Abstract
Anesthesia profoundly impacts peri-infarct depolarizations (PIDs), but only one prior report has described their monitoring during experimental stroke in awake animals. Since temporal patterns of PID occurrence are model specific, the current study examined PID incidence during focal ischemia in the awake Spontaneously Hypertensive Rat (SHR), and documented the impact of both prior and concurrent isoflurane anesthesia. For awake recordings, electrodes were implanted under isoflurane anesthesia 1 day to 5 weeks prior to occlusion surgery. Rats were then subjected to permanent or transient (2 hour) tandem occlusion of the middle cerebral and ipsilateral common carotid arteries, followed by PID monitoring for up to 3 days. Comparison perfusion imaging studies evaluated PID-associated hyperemic transients during permanent ischemia under anesthesia at varied intervals following prior isoflurane exposure. Prior anesthesia attenuated PID number at intervals up to 1 week, establishing 2 weeks as a practical recovery duration following surgical preparation to avoid isoflurane preconditioning effects. PIDs in awake SHR were limited to the first 4 hours after permanent occlusions. Maintaining anesthesia during this interval reduced PID number, and prolonged their occurrence through several hours following anesthesia termination. Although PID number otherwise correlated with infarct size, PID suppression by anesthesia was not protective in the absence of reperfusion. PIDs persisted up to 36 hours after transient occlusions. These results differ markedly from the one previous report of such monitoring in awake Sprague-Dawley rats, which found an extended biphasic PID time course during 24 hours after both permanent and transient filament occlusions. PID occurrence closely reflects the time course of infarct progression in the respective models, and may be more useful than absolute PID number as an index of ongoing pathology.
Keywords: cerebral ischemia, stroke, peri-infarct depolarization, anesthetic preconditioning
INTRODUCTION
Peri-infarct depolarizations (PIDs) have long been recognized to occur in experimental stroke (Branston et al., 1977; Nedergaard and Astrup, 1986), and propagating depolarizations have now been detected in patients following stroke (Dohmen et al., 2008), hemorrhage (Dreier et al., 2006) and trauma (Strong et al., 2002). Their contributions to the pathophysiology of brain injury are increasingly appreciated (Hossmann, 1996; Lauritzen et al., 2011; Leng et al., 2011). Manipulating PID incidence impacts infarct size in many models (Mies et al., 1993; Back et al., 1996; Takano et al., 1996), and cumulative depolarization duration predicts local pathology (Dijkhuizen et al., 1999; Higuchi et al., 2002). PIDs lead to incremental infarct expansion associated with reductions in penumbral perfusion (Higuchi et al., 2002; Shin et al., 2006; Strong et al., 2007; Takeda et al., 2011), which are attributable at least in part to inverse neurovascular coupling in metabolically compromised tissue (Dreier et al., 1998; Dreier et al., 2009; Hinzman et al., 2014).
Essentially all PID studies in animal models have been carried out under anesthesia, which is a critical variable impacting both the initiation and propagation of such depolarizations (Saito et al., 1995; Patel et al., 1998; Kitahara et al., 2001; Kudo et al., 2008; Luckl et al., 2008; Takagaki et al., 2014). In addition, systemic physiology critically influences peri-infarct metabolism and PID incidence (Strong et al., 2000; von Bornstädt et al., 2015), placing considerable demands on its control while under anesthesia. Furthermore, the requirement for sustained anesthesia during invasive monitoring procedures has limited most experimental studies to acute post-occlusion intervals, whereas PIDs can continue in patients for several days following brain injuries (Dohmen et al., 2008; Dreier et al., 2009). Pharmacological management during this time impacts PID number (Sakowitz et al., 2009; Hertle et al., 2012), establishing the need for comparable long term studies in experimental models.
To our knowledge only a single report has described PIDs during stroke in awake animals (Hartings et al., 2003), demonstrating a biphasic time course of PID occurrence persisting through 24 hours, that was comparable after permanent and transient intraluminal filament occlusions in Sprague-Dawley rats. However, there are substantial differences between filament occlusions and direct surgical approaches to the middle cerebral artery (MCA) with regard to the distribution of vascular territories involved (Kanemitsu et al., 2002). In addition, clot formation in the circle of Willis can be a complication unless anticoagulants are present (Ma et al., 2006), and even the most brief filament insertions can lead to microinfarcts (Zhan et al., 2008), presumably due to endothelial damage and secondary embolization. There are also systematic differences in the intrinsic vulnerability of individual rat strains (Brint et al., 1988; Oliff et al., 1997; Ma et al., 2006), and the limited vascular collaterals of the Spontaneously Hypertensive Rat (SHR) have received particular attention in this regard (Coyle, 1986). All of these factors would be expected to contribute to variations in the time course of infarct progression among models.
An additional consideration in awake recordings is the potential impact of prior anesthesia exposure at the time of electrode placement. There is a substantial literature documenting preconditioning effects of volatile anesthetics (Kitano et al., 2007). The previous study of PIDs in awake rats involved a relatively acute 24 hour interval between halothane exposures in the course of surgeries to implant electrodes and produce occlusions (Hartings et al., 2003). We recently observed that prior sham surgery under isoflurane at this interval significantly reduced PID incidence during subsequent stroke when monitored under maintained isoflurane anesthesia (Zhao and Nowak, 2015). It must be asked whether such an effect could continue to impact PID incidence after recovery from anesthesia, introducing a potential anesthesia confound even during awake recordings.
The present study characterized PID incidence in a robust model of surgical MCA occlusion in the awake SHR. Anesthesia effects were investigated in order to define conditions that could minimize its confounds and maximize translational relevance.
EXPERIMENTAL PROCEDURES
Experimental animals and study design
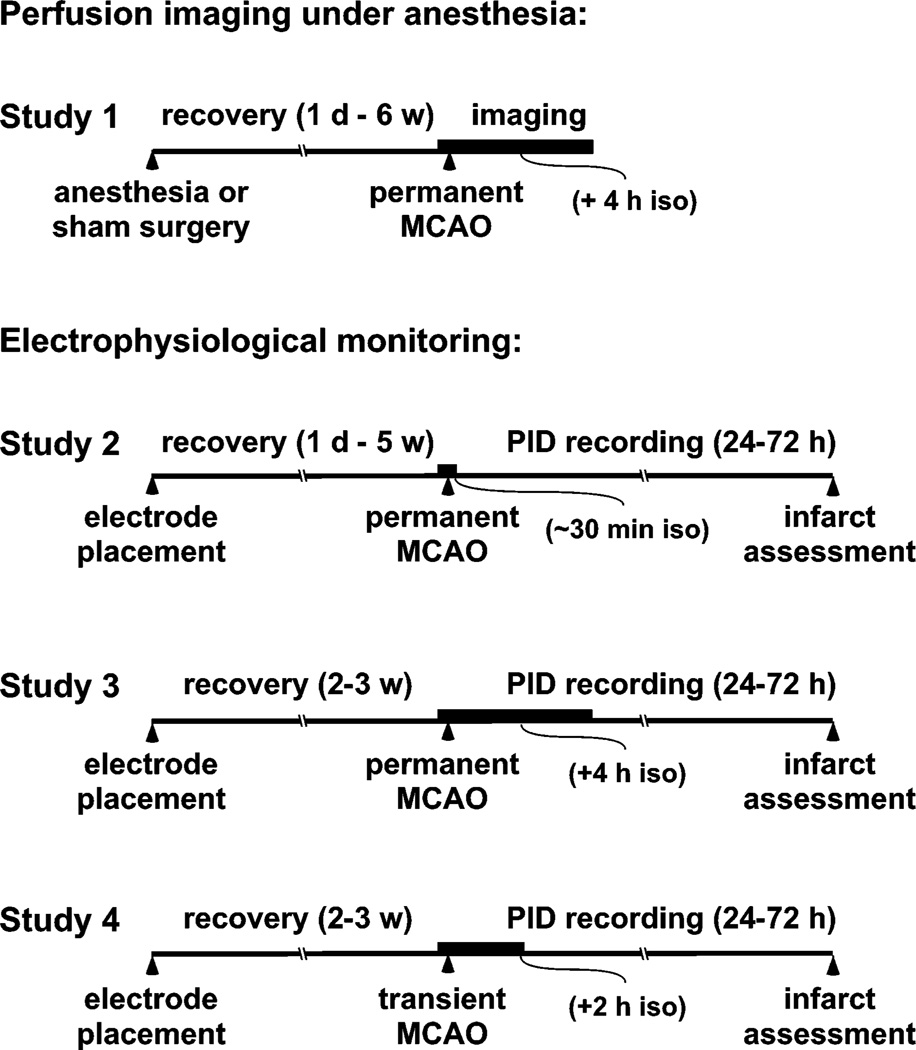
Male SHR (240–260 g, n=88) were acquired from Harlan Laboratories, Inc. (Indianapolis, IN). All experiments were approved by the Institutional Animal Care and Use Committee, University of Tennessee Health Science Center, and were conducted according to United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. The overall design of these studies is illustrated in Fig. 1. Perfusion imaging under anesthesia (Study 1) compared PID incidence in naïve animals (n=12) with those that had experienced isoflurane exposure (n=35) at intervals of 1 day to 6 weeks before occlusion. This expands on recent results showing isoflurane preconditioning effects on PID incidence 1 day after such exposure (Zhao and Nowak, 2015), and the above totals include 5 naïve and 5 sham operated animals from that prior study. The additional animals experienced either sham surgeries or isoflurane exposure alone, to confirm the role of anesthesia per se in producing the effect. For awake recordings (Studies 2–4) animals were fitted with chronic electrode arrays and allowed to recover from the procedure, to be later monitored following MCA occlusion. Study 2 (n=27) established the methodology and documented PID time course and number during permanent occlusions at various intervals after electrode placement, following only brief anesthesia for occlusion surgery. This provides a direct comparison of distal MCA occlusion in the SHR with previous awake recordings after intraluminal occlusions in Sprague-Dawley rats (Hartings et al., 2003), and examines the potential impact of anesthetic preconditioning in this context. Since PIDs have been most extensively investigated acutely under anesthesia, Study 3 (n=6) evaluated the impact of an extended interval of isoflurane exposure on PID incidence and subsequent histopathology. Finally, Study 4 (n=8) examined the response to transient 2-hour occlusions, again to compare with results after transient filament occlusions (Hartings et al., 2003).
Fig. 1.
Study design. Time lines indicate the sequence of procedures experienced by an animal in each of the several experiments. Bold regions illustrate the relative durations of isoflurane anesthesia associated with MCA occlusion in each study. Study 1, in which PIDs were detected by acute perfusion imaging under anesthesia, determined the duration of preconditioning effects induced by prior anesthesia, either alone or in association with a sham surgery. All other studies involved initial surgery for electrode placement, followed by a second surgery for MCA occlusion and prolonged electrophysiological monitoring. Study 2 characterized PID time course after permanent MCA occlusion, and examined whether the recovery interval after electrode placement impacted PID incidence during awake recording. Study 3 assessed the impact of maintained isoflurane anesthesia (4 hours) on PID incidence after permanent occlusions. Study 4 assessed PID incidence after transient occlusions. Overall time lines are not to scale.
Electrode placement and recording
Arrays consisted of 10 mm lengths of 0.125 mm diameter silver wire soldered to a 4-pin segment of in-line strip socket (ED7464-ND, Digi-Key Corporation, Thief River Falls, MN). Electrode tips were rounded to a diameter of ~0.8 mm in a gas flame and electrolytically chloridized. Wires were disinfected with dilute bleach and rinsed in sterile saline prior to use.
Rats were fasted overnight prior to surgery. Anesthesia was induced with 4% isoflurane and maintained at 1–2% in 70% N2 and 30% O2 via a flow through face mask with spontaneous respiration. Rats were positioned in a stereotaxic holder and received subcutaneous Baytril (5 mg/kg) as antibiotic prophylaxis. Body temperature was monitored by rectal probe and maintained at 37 °C with a heating pad. After a midline incision the scalp was retracted to expose the skull, which was cleaned and dried with an alcohol wipe and a warm air stream. Anchor screws were placed and burr holes were made to position electrodes as follows: rostral ipsilateral over right frontal cortex outside the MCA territory (4 mm lateral, 4 mm anterior to bregma), caudal ipsilateral targeting the edge of MCA territory (3 mm lateral, 3 mm posterior), contralateral (4 mm lateral, 3 mm posterior) and reference (midline, caudal to lambda). Targeted recording sites and typical infarct distribution are shown in Fig. 2A. Importantly, adequate signals could be obtained without fully penetrating the skull, avoiding risk of injury to the brain surface. The electrode array was lowered into place with a micromanipulator and fixed in place with dental cement. Rats received 0.05 mg/kg subcutaneous buprenorphine, the scalp was sutured around the connector, and anesthesia was discontinued. Animals recovered for 1 day to 5 weeks prior to occlusion surgery.
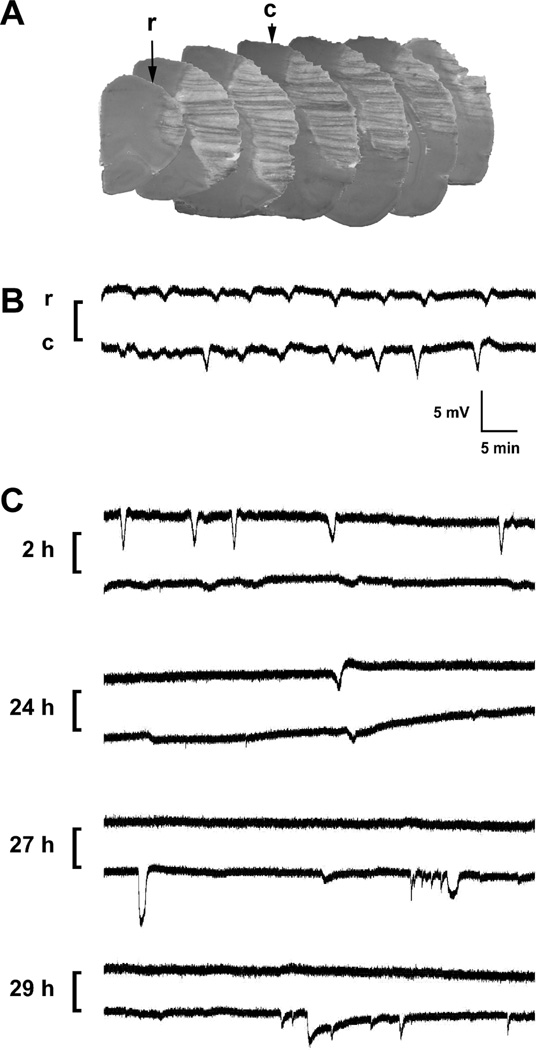
Fig. 2.
Infarct distribution, electrode placement and representative recordings. A) Electrode positions and infarct distribution. Rostral (r) and caudal (c) sites were chosen to overlie positions outside the expected cortical infarct distribution characteristic of the SHR model, shown at 2 mm intervals through the extent of MCA territory in a series of hematoxylin-eosin stained frozen sections from a rat subjected to permanent occlusion. B) Typical paired events. Traces illustrate a series of depolarizations propagating past the two electrode positions during the initial hours after permanent occlusion. C) Atypical dissociated depolarizations. Paired traces are illustrated as in panel B. Prominent PIDs detected at the rostral electrode during the initial hours after transient occlusion were poorly detected at a caudal position overlying the eventual infarct margin. Such events recorded only at the rostral electrode were included in the quantitative assessment. In contrast, depolarizations of variable magnitude and duration restricted to the caudal peri-infarct site at later intervals were not counted in the tally of propagated events.
Data acquisition began within 10 minutes following completion of surgery to produce permanent occlusions, or at a similar interval following the release of transient occlusions. After recovery from anesthesia rats were housed in a round plexiglass chamber lined with bedding and provided standard food pellets and a water bottle. The chamber was placed in a Faraday cage to minimize noise. Signals were routed from the head plug through a 4-channel swivel connector (Plastics One, Roanoke, VA) centered above the chamber on a counterbalanced arm, amplified using Electro 705 electrometers (World Precision Instruments, Sarasota, FL), and digitized and stored via a Micro1401-3 interface and Spike2 software (Cambridge Electronic Design Ltd., Cambridge, UK). The acquisition rate was typically 1 hz for a 24 hour recording, or 0.3 hz when animals were monitored for 3 days.
The majority of PIDs occurred as transient, paired events detected at both ipsilateral electrodes, typically 2–5 mV in magnitude (Fig. 2B). Also counted were depolarizations detected at the rostral electrode alone (9 of 41 animals). This was done if the caudal signal was unstable (n=4, all but one at the late 5 week interval) or when attenuated at positions overlying ischemic or recently ischemic MCA territory (Fig. 2C). Of the 5 such rats, 3 had experienced transient occlusions. Conversely, complex depolarization patterns occasionally recorded from the caudal electrode alone, when positioned at the infarct edge, were ignored in the analysis of propagated PID incidence.
Sham surgery or anesthesia exposure
A sham surgery involving anesthesia, skull exposure and thinning replicated conditions of a previous study that identified preconditioning effects on PID incidence (Zhao and Nowak, 2015). Alternatively, rats were maintained for 2 hours under isoflurane anesthesia, without surgical manipulation, to mimic the duration of anesthesia required for electrode placement described above. Animals were then subjected to experimental stroke 1 day to 6 weeks after the prior treatment, and PID incidence was monitored by perfusion imaging under isoflurane anesthesia.
MCA Occlusion
Focal cerebral ischemia was induced by tandem occlusion of the right MCA and ipsilateral common carotid artery (Brint et al., 1988). In brief, rats were fasted overnight, anesthetized with isoflurane, and positioned on a tooth bar that permitted axial rotation. The tail artery was cannulated for blood pressure monitoring and blood gas sampling during transient occlusions that required prolonged anesthesia intervals, and for studies explicitly investigating the impact of maintained anesthesia during permanent occlusion. The carotid artery was isolated, ligated and cauterized to produce permanent occlusions, or occluded with an aneurysm clip during transient occlusions. The MCA was accessed by a transtemporal approach, exposing the vessel at the level of the rhinal fissure. Rectal temperature was controlled using a circulating water blanket, and brain surface temperature was maintained with a temperature-controlled saline drip throughout the procedure. The MCA was snared and cauterized to produce permanent occlusions, or elevated approximately 1 mm above the brain surface using a micromanipulator-controlled wire hook for the 2 h duration of transient occlusions. Incisions were closed and buprenorphine was administered, after which anesthesia was discontinued. The anesthesia interval required for routine permanent occlusions was approximately 30 minutes. Anesthesia was maintained for 4 hours after occlusion in Study 3 investigating anesthesia effects.
Physiological variables were generally well maintained during spontaneous respiration under anesthesia (Table 1). Prolonged (4 h) anesthesia was associated with greater blood pressure drops and than seen during the 2 h transient occlusions. A lower PaO2 was intentionally targeted in the latter group to maintain consistency with prior transient occlusion studies from this laboratory (Kurasako et al., 2007; Hashimoto et al., 2008).
Table 1.
Physiological variables during spontaneous respiration under prolonged anesthesia
| Experimental group | MABP* (mm Hg) |
pH | PaCO2 (mm Hg) |
PaO2 (mm Hg) |
Glucose (mM) |
|---|---|---|---|---|---|
| Study 3, permanent (n=6) | |||||
| MCAO | 121 ± 12 | 7.35 ± 0.03 | 35 ± 11 | 128 ± 20 | 6.0 ± 1.3 |
| 4 h | 91 ± 13† | 7.33 ± 0.02 | 50 ± 12 | 122 ± 20 | 6.1 ± 1.0 |
| Study 4, transient (n=8) | |||||
| MCAO | 133 ± 22 | 7.36 ± 0.03 | 42 ± 7 | 100 ± 12 | 7.2 ± 1.8 |
| 2 h | 113 ± 11 | 7.37 ± 0.04 | 39 ± 5 | 88 ± 15 | 7.6 ± 0.9 |
MABP, mean arterial blood pressure
Significantly different from initial time point
Perfusion imaging
Hyperemic responses to PIDs were monitored as previously described (Zhao and Nowak, 2015). Surgical preparation for MCA occlusion included thinning of the skull overlying dorsal cortex, and rats were intubated and ventilated to maintain physiological variables. CBF was monitored by laser speckle contrast perfusion imaging (FLPI, Moor Instruments, Inc., Wilmington, DE). After a brief interval of baseline recording vascular occlusions were produced, and propagating peri-infarct hyperemic transients were monitored for 4 hours.
Infarct assessment
Animals were decapitated under isoflurane anesthesia 24 h after permanent occlusion, or 72 hours after transient occlusions. The thinned skull at electrode placement sites was punctured with a needle and ink was introduced to mark the underlying brain. In studies 2 and 3 brains were removed into chilled saline and 2 mm slices were cut on a brain matrix, after which they were incubated in 2 % triphenyltetrazolium chloride (TTC) in saline at ~37 °C until color development was complete, followed by overnight fixation in formalin and saline rinses. In Study 4 brains were frozen in hexane at −40 °C and stored at −70 °C, after which cryostat sections (20 µm) at 1 mm intervals were collected on glass slides and stained with hematoxylin/eosin. In both cases calibrated images were captured (NIH Image) and areas of pallor were measured and summed to yield total infarct volumes. No corrections for edema volume were made in the analyses of pooled data from these studies since it would introduce additional variability, particularly in the fresh slices used for TTC staining.
Statistical analyses
Group values, where given, are stated as mean ± standard deviation. Comparisons were made by Mann-Whitney U or Wilcoxon Signed Rank tests, or by Kruskal-Wallis test followed by Dunn’s Multiple Comparison test, using GraphPad Prism 5.0c (GraphPad Software, San Diego, CA), with P < 0.05 considered significant. Two successfully recorded animals were excluded from the linear regression analysis of the relationship between PID incidence and infarct volume. One exhibited an unusually small and frontally positioned lesion consistent with anomalous MCA branching, and the other had a large infarct and evidence of local trauma at the site of occlusion.
RESULTS
Prior anesthesia and PID number
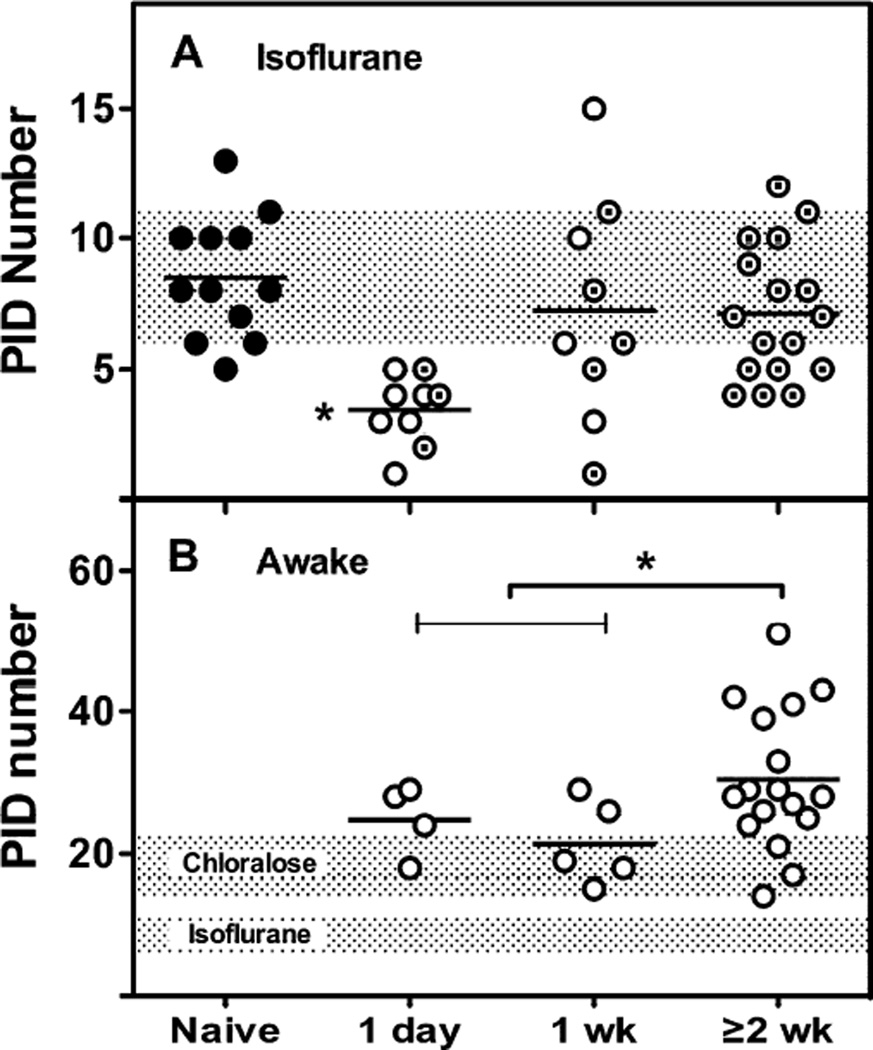
Sham surgery or anesthesia exposure markedly reduced PID incidence during stroke the following day, when monitored by perfusion imaging under sustained isoflurane anesthesia (Fig. 3A). This effect persisted in some animals at 1 week but was no longer evident after recovery intervals of 2 weeks or longer. A similar trend was seen during electrophysiological recording in the awake state, with significantly higher PID incidence for occlusions done at least 2 weeks after electrode placement, relative to pooled 1 day and 1 week groups (Fig. 3B). Subsequent investigations were carried out 2–5 weeks after electrode placement. Total PID number in awake animals was several-fold greater than under isoflurane anesthesia, and also higher than historical data obtained under α-chloralose.
Fig. 3.
Prior isoflurane anesthesia and preconditioning effects. A) PIDs under anesthesia. Propagated hyperemic CBF transients monitored by perfusion imaging during 4 hours under isoflurane anesthesia were markedly attenuated when MCA occlusion occurred 1 day after a previous anesthesia exposure, and this effect persisted in some animals at 1 week. Open circles, anesthesia accompanied by sham surgery; circled dots, anesthesia only; shaded bar, mean ± SD for the Naïve group. * P ≤ 0.05 vs. Naïve and ≥ 2week groups. B) PIDs in awake animals. PIDs were recorded from electrode arrays placed at the indicated intervals prior to MCA occlusion, with subsequent monitoring for up to 3 days. PID incidence was reduced at 1 day and 1 week relative to longer recovery intervals after electrode placement. Shaded bars, mean ± SD for historical results obtained from naïve animals under anesthesia with α-chloralose (Zhao and Nowak, 2015), or isoflurane (from panel A). * P ≤ 0.05 for pooled 1 day and 1 week groups vs. ≥ 2 week group.
PIDs after permanent occlusions and the effect of anesthesia
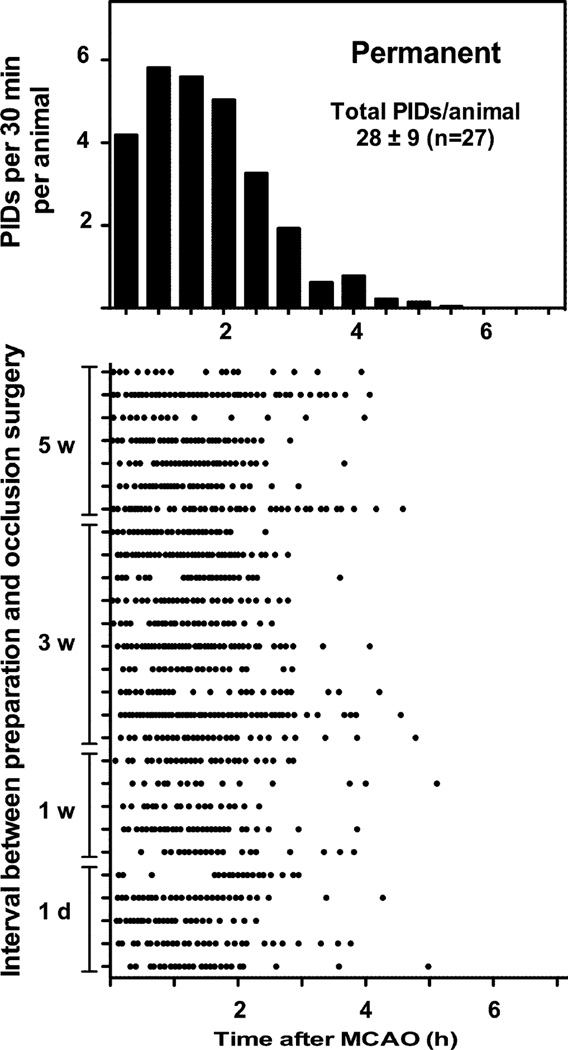
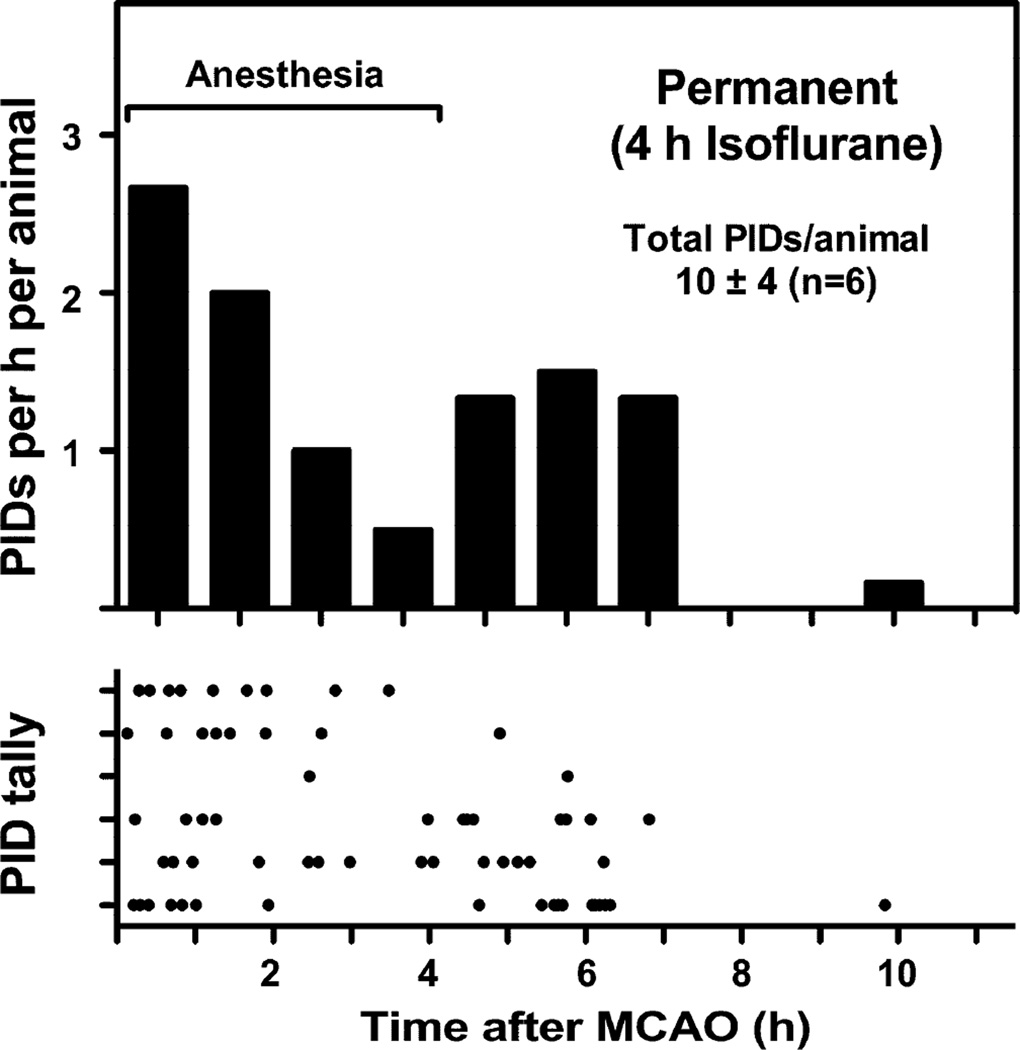
The interval between electrode placement and occlusion surgery did not impact PID time course, and groups were pooled for subsequent analysis. PIDs exhibited a very short time course when anesthesia was terminated immediately after occlusion, essentially all events occurring within 4 hours (Fig. 4). Prolonging anesthesia duration markedly reduced PID incidence during this interval, and in the majority of animals resulted in a second wave of events persisting for several hours after recovery from anesthesia (Fig. 5). Despite the extended time course, total PID number was reduced several-fold by this initial interval of prolonged anesthesia.
Fig. 4.
PID time course after permanent MCA occlusion. Upper panel represents a histogram of data shown in the lower panel, in which each row represents an individual animal and each point a PID. Animals recorded in the awake state following brief anesthesia for occlusion surgery exhibited a rapid time course of PID evolution, essentially all events occurring within 4 hours after the onset of ischemia. The interval between electrode placement and occlusion surgery did not impact PID time course (lower panel).
Fig. 5.
Effect of prolonged isoflurane anesthesia. Upper panel represents a histogram of data shown in the lower panel, in which each row represents an individual animal and each point a PID. Maintaining rats under isoflurane anesthesia during the first 4 hours of permanent occlusion reduced overall PID incidence several fold relative to the awake condition (Fig. 4), and in most animals extended the interval of PID occurrence through several hours after anesthesia termination.
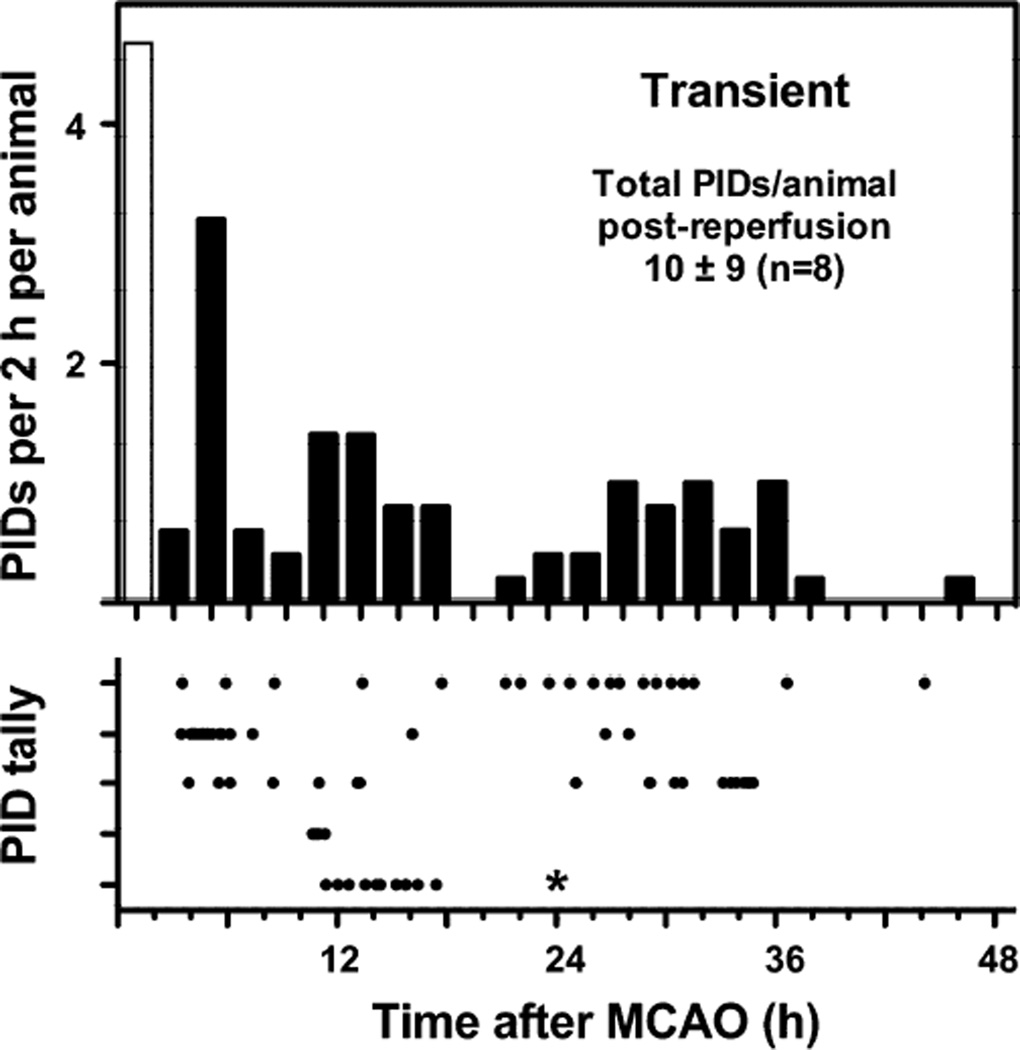
PIDs after transient occlusions
The PID time course was markedly longer following transient MCA occlusions (Fig. 6). In an initial series monitored for only 24 hours reperfusion, 3 of 4 animals failed to exhibit PIDs, and one exhibited a delayed interval of PID incidence during 12–18 hours. All 4 rats of a subsequent series showed an initial phase of depolarizations through approximately 18 hours, followed by a distinct secondary component between 24 and 36 hours. Typically a delay of 1 hour followed release of occlusion and anesthesia termination before the earliest PIDs were detected. However, two rats exhibited much longer delays in PID onset (~10 hours), and this was associated with a reduction in total PID number. No events were observed in any animal beyond 48 hours.
Fig. 6.
Prolonged PID time course after transient ischemia. Upper panel represents a histogram of data shown in the lower panel, in which each row represents an individual animal and each point a PID. Rats were subjected to MCA occlusion for 2 hours under isoflurane anesthesia followed by up to 3 days of awake monitoring. PIDs were observed following release of occlusion in 5 of 8 animals. No events were seen beyond 48 hours. Procedural constraints precluded recording during the occlusion period, so the open bar represents expected PID incidence based on data obtained from other animals during the initial 2 hours of permanent occlusion under anesthesia (Fig. 5). * In one animal recording terminated at 24 hours; for simplicity the histogram was not adjusted for this attrition, and also excluded the 3 rats with no recorded events. However the stated total incidence during the reperfusion period includes all animals.
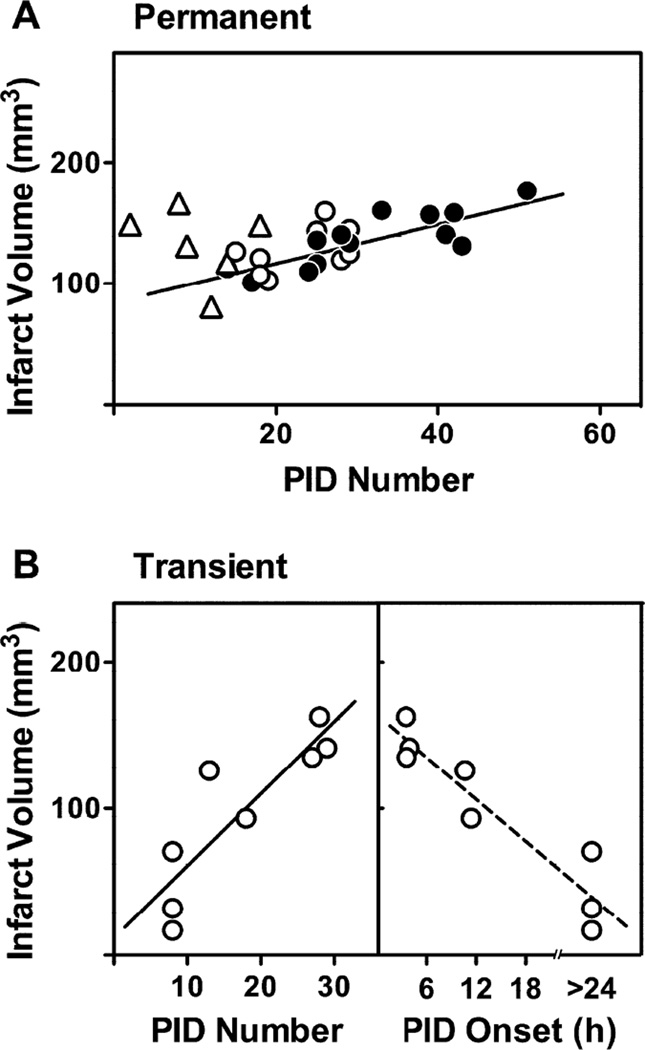
PID number and infarct size
PID number was well correlated with infarct size for rats allowed to recover from anesthesia after permanent occlusions (Fig. 7A). Notably, those in which occlusions were produced within 1 week after electrode placement that exhibited fewer PIDs also tended to have smaller infarcts, although PID number was not a robust predictor of infarct volume for individual animals.
Fig. 7.
Relationship between PID incidence and infarct volume. A) Permanent occlusions. The number of observed PIDs correlated with infarct volume for those animals in which anesthesia was immediately terminated following occlusion. Open and solid circles represent MCA occlusions at ≤1 and ≥2 weeks after electrode placement, respectively; regression line indicates best fit for the combined two groups (r2 = 0.55, P < 0.0001). The marked reduction in total PID incidence observed in animals subjected to prolonged anesthesia (triangles) was not associated with a corresponding reduction in final infarct size. B) Transient occlusions. Infarct volume correlated well with total PID incidence, obtained by summing the number recorded after reperfusion for each animal (Fig. 6) and the mean event number (5) recorded from other animals (Fig. 5) during 2 hours occlusion under anesthesia (r2 = 0.75, P = 0.005). Since increasing PID number was associated with earlier PID onset, infarct volume was inversely related to the delay in initial PID occurrence. The absence of depolarization within a 24 hour recording in some animals precluded defining a specific delay interval, so dotted line is provided for purposes of illustration only.
Furthermore, several hours of maintained isoflurane anesthesia that markedly reduced total PID number did not significantly impact infarct volume. A stronger correlation was evident after transient ischemia (Fig. 7B), particularly in that the several rats in which no PIDs were detected following release of occlusion had consistently smaller infarcts. Conversely, since delayed PID incidence was associated with reduced total number after transient occlusions, there was an inverse relationship between the delay in PID onset and infarct size.
DISCUSSION
Technical considerations
The primary goal was to obtain data on PID incidence in a model distinct from the Sprague-Dawley rat used in previous awake recordings (Hartings et al., 2003). The SHR is characterized by limited collateral vascularization (Coyle, 1986), resulting in both a steep perfusion gradient and a rapid time course of infarct progression following MCA occlusion (Jacewicz et al., 1992). As a relatively inbred strain it yields more consistent infarcts than commonly used outbred rats (Brint et al., 1988; Duverger and MacKenzie, 1988), permitting the use of fewer animals and making it particularly well suited to mechanistic studies. Conversely, the Sprague-Dawley has such robust collateral perfusion that distal MCA occlusions fail to produce consistent infarcts (Coyle, 1986; Ren et al., 2004; Ma et al., 2006). The study of selective cortical injury in the SHR (Fig. 2A) avoids the hypothalamic involvement and associated hyperthermia that can occur following intraluminal filament occlusions (Zhao et al., 1994).
Distributions of PIDs and their associated metabolic and perfusion responses are well established in the SHR (Takeda et al., 2011; Zhao and Nowak, 2015), permitting consistent targeting of informative sites (Fig. 2A,B). Unrecognized variations in fabrication and placement could impact detection sensitivity or the extent of cortex effectively monitored by a given electrode. However, since PID time course was the endpoint of interest, little attention was given to variations in depolarization magnitude. This approach reliably detected the propagated events most closely associated with stepwise infarct expansion (Higuchi et al., 2002; Shin et al., 2006; Takeda et al., 2011). Comprehensive documentation of non-propagating depolarization dynamics at the margin of the ischemic territory (Fig. 2C), undoubtedly important determinants of local pathology and function (Higuchi et al., 2002; Risher et al., 2010), would require alternative approaches. PIDs at the rostral electrode were occasionally absent or greatly attenuated at the infarct margin (Fig. 2C). In rare cases a caudal electrode overlying infarcted cortex confirmed that prior terminal depolarization must have already occurred, precluding PID propagation (Röther et al., 1996; Chen et al., 2006). Baseline drift during long recordings, and signal sampling from a relatively large volume of underlying cortex, made it difficult to unambiguously identify a local transition to persistent ischemic depolarization. However, propagation can also fail in areas of low CBF showing no evidence of prior terminal depolarization (Bere et al., 2014), as also described following electrical stimulation (Koroleva and Bureš, 1979). The blunting of caudal PIDs during early reperfusion, followed much later by evidence of both propagating and local depolarizations at the infarct margin (Fig. 2C), may be consistent with initial failure of PID propagation into recently ischemic cortex. Alternatively, it could simply reflect the medial progression of the active penumbral zone during infarct expansion (Chen et al., 2006), which likely would be more evident after transient occlusions.
Anesthesia preconditioning and inter-operative recovery interval
Acute PID incidence after stroke, monitored under continuous isoflurane anesthesia, is profoundly diminished if rats experienced prior surgery under the same anesthetic (Zhao and Nowak, 2015). Current results confirm that PID attenuation is reproduced by prior anesthesia exposure alone and that it can persist for up to 1 week (Fig. 3A). This is longer than the 1 day interval typically reported for brain protection by anesthetic preconditioning (Kapinya et al., 2002; Kitano et al., 2007), although efficacy was noted 3 days after sevoflurane exposure in one study (Adamczyk et al., 2010). Since anesthetic preconditioning is associated with increased penumbral CBF (Chi et al., 2010; Zhao and Nowak, 2015), the SHR may be particularly sensitive to such effects. The impact on PIDs is much less prominent during subsequent awake recording (Fig. 3B), but its relevance is confirmed by the parallel reduction in infarct volume (Fig. 7A). Halothane also exerts preconditioning (Kapinya et al., 2002), so this agent may have had a similar subtle influence on PID incidence in the previous awake recording study (Hartings et al., 2003). These results establish 2 weeks as a requisite post-surgical interval to avoid confounding effects of prior anesthesia. This could differ after invasive surgical procedures involving penetration of skull and dura, which can result in more persistent inflammatory and microvascular responses (Polikov et al., 2005; Hammer et al., 2014).
PID incidence and time course
As observed in Sprague-Dawley rats (Hartings et al., 2003), more PIDs occurred in awake SHR than seen under anesthesia (Fig. 3B). Given the potential for cyclic propagation of depolarizations around an evolving infarct, trains of multiple PIDs recorded at a given site can arise from a single triggering event (Nakamura et al., 2010). This is undoubtedly sensitive to the attenuating effect of volatile anesthetics (Saito et al., 1995; Saito et al., 1997; Kitahara et al., 2001; Luckl et al., 2008; Nakamura et al., 2010; Takagaki et al., 2014), whereas awake PID incidence is better approximated under α-chloralose anesthesia (Takeda et al., 2011; Zhao and Nowak, 2015). The short 4 hour window during which PIDs occur after permanent occlusions (Fig. 4) differs markedly from results obtained after filament occlusions in Sprague-Dawley rats, which showed a biphasic pattern of PID incidence during 24 hours (Hartings et al., 2003). The abbreviated PID time course in the SHR is consistent with the short temporal threshold for infarction characteristic of this strain (Jacewicz et al., 1992). Total PID number was also several-fold greater in the prior study, largely due to a delayed secondary component (Hartings et al., 2003). Since persistent hyperthermia characteristic of filament occlusions was also noted in that study, the larger depolarization number may in part reflect the temperature dependence of PID incidence reported in such models (Chen et al., 1993).
Prolonged anesthesia is often required in experimental stroke studies for purposes of electrophysiological or perfusion monitoring, but profoundly impacts the endpoints under study. Maintaining isoflurane for several hours not only reduced PID number, but in most animals prolonged their time course (Fig. 5), consistent with the slower infarct progression observed under volatile anesthetics (Saito et al., 1997; Takeda et al., 2011; Zhao and Nowak, 2015). This suppressive effect appears to decay rapidly after anesthesia termination, since the slight lag in PID incidence in awake animals (Fig. 4) is in part attributable to an initial delay in data acquisition.
In agreement with the previous filament occlusion study (Hartings et al., 2003), PIDs after transient occlusions showed a more protracted and apparently biphasic time course (Fig. 6). The 36 hours during which PIDs occurred correlates with the temporal course of infarct evolution in the model, in which detectable lesion expansion can continue after 24 hours (Kurasako et al., 2007). The similarly prolonged time course seen after permanent occlusions in Sprague-Dawley rats (Hartings et al., 2003) suggests that better collateral perfusion in that strain may provide the functional equivalent of partial reperfusion with respect to its impact on PID incidence and infarct progression.
PID number and infarct size
As in many studies, PID numbers after permanent occlusions in the awake SHR increase with infarct size (Fig. 7A). The previous study in Sprague-Dawley rats found a strong relationship between infarct volume and PID incidence in the delayed secondary phase (Hartings et al., 2003), which did not occur after permanent occlusions in the SHR. Importantly, the relationship between PID incidence and infarct volume can be uncoupled in this model. PID number is reduced by hyperglycemia (Nedergaard and Astrup, 1986; Strong et al., 2000; Hopwood et al., 2005), but modest glucose elevation attenuates PID incidence in the SHR without impacting infarct size (Zhao and Nowak, 2015). Likewise, prolonged isoflurane anesthesia during the initial hours of occlusion reduces total PID number, but is not protective in the absence of reperfusion (Fig. 7A). Similar dissociations have been reported following pharmacological interventions in other models (Patel et al., 1998; Christensen et al., 2003; Lu et al., 2005). PIDs only rarely lead to infarct expansion in the SHR (Higuchi et al., 2002; Takeda et al., 2011), so selectively reducing PID number might have little impact on infarct size. As noted previously (von Bornstädt et al., 2015; Zhao and Nowak, 2015), a larger ischemic territory has more extensive penumbra with an increased probability of generating PIDs, potentially reversing the causal relationship. Although each depolarization can contribute to local histopathology and functional deficit (Risher et al., 2010), the temporal intervals during which PID initiation persists appears to be more informative about the status of infarct progression than the absolute number of propagating events.
Transient occlusions were more variable, and the smallest infarcts were found in animals that exhibited no PIDs following release of occlusion (Fig. 7B). Since increasing PID number was associated with their earlier occurrence following reperfusion, infarct volume was inversely related to the delay in PID onset. This replicates observations after filament occlusions that found infarct size correlated with the time of PID onset (Hartings et al., 2003), and again places emphasis on the temporal component of PID incidence.
Implications for stroke modeling and clinical relevance
PIDs can occur in patients over many days (Dohmen et al., 2008), and such intervals are inaccessible to experimental observation in anesthetized animal models. Acute studies under α-chloralose may approximate the awake condition in models with rapidly evolving infarcts (Fig. 3B) (Zhao and Nowak, 2015), but long term monitoring in the awake state is essential if experimental studies are to have broad clinical relevance (Hartings et al., 2003). The current study adds the further requirement for adequate recovery intervals to avoid confounding effects of the anesthesia used during prior surgical preparation.
Differences in anesthesia and analgesia management also impact PID occurrence in human brain. Specifically, N-methyl-D-aspartate antagonists such as ketamine reduce PID incidence (Sakowitz et al., 2009; Hertle et al., 2012). However, although blocking PIDs seems almost certainly beneficial with respect to limiting the functional impact of propagated depolarization, experimental evidence indicates that effects on infarct size should not be expected in the absence of adequate collateral circulation or timely reperfusion (e.g., Fig. 7A). Finally, many interventions documented to reduce PID incidence can also increase CBF (Saito et al., 1997; Kamiya et al., 2005; Takagaki et al., 2014; Zeiler et al., 2015; Zhao and Nowak, 2015). The extent to which such synergy contributes to protection in experimental stroke has yet to be fully defined, and its potential clinical relevance is unknown.
CONCLUSIONS
PID time course varies systematically among stroke models, correlating with recognized variations in the time course of infarct progression, and supporting the suggestion that PID persistence is indicative of ongoing pathophysiology. To date, clinical observations related to PIDs after stroke and other brain injuries have been made in the intensive care setting, but most stroke patients may be assumed to experience unrecognized PIDs. Experimental models exhibiting prolonged intervals of PID occurrence monitored in the awake state are required to evaluate potential interventions targeting their attenuation following stroke.
HIGHLIGHTS.
Peri-infarct depolarizations (PIDs) were recorded in awake rats during stroke.
Recovery for 2 weeks after electrode placement avoided anesthetic preconditioning.
PIDs ceased within 4 or 36 h after permanent or transient occlusions, respectively.
PID suppression by sustained anesthesia was not protective without reperfusion.
PID time course coincides with infarct progression under varied model conditions.
Acknowledgments
Supported by the Ganey Fund, Department of Neurology, University of Tennessee Health Science Center, and by the National Institute of Neurological Disorders and Stroke of the NIH under award number R21NS077039. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also thank Hitoshi Kita and Detlef Heck for helpful suggestions in establishing the awake recording methods.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
KK contributed to study design, optimized electrode placement procedures, performed surgeries for awake recording, analyzed the resulting data and prepared it for presentation. LZ was responsible for perfusion imaging studies and their analysis and presentation. TN designed experiments, assisted in method development, finalized figures and wrote the manuscript.
POTENTIAL CONFLICTS OF INTEREST
None.
REFERENCES
- Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, Bordet R, Lebuffe G. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial KATP channel. Br J Anaesth. 2010;104:191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- Back T, Ginsberg MD, Dietrich WD, Watson BD. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab. 1996;16:202–213. doi: 10.1097/00004647-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Bere Z, Obrenovitch TP, Kozák G, Bari F, Farkas E. Imaging reveals the focal area of spreading depolarizations and a variety of hemodynamic responses in a rat microembolic stroke model. J Cereb Blood Flow Metab. 2014;34:1695–1705. doi: 10.1038/jcbfm.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischemia in baboon cerebral cortex. J Neurol Sci. 1977;32:305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: Methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Chen Q, Chopp M, Bodzin G, Chen H. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: correlation with ischemic injury. J Cereb Blood Flow Metab. 1993;13:389–394. doi: 10.1038/jcbfm.1993.52. [DOI] [PubMed] [Google Scholar]
- Chen S, Li P, Luo W, Gong H, Zeng S, Luo Q. Origin sites of spontaneous cortical spreading depression migrated during focal cerebral ischemia in rats. Neurosci Lett. 2006;403:266–270. doi: 10.1016/j.neulet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. The effects of isoflurane pretreatment on cerebral blood flow, capillary permeability, and oxygen consumption in focal cerebral ischemia in rats. Anesth Analg. 2010;110:1412–1418. doi: 10.1213/ANE.0b013e3181d6c0ae. [DOI] [PubMed] [Google Scholar]
- Christensen T, Bruhn T, Diemer NH. The free radical spin-trap α-PBN attenuates periinfarct depolarizations following permanent middle cerebral artery occlusion in rats without reducing infarct volume. Brain Res. 2003;990:66–76. doi: 10.1016/s0006-8993(03)03439-5. [DOI] [PubMed] [Google Scholar]
- Coyle P. Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke. 1986;17:520–525. doi: 10.1161/01.str.17.3.520. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, van der Worp HB, van der Sprenkel JWB, Tulleken KAF, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus R-I, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhäupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ for the COSBID study group. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann T-N, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: Influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Hammer DX, Lozzi A, Abliz E, Grenbaum N, Agrawal A, Krauthamer V, Welle CG. Longitudinal vascular dynamics following cranial window and electrode implantation measured with speckle variance optical coherence angiography. Biomed Opt Express. 2014;5:2823–2836. doi: 10.1364/BOE.5.002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Rolli ML, Lu X-CM, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Zhao L, Nowak TS., Jr Temporal thresholds for infarction and hypothermic protection in Long-Evans rats. Factors affecting apparent “reperfusion injury” after transient focal ischemia. Stroke. 2008;39:421–426. doi: 10.1161/STROKEAHA.107.495788. [DOI] [PubMed] [Google Scholar]
- Hertle DN, Dreier JP, Woitzik J, Hartings JA, Bullock R, Okonkwo DO, Shutter LA, Vidgeon S, Strong AJ, Kowoll C, Dohmen C, Diedler J, Veltkamp R, Bruckner T, Unterberg AW, Sakowitz OW for the Cooperative Study of Brain Injury Depolarizations (COSBID) Effect of analgesics and sedatives on the occurrrence of spreading depolarizations accompanying acute brain injury. Brain. 2012;135:2390–2398. doi: 10.1093/brain/aws152. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Takeda Y, Hashimoto M, Nagano O, Hirakawa M. Dynamic changes in cortical NADH fluorescence and direct current potential in rat focal ischemia: relationship between propagation of recurrent depolarization and growth of the ischemic core. J Cereb Blood Flow Metab. 2002;22:71–79. doi: 10.1097/00004647-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Hinzman JM, Andaluz N, Shutter LA, Okonkwo DO, Pahl C, Strong AJ, Dreier JP, Hartings JA. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain. 2014;137:2960–2972. doi: 10.1093/brain/awu241. [DOI] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Hossmann K-A. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8:195–208. [PubMed] [Google Scholar]
- Jacewicz M, Tanabe J, Pulsinelli WA. The CBF threshold and dynamics for focal cerebral infarction in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12:359–370. doi: 10.1038/jcbfm.1992.53. [DOI] [PubMed] [Google Scholar]
- Kamiya T, Jacewicz M, Nowak TS, Jr, Pulsinelli WA. Cerebral blood flow thresholds for mRNA synthesis after focal ischemia and the effect of MK-801. Stroke. 2005;36:2463–2467. doi: 10.1161/01.STR.0000185669.60271.78. [DOI] [PubMed] [Google Scholar]
- Kanemitsu H, Nakagomi T, Tamura A, Tsuchiya T, Kono G, Sano K. Differences in the extent of primary ischemic damage between middle cerebral artery coagulation and intraluminal occlusion models. J Cereb Blood Flow Metab. 2002;22:1196–1204. doi: 10.1097/01.wcb.0000037992.07114.95. [DOI] [PubMed] [Google Scholar]
- Kapinya KJ, Löwl D, Fütterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kitahara Y, Taga K, Abe H, Shimoji K. The effects of anesthetics on cortical spreading depression elicitation and c-fos expression in rats. J Neurosurg Anesthesiol. 2001;13:26–32. doi: 10.1097/00008506-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–1128. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva VI, Bureš J. Circulation of cortical spreading depression around electrically stimulated areas and epileptic foci in the neocortex of rats. Brain Res. 1979;173:209–215. doi: 10.1016/0006-8993(79)90622-x. [DOI] [PubMed] [Google Scholar]
- Kudo C, Nozari A, Moskowitz MA, Ayata C. The impact of anesthetics and hyperoxia on cortical spreading depression. Exp Neurol. 2008;212:201–206. doi: 10.1016/j.expneurol.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurasako T, Zhao L, Pulsinelli WA, Nowak TS., Jr Transient cooling during early reperfusion attenuates delayed edema and infarct progression in the Spontaneously Hypertensive Rat. Distribution and time course of regional brain temperature change in a model of postischemic hypothermic protection. J Cereb Blood Flow Metab. 2007;27:1919–1930. doi: 10.1038/sj.jcbfm.9600492. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng LZ, Fink ME, Iadecola C. Spreading depolarization: a possible new culprit in the delayed cerebral ischemia of subarachnoid hemorrhage. Arch Neurol. 2011;68:31–36. doi: 10.1001/archneurol.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-CM, Williams AJ, Wagstaff JD, Tortella FC, Hartings JA. Effects of intrathecal infusion of an NMDA receptor antagonist on ischemic injury and peri-infarct depolarizations. Brain Res. 2005;1056:200–208. doi: 10.1016/j.brainres.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Luckl J, Keating J, Greenberg JH. Alpha-chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: a comparative study. Brain Res. 2008;1191:157–167. doi: 10.1016/j.brainres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhao L, Nowak TS., Jr Selective, reversible occlusion of the middle cerebral artery in rats by an intraluminal approach. Optimized filament design and methodology. J Neurosci Meth. 2006;156:76–83. doi: 10.1016/j.jneumeth.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossman K-A. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sué M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain. 2010;133:1994–2006. doi: 10.1093/brain/awq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab. 1986;6:607–615. doi: 10.1038/jcbfm.1986.108. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Coyle P, Weber E. Rat strain and vendor differences in collateral anastomoses. J Cereb Blood Flow Metab. 1997;17:571–576. doi: 10.1097/00004647-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Patel PM, Drummond GR, Cole DJ, Kelly PJ, Watson M. Isoflurane and pentobarbital reduce the frequency of transient ischemic depolarizations during focal ischemia in rats. Anesth Analg. 1998;86:773–780. doi: 10.1097/00000539-199804000-00018. [DOI] [PubMed] [Google Scholar]
- Polikov VM, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Meth. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ren Y, Hashimoto M, Pulsinelli WA, Nowak TS., Jr Hypothermic protection in rat focal ischemia models: strain differences and relevance to “reperfusion injury”. J Cereb Blood Flow Metab. 2004;24:42–53. doi: 10.1097/01.WCB.0000095802.98378.91. [DOI] [PubMed] [Google Scholar]
- Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci. 2010;30:9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röther J, de Crespigny A, D’Arceuil H, Moseley M. MR detection of cortical spreading depression immediately after focal ischemia in the rat. J Cereb Blood Flow Metab. 1996;16:214–220. doi: 10.1097/00004647-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Saito R, Graf R, Hübel K, Fujita T, Rosner G, Heiss W-D. Reduction of infarct volume by halothane: effect on cerebral blood flow or perifocal spreading depression-like depolarizations. J Cereb Blood Flow Metab. 1997;17:857–864. doi: 10.1097/00004647-199708000-00004. [DOI] [PubMed] [Google Scholar]
- Saito R, Graf R, Hübel K, Taguchi J, Rosner G, Fujita T, Heiss W-D. Halothane, but not α-chloralose, blocks potassium-evoked cortical spreading depression in cats. Brain Res. 1995;699:109–115. doi: 10.1016/0006-8993(95)00898-z. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40:e519–e522. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, Hunter AJ, Patel S. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31:214–221. doi: 10.1161/01.str.31.1.214. [DOI] [PubMed] [Google Scholar]
- Takagaki M, Feuerstein D, Kumagai T, Gramer M, Yoshimine T, Graf R. Isoflurane suppresses cortical spreading depolarizations compared to propofol -- implications for sedation of neurocritical care patients. Exp Neurol. 2014;252:12–17. doi: 10.1016/j.expneurol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Takano K, Latour LL, Formato JE, Carano RAD, Helmer KG, Hasegawa Y, Sotak CH, Fisher M. The role of spreading depression in focal ischemia evaluated by diffusion mapping. Ann Neurol. 1996;39:308–318. doi: 10.1002/ana.410390307. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Zhao L, Jacewicz M, Pulsinelli W, Nowak TS., Jr Metabolic and perfusion responses to peri-infarct depolarization during focal ischemia in the Spontaneously Hypertensive Rat: dominant contribution of sporadic CBF decrements to infarct expansion. J Cereb Blood Flow Metab. 2011;31:1863–1873. doi: 10.1038/jcbfm.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bornstädt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, Sandow N, Kura S, Eikermann-Haerter K, Endres M, Boas DA, Moskowitz MA, Lo EH, Dreier JP, Woitzik J, Sakadži S, Ayata C. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85:1117–1131. doi: 10.1016/j.neuron.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler FA, Sader N, Gillman LM, Teitelbaum J, West M, Kazina CJ. The cerebrovascular response to ketamine: a systematic review of the animal and human literature. J Neurosurg Anesthesiol. 2015 doi: 10.1097/ANA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Nowak TS., Jr Preconditioning cortical lesions reduce the incidence of peri-infarct depolarizations during focal ischemia in the Spontaneously Hypertensive Rat: interaction with prior anesthesia and the impact of hyperglycemia. J Cereb Blood Flow Metab. 2015;35:1181–1190. doi: 10.1038/jcbfm.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Memezawa H, Smith M-L, Siesjö BK. Hyperthermia complicates middle cerebral artery occlusion induced by intraluminal filament. Brain Res. 1994;649:253–259. doi: 10.1016/0006-8993(94)91071-5. [DOI] [PubMed] [Google Scholar]