Abstract
Introduction
TNF superfamily members, including TNF-related weak inducer of apoptosis (TWEAK) and Glucocorticoid-Induced TNFR-Related Protein Ligand (GITRL) have been described as serum based biomarkers for inflammatory and immune mediated diseases. However, up to now the role of TWEAK and GITRL has not been analyzed in critical illness and sepsis.
Methods
GITRL and TWEAK serum concentrations were measured in 121 critically ill patients (84 fulfilled with septic disease), in comparison to 50 healthy controls. Results were correlated with clinical data.
Results
Serum levels of TWEAK and GITRL were strongly decreased in critically ill patients compared with healthy controls. Concentrations of TWEAK (but not GITRL) were further decreased in patients with sepsis and correlated with routinely used markers of inflammation and bacterial infection such as C-reactive protein, procalcitonin and Interleukin-6. Notably, we failed to detect a correlation to other TNFR ligands such as TNF or APRIL. Finally, TWEAK levels of the upper quartile of the cohort were prognostic for mortality during ICU treatment.
Conclusion
TWEAK and GITRL levels were lower in intensive care unit medical patients. Levels of TWEAK were further decreased in septic patients, and alterations in TWEAK concentrations were linked to an unfavorable outcome. Together with recently published results on other TNFR ligands, these data indicate specific functions of the different TNFR ligands in septic diseases.
Introduction
Sepsis and septic shock syndrome are characterized by a highly regulated release of a broad variety of cytokines and chemokines. The tumour necrosis factor (TNF) alpha was among the first soluble proteins factors described in the context of septic disease. Recently other members of the TNF superfamily were shown to fulfil a similar role than TNF in the pathophysiology of infectious or inflammatory diseases [1]. A proliferation-Inducing Ligand (APRIL) has been implicated in the pathogenesis of critical illness and sepsis [2]. APRIL serum concentrations are elevated in critically ill patients [3], and elevated APRIL levels were suggested to be diagnostic for septic disease and were even demonstrated to be predictive for patients survival in the context of critical illness [4]. Moreover, APRIL concentrations were correlated to tumour necrosis factor concentrations in critically ill patients and predicted patients’ outcome with even higher accuracy than TNF. However, the pathophysiological implications of these findings and especially the functional role of TNF superfamily ligands in critical illness and sepsis are not clearly understood.
Similar to APRIL, the Tumor Necrosis Factor Weak Inducer of Apoptosis (TWEAK) is a member of the TNF/TNFR superfamily. TWEAK was first described to induce apoptosis in a human adenocarcinoma cell line [5]. Displaying a much wider tissue distribution than TNF, it has previously gained attention as a pivotal regulator of inflammation and cell death in many cells and pathological conditions [6–9]. TWEAK exists as a membrane-bound form and as a soluble variant, formed after proteolytical cleavage by endoproteinases. Both TWEAK-variants exert their biological activity after binding to the Fibroblast growth factor-inducible 14, representing their bona fide receptor. Alterations of TWEAK serum concentrations have recently be described in the context of various inflammatory and cardiovascular diseases [10–12].
Another TNF/TNFR superfamily member, Glucocorticoid-Induced TNFR-Related Protein Ligand (GITRL), was identified simultaneously with its receptor in 1997 [13]. In humans, GITRL expression was described in a broad panel of immune cells such as antigen-presenting cells, monocytes and B cells as well as in endothelial cells [14, 15]. In response to various inflammatory stimuli, these cell-types were shown to rapidly up-regulate GITRL mRNA. In line, several studies provided evidence for a role of the GITR/GITRL system in regulating inflammation. As such, a pro-inflammatory role of GITRL and its receptor was demonstrated in a mouse model of acute lung and colon injury. Moreover, the GITR/GITRL system was shown to play a regulatory role in leukocyte extravasation [13–16].
Despite the emerging roles of TWEAK and GITRL in inflammation and immunity, no data on a function of these ligands are involved in severe systemic infection in humans are available. To analyse the regulation of TWEAK and GITRL in critically ill patients and to evaluate their potential diagnostic and prognostic potential, TWEAK and GITRL serum levels were measured in 121 critically ill patients at the time point of admission to a intensive care unit.
Materials and Methods
Study design and patient characteristics
TWEAK and GITRL serum levels were analyzed in a recently published cohort of 121 critically ill patients (71 male, 50 female; median age 66 years, range 18 to 90 years; Table 1) (nur das Osteopontin). Patients’ information and samples were acquired prospectively. Follow-up was performed as recently described [17]. Diagnosis of sepsis, severe sepsis and septic shock was performed according to the criteria proposed by the ACCP/SCCM Consensus Conference Committee. Patients that did not fulfill these criteria were categorized as non-sepsis patients [18, 19]. As a control population, we analyzed 50 healthy blood donors (35 male, 15 female, median age 37 years, and range between 18 and 67) free of chronic diseases like diabetes, cardiac and pulmonary diseases and with normal values for blood counts, C-reactive protein and liver enzymes. Patients´ blood was taken ad admission to the ICU and in some cases after 3 days of treatment. Samples were handled on ice, sera were frozen at -80°C. Routine laboratory and experimental parameters were measured as described previously [18, 20–22].
Table 1. Baseline patient characteristics.
| Parameter | all patients | non-sepsis | sepsis |
|---|---|---|---|
| Number | 121 | 37 | 84 |
| Sex (male/female) | 71 / 50 | 23 / 14 | 48 / 36 |
| Age median (range) [years] | 66 (18–90) | 59 (18–84) | 68 (20–90) |
| APACHE-II score median(range) | 19 (3–40) | 17 (4–25) | 19 (3–40) |
| SAPS2 score median(range) | 43 (9–80) | 42 (13–80) | 43.5 (9–65) |
| ICU days median (range) | 9 (1–137) | 7 (1–41) | 12 (1–137) |
| Death during ICU n(%) | 24.0% | 21.6% | 25.0% |
| Death during ICU orfollow-up n(%) | 47.0% | 45.7% | 47.5% |
| Mechanical ventilationn(%) | 88 (72.7%) | 24 (64.9%) | 64 (76.2%) |
| Ventilation time median(range) [h] | 120.5 (0.0–2966.0) | 49,5 (0.0–986.0) | 146,7 (0.0–2966.0) |
| pre-existing diabetes n(%) | 32.8% | 28.6% | 34.6% |
| WBC median (range) [x10³/μl] | 12.7 (0.1–208) | 12.0 (1.8–29.6) | 13.0 (0.1–208) |
| CRP median (range)[mg/dl] | 112 (<5–230) | 17 (5–230) | 181 (<5–230) |
| Procalcitonin median(range) [μg/l] | 1.0 (0.0–125.2) | 0.1 (0.1–36.5) | 1.9 (0–125.2) |
| Interleukin-6 median(range) [pg/ml] | 75 (0–6100) | 29 (7.6–260) | 94 (0–6100) |
APACHE, Acute Physiology and Chronic Health Evaluation; CRP, C-reactive protein; ICU, intensive care unit; SAPS, simplified acute physiology score; WBC, white blood cell count
The study protocol was approved by the local ethics committee and conducted in accordance with the ethical standards laid down in the Declaration of Helsinki (ethics committee of the University Hospital Aachen, RWTH-University, Aachen, Germany, reference number EK 150/06). Written informed consent was obtained from the patient, his or her spouse or the appointed legal guardian.
Determination of TWEAK and GITRL serum concentrations by ELISA
We determined TWEAK and GITRL serum concentrations by using a commercially available enzyme immunoassay (ELISA, both: Ray Biotech, Norcross GA, USA).
Statistical analysis
Statistical analysis have been performed as recently described in detail [18, 20–22]. In brief, data are given as median and range to reflect the skewed distribution of analysis on human samples. The Mann-Whitney- U-test and for multiple comparisons the Kruskal-Wallis-ANOVA was used. Box plot graphics display a statistical summary of the median, quartiles, range and extreme values. Correlations analysis were performed by using the Spearman correlation tests. The prognostic value of the variables was tested by univariate and multivariate analysis in the Cox regression model. Kaplan Meier curves were plotted to display the impact on survival. ROC curves were generated by plotting sensitivity against 1-specificity. All statistical analyses were performed with SPSS (SPSS, Chicago, IL, USA) [23, 24].
Results
TWEAK and GITRL serum concentrations in critically ill patients
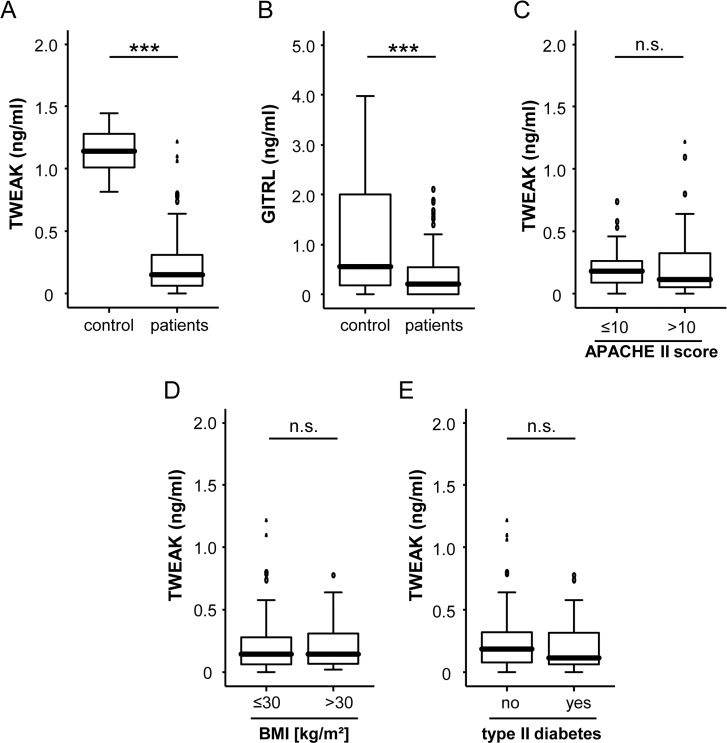
To analyze the significance of these circulating TNF superfamily ligands in critical disease, we measured concentrations of TWEAK and GITRL in sera of 121 critically patients as well as in 50 healthy volunteers by using commercially available ELISA. In contrast to previously published data on TNF and APRIL [2], serum levels of TWEAK were significantly lower in the patients´ group, when compared to the control group (Fig 1A). Similar to TWEAK, the median level of GITRL was lower in critically ill patients than in healthy controls (Fig 1B). However, serum concentrations of GITRL were overall very low and partly close to the detection limit of the assay and we therefore did not pursue it in further analysis as as it could not been excluded that any subsequent regulations may be only artefactual.
Fig 1. Serum TWEAK and GITRL concentrations of critically ill patients at ICU admission.
(a,b) TWEAK and GITRL serum levels at admission to the ICU were significantly (P < 0.001, U-test) lower in critically ill patients (n = 121) compared to healthy controls (n = 50). (c) TWEAK concentrations at admission to the ICU were independent on the severity of disease. (d) Serum TWEAK concentrations were similar in critically ill patients with or without obesity. (e) Serum TWEAK concentrations were similar in critically ill patients with or without diabetes mellitus typ 2. *** p< 0.001
Notably, TWEAK levels were independent of the stage of disease (Fig 1C). Considering the well-known association between TNF superfamily ligands and the metabolic status of patients [25], we next analyzed potential correlations between TWEAK and body mass index (BMI) or the presence of type 2 diabetes. However, TWEAK serum levels were independent on the presence of obesity (Fig 1D) or type 2 diabetes (Fig 1E). Finally, TWEAK serum levels did not differ when patients were analyzed with respect to their age or gender (Table 1 and data not shown).
TWEAK serum levels are decreased in patients with sepsis
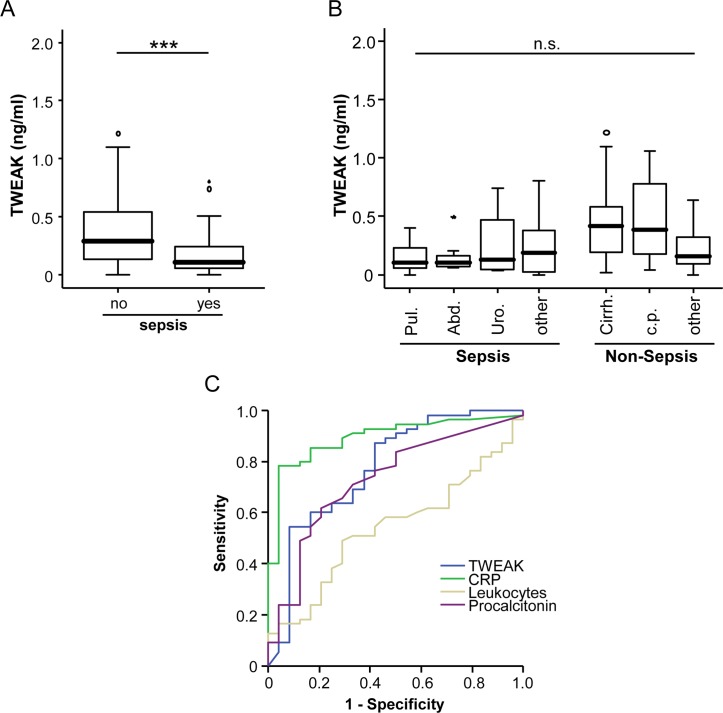
We next analyzed the influence of bacterial infection and sepsis on TWEAK serum levels. TWEAK serum concentrations were decreased in ICU-patients that fulfilled the criteria for septic disease(Fig 2A). We next performed subgroup analysis to understand whether a deregulation of TWEAK might be specific for certain disease etiologies. Among the 84 patients that fulfilled sepsis criteria in our study, 53 were categorized as pulmonary sepsis, 11 abdominal sepsis, 4 urogenital sepsis and 16 as septic disease with a different or unknown infectious focus. Among the non-septic patients, 13 suffered from cardiopulmonary diseases, 10 suffered from decompensated liver cirrhosis and 14 had another diagnosis leading to critical illness (Table 2). Notably, by comparing TWEAK (Fig 2B) concentrations between these distinct patients´ groups we did not find a specific regulation, corroborating that decreased TWEAK is a general feature of sepsis.
Fig 2. Serum TWEAK concentrations are decreased in sepsis.
(a) Critically ill patients with sepsis (n = 84) displayed significantly lower TWEAK serum concentrations (n = 37, U-test) compared to patients without sepsis. (b) TWEAK serum concentrations were not different in patients with different etiologies of critical illness and are highest in patients with cardiac diseases. (c) ROC curve analyses comparing the diagnostic power in predicting sepsis of TWEAK with well-established laboratory markers: C-reactive protein (CRP), procalcitonin, and white blood cell count (leukocytes).
Table 2. Disease etiology of the study population.
| sepsis | non-sepsis | |
|---|---|---|
| n = 84 | n = 37 | |
| Etiology of sepsis critical illness Site of infection n (%) | ||
| Pulmonary | 53 (63.1%) | |
| Abdominal | 11 (13.1%) | |
| Urogenital | 4 (4.8%) | |
| Other | 16 (19.0%) | |
| Etiology of non-sepsis critical illness n (%) | ||
| cardiopulmonary disease | 13 (35.1%) | |
| decompensated liver cirrhosis | 10 (27.0%) | |
| non-sepsis other | 14 (37.8%) |
To further confirm the link between decreased TWEAK concentrations and septic disease we performed correlation analysis revealing that TWEAK concentrations closely correlated to markers of infection in these patients (CRP, PCT and IL-6; Table 3). Comparative ROC-curve analysis on the predictive power for sepsis of TWEAK concentrations and other routine laboratory parameters revealed that TWEAK displayed superior or equal area AUC-statistics compared to leucocyte or PCT, but inferior AUC values compared to CRP (Fig 2C).
Table 3. Correlations of TWEAK serum concentrations at admission day with other laboratory markers.
| ICU patients | ||
|---|---|---|
| TWEAK | ||
| Parameter | r | p |
| Markers of liver function | ||
| Protein | 0.229 | 0.026 |
| Albumin | 0.221 | 0.054 |
| INR | 0.035 | 0.711 |
| AST | 0.264 | 0.006 |
| Bilirubin | 0.075 | 0.505 |
| GLDH | 0.227 | 0.024 |
| Markers of inflammation | ||
| CRP | -0.276 | 0.002 |
| Procalcitonin | -0.205 | 0.048 |
| IL-6 | -0.359 | 0.021 |
| TNF-alpha | -0.056 | 0.732 |
| Markers of renal function | ||
| Creatinine | 0.045 | 0.632 |
| Urea | -0.059 | 0.528 |
| Others variables | ||
| Lactate | 0.210 | 0.028 |
| Resistin | -0.259 | 0.054 |
| LDH | 0.169 | 0.066 |
| Ventilation time | -0.212 | 0.062 |
| Overall survival days | -0.375 | 0.006 |
| ICU days | -0.261 | 0.004 |
| Clinical scoring | ||
| APACHE-II | -0.073 | 0.495 |
r, correlation coefficient; p, p-value; r and p-values by Spearman rank correlation
TWEAK concentrations are associated with ICU survival
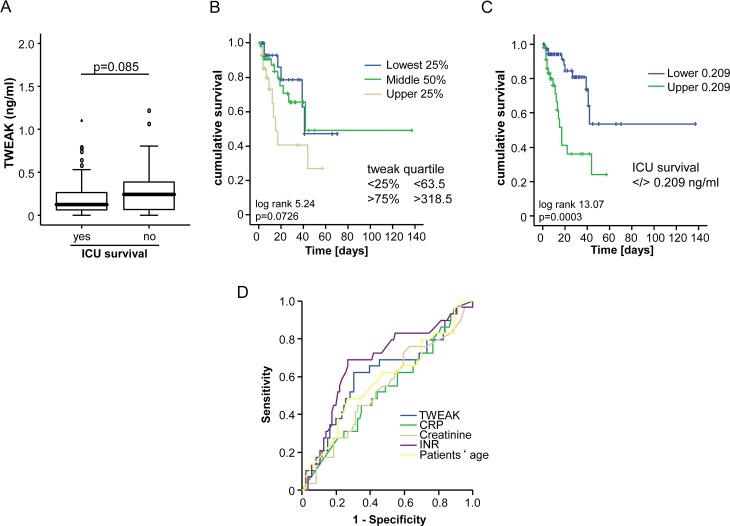
To identify a potential association between TWEAK serum concentrations and patients´ outcome during and after ICU treatment we first compared the concentrations in patients that succumbed to death during ICU treatment and those that survived ICU treatment (Table 1). Notably, serum concentrations of TWEAK were significantly different between these subgroups of patients (Fig 3A). Patients who survived ICU treatment displayed significantly higher TWEAK levels than those patients that succumbed to death (Fig 3A). We next analyzed the prognostic accuracy of TWEAK serum concentrations and therefore performed Cox regression- and Kaplan Meyer curve analyses. These analyses confirmed the unexpected observation that those patients that displayed high TWEAK levels (e.g. TWEAK levels within the upper quartile of the ICU cohort) had a significantly worse prognosis than the others (Fig 3B). Further analyses revealed that the prognostic value of TWEAK was slightly inferior to INR but superior to CRP, creatinine and patients`age according to ROC curve analysis. Moreover, we applied the Youden-index method to define the best cut-off value to discriminate survivors from ICU non-survivors. This analysis revealed that patients with TWEAK levels of lower than 209 pg/ml had a significantly better prognosis compared to the other patients (Fig 3C). We finally applied ROC curve analyses to compare the prognostic value of TWEAK measurements at admission for ICU survival with that of routinely used laboratory parameter (Fig 3D).
Fig 3. Prediction of ICU mortality by TWEAK serum concentrations.
(a) Patients that succumbed to death during ICU treatment displayed higher TWEAK serum levels compared to survivors. (b) Kaplan-Meier survival curves of ICU patients showed that patients with low TWEAK levels an increased short-term survival at the ICU. (c) Kaplan-Meier survival curves of ICU patients revealed that patients with TWEAK levels < 209 ng/ml had an increased ICU survival. (d) ROC curve analyses comparing the prognostic value of TWEAK at admission for ICU survival with the patients´ age and routinely used laboratory parameters.
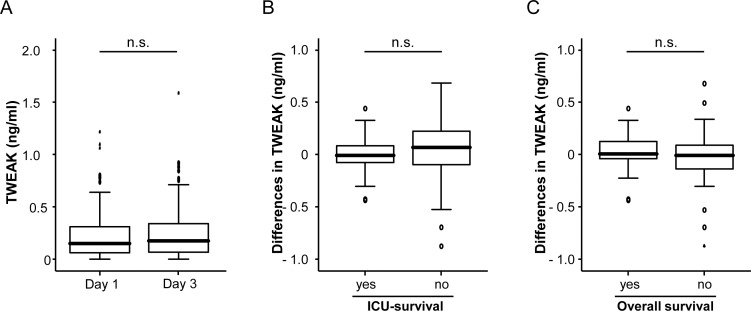
During the follow-up period, 24% of the patients died on the ICU and additional 23% of the patients died after discharge from the ICU. We therefore tested whether TWEAK serum concentrations at admission to ICU could also predict long-term survival. However, patients that died during long-term follow up had a tendency towards higher TWEAK levels than survivors (Fig 4A). Of note, in this setting statistical significance was failed (p = 0.062), which may suggest that patient´s long-term survival is determined by a complex interaction of a network of different factors rather than a single parameter, which is in line to the results of a previous analysis on this patient group [2].
Fig 4. Prediction of long-term survival by analysis of TWEAK serum levels.
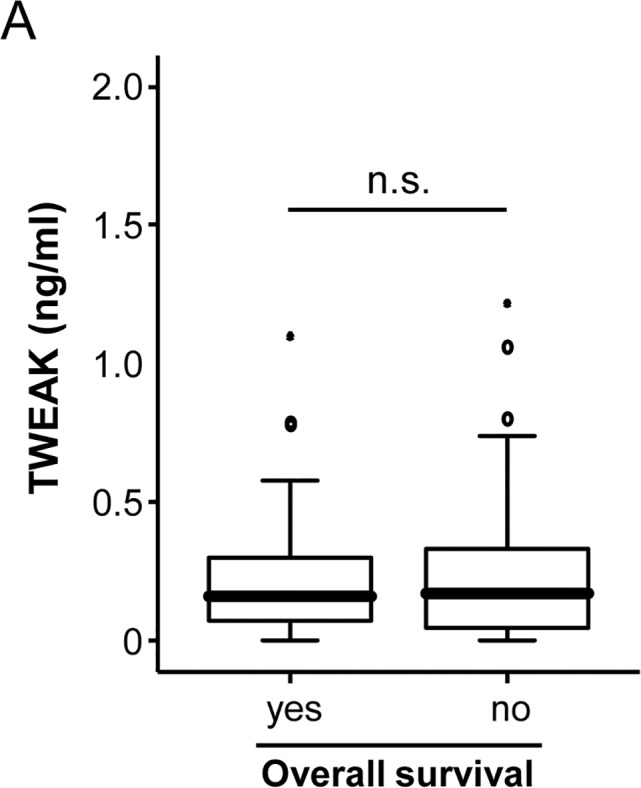
(a) TWEAK levels were unaltered in critically ill patients that survived in the long term follow up compared to patients that succumbed to death (U-test).
Considering the link between ICU survival and TWEAK serum levels, we next examined whether kinetics of TWEAK serum concentrations during the first three days of ICU treatment might indicate patients prognosis. Importantly, longitudinal analysis of TWEAK concentrations revealed no relevant change between the time point of admission and day 3 of ICU-treatment (Fig 5A). Consequently, the differences between admission and day 3 did not reflect patients´ prognosis during or after ICU treatment (Fig 5B and 5C).
Fig 5. Time course of TWEAK concentrations in critically ill patients.
(a) TWEAK serum concentrations are unchanged in critically ill patients at admission to the ICU and after three days of ICU treatment (U-test). (b, c) TWEAK serum concentrations were determined at admission to the ICU and after three days of ICU treatment. The differences in TWEAK levels between these time-points were not predictive for ICU or long-term survival.
Comparison of serum TWEAK, serum APRIL and tumor necrosis factor concentrations
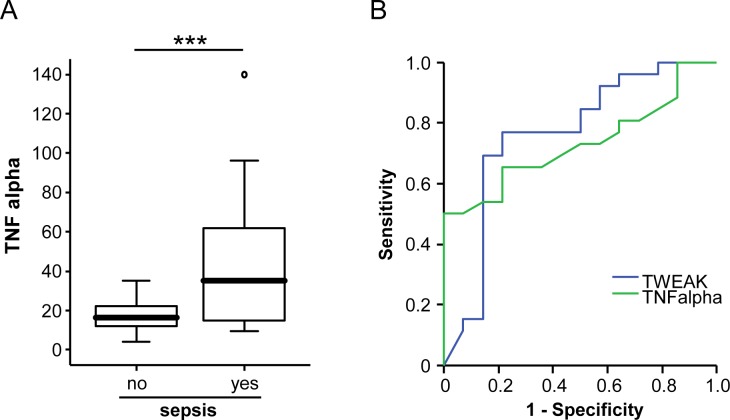
We recently demonstrated a clear correlation of serum levels of TNF and APRIL in critically ill and sepsis patients [2]. We now attempted to compare these markers with TWEAK and GITRL in the more general context of critical illness. These analyses revealed a clear upregulation of TNF (Fig 6A) in sepsis patients. Based on the clear regulations of TNF and TWEAK in sepsis, we next performed correlation analysis between TNF and TWEAK. However, within the 40 patients with available measurements for both markers, we failed to detect a correlation between TNF and TWEAK (Table 3). When comparing the diagnostic accuracy for sepsis, TWEAK displayed a slightly superior AUC value (0.762) compared to TNF (0.731; Fig 6B). Finally, levels of TWEAK and TNF demonstrated a similar accuracy for prediction of patients’ fate during ICU treatment (not shown).
Fig 6. TNF and TWEAK levels for the diagnosis of sepsis.
(a) Patients that fulfilled sepsis criteria had higher TNF levels when compared with critically ill patients without sepsis. (b) ROC analyses comparing the diagnostic power of TWEAK and TNF for sepsis.
Discussion
TNF has been closely associated with the pathogenesis of sepsis [26, 27], and increased levels of TNF were found in the serum of patients with sepsis [3, 4], suggesting that other members of the TNF superfamily might also server as biomarkers in this context. Similar to TNF, we recently demonstrated that elevated APRIL serum levels can be found in patients with sepsis and are associated with a poor prognosis. In the present study we analyzed serum concentrations of circulating TWEAK and GITRL, two other members of the TNF superfamily in critically ill and septic patients. We demonstrate decreased TWEAK serum levels in a large, well characterized cohort of critically ill patients admitted to our ICU. TWEAK levels correlated with markers of inflammation and infection and were further decreased in patients with sepsis. In contrast to our results, Louise Schilder and coworkers recently reported significantly increased TWEAK serum levels in septic patients [28]. We cannot fully explain the apparent discrepancy between the report of Schilder et al. and our study. It may–at least in part- be explained by different group sizes and differences in the experimentsl design (n = 42 in the report of Schilder et al. with n = 18 patients meeting criteria for sepsis vs. n = 121 in our report, of whom 84 fulfilled criteria for sepsis, no healthy control group in the study of Schilder et al.). Moreover, all patients in the report of Schilder et al. received continuous hemofiltration due to acute renal failure, while in our collective of critically ill patients renal failure was only one of several entities that caused critical illness. So maybe further studies on the impact of renal function on serum TWEAK levels are needed to clarify the discrepancies between the work of Schilder et al. and our report.
In contrast to previous results of TNF and APRIL serum levels in this cohort, TWEAK and GITRL serum levels were not elevated. Conversely, TWEAK levels were significantly lower compared with healthy controls. Moreover, we could not demonstrate a clear correlation between TWEAK/GITRL serum levels and disease severity according to the APACHE-II-score. Additionally, while TNF, APRIL and TWEAK serum concentrations reflected the presence or absence of sepsis in critically ill patients we failed to detect a similar correlation for GITRL. These striking differences most likely reflect significant biological differences in their biosynthesis and mechanisms of release into the serum: TNF and TWEAK are both pro-inflammatory cytokines that are able to induce cell death in different tissues. However, while TNF expression and serum release is upregulated during inflammation [29] data from animal experiments suggest that TWEAK is stably expressed in multiple tissues and downregulated on mRNA level in different disease models of sepsis and autoimmune diseases [30]. Although there is an inverse relation between TWEAK mRNA levels and inflammation in these mouse models, the chronological connection between loss of TWEAK and severity of pathological manifestations is not fully understood. In line with these results from mouse models, our patients with critical disease show a decrease in serum TWEAK levels compared to healthy controls. However, in these patients, higher TWEAK levels correlate with a poor prognosis. We cannot fully explain this effect. Our data might reflect the complex role of the inflammatory response during the disease progress of patients suffering from sepsis or SIRS due to other causations (e.g. cardiogenic shock or severe hepatic disease) [2, 31]. Profound inflammatory dysbalance is a pathologic commonality of many critical diseases. One might speculate that while serum TWEAK levels are basically reduced in critically ill patients, different levels of reduction reflect the magnitude of inflammatory imbalance, which might strongly affect the patients`outcome. Thus, our data sound a note of caution that therapies intended to augment TWEAK responses in critically ill patients could carry a significant risk.
Further molecular analyses are clearly needed to more specifically assess the inverse regulation and function of TNF and TWEAK in critical disease. For example, the proinflammatory factor NFκB is a potent executor of both TNF and TWEAK signaling. In heart failure, it has been suggested that differential activation of canonical and non-canonical NFκB signaling might determine different effects of both cytokines [7]. It remains to be assessed if NF-κB also mediates differential roles of TNF and TWEAK in critical ill patients due to other causes.
In contrast to TWEAK, GITRL was not differentially regulated in critical ill patients vs. controls. Off note, our negative findings on GITRL serum levels with regards to disease severity or distinction between septic and non-septic patients probably reflect specific mechanisms of biosynthesis and release into the serum, but do not preclude an important functional role of the GITRL/GITR system in the mediation of sepsis. GITRL expression is strongly increased in response to proinflammatory stimuli, mainly in antigen presenting cells and endothelial cells [32–35]. Our results demonstrate that this putative up-regulation on a cellular level is not reflected in the serum of critically ill and septic patients. However, this up-regulation in endothelium was suggested to control the extravasation process and leucocyte tissue infiltration [16, 36], critical early steps in the pathogenesis of sepsis and shock. In line, functional experiments revealed crucial functions of the GITR/GITRL system in an animal model of acute lung injury [36]. Based on our findings and the previous data, it might therefore be interesting to evaluate expression levels of GITRL and/or GITR on circulating immune cells, e.g. by FACS, rather than levels of free circulating GITRL in order to demonstrate a correlation of critical illness or sepsis with activation of the GITR/GITRL system.
It has been demonstrated in a variety of the disease models that pronounced therapeutic effects are achievable with soluble TWEAK- and Fn14-specific antibodies [37]. Consequently, TWEAK- and Fn14-specific antibodies are currently tested in phase I studies in patients suffering from rheumatoid arthritis, lupus or solid tumors [37]. Based on the strong upregulation of TNF in sepsis, various trials tested the effect of anti-TNF antibodies (such as infliximab) in critically ill patients with severe sepsis. However, in these trials TNF antibodies failed to detect any beneficial effect. Our data on a differential role of the different TGFR ligands in sepsis might therefore be of special clinical relevance, as they suggest that a more differential interventional approach, that affects specific members of the TNF superfamily could potentially be more successful than the use of TNF antibodies.
Guiding early therapeutic decisions represent important challenges in the treatment of critically ill patients [2]. In light of our findings that decreased TWEAK concentrations are indicative for presence of septic disease and prognosis, our results indicate that measurement of circulating TWEAK levels could be considered as a novel element in the diagnostic algorithm of critically ill patients. Of note, although in our patients the prognostic value of TWEAK serum levels for overall survival was comparable to many well established prognostic markers (inferior to INR, but superior to CRP, creatinine and patients`age) we do not intend to imply that well established clinical scores and standard parameters can be replaced by measuring novel biomarkers like TWEAK, but rather could be complemented. Moreover from a basic science view, our findings highlight differential roles of different TNF superfamily members in the pathophysiology of septic disease. Future studies combining prospective clinical trials and experimental animal models are needed to further establish TWEAK measurements in diagnosis of sepsis and to define the exact role of different TNF superfamily members in the pathophysiology of sepsis.
Limitations of this study
Since our report is not a case control study, the patient and control are not fully matched regarding sex and age. However, since in our analysis serum TWEAK or GITRL levels were not correlated to patients’ sex or age, it seems unlikely that these factors have biased our analysis. As a retrospective analysis, our study protocol has to face more possible biases like interpretation bias and selection bias [38]. We tried to rule out theses biases as strictly as possible, however we do not intend to imply that our data on TWEAK serum levels in critically ill patients are sufficient to establish this test as new standard in risk stratification of critically ill and septic patients. Further studies on TWEAK are necessary to establish a role of this molecule in this patient group.
Acknowledgments
The authors would like to express their gratitude to members of the Luedde lab, Michaela Roderburg-Goor and Dr. Jane Beger, for helpful discussions.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by project grants from the German Research Foundation (DFG RO 4317/4-1) and a START grant from the medical faculty RWTH Aachen to C.R. Moreover the work was supported by a Starting Grant from the European Research Council within the FP 7 (ERC-2007-Stg / 208237-Luedde-Med3-Aachen), the German-Research-Foundation (SFB-TRR57, P06+P09), the German Cancer Aid (Deutsche Krebshilfe Grant 110043), the EMBO Young Investigator Program, the Ernst-Jung-Foundation Hamburg, a grant from the Medical Faculty Aachen, from the Interdisciplinary Center of Clinical Research (“IZKF”) Aachen, a grant of the Bundesministerium für Bildung und Forschung, Germany (Nationales Genomforschungsnetz: NGFNplus) and a grant of the Deutsche Stiftung Herzforschung (F12/12). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998; 282(5392):1281–4. . [DOI] [PubMed] [Google Scholar]
- 2.Roderburg C, Koch A, Tacke F, Nieuwenhuijsen L, Bruensing J, Vargas Cardenas D, et al. Serum concentrations of A Proliferation-Inducing Ligand (APRIL) are elevated in sepsis and predict mortality in critically ill patients. J Crit Care. 2013; 28(5):20 10.1016/j.jcrc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 3.De Freitas I, Fernandez-Somoza M, Essenfeld-Sekler E, Cardier JE. Serum levels of the apoptosis-associated molecules, tumor necrosis factor-alpha/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest. 2004; 125(6):2238–46. . [DOI] [PubMed] [Google Scholar]
- 4.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2014; 14(1):R15 10.1186/cc8872 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997; 272(51):32401–10. . [DOI] [PubMed] [Google Scholar]
- 6.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008; 7(5):411–25. 10.1038/nrd2488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novoyatleva T, Sajjad A, Engel FB. TWEAK-Fn14 Cytokine-Receptor Axis: A New Player of Myocardial Remodeling and Cardiac Failure. Front Immunol. 2014; 11;5:50 10.3389/fimmu.2014.00050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwyer BJ, Olynyk JK, Ramm GA, Tirnitz-Parker JE. TWEAK and LTbeta Signaling during Chronic Liver Disease. Front Immunol. 2014; 5(39):2014 10.3389/fimmu.2014.00039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz AB, Izquierdo MC, Sanchez-Nino MD, Ucero AC, Egido J, Ruiz-Ortega M, et al. TWEAK and the progression of renal disease: clinical translation. Nephrol Dial Transplant. 2014; 2014(29):i54–i62. 10.1093/ndt/gft342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chorianopoulos E, Rosenberg M, Zugck C, Wolf J, Katus HA, Frey N. Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail. 2009; 11(11):1050–6. 10.1093/eurjhf/hfp139 . [DOI] [PubMed] [Google Scholar]
- 11.Park MC, Chung SJ, Park YB, Lee SK. Relationship of serum TWEAK level to cytokine level, disease activity, and response to anti-TNF treatment in patients with rheumatoid arthritis. Scandinavian journal of rheumatology. 2008; 37(3):173–8. 10.1080/03009740801898608 . [DOI] [PubMed] [Google Scholar]
- 12.Gungor O, Kismali E, Sisman AR, Kircelli F, Carrero JJ, Tatar E, et al. The relationships between serum sTWEAK, FGF-23 levels, and carotid atherosclerosis in renal transplant patients. Ren Fail. 2013; 35(1):77–81. 10.3109/0886022X.2012.734890 . [DOI] [PubMed] [Google Scholar]
- 13.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A. 1997; 94(12):6216–21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR-GITRL system, a novel player in shock and inflammation. ScientificWorldJournal. 2007; 7:533–66. 10.1100/tsw.2007.106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placke T, Kopp HG, Salih HR . Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin Dev Immunol. 2010; 2010:239083 10.1155/2010/239083 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuzzocrea S, Nocentini G, Di Paola R, Mazzon E, Ronchetti S, Genovese T, et al. Glucocorticoid-induced TNF receptor family gene (GITR) knockout mice exhibit a resistance to splanchnic artery occlusion (SAO) shock. J Leukoc Biol. 2004; 76(5):933–40. 10.1189/jlb.0204110jlb.0204110 . [DOI] [PubMed] [Google Scholar]
- 17.Roderburg C, Benz F, Cardenas DV, Lutz M, Hippe HJ, Luedde T, et al. Persistently elevated osteopontin serum levels predict mortality in critically ill patients. Critical care. 2015; 19:271 10.1186/s13054-015-0988-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacke F, Roderburg C, Benz F, Cardenas DV, Luedde M, Hippe HJ, et al. Levels of circulating miR-133a are elevated in sepsis and predict mortality in critically ill patients. Crit Care Med. 2014; 42(5):1096–104. 10.1097/CCM.0000000000000131 [DOI] [PubMed] [Google Scholar]
- 19.Benz F, Tacke F, Luedde M, Trautwein C, Luedde T, Koch A, et al. Circulating microRNA-223 serum levels do not predict sepsis or survival in patients with critical illness. Dis Markers. 2015; 2015(384208):24 10.1155/2015/384208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011; 15(1):16 10.1186/cc10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch A, Sanson E, Helm A, Voigt S, Trautwein C, Tacke F. Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit Care. 2010; 14(3):25 10.1186/cc9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch A, Gressner OA, Sanson E, Tacke F, Trautwein C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care. 2009; 13(3):19 10.1186/cc7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011; 15(1):R63 10.1186/cc10037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch A, Voigt S, Sanson E, Duckers H, Horn A, Zimmermann HW, et al. Prognostic value of circulating amino-terminal pro-C-type natriuretic peptide in critically ill patients. Crit Care. 2011; 15(1):R45 10.1186/cc10007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegelaar SE, Hickmann M, Hoekstra JB, Holleman F, Devries JH. The effect of diabetes on mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2011; 15(5):R205 10.1186/cc10440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010; 20(2):87–103. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006; 290(4):L622–L45. . [DOI] [PubMed] [Google Scholar]
- 28.Schilder L, Nurmohamed SA, ter Wee PM, Paauw NJ, Girbes AR, Beishuizen A, et al. Putative novel mediators of acute kidney injury in critically ill patients: handling by continuous venovenous hemofiltration and effect of anticoagulation modalities. BMC nephrology. 2015; 16:178 10.1186/s12882-015-0167-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012; 4(3). 10.1101/cshperspect.a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chicheportiche Y, Fossati-Jimack L, Moll S, Ibnou-Zekri N, Izui S. Down-regulated expression of TWEAK mRNA in acute and chronic inflammatory pathologies. Biochem Biophys Res Commun. 2000; 279(1):162–5. [DOI] [PubMed] [Google Scholar]
- 31.Roderburg C, Benz F, Cardenas DV, Lutz M, Hippe HJ, Luedde T, et al. Persistently elevated osteopontin serum levels predict mortality in critically ill patients. Crit Care. 2015; 19(271):015–0988. 10.1186/s13054-015-0988-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003; 100(25):15059–64. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006; 6(8):613–8. . [DOI] [PubMed] [Google Scholar]
- 34.Stephens LA, Anderton SM. Comment on "Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells". J Immunol. 2006; 177(4):2036; author reply 7–8. . [DOI] [PubMed] [Google Scholar]
- 35.Nardelli B, Zaritskaya L, McAuliffe W, Ni Y, Lincoln C, Cho YH, et al. Osteostat/tumor necrosis factor superfamily 18 inhibits osteoclastogenesis and is selectively expressed by vascular endothelial cells. Endocrinology. 2006; 147(1):70–8. . [DOI] [PubMed] [Google Scholar]
- 36.Cuzzocrea S, Nocentini G, Di Paola R, Agostini M, Mazzon E, Ronchetti S, et al. Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation. J Immunol. 2006; 177(1):631–41. . [DOI] [PubMed] [Google Scholar]
- 37.Wajant H. The TWEAK-Fn14 system as a potential drug target. Br J Pharmacol. 2013; 170(4):748–64. 10.1111/bph.12337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mower WR. Evaluating bias and variability in diagnostic test reports. Annals of emergency medicine. 1999; 33(1):85–91. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.