Abstract
Objective
To evaluate an enzyme-linked immunospot assay (ELISpot) for the diagnosis of tuberculosis (TB) in HIV-infected children with suspected TB and to compare the performance of ELISpot with the tuberculin skin test (TST).
Methods
IFN-γ responses to Mycobacterium tuberculosis (MTB)-specific antigens were measured by ELISpot in HIV-infected children with suspected TB. HIV-infected and HIV-uninfected children without TB were used for comparison.
Results
Results were available for 188 children, of which, 139 (74%) were HIV-infected. Of these, 22 were classified as having definite TB, 24 probable TB, 14 possible TB and 128 not having TB. The median (range) age of patients was 20 (10 - 54.1) months. Ninety one percent of ELISpot tests yielded interpretable results. Median IFN-γ responses to early-secreted antigenic target-6 and culture filtrate protein-10 were higher in children with definite or probable TB compared to children without TB (p<0.002). In HIV-infected children with an interpretable result, the ELISpot was positive in 14/21 (66%) with definite TB. A significantly higher proportion of HIV-infected children with definite or probable TB had a positive ELISpot compared to a positive TST (25/39 (64%) vs. 10/34 (29%), p=0.005). In contrast to TST, results from ELISpot were not affected by young age or severe immunosuppression. In HIV-infected children without active TB disease, 27% had a positive ELISpot suggesting latent TB infection.
Conclusions
ELISpot is more sensitive than TST for the detection of active TB in HIV-infected children. However the sensitivity of current ELISpot assays is not sufficiently high to be used as rule out test for TB.
Keywords: tuberculosis, interferon, diagnosis, ESAT-6, CFP-10
Introduction
The Western Cape region of South Africa has one of the highest recorded incidence rates of tuberculosis (TB). Fuelled by the HIV pandemic, rates in excess of 1,600/100,000 have been reported [1]. Historically, it has been thought that children contribute little to the TB burden but recent evidence challenges this dogma [2–4]. It was estimated that 11% of the eight million cases of TB worldwide in 2000 occurred in children [5]. This is likely to be an underestimate because of difficulties with TB diagnosis in children coupled with poor recording and reporting of child TB cases in endemic areas. Childhood TB accounts for 39% of all cases in the Western Cape [6] and the importance of TB as a cause of childhood death in TB endemic areas has also been highlighted in other studies [7, 8].
HIV infection is the major risk factor for the development of TB in sub-Saharan Africa [9]. The risk of TB has recently been reported to be more than 20 fold greater in HIV-infected children compared with uninfected children [10]. The detection of TB in HIV-infected children remains problematic for a number of reasons [11]. There is epidemiological and clinical overlap with the presentation of HIV and TB in children, and investigations such as chest radiography and tuberculin skin testing have reduced specificity and sensitivity [12, 13]. Mycobacterial culture confirms the diagnosis of TB in children and provides important drug susceptibility data [14]. However, culture is not available in most TB/HIV endemic settings. Further, a decision to commence TB treatment usually needs to be made before confirmation of diagnosis by culture, especially in HIV-infected children, as disease progression can be rapid [8, 11, 14].
Interferon gamma (IFN-γ) release assays (IGRA) have emerged as potential tools for the rapid and reliable detection of tuberculosis [15–17]. Few studies have assessed their performance in children [16–19]. Data on the use of IGRA for the diagnosis of TB in HIV-infected children are even more limited [20]. The aims of this study were to evaluate an enzyme-linked immunospot assay (ELISpot) in HIV-infected children with suspected TB in a high TB incidence area; to compare the performance of ELISpot against the tuberculin skin test (TST); and to investigate the effect of age, nutritional status and degree of HIV-associated immune suppression on the performance of both tests.
Methods
Patients
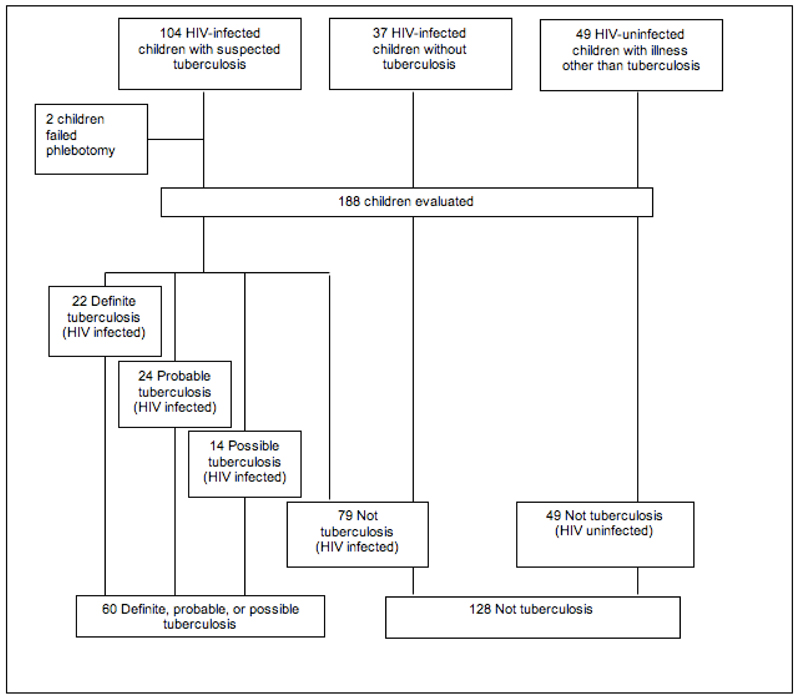
The study was approved by the University of Cape Town Research Ethics Committee. Children known to be HIV-infected and with symptoms suggestive of TB were prospectively recruited from the short stay-unit or general paediatric wards at the Red Cross Children’s Hospital, Cape Town, South Africa. The presence of one or more of the following symptoms was considered suggestive of TB: cough for longer than two weeks, persistent fever, night sweats, weight loss and fatigue. Children already receiving TB treatment for more than seven days were excluded. A second group of known HIV-infected children presenting with an illness other than TB or in whom TB had been actively excluded were recruited from the infectious diseases clinic, short-stay unit as well as from an ongoing efficacy study of isoniazid preventive treatment (IPT) in HIV-infected children. A major entry criteria for participants in the IPT study was exclusion of active TB disease. A group of HIV-uninfected children with an illness not consistent with TB were also prospectively recruited (Figure 1). These children had a range of alternative diagnoses including asthma, malaria, pneumonia, croup and urinary tract infection.
Figure 1:
Flowchart of study recruitment and final diagnostic groups.
Written witnessed informed consent was obtained from a parent in their preferred language. Demographic and clinical details were obtained using a questionnaire and included age, weight, presence of BCG scar, TB contact history, previous history of TB, symptoms suggestive of TB, WHO HIV clinical stage and details of anti-retroviral medications.
Diagnostic tests
Children with suspected TB were clinically assessed, had a TST, chest radiograph, and at least one induced sputum or two gastric washings or other site-specific clinical specimen sent for TB microscopy and culture. TST was performed by intradermal installation of 2 TU (PPD RT-23) and read after 48 to 72 hours. A positive TST was defined as greater than or equal to 5 mm in HIV-infected children and greater than or equal to 10 mm in HIV-uninfected children. HIV ELISA testing was performed in all children, with positive ELISA results confirmed by HIV RNA PCR. A blood sample was obtained from each child for ELISpot and, in HIV-infected children, a concomitant CD4 count. HIV-infected children were classified as having mild, advanced or severe immunodeficiency according to WHO criteria based on age-related CD4% values. All children in the study who qualified for anti-retroviral treatment based on the WHO eligibility guidelines that were in use at the time of the study were referred to the appropriate clinic for assessment. HIV-infected and HIV-uninfected children with illnesses not consistent with TB had specific investigations at the discretion of the child’s physician and their classification subsequently confirmed at a follow-up appointment 14 to 28 days after initial assessment. Patients failing to attend follow-up visits were contacted.
All blood samples were processed within 4 hours of phlebotomy and ELISpot was performed as previously described [21]. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation, washed, re-suspended and counted. PBMC (2 x105/ml) were added to duplicate wells (96-well polyvinylidine fluoride plates pre-coated with anti-IFN-γmAb 1-DIK antibody (Mabtech) containing antigen. Stimulatory antigens were added to PBMC and comprised, early secretory antigen target-6 (ESAT-6; a pool of 15-mer peptides overlapping by 10 amino acids, Peptide Protein Research UK; final concentration 5 μg/ml/peptide) and culture filtrate protein 10 (CFP-10, a pool of 15-mer peptides overlapping by 10 amino acids, Peptide Protein Research, UK; final concentration 5 μg/ml/peptide). All stimulatory antigens were placed in separate wells and samples were processed in duplicate. No antigen was added to the negative control well. Anti CD3 mAb CD3-2 (Mabtech) at a final concentration of 100 ng/ml was included as a positive control. During the study, samples from a subset of 22 HIV-infected children were tested with both the ELISpot assay and the T.SPOT.TB (Oxford Immunotec, UK), a commercially available IGRA.
ELISpots were scored by two independent observers using a stereo microscope (Zeiss). Laboratory scientists were blinded to the final categorisation of patients. Study investigators also confirmed spot counts in a blinded manner. The mean IFN-γ response to each of the antigens (ESAT-6 and CFP-10) and positive control was calculated after subtraction of background IFN-γ responses obtained from the negative control wells. IFN-γ responses were expressed as spot forming cells (SFCs)/million (106) PBMC. The ELISpot was considered positive if the number of SFCs in the antigen-stimulated wells was greater than or equal to 5 SFCs/2 x 105 (25 SFCs/106) above the background response, and, in cases where the background response was greater than or equal to 10 SFCs, more than twice the background response. Responses were classified as indeterminate when, (i) the number of SFCs in the antigen-stimulated wells, corrected for background, were less than 5 (25 SFCs/106) in the presence of a failed positive control response, (ii) the number of SFCs in the antigen-stimulated wells was less than twice the background response (when the background response was greater than or equal to 10 SFCs (50 SFCs/106)), (iii) there was an insufficient number of PBMC to undertake the assay (technical). The overall ELISpot result was considered positive if the response to ESAT-6 and/or CFP-10 was positive.
Children were classified as definite, probable, possible or not TB using the clinical and microbiological criteria detailed in Table 1. TST was not included as part of the diagnostic algorithm in assigning children to clinical categories.
Table 1. Clinical and microbiological criteria used for assigning children to diagnostic groups.
| Diagnostic classification |
Criteria |
|---|---|
| Definite TB (n=22) |
Isolation of Mycobacterium tuberculosis in culture or detection of acid-fast bacilli on microscopy of appropriate site-specific specimen. |
| Probable TB (n=24) |
Symptoms and signs suggestive of TB** AND two or more of the following: • TB contact • chest radiograph findings consistent with TB* • response to TB treatment† |
| Probable TB (n=14) |
Symptoms suggestive of TB AND only one of the following • TB contact • chest radiograph findings consistent with TB* • No alternative definitive diagnosis established |
| Not TB, HIV positive (n=79) |
Confirmed HIV infection AND no symptoms or signs of TB (or symptoms potentially consistent with TB but full resolution on clinical follow-up) AND/OR alternative definitive diagnosis established |
| Not TB, HIV negative (n=49) |
As above but confirmed HIV uninfected |
A chest radiograph with hilar lymphadenopathy, right upper lobe infiltrates, pleural effusion or a miliary pattern
Resolution of clinical symptoms, radiological improvement and weight gain
Symptoms suggestive of TB included; cough for longer than two weeks, persistent fever, night sweats, weight loss and fatigue.
Data analysis
Data were analysed using Prism Graphpad. The Mann-Whitney U test was used to compare nonparametric unpaired data. Fisher’s exact or Chi-squared test was used to compare proportions when test results were analysed as dichotomous outcomes. McNemar’s paired test was used to compare the results of TST and ELISpot. Correlations were assessed using the Spearman’s correlation coefficient. Weight-for-age Z-scores (WAZ) were calculated using Epi-info 2000, version 1.0 (Division of Surveillance and Epidemiology, CDC, Atlanta, Georgia)
Results
A total of 188 children were enrolled between April 2006 and May 2007, of which 139 (74%) were HIV infected (Figure 1). Demographic details are shown in Table 2. The median (IQR) age of patients included in the study was 20 (10.0-54.1) months. Phlebotomy failed in two (1%) children who were subsequently excluded from analysis. Of 104 HIV-infected children presenting with suspected TB, 22 (21%) had definite TB as defined by study criteria. There was no significant difference in age, presence of BCG scar, or absolute CD4 count between HIV-infected children in each diagnostic group (Table 2). HIV-infected children with possible TB had a lower CD4% compared to HIV-infected children with definite TB (14.0% vs. 24.2%, p=0.050). HIV-infected children with definite or probable TB had significantly lower weight-for-age Z-scores than HIV-infected or HIV-uninfected children without TB.
Table 2. Demographic details for patients included in the study.
| All patients (n=188) |
TB |
Not TB |
||||
|---|---|---|---|---|---|---|
| Definite (n=22) |
Probable (n=24) |
Possible (n=14) |
HIV positive (n=79) |
HIV negative (n=49) |
||
| Median age in months (IQR) | 20 (10.0 – 54.1) | 34.7 (14.6 – 75.6) | 17.9 (11.5 – 46.9) | 14.3 (7.1 – 34.9) | 18.0 (9.0 – 45.9) | 24.2 (10.9 – 63.8) |
| Male n(%) | 100 (53) | 14 (64) | 16 (67) | 6 (43%) | 36 (46) | 28 (57.1) |
| Median weight for age score (IQR) | −1.7 (−2.7 – −0.88) | −2.3 (−3.3 – −1.5) | −2.3 (−3.2 – −1.3) c | −2.4 (−2.9 – −1.3) | −1.3 (−2.5 – −0.5) | −0.4 (−1.4 – 0.25)d |
| WHO HIV stage 4 n (%) | 8 (36) | 8 (33) | 4 (28) | 20 (25) | NA | |
| BCG scar present n (%) | 144 (76) | 16 (73) | 17 (71) | 10 (71) | 64 (81) | 37 (76) |
| Median CD4 (IQR) % | 19.9 (14.6 – 29.5) | 24.2 (14.1 – 29.9) | 19.7 (10.7 – 23.5) | 14.8 (8.5 – 20.3) | 22.3 (16.0 – 31.1) | NA |
| Median absolute CD4 count (IQR) cells/ml | 788 (450 – 1258) | 564 (227 – 1312) | 933 (430 – 1417) | 695 (327 – 1232) | 868 (478 – 1279) | NA |
| On anti-retrovirals at diagnosis n (%) | 4 (19)a | 6 (25)b | 5 (36) | 40 (51) | NA | |
| Median TST induration (range) mm | 0 (0 - 27) | 0(0 - 32) | 0 (0) | 0 (0 - 22) | 0 (0 - 22) | |
p =0.007 for difference between HIV positive children with definite TB and HIV positive children without TB
p =0.03 for difference between HIV positive children with probable TB and HIV positive children without TB
p =0.0007 for difference between HIV positive children with definite/probable TB and HIV positive children without TB
p <0.0001 for difference between HIV positive children and HIV negative children without TB
The median (inter quartile range (IQR)) IFN-γ response to ESAT-6 and CFP-10 were higher in children with definite TB compared to children without TB (ESAT-6 30 (2.5–162.5) vs. 5 (0–15) SFCs/106, p=0.002; CFP-10 45 (12.5–407.5) vs. 5 (0-20) SFCs/106, p <0.0001). Similarly, median (IQR) IFN-γ responses to ESAT-6 and CFP-10 were higher in children with definite or probable TB compared to children without TB (ESAT-6 30 (5–110) vs. 5 (0–15) SFCs/106, p<0.0001; CFP-10 45 (10–190) vs. 5 (0–20) SFCs/106, p <0.0001). Furthermore, children with definite or probable TB had higher median IFN-γ responses compared to children with possible TB (ESAT-6 30 (5–110) vs. 5 (0.0–37.5), p =0.03; CFP-10 45 (10–190) vs. 2.5 (0.0–37.5) SFCs/106, p =0.006).
The response rates to the individual antigens alone and in combination are shown in Table 3. Of the 22 children with definite TB, 21 (95%) had an interpretable ELISpot result. Of these 21 children, 14 (66%) had a positive response. Of the 22 children with definite TB, 12 (55%) were sputum smear negative and 8 (67%) of these had a positive ELISpot. ELISpot was positive in 6 (67%) of 9 sputum smear positive children and was indeterminate in one child with sputum smear positive disease. Of 19 children with definite TB who had a TST undertaken, 15 (79%) returned for their TST reading. Of these 15 children, 5 (33%) had induration greater than or equal to 5 mm.
Table 3. Number of children (%) positive to TB antigens by diagnostic group.
| Response rates | Definite TB | Probable TB | Possible TB | Not TB | |
|---|---|---|---|---|---|
| HIV+ (n=22)a | HIV+ (n=24)c | HIV+ (n=14) | HIV+ (n=79)f | HIV− (n=49)h | |
| ESAT-6 | 10 (48) | 9 (50) | 4 (28) | 14 (19) | 3 (7) |
| CFP-10 | 13 (62) | 9 (50) | 3 (21) | 18 (24) | 7 (16) |
| ESAT-6/CFP-10 | 14 (66) | 11(61) | 4 (28) | 20 (27) | 7 (16) |
| TST | 5/15 (33)b | 5 /19(26)d | 0/9 (0)e | 7 (15)g | 1 (10) |
One assay deemed indeterminate (high negative control)
4 children failed to return for TST reading. 3 children did not have TST.
6 assays deemed indeterminate (6 high negative control)
5 children failed to return for TST reading
3 children failed to return for TST reading
5 assays deemed indeterminate (3 high negative control, 1 positive control, 1 technical)
7 children failed to return for TST reading; 24 children did not have TST
5 assays deemed indeterminate (3 high negative control; 2 low cell count)
Combining the results of TST and ELISpot resulted in a marginal increase in sensitivity over the use of ELISpot alone. Of the 22 children with definite TB, 16 (73%) had a positive result in at least one of the two tests. Similarly, of the 46 HIV-infected children with definite or probable TB, 27 (69%) had a positive result in at least of one of the two tests. Overall, a significantly higher number of children with definite or probable TB had a positive ELISpot compared to a positive TST (ELISpot 25/39 (64%) vs. 10/34 (29%) TST positive, p=0.005). Compared to children without TB, children with definite or probable TB were more likely to have a positive ELISpot than a positive TST (ELISPOT: OR 6.0 (95% CI 2.7-13.1, p<0.0001 vs. TST: OR 2.6 (95% CI 0.9-7.2, p=0.12). In HIV-infected children with definite or probable TB, four children were TST positive/ELISpot negative or indeterminate and 11 were ELISpot positive/TST negative.
Of the 128 children without TB, 118 (93%) had an interpretable ELISpot result and 27 (23%) were positive. The median (IQR) IFN-γ responses to ESAT-6 and CFP-10 in children with a positive ELISpot in the not TB group were significantly lower compared to the responses in children with definite or probable TB and a positive ELISpot (ESAT-6 45 (32–65) SFCs/106 vs. 110 (70–280), p=0.003; CFP-10 40 (27–52) SFCs/106 vs. 177 (48–508), p=0.0005). There was poor agreement between the TST and ELISpot in HIV-infected children without TB, (κ = 0.12 (95% CI -0.1– 0.3). A higher proportion of these children had a positive ELISpot than a positive TST (McNemar paired analysis, p=0.06).
We investigated the effect of age, nutritional status and degree of immunosuppression on test performance by comparing the results of TST and ELISpot in children less than or greater than 24 months, in children with CD4 percent greater than or less than 15 and in children with moderate to severe malnutrition.
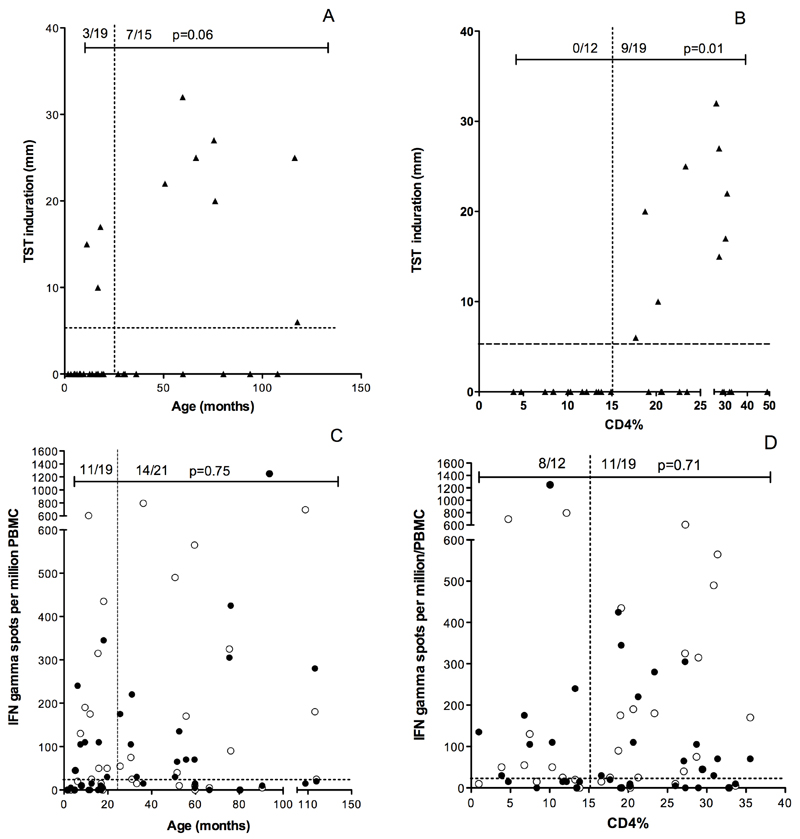
In those with definite or probable TB, children less than 24 months of age were less likely to have a positive TST compared to children 24 months of age or older, (3/19 (16%) vs. 7/15 (47%), p =0.06) (Figure 2, Panel A). The median (IQR) age of children with definite or probable TB and a positive TST was significantly higher than those with a negative TST (63.1 (17.8–86.1) vs. 16.3 (7.6–30.3) months, p=0.01). The magnitude of TST induration correlated with age (Spearman correlation 0.46, p=0.006). In contrast, age did not affect the proportion of children with definite or probable TB having a positive ELISPOT (11/19 (58%) children <24 months vs. 14/21 (67%) children ≥24 months p =0.75) (Figure 2 Panel C).
Figure 2:
TST was frequently negative in HIV-infected children with definite/probable TB aged less than 24 months or with advanced immunosuppression (CD4% less than 15%) (Panel A and B). In contrast ELISpot responses to ESAT-6 (●) or CFP-10 (○) were positive in a similar proportion of HIV-infected children with definite/probable TB aged less than 24 months of age or with a CD4% less than 15% (Panel C and D).
None of 12 (0%) children with a CD4 percent less than 15% had a positive TST compared to 9 of 19 (47%) children with a CD4 percent greater than 15%, p =0.005 (Figure 2, Panel B). However, there was no difference in the proportion of children with a positive ELISpot in those with CD4 percent less than or greater than 15% (8/12 (67%) vs. 11/19 (58%), p = 0.71) (Figure 2 Panel D).
WHO guidelines classify HIV associated immunodeficiency as mild, advanced or severe based on age-related CD4% values. Therefore, we investigated the influence of the degree of immunosuppression on the results of TST and ELISpot in HIV-infected children with definite or probable TB. Of 36 HIV-infected children with definite or probable TB with interpretable results of TST and ELISpot, 15 (42%) were classified as severely immunodeficient. Of these 15 children, none had a positive TST. In contrast, 10 (67%) of these 15 children had a positive ELISpot (p=0.0002).
Only four (9%) HIV-infected children with definite or probable TB with moderate to severe malnutrition (WAZ ≤ – 2) had a positive TST. In contrast, 13 (68%) HIV-infected children with definite and probable TB with moderate to severe malnutrition had a positive ELISpot (TST 4/21(19%) vs. 13/19 (68%) p=0.003).
For the whole cohort, the sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) of ELISpot for the diagnosis TB in children with definite or probable TB was 64% (95% CI 47-79), 77% (95% CI 68-84),48% (95% CI 34-62) and 87% (95% CI 78-92) respectively. For TST the corresponding values in children with definite or probable TB were 29% (95% CI 15-47), 86% (95% CI 75-93), PPV, 55% (95% CI 31-78) and NPV 67% (95% CI 55-78) respectively. In HIV-infected children with definite TB, the sensitivity, specificity, PPV and NPV of ELISpot was 67% (95% CI 43-85), 77% (68-84), 34% (95% CI 21-50) and 93 (95% CI 86-97) respectively. For the TST, the corresponding values in HIV-infected children with definite TB were 33% (95% CI 11-62), 86% (95% CI 74-93), 38% (95% CI 13-68) and 83% (95% CI 71-91) respectively.
The inter-observer agreement in assigning results for the ELISpot assays was high (96%). There was a strong correlation between individual spot counts recorded by both observers (Spearman correlation 0.98, p<0.0001). In the 22 patients who had both ELISpot and T-SPOT.TB performed, IFN-γ responses to ESAT-6 and CFP-10 were significantly correlated between the two assays (ESAT-6: Spearman correlation 0.71, p=0.0002; CFP-10: Spearman correlation 0.72, p=0.0002). Of 188 baseline ELISpot assays, 17 (9.0%) yielded results that were considered indeterminate by study criteria (2 inadequate PBMC, 1 technical, 1 failed positive control, 13 high negative control). Therefore, overall, 171 (91%) ELISpot assays yielded interpretable results. There was no significant difference in the proportion of indeterminate ELISpot results between HIV-infected and HIV uninfected children (12/139 (8.6%) vs. 5/49 (10.2%), p=0.78).
Discussion
The potential for immunodiagnosis of TB has improved with the discovery of the TB specific antigens [22]. IGRA are increasingly being proposed as replacement tests for the TST [23–25]. The majority of studies have evaluated IGRA for the detection of TB in HIV uninfected people and there are several recent reports of their use in HIV-infected adults [26–36]. There are limited data from children and only one study reporting their use in a small number of HIV-infected children [18–20]. This is the first study to describe the effect of immunosuppression on the performance of IGRA in children.
Our study confirms the poor sensitivity of TST in HIV-infected children with TB [13] and shows that sensitivity of ELISpot is significantly higher than TST in children with definite or probable TB including those with malnutrition. These data are consistent with the findings of the previous study in South African children [20]. In addition, we found that compared to TST, ELISpot responses were well maintained in young children with severe immunosuppression. These data are of practical relevance because the diagnosis of TB in children is challenging and important in those at greatest risk of disease: young, HIV-infected children with advanced immunosuppression and malnutrition [11].
The sensitivity of ELISpot in HIV-infected children in this study is inferior to that reported in HIV-infected adults. Chapman et al reported a sensitivity of 90% for ELISpot for the diagnosis of active TB in HIV-infected Zambian patients [27]. In South African, Rangaka et al compared T-SPOT.TB and QuantiFERON-TB Gold for the detection of latent TB infection in both HIV-infected and HIV uninfected adults and found that proportion scored positive by either IGRA was fairly well maintained with advanced immunosuppression [1]. Concerns, however, have been raised about potential false negative IGRA responses in this setting. For example, Brock et al reported a higher rate of indeterminate assay results in individuals with CD4 counts less than 100 cells/mm3 in a low incidence setting [26]. Similarly Jones et al found indeterminate results over-represented in HIV infected individuals with a CD4 count less than 200 cells/mm3 screened for latent TB infection [32]. In contrast, the majority of indeterminate assay results in our study resulted from a high background nil control response in HIV-infected children, a finding that has been reported in other cohorts of HIV-uninfected African children [18, 37].
The sensitivity of ELISpot in our study was consistent with the 73% sensitivity reported by Liebeschuetz et al in a smaller group of 30 HIV infected children (median age 50 months) with confirmed or highly probable TB [20]. A case report detailed the use of an ELISpot for the early detection of TB in an 11-year old HIV-infected child with advanced immunosuppression (CD4 2%) in whom the TST was negative [38]. The detection of an immune response to TB prompted the early introduction of anti-tuberculous treatment prior to culture confirmation and illustrates the potential benefit of IGRA to influence management decisions [39].
A sensitivity of 64% and negative predictive value of 87% is an improvement over TST but indicates that ELISpot cannot be used to definitively exclude a diagnosis of TB in HIV-infected children. The potential for ELISpot, however, to provide an early indication of TB is important in clinical practice and may allow for earlier initiation of anti-tuberculous treatment in this highly vulnerable cohort. In our study, over two-thirds of children with smear negative TB had a positive ELISpot.
Currently available IGRA are unable to discriminate between active TB disease and latent TB infection (LTBI). A relationship between bacillary load and IFN-γ responses may exist, with active TB disease associated with higher IFN-γ responses compared to latent TB infection [40, 41]. An unanticipated finding of our study was that the magnitude of IFN-γ responses to ESAT-6 and CFP-10 was significantly higher in HIV-infected children with definite or probable TB compared to IFN-γ responses in HIV-infected children without TB who had a positive ELISpot. Our data therefore are consistent with the possibility that the magnitude of the IGRA response may help to differentiate active TB disease from latent TB infection in children, as has been suggested in adults [28, 36].
There were several limitations to our study. Firstly, the ELISpot assay used in the study was not the commercially available T-SPOT.TB assay. However, the assay, incorporating pools of peptides of ESAT-6 and CFP-10 as stimulatory antigens, is similar to the commercial T.SPOT.TB assay. In addition, there was a significant correlation in the magnitude of IFN-γ responses to ESAT-6 and CFP-10 between ELISpot and T.SPOT.TB assays. An in house version of the ELISpot assay has been used in many previous published comparison studies [21, 42–46]. A further limitation of our study was that not every child deemed uninfected had a thorough work-up to exclude TB. Follow up of this cohort was for a relatively short period of time and therefore we cannot be certain that some of these children did not develop TB.
In conclusion, we found the ELISpot assay to be superior to TST in the evaluation of HIV-infected children with suspected TB. Performance of ELISpot was relatively unaffected by young age or HIV-associated immunosuppression. However the sensitivity of current ELISpot assays is not sufficiently high to be used as rule out test for TB in children.
Acknowledgements
We thank the parents and children who participated in this study. We thank Marco Zampoli, Patti Apolles, James Nuttall and Wendy Dick for assistance in patient recruitment, as well as Karin McCabe and Glynnis Kossew for laboratory assistance. We also thank Ashleigh Mills for assistance in patient recruitment and data entry. We would like to thank Steve Graham for helpful comments on the manuscript.
Conceived and designed the study: MAD, TGC, RJW, KAW, NC, HZ, BE and DB and MPN. Performed the experiments: KW and SP. Contributed reagents/materials: RJW, KAW, MPN, MAD, DB. Data analysis: MAD,TGC, NC and MPN. Wrote the manuscript: TGC, MAD, NC and MPN. Enrolled patients: MAD, TGC, and CJ.
Funding Sources
The study was funded by a BMS Secure the Future grant and by the South African Medical Research Council. MAD received support from the International Epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) collaboration funded by the National Institutes for Health (NIH; U01 AI069924-01). TGC is supported by Fellowship awards from the European Society of Paediatric Infectious Diseases, the Faculty of Medical and Dental Health Sciences, University of Melbourne and the Nossal Institute of Global Health, University of Melbourne. RJW and MPN received funding from the Wellcome Trust (072065 and 072070) and RJW is supported by the European Union (EuropeAid/121404/C/G/Multi).
Footnotes
Conflict of interest
None
References
- 1.Rangaka MX, Wilkinson KA, Seldon R, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 2.Donald PR. Childhood Tuberculosis: out of control? Current Opinion in Pulmonary Medicine. 2002;8:178–182. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–63. [PubMed] [Google Scholar]
- 4.Zar HJ. Childhood tuberculosis: new recognition of an old disease. Paediatr Respir Rev. 2007;8:97–8. doi: 10.1016/j.prrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636–47. [PubMed] [Google Scholar]
- 6.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child. 1999;80:433–7. doi: 10.1136/adc.80.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360:985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 8.Jeena PM, Pillay P, Pillay T, Coovadia HM. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int J Tuberc Lung Dis. 2002;6:672–8. [PubMed] [Google Scholar]
- 9.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–37. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 10.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–14. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 11.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis. 2007;196(Suppl 1):S76–85. doi: 10.1086/518659. [DOI] [PubMed] [Google Scholar]
- 12.Anastos K, Kalish LA, Palacio H, et al. Prevalence of and risk factors for tuberculin positivity and skin test anergy in HIV-1-infected and uninfected at-risk women. Women's Interagency HIV Study (WIHS) J Acquir Immune Defic Syndr. 1999;21:141–7. [PubMed] [Google Scholar]
- 13.Madhi SA, Gray GE, Huebner RE, Sherman G, McKinnon D, Pettifor JM. Correlation between CD4+ lymphocyte counts, concurrent antigen skin test and tuberculin skin test reactivity in human immunodeficiency virus type 1-infected and -uninfected children with tuberculosis. Pediatr Infect Dis J. 1999;18:800–5. doi: 10.1097/00006454-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Schaaf HS, Marais BJ, Whitelaw A, et al. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis. 2007;7:140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connell TG, Rangaka MX, Curtis N, Wilkinson RJ. QuantiFERON-TB Gold: state of the art for the diagnosis of tuberculosis infection? Expert Rev Mol Diagn. 2006;6:663–677. doi: 10.1586/14737159.6.5.663. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani A, Millington KA. T cell-based diagnosis of childhood tuberculosis infection. Curr Opin Infect Dis. 2007;20:264–71. doi: 10.1097/QCO.0b013e32813e3fd8. [DOI] [PubMed] [Google Scholar]
- 17.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61:616–20. doi: 10.1136/thx.2005.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS ONE. 2008;3:e2624. doi: 10.1371/journal.pone.0002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 21.Nicol MP, Pienaar D, Wood K, et al. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis. 2005;40:1301–8. doi: 10.1086/429245. [DOI] [PubMed] [Google Scholar]
- 22.Andersen P, Munk ME, Pollack JM, Doherty MT. Specific immune-based diagnosis of tuberculosis. The Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 23.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898–906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 24.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. [PubMed] [Google Scholar]
- 25.Shingadia D, Novelli V. The tuberculin skin test: a hundred, not out? Arch Dis Child. 2008;93:189–90. doi: 10.1136/adc.2007.129585. [DOI] [PubMed] [Google Scholar]
- 26.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent Tuberculosis in HIV positive, diagnosed by the M. Tuberculosis Specific Interferon Gamma test. Respir Res. 2006;7:56. doi: 10.1186/1465-9921-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. Aids. 2002;16:2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 28.Clark SA, Martin SL, Pozniak A, et al. Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007;150:238–44. doi: 10.1111/j.1365-2249.2007.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goletti D, Carrara S, Mayanja-Kizza H, et al. Response to M. tuberculosis selected RD1 peptides in Ugandan HIV-infected patients with smear positive pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2008;8:11. doi: 10.1186/1471-2334-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond AS, McConkey SJ, Hill PC, et al. Mycobacterial T Cell Responses in HIV-Infected Patients with Advanced Immunosuppression. J Infect Dis. 2008;197:295–9. doi: 10.1086/524685. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann M, Reichmuth M, Fantelli K, et al. Conventional tuberculin skin testing versus T-cell-based assays in the diagnosis of latent tuberculosis infection in HIV-positive patients. Aids. 2007;21:390–2. doi: 10.1097/QAD.0b013e328012164b. [DOI] [PubMed] [Google Scholar]
- 32.Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold in-tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lung Dis. 2007;11:1190–5. [PubMed] [Google Scholar]
- 33.Karam F, Mbow F, Fletcher H, et al. Sensitivity of IFN-gamma Release Assay to Detect Latent Tuberculosis Infection Is Retained in HIV-Infected Patients but Dependent on HIV/AIDS Progression. PLoS ONE. 2008;3:e1441. doi: 10.1371/journal.pone.0001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn SD, Bangani N, Vogt M, et al. Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa. BMC Infect Dis. 2007;7:99. doi: 10.1186/1471-2334-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med. 2007;175:737–42. doi: 10.1164/rccm.200608-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangaka MX, Diwakar L, Seldon R, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis. 2007;44:1639–46. doi: 10.1086/518234. [DOI] [PubMed] [Google Scholar]
- 37.Nakaoka H, Lawson L, Bertel Squire S, et al. Risk for Tuberculosis among children. Emerg Infect Dis. 2006;12:1383–1388. doi: 10.3201/eid1209.051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spyridis N, Chakraborty R, Sharland M, Heath PT. Early diagnosis of tuberculosis using an INF-gamma assay in a child with HIV-1 infection and a very low CD4 count. Scand J Infect Dis. 2007:1–2. doi: 10.1080/00365540701481537. [DOI] [PubMed] [Google Scholar]
- 39.Gooding S, Chowdhury O, Hinks T, et al. Impact of a T cell-based blood test for tuberculosis infection on clinical decision-making in routine practice. J Infect. 2007;54:e169–74. doi: 10.1016/j.jinf.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higuchi K, Harada N, Fukazawa K, Mori T. Relationship between whole-blood interferon-gamma responses and the risk of active tuberculosis. Tuberculosis (Edinb) 2008 doi: 10.1016/j.tube.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur Respir J. 2007;30:722–8. doi: 10.1183/09031936.00028507. [DOI] [PubMed] [Google Scholar]
- 42.Mantegani P, Piana F, Codecasa L, et al. Comparison of an in-house and a commercial RD1-based ELISPOT-IFN-gamma assay for the diagnosis of Mycobacterium tuberculosis infection. Clin Med Res. 2006;4:266–72. doi: 10.3121/cmr.4.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 44.Fox A, Jeffries DJ, Hill PC, et al. ESAT-6 and CFP-10 can be combined to reduce the cost of testing for Mycobacterium tuberculosis infection, but CFP-10 responses associate with active disease. Trans R Soc Trop Med Hyg. 2007 doi: 10.1016/j.trstmh.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Hill PC, Brookes RH, Adetifa IMO, et al. Comparison of Enzyme-Linked Immunospot Assay and Tuberculin Skin Test in Healthy Children Exposed to Myocbacterium tuberculosis. Pediatrics. 2006;117:1542–1548. doi: 10.1542/peds.2005-2095. [DOI] [PubMed] [Google Scholar]
- 46.Richeldi L, Ewer K, Losi M, et al. T-cell-based diagnosis of neonatal multidrug-resistant latent tuberculosis infection. Pediatrics. 2007;119:e1–5. doi: 10.1542/peds.2006-1057. [DOI] [PubMed] [Google Scholar]