Abstract
Bariatric surgery has come to the forefront of weight loss treatment due to its complex interactions via anatomic, physiologic, and neurohormonal changes leading to sustained weight loss. Unlike lifestyle and pharmacologic options, which fail to show long-term sustained weight loss, bariatric surgery has been shown to decrease overall mortality and morbidity. Bariatric surgery can be purely restrictive, such as laparoscopic adjustable gastric band (LAGB) or laparoscopic sleeve gastrectomy (LSG), or restrictive-malabsorptive, such as Roux-en-Y gastric bypass (RYGB). These surgeries cause specific anatomic changes that promote weight loss; however, they also have unintended effects on the esophagus, particularly in terms of gastroesophageal reflux disease (GERD) and esophageal motility. Via restrictive surgery, LAGB has been widely reported to cause significant weight loss, although studies have also shown an increase and worsening of GERD as well as elevated rates of esophageal dilation, aperistalsis, and alterations in lower esophageal sphincter pressure. Along with LAGB, LSG has shown not only a worsening of GERD, but also the formation of de novo GERD in patients who were asymptomatic before the operation. In a restrictive-malabsorptive approach, RYGB has been reported to improve GERD and preserve esophageal motility. Bariatric surgery is a burgeoning field with immense implications on overall mortality. Future randomized, controlled trials are needed to better understand which patients should undergo particular surgeries, with greater emphasis on esophageal health and prevention of GERD and esophageal dysmotility.
Keywords: Laparoscopic adjustable gastric band, laparoscopic sleeve gastrectomy, Roux-en-Y gastric bypass, esophageal dysmotility, gastroesophageal reflux disease, bariatric surgery, obesity
Rising rates of obesity, a condition defined as having a body mass index (BMI) greater than 30, have led to an increase in the incidence of metabolic syndrome, type 2 diabetes mellitus, hypertension, nonalcoholic fatty liver disease, and hyperlipidemia.1-3 The increase in obesity is complex and involves genetic predisposition, hormonal changes, and dysbiosis.4 Moreover, there is a considerable economic burden associated with obesity, with an average of 5% of the total global health care cost going toward treating obese patients.5 Weight reduction is vital to alter the course of obesity and its related medical conditions, as well as to decrease the medical and economic burdens.
Lifestyle modifications have been utilized for weight loss, but the results have been disappointing. Despite an initial weight loss of typically 5% to 10% in the first 6 months, most weight is regained. Most lifestyle changes encompass a low-calorie diet and increased physical exercise but lack consistent weight loss, which is needed to decrease metabolic syndrome.6-9
An alternative to diet and exercise is medication, such as orlistat, lorcaserin, and phentermine/topiramate extended-release. Similar to lifestyle changes, there have been limited data on prolonged weight loss and long-term improvements in risk for metabolic syndrome due to a lack of substantial weight loss. Additionally, these medications are not without harm; prior weight loss medications have been implicated in pulmonary disorders (eg, pulmonary hypertension) and cardiac disorders (eg, valvulopathies).10-12
The third and most viable option for weight loss is bariatric surgery, which is the only modality that has shown long-term sustained weight loss, reduction in comorbidities, and improvement in all-cause mortality.8,13 Bariatric surgery is reserved for patients with a BMI greater than 40 or a BMI greater than 35 with comorbidities. For instance, one study examining the effect of Roux-en-Y gastric bypass (RYGB) reported a mean loss of 50% to 70% of excess body weight and a decrease in the rate of metabolic syndrome.14 However, bariatric surgery does have complications, including stenosis at anastomotic sites, stomal ulcers, band erosion, and fistulae, all of which have implications on the esophagus.15-17
This article summarizes the relationship of obesity with esophageal health and the physiologic changes that occur after bariatric surgery. The focus will be on gastroesophageal reflux disease (GERD) and esophageal motility as well as the most common forms of bariatric surgery: laparoscopic adjustable gastric band (LAGB), laparoscopic sleeve gastrectomy (LSG), and RYGB.
The Relationship Between Obesity and Esophageal Health
Obesity is associated with an increase in esophageal disorders, which range from GERD to conditions of the lower esophageal sphincter (LES) and motility dysfunction. GERD, as defined by the Montreal Classification, is the reflux of stomach contents that leads to symptoms of heartburn and regurgitation.18-21 The natural antireflux mechanism is composed of the LES, esophageal hiatus of the diaphragm, phrenoesophageal ligaments, and angle of His. Combined, these parts serve as a unit to prevent reflux of stomach contents.22 Breakdown of this barrier leads to reflux of low pH stomach contents and GERD, which damages the esophageal mucosa.
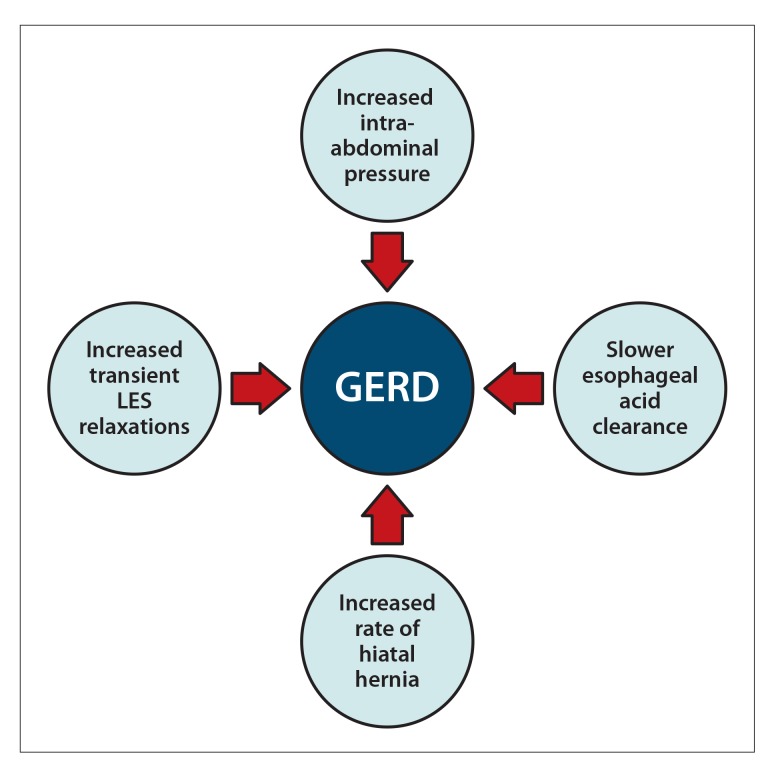
Obesity has been shown to increase the risk of GERD symptoms by 50% as well as cause a 2-fold increase in esophageal adenocarcinoma.23 Furthermore, obese patients have a 2.5-fold increased risk of developing a hiatal hernia, which is a known risk factor for GERD.24 The physiology of increased GERD with obesity is believed to be multifactorial, including increased abdominal girth leading to high intra-abdominal pressure, increased rate of hiatal hernia, high rate of transient LES relaxations, and slower esophageal acid clearance, all of which favor the development of GERD (Figure 1).24-28 Obesity also leads to an increase in fatty tissue, which produces estrogen; in turn, elevated transient LES relaxations cause increased GERD.29,30
Figure 1.
Mechanisms by which obesity leads to gastroesophageal reflux disease (GERD).
LES, lower esophageal sphincter.
Morbid obesity has been associated with an increase in esophageal dysmotility, ranging in prevalence from 20% to 61%.31,32 Studies have shown that morbidly obese patients have higher rates of hypotensive LES, although a lack of subjective symptoms was attributed to decreased sensation via altered sympathetic and parasympathetic innervation.31,33-36 In one prospective study evaluating obese patients selected for bariatric surgery, the presurgical manometric data showed that patients had varied changes to the esophagus, including defective LES (16%), hypertensive LES (18%), diffuse esophageal spasm (3%), nutcracker esophagus (5%), ineffective esophageal disorder (2%), and nonspecific motility disorder (23%).37 Understanding the pretest probability of esophageal motility disorders is important, as underlying motility disorders have been shown to be predictors of the need for LAGB reoperation.38
Overview of Bariatric Surgery
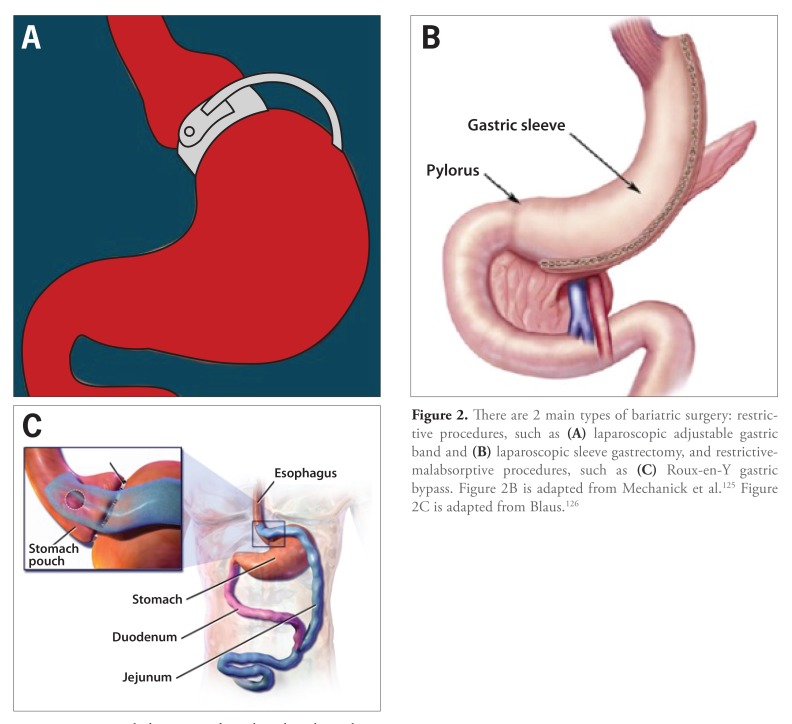
The 2 main types of bariatric surgery, restrictive and restrictive-malabsorptive, are divided by anatomic and functional differences (Figure 2). Restrictive surgeries, which include LAGB and LSG, decrease the functional capacity of the stomach without a notable change in absorption. Restrictive-malabsorptive surgeries, of which RYGB is the most common, both restrict the carrying capacity of the stomach and lead to anatomic resections that serve to limit calorie and, as a byproduct, nutrient absorption.
Figure 2.
There are 2 main types of bariatric surgery: restrictive procedures, such as (A) laparoscopic adjustable gastric band and (B) laparoscopic sleeve gastrectomy, and restrictive-malabsorptive procedures, such as (C) Roux-en-Y gastric bypass. Figure 2B is adapted from Mechanick et al.125 Figure 2C is adapted from Blaus.126
Despite anatomic differences, the bariatric surgeries have in common modifications to the digestive tract and anatomic rearrangement. These modifications are known as the BRAVE effects: bile flow alteration, reduction of gastric size, anatomic gut rearrangement and altered flow of nutrients, vagal manipulation, and enteric gut hormone modulation.39-47 Weight loss due to bariatric surgery has been shown to decrease all-cause mortality at 10 years with significant morbidity improvements. Growing attention has been placed on neurohormonal changes (such as the alteration of peptide-1 and ghrelin) and their positive effects in sustained weight loss.48
The following sections will discuss LAGB, LSG, and RYGB, along with associated changes in GERD and motility. When determining the appropriate surgical approach, clinicians should consider patient history (eg, preexisting esophageal motility) and understand the physiologic changes that result from the surgery.
Laparoscopic Adjustable Gastric Band
Surgical Approach
LAGB is a restrictive surgery that involves placing an adjustable gastric band around the proximal stomach to divide it into a small pouch and a larger pouch, the latter of which connects to the remainder of the small bowel (Figure 2A).43,49 The inflatable band, and thus the size of the pouches, can be adjusted via a subcutaneous access point as needed. A smaller proximal pouch limits food intake due to decreased stomach size and increased food transit time.
Implications With Gastroesophageal Reflux Disease
The relationship between LAGB and GERD changes over time. Many studies show an initial improvement in GERD symptoms in short-term follow-up (<6 months); however, longer follow-up periods show a return of GERD symptoms.50-52 Symptoms are typically heartburn in the preoperative period, as a result of direct reflux, and regurgitation in the postoperative period, in which surgical placement of the band is associated with pouchstasis. Few studies have shown improvement in GERD with this technique and consider LAGB a physical barrier to GERD; however, the burden of evidence supports an increase in GERD, predominantly exhibited via regurgitation.53
Changes in Esophageal Motility
LAGB has been associated with multiple esophageal motility changes primarily related to proximal migration of the inflatable band. The pathology of this migration was elucidated in animal models, in which a nonobstructive band was placed around the esophagogastric junction in a fashion similar to a LAGB and was found to cause esophageal dilation and elevated LES pressure.54,55 Many studies have shown similar esophageal dilations (with rare cases of megaesophagus) and increased LES pressures (Table 1).52,56-61 The etiology of these changes is associated with increases in LES high-pressure zone length, defective propagations, and increased rate of esophageal dilations (Table 2).62-70 There is a lack of randomized, controlled trials for LAGB; one of the largest studies, which evaluated 1232 patients with LAGB over a 9-year period, found anterior and posterior slippage with esophageal dilations in concordance with prior studies’ findings of increased rates of esophageal dilation, achalasia, and pouch dilation.58,68,71 However, due to recent changes in surgical approach and band design, the rates of both anterior and posterior prolapses have decreased significantly. When the band is placed suprabursally, the rate of posterior prolapse approaches 0, while the rate of anterior prolapse is also drastically reduced.72,73
Table 1.
The Relationship Between Laparoscopic Adjustable Gastric Banding and Esophageal Disease
| Study | Pathology | Evaluation | Findings |
|---|---|---|---|
| de Jong et al50 | GERD | Endoscopy, manometry, ambulatory pH monitoring | Reduced GERD |
| Woodman et al51 | GERD | Symptom reporting | Reduced GERD |
| Iovino et al56 | LES pressure | Manometry, questionnaire, 24-hour ambulatory pH-metry | Increase in LES pressure |
| Weiss et al59 | LES pressure | Endoscopy, barium swallow, manometry, 24-hour esophageal monitoring | Impaired LES relaxation, increased esophageal dilation |
| Milone et al65 | Esophageal dilation | Barium swallow | Increased esophageal dilation |
| Dargent68 | Esophageal dilation | Barium swallow, band removal | Increased esophageal dilation |
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter.
Table 2.
Consequences of Esophageal Disorders After Bariatric Surgery
| Bariatric Surgery | Mechanistic Changes to the Esophagus | Esophageal Complications |
|---|---|---|
| Laparoscopic adjustable gastric band | Weak esophageal motility, pouch dilation, preexisting reduced esophageal motility, increased lower esophageal sphincter pressure, increased high-pressure zone length | Worsened gastroesophageal reflux disease, esophageal dilation, esopha geal stasis, achalasia-type symptoms |
| Laparoscopic sleeve gastrectomy | Weak lower esophageal sphincter pressure, decreased gastric compliance leading to high intragastric pressure, breaking of sling fibers disrupting competency of the esophagogastric junction, increased rate of hiatal hernia, surgical damage to phrenoesophageal ligament, sleeve migration | Worsened gastroesophageal reflux disease (recurrent and de novo), increased gastric emptying |
| Roux-en-Y gastric bypass | Malabsorption | Improved gastroesophageal reflux disease, minimal effect on motility |
Laparoscopic Sleeve Gastrectomy
Surgical Approach
Sleeve gastrectomy is a restrictive surgery that involves removing a large portion of the body and all of the fundus of the stomach to create a smaller gastric pouch (Figure 2B). The remaining portion of the stomach is formed into a narrow sleeve and is stapled closed, with the remainder of the stomach connected to the small bowel. The reduced gastric pouch leads to a decrease in acid production.
Implications With Gastroesophageal Reflux Disease
There have been mixed results in determining the relationship of GERD with LSG, likely owing to decreased literature on this surgical approach (Table 3). Studies show both improvement and worsening of GERD symptoms when using symptom reporting and medication.74-89 Many authors think that reducing the gastric pouch with surgical resection should improve GERD. However, other authors propose that de novo formations of hiatal hernias, sleeve migration, and disruption of the esophagogastric junction lead to worsening, and sometimes de novo formation, of GERD symptoms (Table 3).81,90,91 Hence, despite the surgical resection of a proacidic environment, there are many proregurgitant factors that lead to increased GERD.
Table 3.
The Relationship Between Laparoscopic Sleeve Gastrectomy and Esophageal Disease
| Study | Pathology | Evaluation | Findings |
|---|---|---|---|
| Arias et al69 | GERD | Symptom reporting | Increased GERD |
| Lakdawala et al78 | GERD | Symptom reporting | Increased GERD |
| Melissas et al81 | GERD, gastric emptying | Symptom reporting | Increased GERD, increased gastric emptying |
| Melissas et al82 | GERD, gastric emptying | Symptom reporting | Reduced GERD, increased gastric emptying |
| Howard et al91 | GERD | Symptom reporting | Reduced GERD |
| Del Genio et al92 | Peristalsis | High-resolution impedance manometry | Increased ineffective peristalsis |
GERD, gastroesophageal reflux disease.
Changes in Esophageal Motility
LSG has been shown to cause increased rates of global gastrointestinal motility. There is evidence of ineffective peristalsis after LSG with an increase in LES pressures (Table 3).92,93 Moreover, there are global findings of increased rates of gastric and small bowel transit, which contribute to the excision of the gastric fundus, leading to altered intestinal motility.81,82,94 Two studies by the same group evaluating patients after LSG reported increased gastric emptying, which has been postulated to be due to lack of peristalsis in the sleeve portion.81,82,95 This acceleration of gastric emptying has been utilized by combining LSG and fundoplication for patients with GERD and delayed gastric emptying, with improvement in both areas.96 However, a few studies have shown that there is, conversely, a delay in esophageal and gastric emptying, likely owing to the location of the gastrectomy from the gastroesophageal junction.92 Thus, there is a lack of substantial studies evaluating the effects of LSG, although current studies show a trend toward alteration in gastrointestinal motility with increased ineffective peristalsis. This leads to downstream small bowel motility changes, although the effect on the esophagus has not been fully ascertained.
Roux-en-Y Gastric Bypass
Surgical Approach
RYGB is a restrictive-malabsorptive procedure in which the stomach is transected to form a proximal stomach pouch that is connected to a divided jejunal loop called the Roux limb (Figure 2C). The proximal stomach transection leads to the restrictive component of this surgery; the bypassed small bowel with direct connection to the jejunal loop leads to the malabsorption component. Decreased acid production, as seen in the LSG, leads to faster transit times in the stomach.97 Restrictive-malabsorptive surgery leads to larger weight loss than purely restrictive surgeries, likely due to neurohormonal changes; however, there are associated micronutrient deficiencies in vitamin B12, vitamin D, folate, iron, and calcium, which require lifelong supplementation. Moreover, there are associated complications such as anastomotic leakage and marginal ulcers.98-102
Implications With Gastroesophageal Reflux Disease
In patient questionnaires and manometry that evaluates symptoms, RYGB has been shown to decrease symptoms of GERD, the need for proton pump inhibitor therapy, and time with a pH less than 4 (Table 4).86,88,103-108 The decrease in symptoms is due to the combination of altered stomach anatomy via primary resection and a change in the downstream small bowel loop causing both a decrease in acid production and a lack of formation of a proregurgitant environment. There are several studies that show an increase in GERD after RYGB, but in general, RYGB is thought to be a superior surgery in regard to GERD when compared with LAGB and LSG.61,109,110
Table 4.
The Relationship Between Roux-en-Y Gastric Bypass and Esophageal Disease
| Study | Pathology | Evaluation | Findings |
|---|---|---|---|
| Merrouche et al61 | Esophageal dyskinesia | Symptom scoring, endoscopy, manometry, 24-hour pH monitoring | No esophageal dyskinesia |
| Peterli et al88 | GERD | Symptom reporting | Reduced GERD |
| Madalosso et al107 | GERD | Symptom reporting, pH monitoring | Reduced GERD |
| Cassao et al111 | Esophageal sphincter pressure | High-resolution manometry | Increased LES hypotonia |
| Valezi et al112 | LES pressure | High-resolution manometry | Increased LES hypotonia |
| Clements et al113 | Dysphagia | Symptom scoring | Reduced GERD, worsening of dysphagia |
| Foster et al114 | Dysphagia | Symptom scoring | Reduced GERD, no change in dysphagia |
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter.
Changes in Esophageal Motility
There has been little evidence of esophageal dysmotility after RYGB (Table 4). There are a few retrospective studies with relatively low numbers of patients that have shown a trend toward increased frequency of hypotonic LES, hypertonic upper esophageal sphincter, and esophageal wave duration and wave amplitude after RYGB.62,106,111,112 There is also mixed evidence on dysphagia and esophageal contractility; multiple studies report conflicting results.61,104,106,113,114 One study reported an improvement in esophageal dysmotility after RYGB.106 Thus, the lack of evidence supporting overt esophageal dysmotility after RYGB is a large reason why it is the preferred surgery for patients with known esophageal disorders prior to bariatric surgery.61,115
Hernia Repair
In addition to the primary goal of weight loss, the timing of hernia closure in morbidly obese patients is an increasingly important topic. Many studies have shown repair of hernias during bariatric surgery, and some studies have shown no recurrence of hernia.116-118 However, there are side effects of hernia repair, including seroma (as high as 18%) and mesh infections (4.4%).116,119 The ultimate decision of hernia repair becomes a risk-benefit calculation of the risk of recurrence and perioperative complications and the risk of hernia-associated complications. With improving techniques, recent studies have moved toward concomitant hernia repair, but the literature is still in its infancy, and additional, high-quality studies are needed.120
Conclusion
Given the limited effectiveness of lifestyle modifications and medications, bariatric surgery has come to the forefront for sustained weight loss therapies. Due to the anatomic, neurohormonal, and microbiota changes associated with these surgeries, weight loss is profound and sustainable, and leads to a reduction in morbidity as a result of a decrease in type 2 diabetes mellitus, hypertension, hyperlipidemia, and mortality.121 One study comparing all 3 bariatric procedures found LAGB to be inferior to LSG and RYGB in terms of weight reduction, number of long-term complications, and need for revisional surgery; LSG and RYGB reported similar complication rates, although 9% of LSG patients had to be converted to RYGB due to insufficient weight loss.122
RYGB has been shown to improve GERD, whereas restrictive surgeries generally lead to new or worsening GERD through hypotensive LES, decreased gastric compliance and volume, esophageal dilation (via proximal migration in LAGB), or aperistalsis (via surgical resection in LSG).113,114 The largest limitation of these findings is that most studies are retrospective reviews or analyses of prospective cohorts; even randomized, controlled trials typically feature a small number of patients and make general statements. Hence, high-quality studies are needed to better elucidate these findings.
In regard to esophageal motility, LAGB is also associated with worsening motility via surrogates such as esophageal stasis, dilation, and esophagitis.56,65,68 Although preoperative manometry is not generally performed, it could be helpful, as preexisting dysmotility would favor RYGB over LAGB.71,112,123,124 There are limited trials on the effect of LSG, and current studies are focused on the global motility changes of LSG without focusing on the esophagus.82,92 Current data show that RYGB is the safest surgery for patients with known esophageal dysmotility, as it has been shown not only to prevent, but also improve, esophageal disorders.88,114 LAGB should be avoided in patients with known preoperative esophageal concerns because it can lead to proximal migration of the band, causing esophageal dilation mimicking achalasia.56
Therefore, bariatric surgery is an important surgical tool for the treatment of obesity. Each bariatric surgery has key esophageal changes in regard to GERD and esophageal motility; these are important characteristics to understand prior to recommending particular bariatric surgeries and when taking care of these patients postoperatively. LAGB leads to pronounced increases in GERD and esophageal motility issues, whereas RYGB shows continued improvement in GERD symptoms without overt effects on the esophagus.113,114 LSG is discussed less frequently in the literature, so any conclusions on its effects on GERD and motility are premature. This field needs large-volume, randomized, controlled studies to better characterize the limitations of each surgery; elucidate mechanisms of improvement for the surgical technique; and help understand the neurohormonal changes, which have been vastly underappreciated in the current body of evidence. Such studies can offer better evidence on the role of preoperative manometry, indications and restrictions on each bariatric surgery, and maintenance of the health of the esophagus while still promoting improvements in morbidity and mortality.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–2005. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Jones K, Ruotolo G, et al. Strong Heart Study. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care. 2003;26(3):861–2003. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–2005. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9(10):590–2012. doi: 10.1038/nrgastro.2012.161. [DOI] [PubMed] [Google Scholar]
- 5.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 6.Dyson PA. The therapeutics of lifestyle management on obesity. Diabetes Obes Metab. 2010;12(11):941–2010. doi: 10.1111/j.1463-1326.2010.01256.x. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 suppl):222S–2005. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 8.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2004. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Astrup A, Dyerberg J, Selleck M, Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 2008;9(suppl 1):48–2008. doi: 10.1111/j.1467-789X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 10.Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest. 2000;117(3):870–2000. doi: 10.1378/chest.117.3.870. [DOI] [PubMed] [Google Scholar]
- 11.Frachon I, Etienne Y, Jobic Y, Le Gal G, Humbert M, Leroyer C. Ben-fluorex and unexplained valvular heart disease: a case-control study. PLoS One. 2010;5(4):e10128. doi: 10.1371/journal.pone.0010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002;144(6):1065–2002. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–2004. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 14.Balsiger BM, Murr MM, Poggio JL, Sarr MG. Bariatric surgery. Surgery for weight control in patients with morbid obesity. Med Clin North Am. 2000;84(2):477–2000. doi: 10.1016/s0025-7125(05)70232-7. [DOI] [PubMed] [Google Scholar]
- 15.Westling A, Bjurling K, Ohrvall M, Gustavsson S. Silicone-adjustable gastric banding: disappointing results. Obes Surg. 1998;8(4):467–1998. doi: 10.1381/096089298765554386. [DOI] [PubMed] [Google Scholar]
- 16.Filho AJ, Kondo W, Nassif LS, Garcia MJ, Tirapelle Rde A, Dotti CM. Gastrogastric fistula: a possible complication of Roux-en-Y gastric bypass. JSLS. 2006;10(3):326–2006. [PMC free article] [PubMed] [Google Scholar]
- 17.Gumbs AA, Duffy AJ, Bell RL. Incidence and management of marginal ulceration after laparoscopic Roux-Y gastric bypass. Surg Obes Relat Dis. 2006;2(4):460–2006. doi: 10.1016/j.soard.2006.04.233. [DOI] [PubMed] [Google Scholar]
- 18.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–2006. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 19.Spechler SJ. Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion. 1992;51(suppl 1):24–1992. doi: 10.1159/000200911. [DOI] [PubMed] [Google Scholar]
- 20.Vaezi MF. Therapy insight: gastroesophageal reflux disease and laryngopharyngeal reflux. Nat Clin Pract Gastroenterol Hepatol. 2005;2(12):595–2005. doi: 10.1038/ncpgasthep0358. [DOI] [PubMed] [Google Scholar]
- 21.Poelmans J, Tack J. Extraoesophageal manifestations of gastro-oesophageal reflux. Gut. 2005;54(10):1492–1499. doi: 10.1136/gut.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trus TL, Hunter JG. Minimally invasive surgery of the esophagus and stomach. Am J Surg. 1997;173(3):242–1997. doi: 10.1016/s0002-9610(97)89601-8. [DOI] [PubMed] [Google Scholar]
- 23.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199–2005. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 24.Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94(10):2840–1999. doi: 10.1111/j.1572-0241.1999.01426.x. [DOI] [PubMed] [Google Scholar]
- 25.Zacchi P, Mearin F, Humbert P, Formiguera X, Malagelada JR. Effect of obesity on gastroesophageal resistance to flow in man. Dig Dis Sci. 1991;36(10):1473–1991. doi: 10.1007/BF01296818. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100(6):1243–2005. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 27.Kitchin LI, Castell DO. Rationale and efficacy of conservative therapy for gastroesophageal reflux disease. Arch Intern Med. 1991;151(3):448–1991. [PubMed] [Google Scholar]
- 28.Mercer CD, Rue C, Hanelin L, Hill LD. Effect of obesity on esophageal transit. Am J Surg. 1985;149(1):177–1985. doi: 10.1016/s0002-9610(85)80029-5. [DOI] [PubMed] [Google Scholar]
- 29.Yang SS, Cheng KS, Lai YC, et al. Decreasing serum alpha-fetoprotein levels in predicting poor prognosis of acute hepatic failure in patients with chronic hepatitis B. J Gastroenterol. 2002;37(8):626–2002. doi: 10.1007/s005350200099. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290(1):66–2003. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 31.Jaffin BW, Knoepflmacher P, Greenstein R. High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg. 1999;9(4):390–1999. doi: 10.1381/096089299765552990. [DOI] [PubMed] [Google Scholar]
- 32.Almogy G, Anthone GJ, Crookes PF. Achalasia in the context of morbid obesity: a rare but important association. Obes Surg. 2003;13(6):896–2003. doi: 10.1381/096089203322618731. [DOI] [PubMed] [Google Scholar]
- 33.Granström L, Backman L. Stomach distension in extremely obese and in normal subjects. Acta Chir Scand. 1985;151(4):367–1985. [PubMed] [Google Scholar]
- 34.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–1988. doi: 10.1093/ajcn/48.3.592. [DOI] [PubMed] [Google Scholar]
- 35.Backman L, Granström L, Lindahl J, Melcher A. Manometric studies of lower esophageal sphincter in extreme obesity. Acta Chir Scand. 1983;149(2):193–1983. [PubMed] [Google Scholar]
- 36.O’Brien TF., Jr. Lower esophageal sphincter pressure (LESP) and esophageal function in obese humans. J Clin Gastroenterol. 1980;2(2):145–1980. [PubMed] [Google Scholar]
- 37.Hong D, Khajanchee YS, Pereira N, Lockhart B, Patterson EJ, Swanstrom LL. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg. 2004;14(6):744–2004. doi: 10.1381/0960892041590854. [DOI] [PubMed] [Google Scholar]
- 38.Greenstein RJ, Nissan A, Jaffin B. Esophageal anatomy and function in lapa roscopic gastric restrictive bariatric surgery: implications for patient selection. Obes Surg. 1998;8(2):199–1998. doi: 10.1381/096089298765554818. [DOI] [PubMed] [Google Scholar]
- 39.Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117(9):1788–2011. doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 40.Ashrafian H, Athanasiou T, Li JV, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev. 2011;12(5):e257–2011. doi: 10.1111/j.1467-789X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 41.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110(4):571–2010. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–2014. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo Menzo E, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand J Surg. 2015;104(1):18–2015. doi: 10.1177/1457496914552344. [DOI] [PubMed] [Google Scholar]
- 44.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33(7):786–2009. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–2007. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 46.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–2002. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 47.Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13(1):17–2003. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 48.Ochner CN, Gibson C, Carnell S, Dambkowski C, Geliebter A. The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol Behav. 2010;100(5):549–2010. doi: 10.1016/j.physbeh.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller K. Obesity: surgical options. Best Pract Res Clin Gastroenterol. 2004;18(6):1147–2004. doi: 10.1016/j.bpg.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 50.de Jong JR, Besselink MG, van Ramshorst B, Gooszen HG, Smout AJ. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11(4):297–2010. doi: 10.1111/j.1467-789X.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 51.Woodman G, Cywes R, Billy H, Montgomery K, Cornell C, Okerson T APEX Study Group. Effect of adjustable gastric banding on changes in gastroesophageal reflux disease (GERD) and quality of life. Curr Med Res Opin. 2012;28(4):581–2012. doi: 10.1185/03007995.2012.666962. [DOI] [PubMed] [Google Scholar]
- 52.Gamagaris Z, Patterson C, Schaye V, et al. Lap-band impact on the function of the esophagus. Obes Surg. 2008;18(10):1268–2008. doi: 10.1007/s11695-008-9601-0. [DOI] [PubMed] [Google Scholar]
- 53.Dixon JB, O’Brien PE. Gastroesophageal reflux in obesity: the effect of lap-band placement. Obes Surg. 1999;9(6):527–1999. doi: 10.1381/096089299765552602. [DOI] [PubMed] [Google Scholar]
- 54.Tung HN, Schulze-Delrieu K, Shirazi S, Noel S, Xia Q, Cue K. Hypertrophic smooth muscle in the partially obstructed opossum esophagus. The model: histological and ultrastructural observations. Gastroenterology. 1991;100(4):853–1991. doi: 10.1016/0016-5085(91)90256-k. [DOI] [PubMed] [Google Scholar]
- 55.O’Rourke RW, Seltman AK, Chang EY, et al. A model for gastric banding in the treatment of morbid obesity: the effect of chronic partial gastric outlet obstruction on esophageal physiology. Ann Surg. 2006;244(5):723–2006. doi: 10.1097/01.sla.0000218082.12999.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iovino P, Angrisani L, Tremolaterra F, et al. Abnormal esophageal acid exposure is common in morbidly obese patients and improves after a successful Lap-band system implantation. Surg Endosc. 2002;16(11):1631–2002. doi: 10.1007/s00464-001-9225-0. [DOI] [PubMed] [Google Scholar]
- 57.de Jong JR, van Ramshorst B, Timmer R, Gooszen HG, Smout AJ. Effect of laparoscopic gastric banding on esophageal motility. Obes Surg. 2006;16(1):52–2006. doi: 10.1381/096089206775222005. [DOI] [PubMed] [Google Scholar]
- 58.Klaus A, Gruber I, Wetscher G, et al. Prevalent esophageal body motility disorders underlie aggravation of GERD symptoms in morbidly obese patients following adjustable gastric banding. Arch Surg. 2006;141(3):247–2006. doi: 10.1001/archsurg.141.3.247. [DOI] [PubMed] [Google Scholar]
- 59.Weiss HG, Nehoda H, Labeck B, et al. Adjustable gastric and esophagogastric banding: a randomized clinical trial. Obes Surg. 2002;12(4):573–2002. doi: 10.1381/096089202762252370. [DOI] [PubMed] [Google Scholar]
- 60.Weiss HG, Nehoda H, Labeck B, et al. Treatment of morbid obesity with laparoscopic adjustable gastric banding affects esophageal motility. Am J Surg. 2000;180(6):479–2000. doi: 10.1016/s0002-9610(00)00511-0. [DOI] [PubMed] [Google Scholar]
- 61.Merrouche M, Sabaté JM, Jouet P, et al. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17(7):894–2007. doi: 10.1007/s11695-007-9166-3. [DOI] [PubMed] [Google Scholar]
- 62.Tolonen P, Victorzon M, Niemi R, Mäkelä J. Does gastric banding for morbid obesity reduce or increase gastroesophageal reflux? . Obes Surg. 2006;16(11):1469–2006. doi: 10.1381/096089206778870120. [DOI] [PubMed] [Google Scholar]
- 63.Gutschow CA, Collet P, Prenzel K, Hölscher AH, Schneider PM. Long-term results and gastroesophageal reflux in a series of laparoscopic adjustable gastric banding. J Gastrointest Surg. 2005;9(7):941–2005. doi: 10.1016/j.gassur.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Suter M, Dorta G, Giusti V, Calmes JM. Gastric banding interferes with esophageal motility and gastroesophageal reflux. Arch Surg. 2005;140(7):639–2005. doi: 10.1001/archsurg.140.7.639. [DOI] [PubMed] [Google Scholar]
- 65.Milone L, Daud A, Durak E, et al. Esophageal dilation after laparoscopic adjustable gastric banding. Surg Endosc. 2008;22(6):1482–2008. doi: 10.1007/s00464-007-9651-8. [DOI] [PubMed] [Google Scholar]
- 66.DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809–2001. doi: 10.1097/00000658-200106000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naef M, Mouton WG, Naef U, van der Weg B, Maddern GJ, Wagner HE. Esophageal dysmotility disorders after laparoscopic gastric banding—an underestimated complication. Ann Surg. 2011;253(2):285–2011. doi: 10.1097/SLA.0b013e318206843e. [DOI] [PubMed] [Google Scholar]
- 68.Dargent J. Esophageal dilatation after laparoscopic adjustable gastric banding: definition and strategy. Obes Surg. 2005;15(6):843–2005. doi: 10.1381/0960892054222795. [DOI] [PubMed] [Google Scholar]
- 69.Arias IE, Radulescu M, Stiegeler R, et al. Diagnosis and treatment of megaesophagus after adjustable gastric banding for morbid obesity. Surg Obes Relat Dis. 2009;5(2):156–2009. doi: 10.1016/j.soard.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Khan A, Ren-Fielding C, Traube M. Potentially reversible pseudoachalasia after laparoscopic adjustable gastric banding. J Clin Gastroenterol. 2011;45(9):775–2011. doi: 10.1097/MCG.0b013e318226ae14. [DOI] [PubMed] [Google Scholar]
- 71.Klaus A, Weiss H. Is preoperative manometry in restrictive bariatric procedures necessary? . Obes Surg. 2008;18(8):1039–2008. doi: 10.1007/s11695-007-9399-1. [DOI] [PubMed] [Google Scholar]
- 72.Wiesner W, Weber M, Hauser RS, Hauser M, Schoeb O. Anterior versus posterior slippage: two different types of eccentric pouch dilatation in patients with adjustable laparoscopic gastric banding. Dig Surg. 2001;18(3):182–2001. doi: 10.1159/000050127. [DOI] [PubMed] [Google Scholar]
- 73.Lee WK, Kim SM. Three-year experience of pouch dilatation and slippage management after laparoscopic adjustable gastric banding. Yonsei Med J. 2014;55(1):149–2014. doi: 10.3349/ymj.2014.55.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arias E, Martinez PR, Ka Ming Li V, Szomstein S, Rosenthal RJ. Mid-term follow-up after sleeve gastrectomy as a final approach for morbid obesity. Obes Surg. 2009;19(5):544–2009. doi: 10.1007/s11695-009-9818-6. [DOI] [PubMed] [Google Scholar]
- 75.Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012;22(6):479–2012. doi: 10.1097/SLE.0b013e318262dc29. [DOI] [PubMed] [Google Scholar]
- 76.Carter PR, LeBlanc KA, Hausmann MG, Kleinpeter KP, deBarros SN, Jones SM. Association between gastroesophageal reflux disease and laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(5):569–2011. doi: 10.1016/j.soard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 77.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–2010. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 78.Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg. 2010;20(1):1–2010. doi: 10.1007/s11695-009-9981-9. [DOI] [PubMed] [Google Scholar]
- 79.Nocca D, Krawczykowsky D, Bomans B, et al. A prospective multicenter study of 163 sleeve gastrectomies: results at 1 and 2 years. Obes Surg. 2008;18(5):560–2008. doi: 10.1007/s11695-007-9288-7. [DOI] [PubMed] [Google Scholar]
- 80.Tai CM, Huang CK, Lee YC, Chang CY, Lee CT, Lin JT. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg Endosc. 2013;27(4):1260–2013. doi: 10.1007/s00464-012-2593-9. [DOI] [PubMed] [Google Scholar]
- 81.Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy: a restrictive procedure? . Obes Surg. 2007;17(1):57–2007. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 82.Melissas J, Daskalakis M, Koukouraki S, et al. Sleeve gastrectomy—a “food limiting” operation. Obes Surg. 2008;18(10):1251–2008. doi: 10.1007/s11695-008-9634-4. [DOI] [PubMed] [Google Scholar]
- 83.Chopra A, Chao E, Etkin Y, Merklinger L, Lieb J, Delany H. Laparoscopic sleeve gastrectomy for obesity: can it be considered a definitive procedure? . Surg Endosc. 2012;26(3):831–2012. doi: 10.1007/s00464-011-1960-2. [DOI] [PubMed] [Google Scholar]
- 84.Rawlins L, Rawlins MP, Brown CC, Schumacher DL. Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis. 2013;9(1):21–2013. doi: 10.1016/j.soard.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Weiner RA, Weiner S, Pomhoff I, Jacobi C, Makarewicz W, Weigand G. Laparoscopic sleeve gastrectomy—influence of sleeve size and resected gastric volume. Obes Surg. 2007;17(10):1297–2007. doi: 10.1007/s11695-007-9232-x. [DOI] [PubMed] [Google Scholar]
- 86.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2. Obes Surg. 2011;21(11):1650–2011. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 87.Sieber P, Gass M, Kern B, Peters T, Slawik M, Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(2):243–2014. doi: 10.1016/j.soard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 88.Peterli R, Borbély Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and gastric bypass. Ann Surg. 2013;258(5):690–2013. doi: 10.1097/SLA.0b013e3182a67426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DuPree CE, Blair K, Steele SR, Martin MJ. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149(4):328–2014. doi: 10.1001/jamasurg.2013.4323. [DOI] [PubMed] [Google Scholar]
- 90.Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–2006. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 91.Howard DD, Caban AM, Cendan JC, Ben-David K. Gastroesophageal reflux after sleeve gastrectomy in morbidly obese patients. Surg Obes Relat Dis. 2011;7(6):709–2011. doi: 10.1016/j.soard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24(1):71–2014. doi: 10.1007/s11695-013-1046-4. [DOI] [PubMed] [Google Scholar]
- 93.Petersen WV, Meile T, Küper MA, Zdichavsky M, Königsrainer A, Schneider JH. Functional importance of laparoscopic sleeve gastrectomy for the lower esophageal sphincter in patients with morbid obesity. Obes Surg. 2012;22(3):360–2012. doi: 10.1007/s11695-011-0536-5. [DOI] [PubMed] [Google Scholar]
- 94.Bernstine H, Tzioni-Yehoshua R, Groshar D, et al. Gastric emptying is not affected by sleeve gastrectomy—scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obes Surg. 2009;19(3):293–2009. doi: 10.1007/s11695-008-9791-5. [DOI] [PubMed] [Google Scholar]
- 95.Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy— volume and pressure assessment. Obes Surg. 2008;18(9):1083–2008. doi: 10.1007/s11695-008-9576-x. [DOI] [PubMed] [Google Scholar]
- 96.Le Page PA, Martin D. Laparoscopic partial sleeve gastrectomy with fundoplication for gastroesophageal reflux and delayed gastric emptying. World J Surg. 2015;39(6):1460–2015. doi: 10.1007/s00268-015-2981-0. [DOI] [PubMed] [Google Scholar]
- 97.El Oufir L, Flourié B, Bruley des Varannes S, et al. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38(6):870–1996. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hell E, Miller KA, Moorehead MK, Norman S. Evaluation of health status and quality of life after bariatric surgery: comparison of standard Roux-en-Y gastric bypass, vertical banded gastroplasty and laparoscopic adjustable silicone gastric banding. Obes Surg. 2000;10(3):214–2000. doi: 10.1381/096089200321643485. [DOI] [PubMed] [Google Scholar]
- 99.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obes Surg. 2000;10(3):233–2000. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 100.Wittgrove AC, Clark GW, Schubert KR. Laparoscopic gastric bypass, Roux-en-Y: technique and results in 75 patients with 3-30 months follow-up. Obes Surg. 1996;6(6):500–1996. doi: 10.1381/096089296765556412. [DOI] [PubMed] [Google Scholar]
- 101.Pories WJ, MacDonald KG, Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(2 suppl):582S–1992. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 102.Cleator IG, Litwin D, Phang P, Brosseuk D, Rae A. Laparoscopic ileogastrostomy for morbid obesity. Obes Surg. 1994;4(4):358–1994. doi: 10.1381/096089294765558340. [DOI] [PubMed] [Google Scholar]
- 103.Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2002;16(7):1027–2002. doi: 10.1007/s00464-001-8313-5. [DOI] [PubMed] [Google Scholar]
- 104.Ortega J, Escudero MD, Mora F, et al. Outcome of esophageal function and 24-hour esophageal pH monitoring after vertical banded gastroplasty and Roux-en-Y gastric bypass. Obes Surg. 2004;14(8):1086–2004. doi: 10.1381/0960892041975497. [DOI] [PubMed] [Google Scholar]
- 105.Perry Y, Courcoulas AP, Fernando HC, Buenaventura PO, McCaughan JS, Luketich JD. Laparoscopic Roux-en-Y gastric bypass for recalcitrant gastroesophageal reflux disease in morbidly obese patients. JSLS. 2004;8(1):19–2004. [PMC free article] [PubMed] [Google Scholar]
- 106.Mejía-Rivas MA, Herrera-López A, Hernández-Calleros J, Herrera MF, Valdovinos MA. Gastroesophageal reflux disease in morbid obesity: the effect of Roux-en-Y gastric bypass. Obes Surg. 2008;18(10):1217–2008. doi: 10.1007/s11695-008-9474-2. [DOI] [PubMed] [Google Scholar]
- 107.Madalosso CA, Gurski RR, Callegari-Jacques SM, Navarini D, Thiesen V, Fornari F. The impact of gastric bypass on gastroesophageal reflux disease in patients with morbid obesity: a prospective study based on the Montreal Consensus. Ann Surg. 2010;251(2):244–2010. doi: 10.1097/SLA.0b013e3181bdff20. [DOI] [PubMed] [Google Scholar]
- 108.Nelson LG, Gonzalez R, Haines K, Gallagher SF, Murr MM. Amelioration of gastroesophageal reflux symptoms following Roux-en-Y gastric bypass for clinically significant obesity. Am Surg. 2005;71(11):950–2005. [PubMed] [Google Scholar]
- 109.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232(4):515–2000. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pallati PK, Shaligram A, Shostrom VK, Oleynikov D, McBride CL, Goede MR. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2014;10(3):502–2014. doi: 10.1016/j.soard.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 111.Cassão BD, Herbella FA, Silva LC. Vicentine FP Esophageal motility after gastric bypass in Roux-en-Y for morbid obesity: high resolution manometry findings. Arq Bras Cir Dig. 2013;26(suppl 1):22–2013. doi: 10.1590/s0102-67202013000600006. [DOI] [PubMed] [Google Scholar]
- 112.Valezi AC, Herbella FA, Junior JM, de Almeida Menezes M. Esophageal motility after laparoscopic Roux-en-Y gastric bypass: the manometry should be preoperative examination routine? . Obes Surg. 2012;22(7):1050–2012. doi: 10.1007/s11695-012-0613-4. [DOI] [PubMed] [Google Scholar]
- 113.Clements RH, Gonzalez QH, Foster A, et al. Gastrointestinal symptoms are more intense in morbidly obese patients and are improved with laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(4):610–2003. doi: 10.1381/096089203322190835. [DOI] [PubMed] [Google Scholar]
- 114.Foster A, Laws HL, Gonzalez QH, Clements RH. Gastrointestinal symptomatic outcome after laparoscopic Roux-en-Y gastric bypass. J Gastrointest Surg. 2003;7(6):750–2003. doi: 10.1016/s1091-255x(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 115.Weber M, Müller MK, Michel JM, et al. Laparoscopic Roux-en-Y gastric bypass, but not rebanding, should be proposed as rescue procedure for patients with failed laparoscopic gastric banding. Ann Surg. 2003;238(6):827–2003. doi: 10.1097/01.sla.0000098623.53293.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Praveenraj P, Gomes RM, Kumar S, et al. Concomitant bariatric surgery with laparoscopic intra-peritoneal onlay mesh repair for recurrent ventral hernias in morbidly obese patients: an evolving standard of care [published online September 3][2015] Obes Surg. doi: 10.1007/s11695-015-1875-4. doi:10.1007/s11695-015-1875-4. [DOI] [PubMed] [Google Scholar]
- 117.Long AJ, Burton PR, Laurie CP, et al. Concurrent large para-oesophageal hiatal hernia repair and laparoscopic adjustable gastric banding: results from 5-year follow up [published online October 10][2015] Obes Surg. doi: 10.1007/s11695-015-1881-6. doi:10.1007/s11695-015-1881-6. [DOI] [PubMed] [Google Scholar]
- 118.Ardestani A, Tavakkoli A. Hiatal hernia repair and gastroesophageal reflux disease in gastric banding patients: analysis of a national database. Surg Obes Relat Dis. 2014;10(3):438–2014. doi: 10.1016/j.soard.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 119.Chan DL, Talbot ML, Chen Z, Kwon SC. Simultaneous ventral hernia repair in bariatric surgery. ANZ J Surg. 2014;84(7-8):581–2014. doi: 10.1111/ans.12174. [DOI] [PubMed] [Google Scholar]
- 120.Bonatti H, Hoeller E, Kirchmayr W, et al. Ventral hernia repair in bariatric surgery. Obes Surg. 2004;14(5):655–2004. doi: 10.1381/096089204323093444. [DOI] [PubMed] [Google Scholar]
- 121.Puzziferri N, Roshek TB III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–2014. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dogan K, Gadiot RP, Aarts EO, et al. Effectiveness and safety of sleeve gastrectomy, gastric bypass, and adjustable gastric banding in morbidly obese patients: a multicenter, retrospective, matched cohort study. Obes Surg. 2015;25(7):1110–2015. doi: 10.1007/s11695-014-1503-8. [DOI] [PubMed] [Google Scholar]
- 123.Suter M, Giusti V, Calmes JM, Paroz A. Preoperative upper gastrointestinal testing can help predicting long-term outcome after gastric banding for morbid obesity. Obes Surg. 2008;18(5):578–2008. doi: 10.1007/s11695-007-9341-6. [DOI] [PubMed] [Google Scholar]
- 124.Mion F, Roman S, Lindecker V. Esophageal dilation after gastric banding: to test or not to test before surgery? . Surg Endosc. 2010;24(4):972–2010. doi: 10.1007/s00464-009-0706-x. [DOI] [PubMed] [Google Scholar]
- 125.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013;21(0 1):S1–2013. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blaus B. Blausen Medical Communications. Wikiversity Journal of Medicine. [November 9, 2015]. https://en.wikiversity.org/wiki/Wikiversity_Journal_of_Medicine/Blausen_gal-lery_2014 Published October 8, 2013.