide bonds that are crucial for protein folding, dimerization, and cellular trafficking.95 One study showed that reduction of PLP1 levels greatly reduced conduction velocity of myelinated axons in mice.96 Moreover, these mice displayed anxiety-like behaviors, reduced pre-pulse inhibition, spatial learning deficits, and working memory deficits, as found in SCZ.
MAG
MAG is a cell adhesion protein found in myelin in both the CNS and peripheral nervous system (PNS) in non-compact regions of the myelin sheath.97 This protein is extensively glycosylated on its extracellular loops, which are composed of five tandem immunoglobulin-like domains. The cytoplasmic domains have different intracellular binding sites and properties, suggesting an intracellular signaling function and the capability of interacting with cytoskeletal elements.98 Aiming to localize the effects of risk variants in MAG gene on brain morphometry in SCZ patients, Felsky et al. 99 showed that the temporal and parietal cortices were the areas that were most affected by MAG gene polymorphisms.
MOG
MOG is also a myelin-associated protein related to the immunoglobulin family and is comprised of 12 isoforms. As the extracellular domain of MOG is capable of undergoing dimerization, Clements et al. 100 suggested that this assembly could represent a homophilic adhesion complex in myelin sheaths. Other researchers investigated the contribution of genotypic variation of a single-nucleotide polymorphism in MOG (rs2857766) to white matter volumes in psychotic disorders.101 They found that healthy G-homozygotes of the MOG single-nucleotide polymorphism had greater white matter volume in the brainstem and cerebellum compared with those with a psychotic disorder.
Other proteins
Besides the classical myelin proteins, other proteins related to oligodendocyte function and myelination have been found to be affected in different brain regions from SCZ patients (Table 1). Ermin is an oligodendrocyte-specific protein that appears at a late stage during myelination of mature nerves, and is localized to the outer cytoplasmic lip of the myelin sheath and paranodal loops.102 It has been proposed that this protein is involved in regulating cytoskeletal rearrangements by binding F-actin during the late wrapping and/or compaction phases of myelinogenesis.102 Gelsolin is a Ca2+-dependent actin-binding protein that is found in many types of cells and produced by oligodendrocytes in the CNS. This protein has also been proposed to have role in myelinogenesis it has cleavage, capping, and nucleating activities that might be important in initiating lamellipodia-like protrusive movements of myelin along axonal segments.103 Hirakawa et al. 104 found that the protein hyaluronan and proteoglycan link hyaluprotein 2 (HAPLN2) could have a pivotal role in the formation of the hyaluronan-associated matrix in the CNS, which facilitates neuronal conduction and structural stabilization by mediating binding of versican V2 to hyaluronic acid. Another protein that has been linked to myelination functions is transferrin. This is an iron-binding glycoprotein that controls the levels of free iron in cells, tissues, and body fluids.105 In the CNS, transferrin is expressed mainly by oligodendrocytes and found to be differentially expressed in many transcriptomic60 and proteomic profiling studies71,106,107 of post-mortem brain samples from SCZ patients.
Myelination pathways mediated by oligodendrocytes
CNS myelination of neuronal axons is mediated by oligodendrocytes in a process involving complex interactions with the extracellular matrix andaxolemma.108 The process proceeds in a posterior to anterior gradient across the brain after the neuronal circuitry has been laid down with a peak in early postnatal life and culminating in late-maturing brain structures such as the prefrontal cortex around the time of early adulthood. The key molecules that control myelination are described below.
NRG1/ErbB
Several isoforms of the NRG1 protein have been shown to be differentially expressed in post-mortem brains of SCZ patients109 and mice overexpressing NRG1 types I and III have shown deficits in pre-pulse inhibition, a behavioral test with relevance to symptoms found in SCZ.110–112 NRG1 is involved in regulation of glutamatergic receptors, oligodendrocyte proliferation, and myelination,113 and can therefore also be linked to brain connectivity. Indeed, myelination is triggered mainly through NRG1/ErbB signaling in oligodendrocytes. The NRG1 family is composed of four proteins belonging to the superfamily of epidermal growth factor-like ligands. Most of these are synthesized as transmembrane precursor polypeptides (pro-NRG1s) with the epidermal growth factor domain located outside the cell. This domain is cleaved by proteases such as tumor necrosis factor-alpha-converting enzyme. This leads to production of mature NRG1s that are soluble, except in the case of NRG1-III. Both the N- and C-terminal regions of NRG1-III are located inside the cell. Thus, NRG1-III may require cell contact to exert its function.114
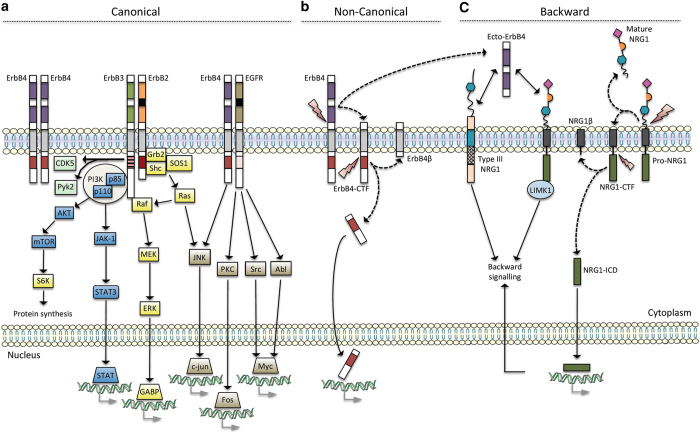
NRG1 proteins released from neurons bind to ErbB receptors on oligodendrocytes, which initiates several intracellular signaling cascades. The ErbB receptor family is comprised of four structurally related receptor tyrosine kinases (ErbB1–4).115 NRG1 binds to ErbB3 and ErbB4 directly. ErbB4 exists as a homodimer116,117 and ErbB1 and ErbB2 form heterodimers with ErbB4 and ErbB3, respectively. Dimerization of these proteins triggers NRG1–ErbB signaling in oligodendrocytes as summarized in Figure 1.
Figure 1.
NRG1 and ErbB signaling (adapted from Mei and Xiong117). The scheme summarizes the different types of signaling - canonical (a), non-canonical (b) and backward (c) - when NRG1 or ErbB proteins are stimulated. The black boxes indicate that ErbB2 and EGFR do not bind to NRG1. The striped pink box indicates the impaired tyrosine kinase domain of ErbB3 Black arrows indicate downstream signaling pathways when ErbB or NRG1 proteins are activated. Moreover, dashed arrows display the release of protein products from transmembrane proteins that were cleaved by proteases (represented by lightning). Gray arrows indicate gene transcription. NRG1, neuregulin 1.
Regulation of the NRG1/ErbB pathway in the CNS is achieved mainly through proteolytic cleavage of membrane-bound NRGs by enzymes, such asγ-secretase,118,119 β-secretase and a disintegrin, and metalloproteinase domain-containing protein 10 (ADAM10).120 Cleavage of NRG1 by ADAM10 and β-secretase releases N-terminal fragments that activate ErbB receptors. However, one study showed that ADAM10 inhibition did not affect normal myelination in a co-culture system, whereas β-secretase inhibition impaired this process.120 γ-Secretase can cleave NRG1 and the intracellular domain of the ErbB4 receptor, promoting oligodendrocyte maturation in primary cultures. Moreover, it has been shown that cleavage is likely to block myelination, as inhibitors of this activity accelerate and enhance myelination.118,121
Extracellular matrix components
Several components of the extracellular matrix such as laminin, insulin-like growth factor 1 (IGF-I), and the fibroblast growth factors (FGFs) are essential in the development and function of myelinating cells in the CNS.122–124 Laminin receptors such as α6β1-integrin and dystroglycan in oligodendrocyte lineage cells have been shown to mediate oligodendrocyte survival, differentiation, and spatiotemporal targeting in coordination with axonal NRG1 signaling.125 Chun et al. 126 showed that laminin-deficient mice developed demyelinated axons and reduced sheath thickness during the early stages of myelination. In this manner, extracellular laminin could contribute for creating an environment that facilitates myelin production through activation of appropriate signaling pathways.108
IGF-I is an anabolic peptide that shares homology with proinsulin.123 IGF-I is produced by all major neuronal cell types in the brain and its expression is partly regulated by pituitary growth hormone during development.127 IGF-I exerts its action by binding to the IGF-I receptor (IGF-IR), a heterotetrameric glycoprotein composed of two alpha (α) and two beta (β) subunits. The α-subunits constitute the extracellular portion of the receptor with IGF-I-binding sites, whereas the β-subunits span the membrane and initiate intracellular signal transduction via a long intracytoplasmic domain, containing intrinsic tyrosine kinase activity and other critical phosphorylation sites. Studies have shown that the overexpression of IGF-I in the mouse CNS results in increased brain growth, oligodendrocyte number, myelination, and associated myelin protein expression.128–130 This is supported by IGF-I knockout mouse studies, which showed reduced numbers of oligodendrocytes and oligodendrocyte precursor cells and decreased myelin-related proteins,131 along with decreased neuronal survival.132
FGFs are involved in many cell processes and functions, such as proliferation, differentiation, organogenesis, and myelination. FGF-1 and FGF-2 can be produced by neurons and astrocytes,133,134 and the levels of both proteins are increased during active myelination.135 Consistent with these findings, expression of FGF receptor 1 (FGFR1), FGFR2, and FGFR3 have been detected in oligodendrocytes.136,137 These receptors are composed of three immunoglobulin-like domains, a single-transmembrane helical region, and an intracellular domain with tyrosine kinase activity. A recent report showed that oligodendrocytes require FGF receptor signaling to assemble the normal number of myelin membrane wrappings around axons.124 However, this is not consistent with the findings of some previous studies, which found that FGF-2 treatment resulted in reduction of myelin formation in rats and cultured neuronal cells.138,139
PI3K/AKT/mTORC signaling
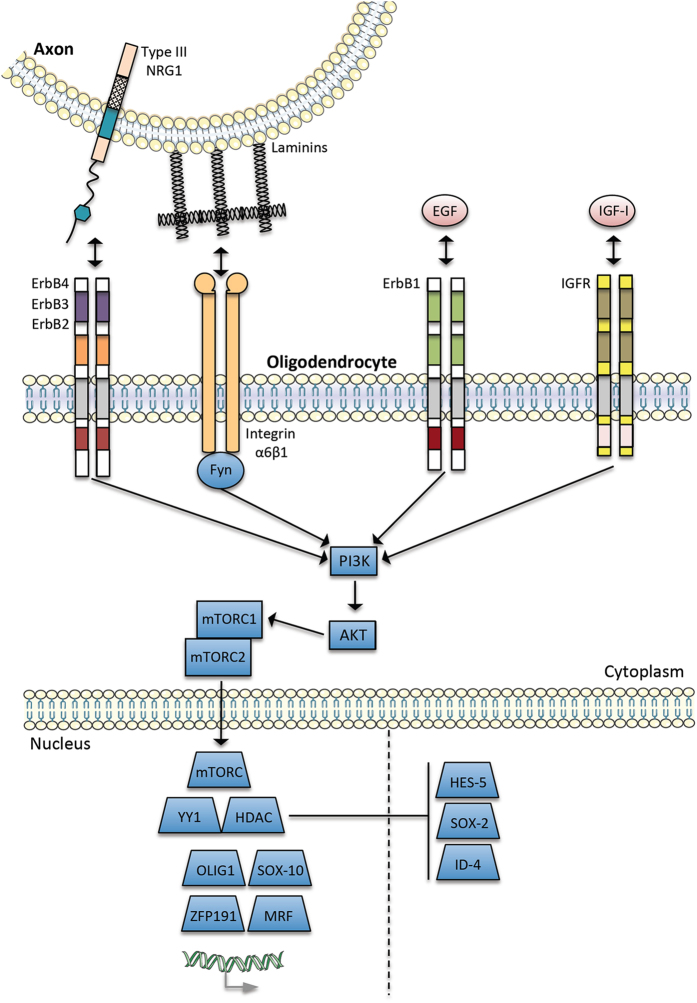
Regarding myelinating factors from the axolemma and extracellular matrix, the phosphatidylinositol 3 kinase/serine–threonine-specific protein kinase/mammalian target of rapamycin complex (PI3K/AKT/mTORc) appears to be a point of convergence in myelination processes (Figure 2). For example, several reports have demonstrated that PI3K/AKT/mTORC signaling is activated by the neuregulins,140 integrins,141 and IGF-I.128,142 Moreover, it has been shown that the expression of constitutively active AKT1 leads to an increase of myelination in mouse oligodendrocytes.143 In terminal differentiation of oligodendrocytes, myelin protein and lipid expression can be activated by mTORC1 and mTORC2 complexes induced via PI3K/AKT/mTORC signaling.144 A study carried out by Wesseling et al. 145 identified increased levels of mTOR kinase in the ketamine rat model of SCZ. Apart from effects on myelination, the mTOR kinase is also crucial for regulating expression of proteins involved in synaptic plasticity.146 In addition, mTOR signaling has been reported disrupted in SCZ patients147 and reduced levels of synaptic proteins normally induced by mTOR signaling have been reported in the prefrontal cortex of depressed patients.148 Therefore, these findings support the possibility of targeting components of the mTOR signaling pathway as a potential new approach in the development of antipsychotic drugs. Other nuclear proteins involved in activation and inhibition of myelin gene transcription are also shown in Figure 2.
Figure 2.
Convergence and integration of external signals in myelination processes by oligodendrocytes (adapted from Taveggia et al.159). As explained in the text, the PI3K/AKT/mTORC pathway may be the point of convergence and integration of these external signals in myelination process.159 The dashed line in the nucleus separates proteins involved in activation and inhibition of myelin gene transcription.
Future work: identification of novel targets for schizophrenia associated with oligodendrocyte and myelin function
Considering that proteins do not work alone but rather as multi-protein complexes, pathways, or signaling cascades, further studies should be carried out to map the protein and small molecule networks associated with the oligendrocyte-related proteins discussed in this review. This could be achieved using laboratory techniques such as tandem affinity purification, which employs a tagged protein bait for co-purification of interacting proteins in cells and mass spectrometry for identification.149 In addition, in silico methods could be applied such as Ingenuity Pathway Analysis150 or GeneMANIA,151 which can identify potential interaction partners in protein networks by superimposing laboratory data on to pre-existing networks in interaction databases. Subsequently, further mapping studies known as ‘pathway walking’ can be performed as more SCZ-related molecules are identified, thereby extending networks to include key regulatory points, which may be druggable.
As inflammation and immune activation have been implicated in the development and progression of SCZ, it follows that compounds capable of normalizing such imbalances may be helpful in treating the disease. For example, a study using a rat model of impaired myelin production found that oligodendrocytes recovered their function after restoration of immunoglobulin Fc receptor gamma/Fyn signaling.152 Another study showed that the antibiotic minocyclin was capable of reducing oligodendrocyte damage caused by gamma-interferon-stimulated microglia in a cell co-culture investigation153 and the same compound was used inhibit cytokine release and increase oligodendrocyte precursor cell survival in a hypoxia-based animal model, as described above.51 A recent clinical study found that adjunctive minocycline with clozapine treatment helped to relieve impaired working memory, avolition, and depression/anxiety in chronic SCZ patients with persistent symptoms.154 On the basis of studies such as these, researchers have suggested that the best approach would be to combine the use of anti-inflammatory substances with standard antipsychotic treatment for improved treatment of those SCZ patients who also display an inflammatory phenotype.155 Furthermore, growth factors, such as IGF-I, are also under consideration as new therapeutic candidates, as the IGF pathway is known to be involved in the regulation of oligodendrocyte development and repair.156 In line with this, a recent study published in Nature showed that IGF-I treatment restored synaptic deficits in neurons from 22q11.2 deletion patients, a syndrome characterized by an increased risk of SCZ and other psychiatric conditions.156 According to some studies, the initiation of treatment is likely to be more effective at the onset of symptoms,157 suggesting that it may be more appropriate for testing in patients with first episode psychosis and as an adjuvant treatment to antipsychotic agents, as described above for the anti-inflammatory agents.
Final remarks
The study of SCZ is challenging owing to its complex nature. However, breakthroughs are essential to potential shed light on new possible treatment avenues. Numerous clinical studies using imaging techniques have reported white matter changes in SCZ indicative of perturbations in connectivity within and across different brain regions. Furthermore, recent molecular studies, such as transcriptomic and proteomic profiling analyses of brain tissues and cell culture models have implicated oligodendrocyte and myelination dysfunction as significant features of the disease.158 The present review has linked imaging and molecular data on these effects in SCZ, providing an impetus for further studies in this area. It is only through an increased understanding of the disease pathways that much-needed novel drug targets can be identified.
Acknowledgments
We dedicate our work to psychiatric patients and their families and thank them for understanding the importance of science and for having made our research possible.
Funding
DMS and JSC are funded by FAPESP (São Paulo Research Foundation, grants 2013/08711-3, 2014/10068-4 and 2014/14881-1).
AS was an honorary speaker for TAD Pharma and Roche and has been a member of advisory boards for Roche. PF has been an honorary speaker for AstraZeneca, Bristol Myers Squibb, Eli Lilly, Essex, GE Healthcare, GlaxoSmithKline, Janssen Cilag, Lundbeck, Otsuka, Pfizer, Servier, and Takeda. During the past 5 years, but not presently, PF has been a member of the advisory boards of Janssen Cilag, AstraZeneca, Eli Lilly, and Lundbeck. The remaining authors declare no conflict of interest.
References
- Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci 2000; 250: 274–285. [DOI] [PubMed] [Google Scholar]
- Häfner H, an der Heiden W. Schizophrenia. Blackwell Science: 2007; 101–141. [Google Scholar]
- WHO. International Statistical Classification of Diseases and Related Health Problems 10th Revision 4th edn. WHO Library: Geneva, Switzerland, 2010. [Google Scholar]
- Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients' perspective 5 years later. Dialogues Clin Neurosci 2010; 12: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 2012; 13: 318–378. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci 2014; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TA, Christensen BK, Rizvi S. Visual-spatial episodic memory in schizophrenia: A multiple systems framework. Neuropsychology 2010; 24: 368–378. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schmitt A, Cannon TD . Pathophysiology of schizophrenia In: Gaebel W (ed). Schizophrenia: Current Science and Clinical Practice. Wiley-Blackwell: Oxford, UK, 2011; 31–65. [Google Scholar]
- Hunter R, Barry S. Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. Eur Psychiatry 2012; 27: 432–436. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3: 89–97. [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler's ‘fragmented phrene’ as schizencephaly. Arch Gen Psychiatry 1999; 56: 781–787. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Paez P, Chance SA. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry 2007; 19: 449–457. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108: 3–10. [DOI] [PubMed] [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 100–106. [DOI] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry 2003; 8: 811–820. [DOI] [PubMed] [Google Scholar]
- Hoistad M, Segal D, Takahashi N, Sakurai T, Buxbaum JD, Hof PR. Linking white and grey matter in schizophrenia: oligodendrocyte and neuron pathology in the prefrontal cortex. Front Neuroanat 2009; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 2008; 31: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL Jr , Byne W, Buitron C, Perl DP et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003; 53: 1075–1085. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 2004; 67: 269–275. [DOI] [PubMed] [Google Scholar]
- Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res 2007; 94: 273–280. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 2009; 117: 395–407. [DOI] [PubMed] [Google Scholar]
- Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 2006; 85: 245–253. [DOI] [PubMed] [Google Scholar]
- Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry 2008; 9: 34–42. [DOI] [PubMed] [Google Scholar]
- Segal D, Schmitz C, Hof PR. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol 2009; 117: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013; 263: 41–52. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol 1988; 278: 344–352. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010; 468: 203–212. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 2013; 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry 2008; 13: 873–877. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2012; 71: 444–457. [DOI] [PubMed] [Google Scholar]
- Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci 2013; 35: 102–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Lex A, Falkai P, Henn FA, Schmitt A. Behavioural alterations in rats following neonatal hypoxia and effects of clozapine: implications for schizophrenia. Pharmacopsychiatry 2008; 41: 138–145. [DOI] [PubMed] [Google Scholar]
- Sommer JU, Schmitt A, Heck M, Schaeffer EL, Fendt M, Zink M et al. Differential expression of presynaptic genes in a rat model of postnatal hypoxia: relevance to schizophrenia. Eur Arch Psychiatry Clin Neurosci 2010; 260 (Suppl 2): S81–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HWM, Rutten BPF. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry 2012; 17: 1194–1205. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK et al. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 2001; 220: 175–186. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Simon MC. Oxygen, genes, and development: an analysis of the role of hypoxic gene regulation during murine vascular development. J Mol Med (Berl) 1998; 76: 391–401. [DOI] [PubMed] [Google Scholar]
- Kaur C, Sivakumar V, Ang LS, Sundaresan A. Hypoxic damage to the periventricular white matter in neonatal brain: role of vascular endothelial growth factor, nitric oxide and excitotoxicity. J Neurochem 2006; 98: 1200–1216. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia 2002; 40: 140–155. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 42: 115–121. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 2005; 43: 81–89. [PubMed] [Google Scholar]
- Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 2012; 26: 1273–1279. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 2006; 112: 305–316. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 2008; 42: 151–157. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry 2011; 69: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- Kato T, Monji A, Hashioka S, Kanba S. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res 2007; 92: 108–115. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci 2010; 64: 217–230. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 2015; 161: 102–112. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Krabbe G, Weikert G, Scheuer T, Matheus F, Wang Y et al. Minocycline protects the immature white matter against hyperoxia. Exp Neurol 2014; 254: 153–165. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001; 98: 4746–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res 2004; 77: 858–866. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 2004; 9: 684–697 43. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003; 362: 798–805. [DOI] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 2009; 112: 54–64. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology 2008; 33: 2993–3009. [DOI] [PubMed] [Google Scholar]
- Kerns D, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P et al. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res 2010; 120: 150–158. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Kelly F, Taylor A, Bates S, Reid J, Logendra R et al. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol Psychiatry 2009; 14: 1083–1094. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Wang X, Beveridge NJ, Tooney PA, Scott RJ, Carr VJ et al. Transcriptome sequencing revealed significant alteration of cortical promoter usage and splicing in schizophrenia. PLoS One 2012; 7: e36351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res 2005; 79: 157–173. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis 2006; 21: 531–540. [DOI] [PubMed] [Google Scholar]
- Weidenhofer J, Bowden NA, Scott RJ, Tooney PA. Altered gene expression in the amygdala in schizophrenia: up-regulation of genes located in the cytomatrix active zone. Mol Cell Neurosci 2006; 31: 243–250. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL et al. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res 2007; 90: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Tiwari-Woodruff S, Buznikov AG, Stevens DB. Involvement of OSP/claudin-11 in oligodendrocyte membrane interactions: role in biology and disease. J Neurosci Res 2000; 59: 706–711. [DOI] [PubMed] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99: 649–659. [DOI] [PubMed] [Google Scholar]
- Aberg K, Saetre P, Jareborg N, Jazin E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc Natl Acad Sci USA 2006; 103: 7482–7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res 2004; 29: 2293–2302. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyas E, Eberlin MN et al. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res 2009; 43: 978–986. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Maccarrone G, Dias-Neto E et al. Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2009; 259: 151–163. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Marangoni S, Novello JC et al. Alterations in oligodendrocyte proteins, calcium homeostasis and new potential markers in schizophrenia anterior temporal lobe are revealed by shotgun proteome analysis. J Neural Transm 2009; 116: 275–289. [DOI] [PubMed] [Google Scholar]
- English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics 2009; 9: 3368–3382. [DOI] [PubMed] [Google Scholar]
- Chan MK, Tsang TM, Harris LW, Guest PC, Holmes E, Bahn S. Evidence for disease and antipsychotic medication effects in post-mortem brain from schizophrenia patients. Mol Psychiatry 2011; 16: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Maccarrone G, Wobrock T, Zerr I, Gormanns P, Reckow S et al. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res 2010; 44: 1176–1189. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GI, Iyer NT, Keith J. Hydrolysis of Ribonucleoside 2',3'-Cyclic Phosphates by a Diesterase from Brain. J Biol Chem 1962; 237: 3535–3539. [Google Scholar]
- Mazumder R, Iyer LM, Vasudevan S, Aravind L. Detection of novel members, structure-function analysis and evolutionary classification of the 2H phosphoesterase superfamily. Nucleic Acids Res 2002; 30: 5229–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Bernier L, Andrews SB, Colman DR. Cellular and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphodiesterase and its mRNA in the rat central nervous system. J Neurochem 1988; 51: 859–868. [DOI] [PubMed] [Google Scholar]
- Agrawal HC, Sprinkle TJ, Agrawal D. 2',3'-cyclic nucleotide-3'-phosphodiesterase in the central nervous system is fatty-acylated by thioester linkage. J Biol Chem 1990; 265: 11849–11853. [PubMed] [Google Scholar]
- Braun PE, De Angelis D, Shtybel WW, Bernier L. Isoprenoid modification permits 2',3'-cyclic nucleotide 3'-phosphodiesterase to bind to membranes. J Neurosci Res 1991; 30: 540–544. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Stingo S, Wolff J. 2',3'-Cyclic nucleotide 3'-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc Natl Acad Sci USA 2002; 99: 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Gravel M, Zhang R, Thibault P, Braun PE. Process outgrowth in oligodendrocytes is mediated by CNP, a novel microtubule assembly myelin protein. J Cell Biol 2005; 170: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A et al. Convergent evidence for 2',3'-cyclic nucleotide 3'-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry 2006; 63: 18–24. [DOI] [PubMed] [Google Scholar]
- Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L et al. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci USA 2006; 103: 12469–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar NM, Touma C, Palme R, Sibille E. Resilient emotionality and molecular compensation in mice lacking the oligodendrocyte-specific gene Cnp1. Transl Psychiatry 2011; 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G, Ladizhansky V, Boggs JM. Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry 2009; 48: 8094–8104. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 2001; 81: 871–927. [DOI] [PubMed] [Google Scholar]
- Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C et al. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann N Y Acad Sci 2004; 1025: 84–91. [DOI] [PubMed] [Google Scholar]
- Parlapani E, Schmitt A, Erdmann A, Bernstein HG, Breunig B, Gruber O et al. Association between myelin basic protein expression and left entorhinal cortex pre-alpha cell layer disorganization in schizophrenia. Brain Res 2009; 1301: 126–134. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honer WG, Bergmann K, Falkai P, Lutjohann D, Bayer TA. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord 2005; 7: 449–455. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999; 19: 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol 2009; 40: 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JM, Lees MB. Myelin proteolipid protein--the first 50 years. Int J Biochem Cell Biol 2002; 34: 211–215. [DOI] [PubMed] [Google Scholar]
- Daffu G, Sohi J, Kamholz J. Proteolipid protein dimerization at cysteine 108: Implications for protein structure. Neurosci Res 2012; 74: 144–155. [DOI] [PubMed] [Google Scholar]
- Dhaunchak AS, Nave KA. A common mechanism of PLP/DM20 misfolding causes cysteine-mediated endoplasmic reticulum retention in oligodendrocytes and Pelizaeus-Merzbacher disease. Proc Natl Acad Sci USA 2007; 104: 17813–17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Ma J, Tanaka KF, Takao K, Komada M, Tanda K et al. Mice with altered myelin proteolipid protein gene expression display cognitive deficits accompanied by abnormal neuron-glia interactions and decreased conduction velocities. J Neurosci 2009; 29: 8363–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Quarles RH. Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. J Cell Biol 1982; 92: 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursula P, Lehto VP, Heape AM. The small myelin-associated glycoprotein binds to tubulin and microtubules. Brain Res Mol Brain Res 2001; 87: 22–30. [DOI] [PubMed] [Google Scholar]
- Felsky D, Voineskos AN, Lerch JP, Nazeri A, Shaikh SA, Rajji TK et al. Myelin-associated glycoprotein gene and brain morphometry in schizophrenia. Front Psychiatry 2012; 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CS, Reid HH, Beddoe T, Tynan FE, Perugini MA, Johns TG et al. The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis. Proc Natl Acad Sci USA 2003; 100: 11059–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Walshe M, Dempster E, Collier DA, Marshall N, Bramon E et al. The association of white matter volume in psychotic disorders with genotypic variation in NRG1, MOG and CNP: a voxel-based analysis in affected individuals and their unaffected relatives. Transl Psychiatry 2012; 2: e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D, Sabanay H, Riethmacher D, Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci 2006; 26: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouyiouklis DA, Brophy PJ. A novel gelsolin isoform expressed by oligodendrocytes in the central nervous system. J Neurochem 1997; 69: 995–1005. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Oohashi T, Su WD, Yoshioka H, Murakami T, Arata J et al. The brain link protein-1 (BRAL1): cDNA cloning, genomic structure, and characterization as a novel link protein expressed in adult brain. Biochem Biophys Res Commun 2000; 276: 982–989. [DOI] [PubMed] [Google Scholar]
- Crichton RR, Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem 1987; 164: 485–506. [DOI] [PubMed] [Google Scholar]
- Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry 2006; 11: 459–470, 23. [DOI] [PubMed] [Google Scholar]
- Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry 2008; 13: 1102–1117. [DOI] [PubMed] [Google Scholar]
- Ahrendsen JT, Macklin W. Signaling mechanisms regulating myelination in the central nervous system. Neurosci Bull 2013; 29: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Parlapani E, Gruber O, Wobrock T, Falkai P. Impact of neuregulin-1 on the pathophysiology of schizophrenia in human post-mortem studies. Eur Arch Psychiatry Clin Neurosci 2008; 258 (Suppl 5): 35–39. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci 2008; 28: 6872–6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ et al. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport 2009; 20: 1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Zhang M, Trembak-Duff I, Unterbarnscheidt T, Radyushkin K, Dibaj P et al. Dysregulated expression of neuregulin-1 by cortical pyramidal neurons disrupts synaptic plasticity. Cell Rep 2014; 8: 1130–1145. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Koschel J, Zink M, Bauer M, Sommer C, Frank J et al. Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 2010; 260: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE et al. Isoform-specific expression and function of neuregulin. Development 1997; 124: 3575–3586. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res 2009; 315: 611–618. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 2003; 284: 14–30. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 2008; 9: 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Feng L. Implication of gamma-secretase in neuregulin-induced maturation of oligodendrocytes. Biochem Biophys Res Commun 2004; 314: 535–542. [DOI] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol 2003; 161: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Prior M, He W, Hu X, Tang X, Shen W et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem 2011; 286: 23967–23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 2008; 60: 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Tzvetanova ID. Glia unglued: how signals from the extracellular matrix regulate the development of myelinating glia. Dev Neurobiol 2011; 71: 924–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kusky J, Ye P. Neurodevelopmental effects of insulin-like growth factor signaling. Front Neuroendocrinol 2012; 33: 230–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Dupree JL, Nave KA, Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J Neurosci 2012; 32: 6631–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002; 4: 833–841. [DOI] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol 2003; 163: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Umayahara Y, Ritter D, Bunting T, Auman H, Rotwein P et al. Regulation of insulin-like growth factor I (IGF-I) gene expression in brain of transgenic mice expressing an IGF-I-luciferase fusion gene. Endocrinology 1997; 138: 5466–5475. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 1993; 10: 729–740. [DOI] [PubMed] [Google Scholar]
- Ye P, Carson J, D'Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J Neurosci 1995; 15: 7344–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Butt AM. In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-I on oligodendrocyte development and myelination in the central nervous system. J Neurosci Res 1999; 57: 74–85. [DOI] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci 2002; 22: 6041–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia 2007; 55: 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva MA, Mocchetti I. Developmental expression of the basic fibroblast growth factor gene in rat brain. Brain Res Dev Brain Res 1991; 62: 45–50. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Iwata H, Okumura N, Yoshida S, Imaizumi K, Lee Y et al. Localization of basic fibroblast growth factor-like immunoreactivity in the rat brain. Brain Res 1992; 587: 49–65. [DOI] [PubMed] [Google Scholar]
- Ratzka A, Baron O, Grothe C. FGF-2 deficiency does not influence FGF ligand and receptor expression during development of the nigrostriatal system. PLoS One 2011; 6: e23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J Neurosci 2005; 25: 7470–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J Neurosci 2011; 31: 5055–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, de Jonge JC, de Vries H, Hoekstra D. Perturbation of myelination by activation of distinct signaling pathways: an in vitro study in a myelinating culture derived from fetal rat brain. J Neurosci Res 2000; 59: 74–85. [PubMed] [Google Scholar]
- Wang Z, Colognato H, Ffrench-Constant C. Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia 2007; 55: 537–545. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005; 47: 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 2009; 136: 2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness JK, Mitchell NE, Wood TL. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: differential regulation and activation of receptors and the downstream effector Akt. Dev Neurosci 2002; 24: 437–445. [DOI] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci 2008; 28: 7174–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW et al. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci 2009; 29: 6367–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling H, Rahmoune H, Tricklebank M, Guest PC, Bahn S. A targeted multiplexed proteomic investigation identifies ketamine-induced changes in immune markers in rat serum and expression changes in protein kinases/phosphatases in rat brain. J Proteome Res 2015; 14: 411–421. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 2013; 73: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A, van den Buuse M. Is the mTOR-signalling cascade disrupted in Schizophrenia? J Neurochem 2014; 129: 377–387. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace E, Moazed D. Affinity Purification of Protein Complexes Using TAP Tags. Methods Enzymol 2015; 559: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Bonchev D. A survey of current software for network analysis in molecular biology. Hum Genomics 2010; 4: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010; 38: W214–W220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwa C, Yamamoto M, Tanaka K, Fukutake M, Ueki T, Takeda S et al. Restoration of FcRgamma/Fyn signaling repairs central nervous system demyelination. J Neurosci Res 2007; 85: 954–966. [DOI] [PubMed] [Google Scholar]
- Seki Y, Kato TA, Monji A, Mizoguchi Y, Horikawa H, Sato-Kasai M et al. Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-gamma-stimulated microglia in co-culture model. Schizophr Res 2013; 151: 20–28. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Sullivan KM, McEvoy JP, McMahon RP, Wehring HJ, Gold JM et al. Adjunctive Minocycline in Clozapine-Treated Schizophrenia Patients With Persistent Symptoms. J Clin Psychopharmacol 2015; 35: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leza JC, Garcia-Bueno B, Bioque M, Arango C, Parellada M, Do K et al. Inflammation in schizophrenia: A question of balance. Neurosci Biobehav Rev 2015; 55: 612–626. [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 2014; 276: 29–47. [DOI] [PubMed] [Google Scholar]
- Bou Khalil R. Recombinant human IGF-1 for patients with schizophrenia. Med Hypotheses. 2011; 77: 427–429. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 2015; 161: 4–18. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol 2010; 6: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]