Abstract
Noncardiac chest pain is a term that encompasses all causes of chest pain after a cardiac source has been excluded. This article focuses on esophageal sources for chest pain. Esophageal chest pain (ECP) is common, affects quality of life, and carries a substantial health care burden. The lack of a systematic approach toward the diagnosis and treatment of ECP has led to significant disability and increased health care costs for this condition. Identifying the underlying cause(s) or mechanism(s) for chest pain is key for its successful management. Common etiologies include gastroesophageal reflux disease, esophageal hypersensitivity, dysmotility, and psychological conditions, including panic disorder and anxiety. However, the pathophysiology of this condition is not yet fully understood. Randomized controlled trials have shown that proton pump inhibitor therapy (either omeprazole, lansoprazole, or rabeprazole) can be effective. Evidence for the use of antidepressants and the adenosine receptor antagonist theophylline is fair. Psychological treatments, notably cognitive behavioral therapy, may be useful in select patients. Surgery is not recommended. There remains a large unmet need for identifying the phenotype and prevalence of pathophysiologic mechanisms of ECP as well as for well-designed multicenter clinical trials of current and novel therapies.
Keywords: Noncardiac chest pain, esophageal chest pain, pathophysiology, treatment
Noncardiac chest pain is a nonspecific term that encompasses all causes of chest pain after cardiovascular disease has been definitively excluded. The term therefore includes etiologies such as esophageal diseases, musculoskeletal or inflammatory disorders, pulmonary diseases, neurologic causes such as entrapment neuropathy, and psychological disorders.1 In clinical practice, esophageal chest pain (ECP) is a common problem whose diagnosis and treatment remain challenging.
Functional chest pain of esophageal origin is part of the spectrum of ECP that is diagnosed when other organic etiologies have been ruled out. Rome III criteria define this condition as episodes of unexplained chest pain that are usually midline in location and of visceral quality; therefore, these episodes are potentially of esophageal origin.2 The nature of ECP is identical. In this article, we provide an update on the epidemiology, diagnostic strategies, and treatment of ECP.
Epidemiology
The exact prevalence of ECP is difficult to estimate because its diagnosis requires the use of multiple diagnostic tests to exclude other conditions. However, several studies have reported the prevalence of ECP based on symptom patterns. In a population-based survey from Olmsted County, Minnesota, the annual prevalence was estimated to be 23%, with an equal sex distribution.3 A validated chest pain questionnaire used in 1000 randomly selected individuals in Australia reported the prevalence of ECP to be 33%, with a nearly equal prevalence in either sex (32% in men vs 33% in women). Interestingly, the prevalence of ECP decreased with advancing age.4 Compared with younger women (<25 years), older women (45-55 years) reported less frequent ECP.4 In Argentina, a population-based survey estimated the annual prevalence of ECP to be 23.5%, and the condition was most frequently associated with symptoms of gastroesophageal reflux disease (GERD) at least once a week.5 In China, the annual prevalence of ECP was 19%.6 Reports using Rome I criteria for ECP have shown a prevalence of 13.6% among householders in the United States,7 but other causes of chest pain were not excluded due to methodologic limitations of the study.
There are several risk factors associated with ECP that influence the frequency with which symptoms are reported, the number of prescriptions that are written, and the health care resources that are utilized. In one study, patients with ECP were younger, consumed more alcohol, smoked more frequently, and reported more anxiety when compared with other causes of chest pain.8 ECP also accounted for approximately 2% to 5% of all emergency room visits in the United States, and more than 6 million patients were admitted to a hospital for the evaluation of chest pain.9 Overlap with other functional gastrointestinal disorders is common in patients with ECP. One study reported that 80% of patients with ECP had an overlap with another functional gastrointestinal disorder such as irritable bowel syndrome (27%) or functional bloating (22%).10
Patients often report ECP as a debilitating medical condition due to the physical pain involved, and this is further compounded by the psychological distress associated with the condition. Consequently, these patients report a diminished quality of life. Patients with ECP also seek health care from a variety of settings. In a survey of primary care physicians (PCPs), 79.5% of patients with noncardiac chest pain, including those with ECP, were treated by PCPs. Interestingly, PCPs referred these patients most frequently to a gastroenterologist (75.6%), followed by a cardiologist (7.8%) or a pulmonologist (1.6%). Almost half of these patients (45.6%) were treated with proton pump inhibitors (PPIs).11
Costs associated with ECP have not been systematically assessed. In the United States, it has been estimated that the annual cost of admission and investigation of acute chest pain with normal coronaries is $8 billion.12 Indirect costs associated with prescriptions and the loss of work-related productivity were not included, but there are data showing that work absenteeism rates (29%) and interruptions to daily activities (63%) are quite high in these patients.13 A recent Irish study found that the cost per hospitalization in patients with nonspecific chest pain was €3729, and the annual national cost for providing health care for ECP was €71 million.14 ECP patients make numerous visits to the emergency room, have greater utilization of health care and greater costs, report significant psychological distress, and demonstrate diminished quality of life.2
Pathophysiology
Several mechanisms have been proposed, and it is likely that the pathogenesis of ECP is multifactorial and heterogeneous. In a given patient, one or more mechanisms may be involved.
Gastroesophageal Reflux Disease
ECP is often presumed to be due to GERD, and the pain may be mediated by activation of esophageal chemoreceptors.15 DeMeester and colleagues showed that 46% of patients with noncardiac chest pain had acid reflux during ambulatory pH studies.16 In another study, pH testing yielded a positive symptom index and/or pathologic acid reflux in 50% of individuals with chest pain.17 A different study showed that acid reflux may cause ECP in 30% to 60% of patients.18 Nonacid (or weakly acidic) reflux may also cause chest pain, but the mechanism leading to this symptom is not clearly known.19 In a study of patients on and off PPI therapy, heartburn—but not regurgitation or chest pain—decreased significantly during PPI therapy, suggesting that nonacid reflux may cause ECP.20 However, more evidence is needed to establish the association of nonacid reflux and ECP. Thus, both acid and nonacid reflux may be involved in the pathogenesis of ECP, and the latter may include bile acid reflux.
Esophageal Dysmotility
Several motility disorders have been implicated in the pathogenesis of ECP, including diffuse esophageal spasm, nutcracker esophagus, achalasia, scleroderma, and nonspecific motility disorders.21 However, the evidence is conflicting. In one study, although 32% of patients had dysmotility, none experienced pain.17 A study of 10 patients who underwent 24-hour endoluminal ultrasonography found sustained esophageal contractions during episodes of spontaneous chest pain.22 However, this activity, mediated by longitudinal muscle contractions, occurred only in a subset of patients and only during some of the pain episodes. In another study, heartburn with acid reflux was shown to be triggered by these sustained contractions.23 Esophageal spasm may cause ECP and may occur either spontaneously or as a result of noxious stimuli such as acid reflux or ingestion of corrosive materials.24
Esophageal Hypersensitivity
Esophageal hypersensitivity refers to a sensory dysfunction in which patients experience discomfort or pain at thresholds that are significantly lower than those experienced by healthy controls. Esophageal hypersensitivity is believed to be a key mechanism for ECP and considered a hallmark of this condition.10,25 Patients with ECP demonstrated a hyperreactive and poorly compliant esophagus and 50% lower sensory thresholds during esophageal balloon distension studies when compared with healthy controls.25,26 In one study, 83% of ECP patients reported discomfort at pressure distensions less than 50 cm H2O, whereas none of the healthy controls reported discomfort at this distension threshold.26 In addition, 80% of ECP patients reported that their usual chest pain was reproduced during the test. More significantly, in another study, smooth muscle relaxation with atropine did not improve esophageal sensory thresholds or chest pain, suggesting that muscle spasm is not the main cause of ECP.27 In yet another study, esophageal hypersensitivity was seen in 90% of patients with nutcracker esophagus, suggesting that sensory dysfunction causes chest pain in hypercontractile esophagus.24 Together, these findings suggest that esophageal hypersensitivity, rather than a primary motor dysfunction, is more important in the pathogenesis of symptoms in ECP. Furthermore, these findings explain why smooth muscle relaxants by themselves are generally ineffective for relieving ECP.21
Recent studies suggest that pain perception in ECP may be due to central sensitization28 and that N-methyl-D-aspartate (NMDA) blockers may alter chest pain. This was elegantly demonstrated with ketamine (an NMDA blocker), which reversed visceral hypersensitivity.29 In a controlled study of healthy subjects, citalopram, a selective serotonin reuptake inhibitor given intravenously, significantly increased sensory thresholds and prolonged the time for the perception of heartburn after acid infusion,30 implying that ECP may be centrally mediated. More recently, adenosine has been implicated in the pathogenesis of chest pain; adenosine infusion decreased esophageal sensory thresholds in both healthy controls and ECP patients, suggesting that both peripheral and central sensitization may play a role.31
Diagnostic Approaches
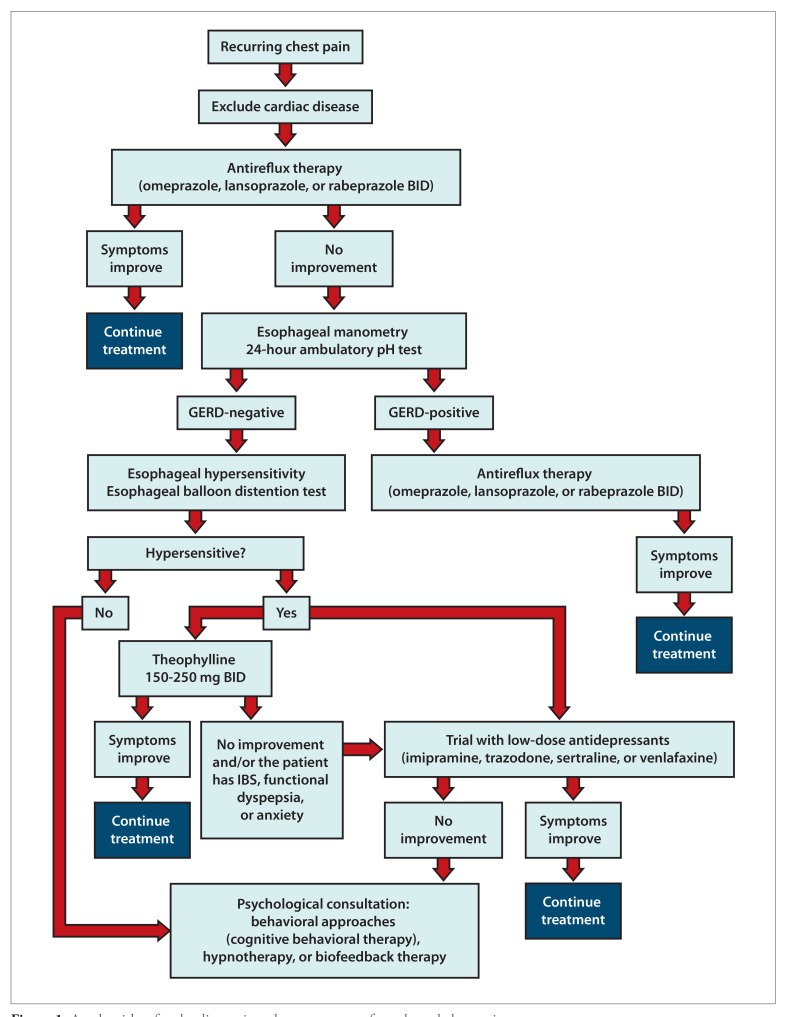
There is little consensus on how to diagnose a patient with ECP. A practical approach is to identify and/or rule out mechanisms that cause ECP. An algorithm for the diagnosis and management of ECP is shown in Figure 1. GERD is a widely recognized cause of ECP, and a logical first step is to perform diagnostic studies directed toward identifying this problem.
Figure 1.
An algorithm for the diagnosis and management of esophageal chest pain.
GERD, gastroesophageal reflux disease; IBS, irritable bowel syndrome.
Modified with permission from Coss-Adame E, et al. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
Several studies have proposed a high-dose PPI trial (using omeprazole, rabeprazole, or lansoprazole) as a tool for the diagnosis of GERD-related ECP. This approach has shown similar levels of sensitivity and specificity for the diagnosis of ECP that is not related to GERD. The optimal dose and duration of the PPI trial remain controversial and heterogeneous. Double-dose PPI trials were assessed for 7 days in 3 studies.18,32,33 Alternatively, omeprazole 40 mg twice daily for 2 weeks34 and lansoprazole 30 mg once daily for 4 weeks35 were found to have a sensitivity of 78% to 92% and a specificity of 67% to 87% for identifying GERD-related chest pain. It is important to emphasize that in the aforementioned studies, the standard test for GERD diagnosis was endoscopy (erosive esophagitis) and 24-hour pH metry. In addition, responders to the PPI trial were predominantly patients who had documented erosive esophagitis or greater acid exposure on 24-hour pH metry.
Two meta-analyses have shown that a PPI therapeutic trial adequately identified patients with ECP caused by GERD.36,37 When a therapeutic PPI trial fails or clinical suspicion remains high favoring GERD as a cause of ECP, then further evaluation is recommended. Although erosive esophagitis is being seen less frequently in clinical practice, an upper endoscopy is the first diagnostic step, particularly in the presence of alarm symptoms (dysphagia, weight loss, bleeding). However, erosive esophagitis is infrequently found in patients with ECP. In addition, strictures or signs suggestive of eosinophilic esophagitis that may be associated with ECP can also be identified.
Nonerosive reflux disease (NERD) is the most frequent type of GERD,38 particularly with widespread use of PPIs. Patients with NERD are challenging because they can show true reflux (excessive acid exposure, nonacid reflux, or symptom correlation) or they may have no acid exposure or symptom correlation; this subtype is also frequently associated with functional heartburn.2 In addition, ECP is more frequently seen in NERD. In this group of patients, 24-hour pH metry can detect true reflux even when a PPI trial is negative. Studies that have addressed therapy with a PPI trial have shown that most patients who respond to treatment have abnormal pH metry results (39%-75% in responders).18,33,34
Esophageal motility disorders are associated with ECP. High-resolution esophageal manometry with impedance is the standard test for identifying motility disorders. It should be performed in patients complaining of ECP, especially when endoscopic evaluation is negative. Approximately 30% of patients with ECP show abnormal esophageal manometry, and the most common disorder, seen in approximately 14.4% of patients, is nutcracker esophagus.39 The frequency of achalasia or distal esophageal spasm is very low. A study of ECP from South America showed that only 8% of subjects had abnormal esophageal manometry, and nutcracker esophagus was the most frequent motor abnormality.40
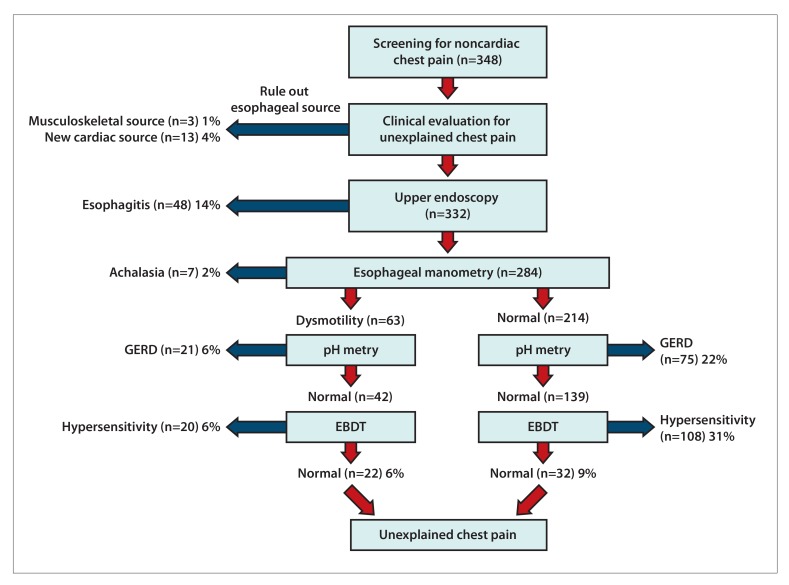
When reflux or motility dysfunction has been excluded by the aforementioned diagnostic approaches, esophageal hypersensitivity will explain the presence of ECP. Esophageal hypersensitivity is best identified with an esophageal balloon distention test. In a study of 348 patients with chest pain of presumed esophageal origin, after excluding GERD (via a normal upper endoscopy and normal 24-hour pH metry) and dysmotility, an esophageal balloon distension test was performed in 181 subjects. A positive balloon distention test result identifying esophageal hypersensitivity as a source of ECP was seen in 128 of 178 (72%) patients, and in 75% of these patients (97 patients), the chest pain was reproduced during the test.41 Although the esophageal balloon distension test can be a useful diagnostic test in patients with unexplained chest pain, more studies are needed to confirm and assess its utility in clinical practice, especially as this test is typically performed in specialized centers. A diagnostic approach for ECP is shown in Figure 2.
Figure 2.
Diagnostic yield of testing for esophageal chest pain.
EBDT, esophageal balloon distention test; GERD, gastroesophageal reflux disease.
Modified with permission from Nasr I et al. Aliment Pharmacol Ther. 2012;35(12):1474 1481.
Clearly, judicious use of the aforementioned diagnostic strategies could lead to a better understanding of the etiology of ECP and facilitate optimal management. In addition, these approaches should be individualized based on the clinical features and the available resources.
Table.
Common Etiologies, Pathophysiologies, and Treatments of Esophageal Chest Pain
| Etiology | Pathophysiology | Treatment |
|---|---|---|
| Gastroesophageal reflux disease |
|
|
| Dysmotility |
|
|
| Visceral hypersensitivity |
|
|
| Psychiatric disturbances |
|
|
Treatment
Treatment strategies should start with an empiric PPI trial. If there is no response, the clinician should consider grouping patients according to their underlying pathophysiology, and then should use this information to direct treatment. The Table shows common etiologies, pathophysiologies, and treatments of esophageal chest pain.
Treatment of Gastroesophageal Reflux Disease-Related Esophageal Chest Pain
Acid reflux causes ECP, but it is only one of many components of a complex, multifactorial disorder.42,43 In a meta-analysis of 8 studies, the pooled sensitivity, specificity, and diagnostic odds ratio for a PPI test vs 24-hour pH study and endoscopy were 80%, 74%, and 13.8% (95% CI, 5.48-34.91), respectively. The pooled risk ratio for continued chest pain was 0.54 (95% CI, 0.41-0.71).36 A systematic review that included 7 randomized controlled trials comparing PPI use vs placebo found a therapeutic gain of 56% to 85% and a relative risk of 4.3 (95% CI, 2.8-6.7; P<.001) in GERD-positive patients, along with a therapeutic gain of 0% to 17% and a relative risk of 0.4 (95% CI, 0.3-0.7; P<.0004) in GERD-negative patients.37 These data suggest that patients with acid reflux and ECP may improve with PPI therapy; hence, a PPI trial is the first-line approach.
Several PPIs, including omeprazole, lansoprazole, and rabeprazole, have been examined. In one study, ECP patients with acid reflux were more likely to respond to PPI therapy than ECP patients without reflux.18 However, the literature on GERD and ECP is inconsistent. This is because patients with NERD, who represent 70% of the GERD population, may not respond to PPIs. Studies have shown that approximately 50% of these individuals may experience heartburn without acid reflux,2 and at least one-third of these patients have physiologically normal levels of acid reflux. It is likely that these individuals have either altered afferent receptor dysfunction or aberrant central modulation of pain.
Treatment of Dysmotility-Related Esophageal Chest Pain
Several drugs with different mechanisms of action have been studied, including calcium channel blockers, nitrates, and botulinum toxin.44-47 However, only small numbers of patients were evaluated, and overall the studies showed either no benefit or only a partial response.
Sildenafil, a phosphodiesterase type 5 inhibitor, was examined in an uncontrolled, small study of patients with spastic esophageal motor disorders.48 The results were inconsistent, and both acid reflux and cardiac disease were not excluded. Thus, antispasmodics and muscle relaxants are either ineffective or there is poor evidence to support their use.
Treatment of Hypersensitivity-Related Esophageal Chest Pain
Various classes of drugs have been studied, including the antidepressants imipramine and trazodone, selective serotonin reuptake inhibitors such as citalopram and sertraline, and the adenosine receptor antagonist theophylline.42,49-57
In a 3-week trial of imipramine, clonidine, and placebo, chest pain decreased in 52%, 39%, and 1%, respectively, but the reduction was significant (P<.03) only in the imipramine group.49 Likewise, a 6-week randomized controlled trial of trazodone (100-150 mg/day) showed greater global improvement (P=.02) than placebo.50 In another study, sertraline was titrated up to 200 mg daily in 30 patients for 8 weeks, which resulted in a significant reduction in pain (P<.02) when compared with placebo.51
Psychological treatment (coping skills) plus sertraline, sertraline alone, coping skills alone, or placebo was also effective in ECP, with the highest response occurring in the combined therapy group (coping skills plus sertraline).52 Interestingly, antidepressants were effective even in the absence of psychological comorbidity, suggesting that most patients with ECP may benefit from these drugs, while treatment with coping skills may be useful in patients with increased levels of anxiety and catastrophizing.52 In one study, venlafaxine improved symptoms in 52% of patients compared with 4% in the placebo group.53 Paroxetine also improved physician-rated symptoms, but not patient-rated chest pain.54 In another study, paroxetine was no more effective than placebo.55
Theophylline, an adenosine receptor antagonist, has been shown to have both visceral analgesic properties and smooth muscle relaxant properties.56 In a randomized, placebo-controlled, crossover study of theophylline given 200 mg orally twice daily, 58% of patients with ECP showed improvement in chest pain compared with 6% in the placebo group.57 More studies are needed to confirm the safety and efficacy of theophylline in clinical practice.
Psychological Treatments for Esophageal Chest Pain
Several approaches have been tested, but cognitive behavioral therapy (CBT) has shown the best results. Four randomized controlled trials of CBT have been reported,42,58-61 comparing it with conventional treatment, usual care, or a control. Overall, pain severity decreased, depression and anxiety improved, and some quality-of-life domains improved.43 However, GERD was not excluded in these patients,42 questioning the efficacy and applicability of CBT to routine practice.
In a single-blind, randomized, controlled trial, hypnotherapy showed greater improvement when compared with supported listening plus placebo medication.58 An open-label study administered a psychological treatment combination (breathing exercises, education, relaxation, and graded exposure to activity) to 60 patients with ECP, and showed a significant reduction in median chest pain episodes, as well as anxiety and depression scores that were maintained for 6 months.59 However, the study was not blinded, and GERD and other sources of chest pain were not excluded.
A study of 9 patients compared biofeedback (diaphragmatic exercises), breathing techniques, and self-control of stress by using galvanic skin resistance feedback. Symptoms improved in 5 of 9 patients with functional chest pain but not in patients with functional heartburn.60 Finally, 20 minutes of Johrei treatment (spiritual energy healing) was compared in a randomized controlled trial of 6 weeks to a wait-list control.61 There was a significant reduction in chest pain intensity scores (P<.0002) in the Johrei group but not in the control group.
Most of the aforementioned studies did not include either sham treatment or a credible control; hence, these preliminary observations need further confirmation with carefully designed controlled trials.
Surgical Treatments for Esophageal Chest Pain
Several surgical approaches have been tried, including long esophageal myotomy62 or thoracoscopic vs laparoscopic myotomy,63 but the efficacy of these procedures is unclear, and randomized controlled trials are lacking. Achalasia may also cause chest pain. Recently, peroral endoscopic myotomy (POEM) has evolved as a treatment option for achalasia, and one center has reported a success rate of 82.4% 1 year after the procedure.64 In a retrospective study, a POEM procedure was performed in 33 patients with achalasia, 8 of whom had chest pain, with complete resolution of symptoms.65
Summary
ECP is a common problem. After ruling out life-threatening causes of chest pain, notably heart disease, several diagnostic modalities are available, including upper endoscopy, highresolution esophageal manometry, pH metry, and esophageal balloon distention testing. These tests should facilitate an accurate diagnosis of ECP. Treatment is best directed toward the underlying mechanism(s) that may be identified by the aforementioned tests. If drug therapy fails, psychological treatments, especially CBT, may be considered.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Chahal PS, Rao SS. Functional chest pain: nociception and visceral hyperalgesia. J Clin Gastroenterol. 2005;39(5) suppl 3:S204–S209. doi: 10.1097/01.mcg.0000156108.20871.bb. [DOI] [PubMed] [Google Scholar]
- 2.Galmiche JP, Clouse RE, Bálint A, et al. Functional esophageal disorders. Gastroenterology. 2006;130(5):1459–1465. doi: 10.1053/j.gastro.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Locke GR, III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 4.Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting—a population-based study. Aliment Pharmacol Ther. 2003;17(9):1115–1124. doi: 10.1046/j.1365-2036.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiocca JC, Olmos JA, Salis GB, Soifer LO, Higa R, Marcolongo M Argentinean Gastro-Oesophageal Reflux Study Group. Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: a nationwide population-based study. Aliment Pharmacol Ther. 2005;22(4):331–342. doi: 10.1111/j.1365-2036.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong WM, Lai KC, Lam KF, et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther. 2003;18(6):595–604. doi: 10.1046/j.1365-2036.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 8.Tew R, Guthrie EA, Creed FH, Cotter L, Kisely S, Tomenson B. A long-term follow-up study of patients with ischaemic heart disease versus patients with nonspecific chest pain. J Psychosom Res. 1995;39(8):977–985. doi: 10.1016/0022-3999(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Eslick GD, Coulshed DS, Talley NJ. Review article: the burden of illness of non-cardiac chest pain. Aliment Pharmacol Ther. 2002;16(7):1217–1223. doi: 10.1046/j.1365-2036.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 10.Mudipalli RS, Remes-Troche JM, Andersen L, Rao SSC. Functional chest pain: esophageal or overlapping functional disorder. J Clin Gastroenterol. 2007;41(3):264–269. doi: 10.1097/01.mcg.0000225521.36160.1b. [DOI] [PubMed] [Google Scholar]
- 11.Wong WM, Beeler J, Risner-Adler S, Habib S, Bautista J, Fass R. Attitudes and referral patterns of primary care physicians when evaluating subjects with noncardiac chest pain—a national survey. Dig Dis Sci. 2005;50(4):656–661. doi: 10.1007/s10620-005-2552-6. [DOI] [PubMed] [Google Scholar]
- 12.Eslick GD, Talley NJ. Non-cardiac chest pain: predictors of health care seeking, the types of health care professional consulted, work absenteeism and interruption of daily activities. Aliment Pharmacol Ther. 2004;20(8):909–915. doi: 10.1111/j.1365-2036.2004.02175.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SE. The challenge of evaluating the patient with chest pain. Arch Pathol Lab Med. 2000;124(10):1418–1419. doi: 10.5858/2000-124-1418-TCOETP. [DOI] [PubMed] [Google Scholar]
- 14.Groarke J, O’Brien J, Go G, Susanto M, Owens P, Maree AO. Cost burden of non-specific chest pain admissions. Ir J Med Sci. 2013;182(1):57–61. doi: 10.1007/s11845-012-0826-5. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Richter JE, Hewson EG, Sinclair JW, Hackshaw BT. The contribution of gastroesophageal reflux to chest pain in patients with coronary artery disease. Ann Intern Med. 1992;117(10):824–830. doi: 10.7326/0003-4819-117-10-824. [DOI] [PubMed] [Google Scholar]
- 16.DeMeester TR, O’Sullivan GC, Bermudez G, Midell AI, Cimochowski GE, O’Drobinak J. Esophageal function in patients with angina-type chest pain and normal coronary angiograms. Ann Surg. 1982;196(4):488–498. doi: 10.1097/00000658-198210000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med. 1991;90(5):576–583. [PubMed] [Google Scholar]
- 18.Fass R, Fennerty MB, Ofman JJ, et al. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology. 1998;115(1):42–49. doi: 10.1016/s0016-5085(98)70363-4. [DOI] [PubMed] [Google Scholar]
- 19.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61(9):1340–1354. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 20.Hemmink GJ, Bredenoord AJ, Weusten BL, Monkelbaan JF, Timmer R, Smout AJ. Esophageal pH-impedance monitoring in patients with therapyresistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103(10):2446–2453. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 21.Rao SSC. How to treat esophageal chest pain. In: Mittal RK, editor. Esophageal Pain. 1st ed. San Diego, CA: Plural Publishing; 2010. pp. 149–159. [Google Scholar]
- 22.Balaban DH, Yamamoto Y, Liu J, et al. Sustained esophageal contraction: a marker of esophageal chest pain identified by intraluminal ultrasonography. Gastroenterology. 1999;116(1):29–37. doi: 10.1016/s0016-5085(99)70225-8. [DOI] [PubMed] [Google Scholar]
- 23.Pehlivanov N, Liu J, Mittal RK. Sustained esophageal contraction: a motor correlate of heartburn symptom. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G743–G751. doi: 10.1152/ajpgi.2001.281.3.G743. [DOI] [PubMed] [Google Scholar]
- 24.Mujica VR, Mudipalli RS, Rao SS. Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol. 2001;96(5):1371–1377. doi: 10.1111/j.1572-0241.2001.03791.x. [DOI] [PubMed] [Google Scholar]
- 25.Hobson AR, Furlong PL, Sarkar S, et al. Neurophysiologic assessment of esophageal sensory processing in noncardiac chest pain. Gastroenterology. 2006;130(1):80–88. doi: 10.1053/j.gastro.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Rao SS, Gregersen H, Hayek B, Summers RW, Christensen J. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124(11):950–958. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Rao SS, Hayek B, Summers RW. Functional chest pain of esophageal origin: hyperalgesia or motor dysfunction. Am J Gastroenterol. 2001;96(9):2584–2589. doi: 10.1111/j.1572-0241.2001.04101.x. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356(9236):1154–1159. doi: 10.1016/S0140-6736(00)02758-6. [DOI] [PubMed] [Google Scholar]
- 29.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126(3):683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a doubleblind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23(3):365–370. doi: 10.1111/j.1365-2036.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 31.Remes-Troche JM, Chahal P, Mudipalli R, Rao SS. Adenosine modulates oesophageal sensorimotor function in humans. Gut. 2009;58(8):1049–1055. doi: 10.1136/gut.2006.116699. [DOI] [PubMed] [Google Scholar]
- 32.Bautista J, Fullerton H, Briseno M, Cui H, Fass R. The effect of an empirical trial of high-dose lansoprazole on symptom response of patients with non-cardiac chest pain—a randomized, double-blind, placebo-controlled, crossover trial. Aliment Pharmacol Ther. 2004;19(10):1123–1130. doi: 10.1111/j.1365-2036.2004.01941.x. [DOI] [PubMed] [Google Scholar]
- 33.Dickman R, Emmons S, Cui H, et al. The effect of a therapeutic trial of highdose rabeprazole on symptom response of patients with non-cardiac chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Aliment Pharmacol Ther. 2005;22(6):547–555. doi: 10.1111/j.1365-2036.2005.02620.x. [DOI] [PubMed] [Google Scholar]
- 34.Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35(4):307–314. doi: 10.1097/00004836-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17(3):369–377. doi: 10.1046/j.1365-2036.2003.01436.x. [DOI] [PubMed] [Google Scholar]
- 36.Cremonini F, Wise J, Moayyedi P, Talley NJ. Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a meta-analysis. Am J Gastroenterol. 2005;100(6):1226–1232. doi: 10.1111/j.1572-0241.2005.41657.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain? A meta-analysis. Arch Intern Med. 2005;165(11):1222–1228. doi: 10.1001/archinte.165.11.1222. [DOI] [PubMed] [Google Scholar]
- 38.Fass R, Ofman JJ. Gastroesophageal reflux disease—should we adopt a new conceptual framework? Am J Gastroenterol. 2002;97(8):1901–1909. doi: 10.1111/j.1572-0241.2002.05912.x. [DOI] [PubMed] [Google Scholar]
- 39.Katz PO, Dalton CB, Richter JE, Wu WC, Castell DO. Esophageal testing of patients with noncardiac chest pain or dysphagia. Results of three years’ experience with 1161 patients. Ann Intern Med. 1987;106(4):593–597. doi: 10.7326/0003-4819-106-4-593. [DOI] [PubMed] [Google Scholar]
- 40.Rencoret G, Csendes A, Henríquez A. Esophageal manometry in patients with noncardiac chest pain [in Spanish] Rev Med Chil. 2006;134(3):291–298. doi: 10.4067/s0034-98872006000300004. [DOI] [PubMed] [Google Scholar]
- 41.Nasr I, Attaluri A, Coss-Adame E, Rao SS. Diagnostic utility of the oesophageal balloon distension test in the evaluation of oesophageal chest pain. Aliment Pharmacol Ther. 2012;35(12):1474–1481. doi: 10.1111/j.1365-2036.2012.05103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224–1245. doi: 10.1016/j.cgh.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fass R, Achem SR. Noncardiac chest pain: epidemiology, natural course and pathogenesis. J Neurogastroenterol Motil. 2011;17(2):110–123. doi: 10.5056/jnm.2011.17.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cattau EL Jr, Castell DO, Johnson DA, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol. 1991;86(3):272–276. [PubMed] [Google Scholar]
- 45.Swamy N. Esophageal spasm: clinical and manometric response to nitroglycerine and long acting nitrites. Gastroenterology. 1977;72(1):23–27. [PubMed] [Google Scholar]
- 46.Miller LS, Pullela SV, Parkman HP, et al. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol. 2002;97(7):1640–1646. doi: 10.1111/j.1572-0241.2002.05821.x. [DOI] [PubMed] [Google Scholar]
- 47.Storr M, Allescher HD, Rösch T, Born P, Weigert N, Classen M. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc. 2001;54(6):754–759. [PubMed] [Google Scholar]
- 48.Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut. 2002;50(6):758–764. doi: 10.1136/gut.50.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cannon RO, III, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330(20):1411–1417. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 50.Clouse RE, Lustman PJ, Eckert TC, Ferney DM, Griffith LS. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology. 1987;92(4):1027–1036. doi: 10.1016/0016-5085(87)90979-6. [DOI] [PubMed] [Google Scholar]
- 51.Varia I, Logue E, O’Connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140(3):367–372. doi: 10.1067/mhj.2000.108514. [DOI] [PubMed] [Google Scholar]
- 52.Keefe FJ, Shelby RA, Somers TJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain. 2011;152(4):730–741. doi: 10.1016/j.pain.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H, Kim JH, Min BH, et al. Efficacy of venlafaxine for symptomatic relief in young adult patients with functional chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2010;105(7):1504–1512. doi: 10.1038/ajg.2010.82. [DOI] [PubMed] [Google Scholar]
- 54.Doraiswamy PM, Varia I, Hellegers C, et al. A randomized controlled trial of paroxetine for noncardiac chest pain. Psychopharmacol Bull. 2006;39(1):15–24. [PubMed] [Google Scholar]
- 55.Spinhoven P, Van der Does AJ, Van Dijk E, Van Rood YR. Heart-focused anxiety as a mediating variable in the treatment of noncardiac chest pain by cognitivebehavioral therapy and paroxetine. J Psychosom Res. 2010;69(3):227–235. doi: 10.1016/j.jpsychores.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Rao SS, Mudipalli RS, Mujica V, Utech CL, Zhao X, Conklin JL. An open-label trial of theophylline for functional chest pain. Dig Dis Sci. 2002;47(12):2763–2768. doi: 10.1023/a:1021017524660. [DOI] [PubMed] [Google Scholar]
- 57.Rao SS, Mudipalli RS, Remes-Troche JM, Utech CL, Zimmerman B. Theophylline improves esophageal chest pain—a randomized, placebo-controlled study. Am J Gastroenterol. 2007;102(5):930–938. doi: 10.1111/j.1572-0241.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- 58.Jones H, Cooper P, Miller V, Brooks N, Whorwell PJ. Treatment of non-cardiac chest pain: a controlled trial of hypnotherapy. Gut. 2006;55(10):1403–1408. doi: 10.1136/gut.2005.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klimes I, Mayou RA, Pearce MJ, Coles L, Fagg JR. Psychological treatment for atypical non-cardiac chest pain: a controlled evaluation. Psychol Med. 1990;20(3):605–611. doi: 10.1017/s0033291700017116. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro M, Shanani R, Taback H, Abramowich D, Scapa E, Broide E. Functional chest pain responds to biofeedback treatment but functional heartburn does not: what is the difference? Eur J Gastroenterol Hepatol. 2012;24(6):708–714. doi: 10.1097/MEG.0b013e3283525a0c. [DOI] [PubMed] [Google Scholar]
- 61.Gasiorowska A, Navarro-Rodriguez T, Dickman R, et al. Clinical trial: the effect of Johrei on symptoms of patients with functional chest pain. Aliment Pharmacol Ther. 2009;29(1):126–134. doi: 10.1111/j.1365-2036.2008.03859.x. [DOI] [PubMed] [Google Scholar]
- 62.Patti MG, Gorodner MV, Galvani C, et al. Spectrum of esophageal motility disorders: implications for diagnosis and treatment. Arch Surg. 2005;140(5):442–448. doi: 10.1001/archsurg.140.5.442. [DOI] [PubMed] [Google Scholar]
- 63.Henderson RD, Ryder D, Marryatt G. Extended esophageal myotomy and short total fundoplication hernia repair in diffuse esophageal spasm: five-year review in 34 patients. Ann Thorac Surg. 1987;43(1):25–31. doi: 10.1016/s0003-4975(10)60161-0. [DOI] [PubMed] [Google Scholar]
- 64.Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145(2):309–311.e1, .e3. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 65.Hoppo T, Thakkar SJ, Schumacher LY, et al. A utility of peroral endoscopic myotomy (POEM) across the spectrum of esophageal motility disorders [published online April 7][2015] Surg Endosc. :doi:10.1007/s00464–015-4193-y. doi: 10.1007/s00464-015-4193-y. [DOI] [PubMed] [Google Scholar]