Abstract
Abies alba (European silver fir) was used to investigate possible effects of simulated browsing on C allocation belowground by 13CO2 pulse-labelling at spring, summer or autumn, and by harvesting the trees at the same time point of the labelling or at a later season for biomass and for 13C-allocation into the fine-root system. Before budburst in spring, the leader shoots and 50% of all lateral shoots of half of the investigated 5-year old Abies alba saplings were clipped to simulate browsing. At harvest, different fine-root classes were separated, and starch as an important storage compartment was analysed for concentrations. The phenology had a strong effect on the allocation of the 13C-label from shoots to roots. In spring, shoots did not supply the fine-roots with high amounts of the 13C-label, because the fine-roots contained less than 1% of the applied 13C. In summer and autumn, however, shoots allocated relatively high amounts of the 13C-label to the fine roots. The incorporation of the 13C-label as structural C or as starch into the roots is strongly dependent on the root type and the root diameter. In newly formed fine roots, 3–5% of the applied 13C was incorporated, whereas 1–3% in the ≤0.5 mm root class and 1–1.5% in the >0.5–1.0 mm root class were recorded. Highest 13C-enrichment in the starch was recorded in the newly formed fine roots in autumn. The clipping treatment had a significant positive effect on the amount of allocated 13C-label to the fine roots after the spring labelling, with high relative 13C-contents observed in the ≤0.5 mm and the >0.5–1.0 mm fine-root classes of clipped trees. No effects of the clipping were observed after summer and autumn labelling in the 13C-allocation patterns. Overall, our data imply that the season of C assimilation and, thus, the phenology of trees is the main determinant of the C allocation from shoots to roots and is clearly more important than browsing.
Introduction
European silver fir (Abies alba Mill.), a typical tree of montane and lower subalpine forests in the Alps, has a highly relevant protective function against landslide due to its deep rooting system [1], its high resistance against bark beetles (Ips typographus L.), and its general ease of natural regeneration [2]. It produces high quality wood that is used for furniture, construction timber and pulp. However, A. alba has been declining throughout its natural range since the 18th century due to forest management practices [2] and anthropogenic air pollution. Moreover, the high population number of game animals, e.g. deer, and, therefore, high grazing pressure on young A. alba trees is thought to prevent regeneration [3,4], especially due to browsing of the leader shoot, reducing A. alba height growth for several years [5].
Although stress responses of different conifers to either artificial or natural defoliation through clipping or herbivory have been studied extensively [6–10], information on belowground responses is scarce. Based on studies with non-woody plants, it has been suggested that herbivore-mediated shifts in belowground carbon (C) allocation patterns of plants are the result of a close coupling of above- and belowground processes in terrestrial ecosystems, induced by plant defense mechanisms [11,12]. The first studies on belowground responses of trees focussed on root growth, either directly influenced by mammalian browsing of shoots or indirectly by mammalian trampling [13,14]. Hester et al. [9] were the first studying the effect of simulated browsing on above- and belowground growth of different tree species in a pot-experiment and reported not only negative above- but also negative belowground responses due to simulated browsing by clipping. Recent studies also addressed the fate of assimilated C belowground and the belowground starch storage responses to browsing events [15–20], with only one study so far applying isotopically labelled C [21]. Although the 13C-isotope label in that latter study was measured only in the tree rings of the stems without observing any significant effects of the browsing, this study showed the potential of the 13C-isotope application for tracking recently fixed photoassimilates within the plants. Moreover, the 13C-isotope method provides the location of 13C-incorporation (e.g. root tips, coarse roots) as well as the form of the incorporated 13C-label (e.g. starch, cellulose) [22].
The main objectives of the present study were to investigate the effects of browsing, simulated through clipping, on the C allocation belowground at different times during the growing season, to distinguish C incorporation among different types of fine roots, and to investigate the changes in starch storage patterns in the fine roots due to clipping. Deduced from these objectives we tested the following hypotheses: (1) the time point (seasons) of the 13C-pulse-labelling and, thus, phenology has a strong effect on the allocation of the 13C-label from the shoot to the roots, (2) the incorporation of the 13C-label as structural C or as starch into the roots is strongly dependent on the root type and the root diameter, and (3) clipping strongly influences the amount of allocated 13C-label as well as the site of incorporation and the form of the incorporated 13C-label (structural C versus starch) within the root system. Therefore, 5-year-old A. alba saplings, both clipped and control trees, were pulse-labelled with 13CO2, either in spring, summer, or autumn, and the assimilated 13C was traced into the fine-root systems, with specific emphasis on the incorporation of 13C into starch as the main storage carbohydrate.
Material and Methods
Experimental design and plant material
The experiment was carried out in 2008 in the tree nursery of the Swiss Federal Research Institute (WSL) in the Swiss lowlands (Birmensdorf, 47°22 N, 08°27 E, 550 m a.s.l.). According to Palacio et al. [15], saplings of an approximate height of 30–50 cm height are considered to be one of the most vulnerable stages of a young tree in relation to large herbivore browsing (compare also [6,9]). Thus, in total, 68 five-year-old nursery-grown A. alba saplings (height ±30 cm; provenance Beggingen, 47°47 N, 08°33 E, 650 m a.s.l.) were grown in a nursery soil with a pH of 7.2 and were fertilised once at the age of four years with organic NPK fertiliser (Unikorn II, Hauert, Switzerland; 20 g m–2).
In the beginning of April 2008, the tree saplings were excavated with their root systems attached, using a root-cutting machine ('Beetroder', Baertschi Agrartechnik AG, Hüswil, Switzerland). The saplings were then replanted into pots (22.5 cm height, 26 cm in diameter) and assigned to two treatments, 35 plants in a browsing treatment and 33 plants in an untreated control. The browsing treatment was performed at the end of April, just prior to bud burst, when a maximum of browsing by wild ungulates can be assumed [23]. The browsing was carried out according to Häsler et al. [10], with clipping two-thirds of the previous year’s leader shoots and one-third of the previous year’s shoot growth at every second lateral branch (proportion of buds removed: 56±1.3%), using diagonal pliers to break the plant tissue in a comparable way to ungulate browsing.
All saplings were randomly arranged in four rows of 17 trees and shaded with wooden frames above the trees and shading nets at the sides. Thus, the trees received only about one-third of direct sunlight, simulating conditions as in the forest understory [10].
13CO2 pulse-labelling
The 13CO2 pulse-labelling was conducted with three randomly assembled groups of A. alba tree individuals. Each group was labelled during one physiologically significant phase, i.e., in spring (early May 2008, subsequent to sprouting; 28 trees), in summer (early July 2008, period of stem growth; 20 trees) and in autumn (end of September 2008, end of the growing season; 12 trees). In total, 60 trees were labelled with 13CO2. In addition to these 60 trees, eight trees (four clipped and four controls) remained unlabelled and were used to calculate the respective 13C enrichment in the trees (Table 1).
Table 1. Number of harvested and 13CO2 pulse-labelled trees during the seasons in two years for two clipping treatments and three 13CO2 labelling seasons.
Treatments are 'Control' = unclipped trees, and 'Clipped' = clipped trees.
| Treatment | 13CO2 labelling season | Harvesting season | Sum of trees | |||
|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring+1 yr | |||
| Control | Spring | 4 | 4 | 4 | 1 | 13 |
| Summer | - | 4 | 4 | 2 | 10 | |
| Autumn | - | - | 4 | 2 | 6 | |
| Sum of trees | 4 | 8 | 12 | 5 | 29 | |
| Clipped | Spring | 4 | 4 | 4 | 3 | 15 |
| Summer | - | 4 | 4 | 2 | 10 | |
| Autumn | - | - | 4 | 2 | 6 | |
| Sum of trees | 4 | 8 | 12 | 7 | 31 | |
At each labelling date, randomly chosen groups of four A. alba trees were lined up side by side, and the shoots of the four trees (two clipped and two control trees) were covered with one polyethylene bag (volume approx. 300 l, 100 μm thickness) and pulse-labelled with 13CO2. The polyethylene bags were then tightly wrapped at the stems, below the crowns, and sealed with string and adhesive tape. With a syringe, 480 ml of gaseous 13CO2 (CIL Cambridge Isotope Laboratories, Switzerland; 280 mg 13C) were added to each polyethylene bag. This equalled 120 ml 13CO2 (70 mg 13C) for each tree individual. In spring, 7 polyethylen bags with total 28 trees were labelled, in summer 5 bags with 20 trees, and in autumn 3 bags with 12 trees.
The addition of the gas was performed following Kagawa et al. [24] and Endrulat et al. [22] to ensure efficient incorporation of the label. After closure of the bags, 2 x 40 ml 13CO2 were injected into each bag, followed by another 2 x 40 ml after 15 minutes. The trees were left in the bags for 1 h, before bags were opened for another hour. This procedure was repeated three times within one day. The labelling always started around 11 a.m. and lasted until 4 p.m. (summertime) in the afternoon. Since we aimed that plants were exposed to full sunlight for all three labelling dates, we started close to noon because in autumn fog prevails in the mornings. To reduce water condensation and thus limitation of CO2 incorporation rate, two electric fans per bag were used to ensure continuous circulation within the bag. The fans were equipped with 80 g silica gel in the front to additionally reduce humidity in the bags. Maximum temperatures at the labelling dates were 21.3°C (May), 23.8°C (July) and 14.5°C (September).
Tree measurements
Before and after the clipping treatment and immediately before each sapling was harvested, non-destructive morphological measurements were carried out for each tree. These measurements included sapling height, length of new leader shoot, number of buds, number of new shoots (terminals and laterals), and number of branches off the main stem. Furthermore, the origin of the new leader was recorded as originating from an apical, interwhorl or secondary whorl bud according to Häsler et al. [10] (S1 Table).
Sampling and harvest
Immediately after each of the last labelling exposures, 10 needles of every labelled tree were randomly sampled, milled and analyzed for the maximum 13C label in each tree. The maximum 13C contents in the needles were then used to calculate the relative 13C contents in the roots. Whole trees were harvested at one to four different time points after the three 13C-labelling dates: In spring, summer, and autumn 2008, and as well as in spring 2009, i.e., 1, 3, 5, and 12 months after a labelling event (Table 1). Of each harvest, four replicate saplings of both treatments (clipped and control) were harvested, except in the spring 2009, one year after the start (spring+1 yr), when a maximum of three replicates was harvested. At each harvest, two non-labelled control trees were sampled as well.
The whole root systems were carefully washed and partitioned into various root categories using a vernier caliper according to Endrulat et al. [22] to keep track of the spatial distribution of the 13C-label within the roots; new roots (newly formed white-coloured fine roots), fine roots of various size classes (≤0.5 mm in diameter, >0.5–1 mm in diameter, >1.0–1.5 in diameter mm, >1.5–2 mm in diameter; brown-coloured), and coarse roots (>2 mm in diameter; brown-coloured).
Branches and needles as well as stems were weighed (fresh weight), frozen in liquid nitrogen, and then stored at -20°C until lyophilisation and biomass (dry weight) determination.
Starch extraction
Starch was extracted from two fine-root classes, from the newly formed fine roots and the fine roots with a diameter ≤0.5 mm, and in spring, summer, and autumn of 2008. Starch extraction followed a protocol of Smith and Zeeman [25], modified according to Regier et al. [26] and Endrulat et al. [22]. Approximately 60 mg (dry weight; DW) of lyophilized and milled A. alba fine roots were extracted with 80% ethanol, and boiled to gelatinise the starch. Starch was then digested with α-amylase and amyloglucosidase (Roche, Switzerland) in water solution. After digestion, the starch amount was measured as released glucose with a spectrophotometer (Tecan, Switzerland) at 340 nm. For 13C isotopic measurements, the samples were filtrated with centrifugal Filter Devices (Microcon YM-10; Millipore, Switzerland) in order to take out the enzymes according to Göttlicher et al. [27], and then dried in tin capsules (Säntis Analytical AG, Switzerland) in a vacuum centrifuge (Heto Lab Equipment, Denmark).
δ13C analyses and isotope calculations
Carbon isotope and total C concentration analyses in needles, bulk fine-roots and starch were carried out with an Elemental Analyser (EA-1110, Carlo Erba, Italy; Euro EA 3000, Hekatech, Germany) connected to a continuous flow mass spectrometer (Delta V Advantage, Thermo Fisher Scientific, Germany) at WSL. The measured isotope ratios are expressed in δ-notation in permil units (‰), using Vienna-PeeDee Belemnite (V-PDB) as standard. The precision for 13C analysis was ±0.2‰.
The amounts of assimilated 13C were calculated according to Philip and Simard [28] and Keel et al. [29]. δ13C (‰) and C (%) of labelled samples and unlabelled controls were used to calculate the 13C enrichment as described in Endrulat et al. [22]. The needle δ13C values immediately after each last pulse-labelling exposure were used as maximum signal of each tree individual according to Keel et al. [29]. The 13C enrichment (mg) of the needles was set to 100%, and the measured 13C contents in all fine-root samples were expressed in percentage of the maximum needle signals, and referred to as relative 13C content (%). This approach enables the comparison of all tree individuals due to the elimination of the signal strength variation caused by variable CO2 uptake during the labelling.
For 13C analyses in roots, three fine-root classes were analysed: newly formed fine roots, fine roots of ≤0.5 mm in diameter, and fine roots of >0.5–1 mm in diameter. For 13C labelled starch of fine roots, only the newly formed fine roots and the fine roots of ≤0.5 mm in diameter were analysed.
Statistical analyses
All statistical analyses were performed using STATVIEW 5.0 (SAS Institute Inc., Cary, NC, USA). The effects of the clipping treatment and the various harvesting seasons on plants and root properties were analysed by two-way analyses of variance (ANOVA) with using Fisher’s protected least significant difference (PLSD). The data were tested for normal distribution and homogeneity of variance. Starch and relative 13C content data were log-transformed in order to meet the requested criteria. One data set was not normally distributed, therefore, the non-parametric Mann-Whitney (for two groups) and Kruskal-Wallis tests (for more than two groups) were applied.
Results
Biomass partitioning
The clipping of the trees caused over the whole investigation period a significant decrease of aboveground biomass (-5.8%) as well as of the needles (-13.8%; Tables 2 and 3). Subsequently, this loss resulted in a significant increase of the percentage of belowground biomass (+11.9%; Table 3), as well as in a significant increase of the proportion of biomass in coarse (+8.6%) and fine roots (+16%) (Tables 2 and 3). The root/shoot ratio of clipped trees remained more or less on a constant level (0.56–0.63), whereas the ratio of control trees at the beginning in spring was low (0.41) and increased constantly until autumn (to 0.60; Table 2).
Table 2. Total biomass (g±SE), root/shoot ratio, and percentage of the various tree components of control (unclipped) and clipped trees for two clipping treatments and four harvesting seasons.
Aboveground is the sum of stem, branches, and needles, belowground is the sum of coarse and fine roots. Different letters indicate significant differences among harvesting seasons within one treatment (P<0.05). * P<0.05, ** P<0.01, and *** P<0.001 indicate significant differences between 'Control' and 'Clipped' trees.
| Treatment | Harvesting season | Trees (n) | Total biomass (g; ±SE) | Root / shoot ratio | Tree components (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem | Branches | Needles | Coarse roots | Fine roots | Above-ground | Below-ground | |||||
| Control | Spring | 5 | 48.9±7.0c | 0.41b | 17.3 | 14.2 | 39.4 | 17.2 | 11.9 | 70.9 | 29.1 |
| Summer | 9 | 70.5±6.4bc | 0.44b | 18.7 | 14.5 | 36.5 | 18.8 | 11.4 | 69.8 | 30.2 | |
| Autumn | 13 | 81.6±6.4b | 0.60a | 16.4 | 14.4 | 31.6 | 19.5 | 18.0 | 62.5 | 37.5 | |
| Spring+1 yr | 6 | 104.8±10.5a | 0.54a | 13.4 | 11.8 | 40.3 | 18.2 | 16.3 | 65.5 | 34.5 | |
| Mean | 77.8±4.6 | 0.52 | 16.6 | 13.9 | 35.7 | 18.8 | 15.0 | 66.3 | 33.7 | ||
| Clipped | Spring | 5 | 41.2±8.5b | 0.59 | 18.1 | 12.4 | 32.8** | 19.7 | 16.9 | 63.4* | 36.6* |
| Summer | 9 | 59.2±9.3ab | 0.57*** | 21.4* | 14.2 | 28.4*** | 20.8 | 15.3* | 64.0** | 36.0** | |
| Autumn | 13 | 72.6±4.8a | 0.63 | 18.4* | 15.6 | 27.7*** | 22.2* | 16.2 | 61.7 | 38.4 | |
| Spring+1 yr | 8 | 67.6±8.4a* | 0.56 | 14.2 | 10.7 | 39.2 | 17.6 | 18.3 | 64.1 | 35.9 | |
| Mean | 63.5±4.0 | 0.59 | 18.2 | 13.7 | 31.2 | 20.0 | 16.5 | 63.1 | 36.9 | ||
Table 3. Results of two-way ANOVA testing the effect of the clipping treatment (C) and the harvesting seasons (H) (spring, summer, autumn, spring+1 yr) on total biomass and root/shoot ratio as well as on the percentage of the various tree components.
Significant effects (P<0.05) are given in bold.
| Tree components | Clipping (C) | Harvesting season (H) | C x H | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | |
| Total biomass | 1 | 8.31 | 0.006 | 3 | 8.02 | <0.001 | 3 | 1.47 | 0.231 |
| Root/shoot ratio | 1 | 14.57 | <0.001 | 3 | 7.24 | <0.001 | 3 | 2.78 | 0.049 |
| Stem | 1 | 5.34 | 0.024 | 3 | 15.09 | <0.001 | 3 | 0.48 | 0.698 |
| Branches | 1 | 0.75 | 0.391 | 3 | 9.52 | <0.001 | 3 | 1.45 | 0.237 |
| Needles | 1 | 47.35 | <0.001 | 3 | 43.28 | <0.001 | 3 | 4.76 | 0.005 |
| Coarse roots | 1 | 4.40 | 0.040 | 3 | 3.54 | 0.020 | 3 | 1.02 | 0.390 |
| Fine roots | 1 | 5.93 | 0.018 | 3 | 5.24 | 0.003 | 3 | 3.31 | 0.026 |
| Aboveground | 1 | 17.02 | <0.001 | 3 | 8.28 | <0.001 | 3 | 3.15 | 0.031 |
| Belowground | 1 | 17.02 | <0.001 | 3 | 8.28 | <0.001 | 3 | 3.15 | 0.031 |
Both, clipping treatment and harvesting season, influenced the tree parameters significantly, with the one exception of the branches after the clipping treatment (Table 3). Interactions of the two factors, clipping and harvesting, occurred for the root/shoot ratio, the above- and belowground biomass, the needles, and the fine roots (Table 3). This fact indicates that the effects of clipping upon these tree parameters depend on the harvesting season.
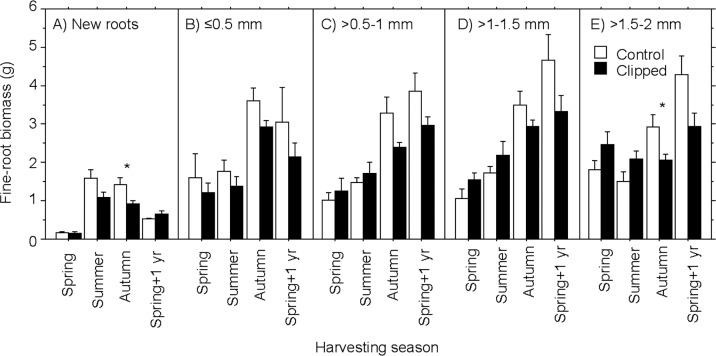
Separating the fine roots into newly formed roots and into different diameter classes (Fig 1), we found that root biomass differed among various fine-root classes in all harvesting seasons with the exception of summer (Table 4). These significant effects were mainly caused by the small biomass of the newly formed fine roots (Fig 1). The clipping treatment caused a significant reduction of fine-root biomass in autumn and spring+1 yr (Table 4, Fig 1), but not in spring or summer (Table 4).
Fig 1.
Mean biomass (g, ±SE; n = 5–13, see S1 Table) of fine-root classes of control and clipped trees: (A) new roots, (B) ≤0.5 mm, (C) >0.5–1.0 mm, (D) >1.0–1.5 mm, (E) >1.5–2.0 mm. Significant differences between control and clipped trees are given with * (P<0.05).
Table 4. Results of two-way ANOVA testing the effect of the clipping treatment (C) and the various fine-root classes (R; new roots, size classes ≤0.5 mm, >0.5–1.0 mm, >1.0–1.5 mm, and >1.5–2.0 mm) on fine-root biomass at various harvesting seasons.
Significant effects (P<0.05) are given in bold (autumn data were tested with non-parametric tests due to non-normal distribution).
| Harvesting season | Clipping (C) | Fine-root classes (R) | C x R | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | |
| Spring | 1 | 1.00 | 0.324 | 4 | 11.41 | <0.001 | 4 | 0.98 | 0.429 |
| Summer | 1 | 0.26 | 0.609 | 4 | 1.90 | 0.1181 | 4 | 2.14 | 0.083 |
| Autumn | 1 | - | 0.007 | 4 | - | <0.001 | - | - | - |
| Spring+1 yr | 1 | 9.77 | 0.003 | 4 | 19.10 | <0.001 | 4 | 0.94 | 0.446 |
13C in needles and fine-roots
The mean recovery of the 13C isotope (ratio of incorporated 13Cneedles to the amount of 13C added to one tree) after the pulse-labelling was 71% (±8.2%) for control and 55% (±6.2%) for clipped trees. However, mean δ13C values in needles directly after the last labelling exposure varied significantly among trees of the same labelling bag (P≤0.019 for all bags) and among the three labelling dates (P<0.001). Clipped and unclipped control trees did not differ significantly in their foliar δ13C values (P = 0.391), and no significant interaction between treatment and labelling date was found (P = 0.078), indicating similar conditions for all labelling dates and no difference in uptake between the two treatments.
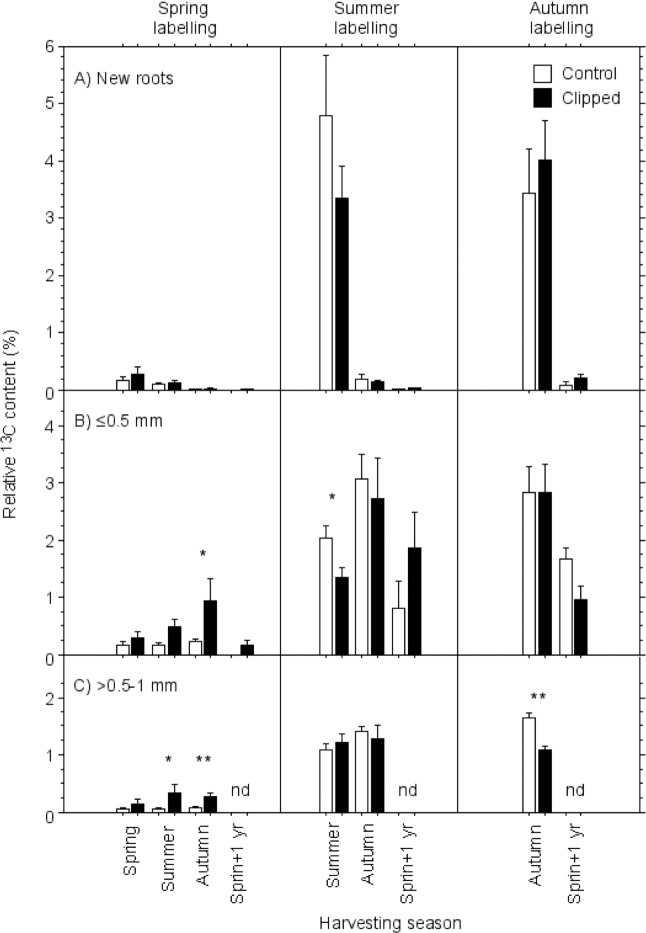
Fine roots of the same diameter class of clipped and control trees had variable values in terms of 13C enrichment, expressed as relative 13C content (%), and the 13C enrichment differed strongly among seasons of labelling (Fig 2). Spring labelled trees had the lowest relative 13C contents (<1%) in the fine roots in comparison to summer and autumn labelled fine roots (Fig 2). Highest relative 13C contents (3–5%) were observed only in newly formed roots of the summer or the autumn harvests immediately after the 13CO2-labelling (Fig 2). Medium high 13C contents (1–3%) were recorded in fine roots of ≤0.5 mm and of >0.5–1.0 mm after the summer and the autumn labellings in all harvesting seasons including spring+1 yr (Fig 2).
Fig 2.
Means of relative 13C contents (%, ±SE; n = 4 for spring, summer, and autumn, n = 1–3 for spring+1 yr, see Table 1) recovered from bulk fine-roots of (A) new fine roots, (B) fine roots of class ≤0.5 mm, and (C) fine roots of class >0.5–1.0 mm for different seasons of 13CO2 pulse-labelling (spring, summer, autumn) and for different harvesting seasons. Relative 13C contents are expressed in percentage of the maximum needle signals. Significant differences between control and clipped trees are given with * (P<0.05) and ** (P<0.01).
Clipping the trees had significantly positive effects on the relative 13C content of the roots of the ≤0.5 mm and >0.5–1.0 mm root classes when the trees were labelled in spring (Fig 2), whereas clipping had significantly negative effects on the >0.5–1.0 mm root class when trees were labelled in autumn (Table 5). The harvesting season had a significantly positive effect on new roots and the ≤0.5 mm root class after summer labelling, with much higher relative 13C contents after the summer and autumn labelling (Table 5, Fig 2).
Table 5. Results of two-way ANOVA testing the effect of the clipping treatment (C) and the harvesting seasons (H) (spring, summer, autumn) on relative 13C contents (%) of three fine-root classes.
Significant effects (P<0.05) are given in bold. (-: data not available).
| Labelling season | Fine-root class | Clipping (C) | Harvesting season (H) | C x H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | ||
| Spring | New roots | 1 | 0.05 | 0.833 | 2 | 5.75 | 0.012 | 2 | 0.30 | 0.747 |
| ≤0.5 mm | 1 | 9.08 | 0.008 | 2 | 2.91 | 0.080 | 2 | 0.71 | 0.504 | |
| >0.5–1.0 mm | 1 | 14.16 | 0.001 | 2 | 3.78 | 0.043 | 2 | 0.09 | 0.914 | |
| Summer | New roots | 1 | 1.00 | 0.337 | 1 | 222.34 | <0.001 | 1 | 0.36 | 0.559 |
| ≤0.5 mm | 1 | 2.09 | 0.172 | 1 | 8.99 | 0.010 | 1 | 0.74 | 0.405 | |
| >0.5–1.0 mm | 1 | 0.05 | 0.836 | 1 | 1.00 | 0.338 | 1 | 0.79 | 0.393 | |
| Autumn | New roots | 1 | 0.31 | 0.598 | - | - | - | - | - | - |
| ≤0.5 mm | 1 | <0.01 | 0.994 | - | - | - | - | - | - | |
| >0.5–1.0 mm | 1 | 25.76 | 0.002 | - | - | - | - | - | - | |
Starch concentrations and recovered 13C label in starch
Highest starch concentrations (20–24 mg g-1) were found in newly grown fine roots in autumn (S2 Table). The clipping treatment did not significantly influence the starch concentrations in the new roots and the ≤0.5 mm fine root class, whereas the harvesting season influenced them in both root classes significantly (Table 6).
Table 6. Results of two-way ANOVA of the effect of the clipping treatment (C) and the harvesting seasons (H) (spring, summer, autumn) on the starch concentration and on the relative 13C content of the starch of two fine-root classes.
Significant effects (P<0.05) are given in bold. (-: data not available).
| Starch | Labelling season | Fine-root class | Clipping (C) | Harvesting season (H) | C x H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | |||
| Concentration | - | New roots | 1 | 0.67 | 0.418 | 2 | 25.05 | <0.001 | 2 | 0.93 | 0.403 |
| ≤0.5 mm | 1 | <0.01 | 0.973 | 2 | 6.01 | 0.005 | 2 | 0.32 | 0.731 | ||
| Relative 13C content | Spring | New roots | 1 | 2.81 | 0.116 | 2 | 11.87 | 0.001 | 2 | 0.48 | 0.632 |
| ≤0.5 mm | 1 | 7.13 | 0.016 | 2 | 2.39 | 0.121 | 2 | 0.76 | 0.483 | ||
| Summer | New roots | 1 | 2.58 | 0.134 | 1 | 61.16 | <0.001 | 1 | 0.84 | 0.377 | |
| ≤0.5 mm | 1 | 0.09 | 0.771 | 1 | 0.67 | 0.434 | 1 | 2.00 | 0.188 | ||
| Autumn | New roots | 1 | 3.00 | 0.134 | - | - | - | - | - | - | |
| ≤0.5 mm | 1 | 0.19 | 0.668 | - | - | - | - | - | - | ||
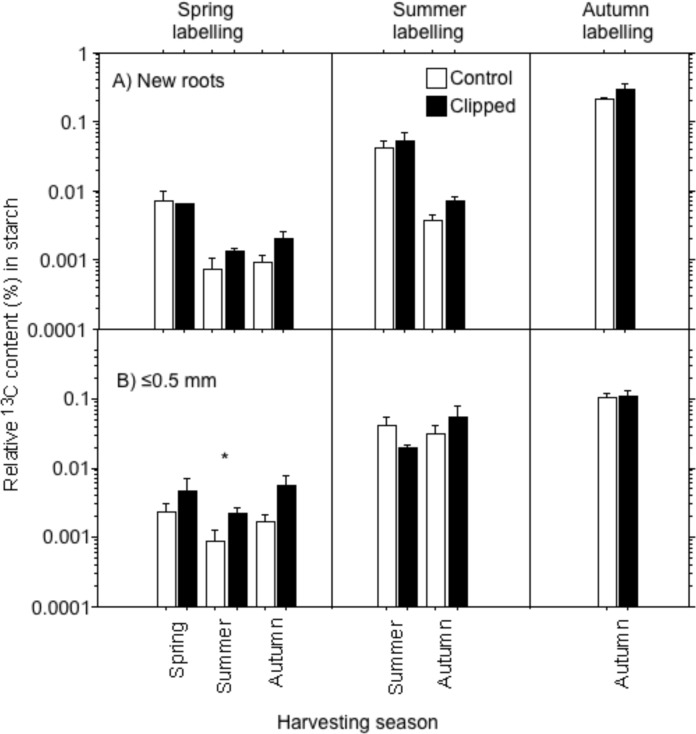
Starch in the fine roots of spring 13CO2-labelled trees was only a little labelled with 13C, with mean relative 13C contents in starch below 0.01% (Fig 3). In contrast, plants labelled in summer allocated more label into the roots than those labelled in spring: mean relative 13C contents of new fine roots were up to 0.06% at the summer harvest (Fig 3). Highest starch 13C enrichments were found after the autumn labelling, with up to 0.3% in new roots and 0.1% in the ≤0.5 mm root class (Fig 3). Clipping had no significant effect on the relative 13C content of fine roots, except for the ≤0.5 mm root class after spring labelling (Table 6). In contrast, harvesting season had significant effects on 13C in new roots for the spring labelling as well as for the summer labelling (Table 6).
Fig 3.
Means of relative 13C content (%, ± SE, n = 4) recovered from starch of (A) new fine roots and (B) of fine roots of class ≤0.5 mm for different seasons of 13CO2 pulse-labelling (spring, summer, autumn) and for different harvesting seasons (spring, summer, autumn) in controls and in clipped trees (logarithmic display). Relative 13C contents are expressed in percentage of the maximum needle signals. Significant differences between control and clipped trees are given with * (P<0.05).
Discussion
The aim of the present study was to investigate the effects of browsing, simulated through clipping, and of different seasons on the C allocation to the root systems of young Abies alba trees. The simulated browsing reduced the aboveground biomass immediately, since a large proportion of the branches have been cut off. The aboveground biomass was reduced mainly due to the clipped branches and due to the proportion of buds removed, and as a consequence, due to the lower number of new shoots produced in the clipped trees. This reduction is in accordance with other studies, e.g. the study of Hester et al. [9], reporting Pinus sylvestris trees having lost a similar amount of buds (58%) due to browsing, whereas the amount was lower in deciduous trees (43% in Betula pendula and 29% in Sorbus aucuparia, respectively). Nevertheless, we found compensatory growth of aboveground parts, e.g. shoots and needles, one year after clipping, as also reported by others [7,9,30]. More than half of the clipped trees built multiple stem forms, similar to results of Häsler et al. [10]. Clipping also affected belowground growth. Evidence of a short-term root growth due to the clipping of shoots was an up to 46% increased biomass in the fine-roots of the classes >0.5–1.0 mm, >1.0–1.5 mm, and >1.5–2.0 mm at spring and summer harvests. Babst et al. [31] as well found increased export of newly assimilated 11C (t1/2: 20.4 min) to roots in Populus tremuloides following jasmonic acid application (simulating a mechanical damage to leaves after feeding by insects). However, after a longer time period, as in our study after half a year or longer, simulated browsing usually results in a significantly reduced fine-root biomass of treated trees compared to control trees. Such belowground reductions were found as well in other tree species, such as Quercus spp., Betula pubescens, Fagus sylvatica, and Abies alba, after herbivore damage or defoliation [15,20,21,32,33]. Only a few studies, however, observed no belowground changes [18,34].
Starch concentrations in the fine roots of our study, in contrast to the biomass, did not show any changes due to the clipping treatment. Other studies made the same observation, that a 50% defoliation does not affect the amount of root starch [15,17,18,20,21,35]. Only a severe defoliation (100%), however, is able to diminish root starch significantly as shown by Piper et al. [18] with Nothofagus spp. or Jacquet et al. [17] with Pinus pinaster. Kosola et al. [35] additionally found that starch accumulation after defoliation occurred mainly in August, but not during the other measured seasons (early summer, late autumn). Overall, the above mentioned reports on root biomass and root starch showed that the severity of defoliation and the environmental circumstances (e.g. season of measurement) matter strongly.
Pulse-labelling with 13CO2 offers a straightforward method to track recently fixed photoassimilates from the shoots to the roots (e.g. [22,27,36,37]). Concerning our three hypotheses (1–3) postulated earlier, our data supported (1) and (2), but (3) only partly:
According to our first hypothesis, the seasons of the 13C-pulse-labelling and, thus, phenology had a strong effect on the allocation of the 13C-label from the shoot to the roots. Shoots labelled in spring did not supply the fine-roots with high amounts of the 13C-label, because the fine-roots had a relatively low 13C-content (<1%). Labelling in summer and autumn, however, revealed that relatively high amounts of the 13C-label (1–5%) were allocated to the fine roots. Thus, it can be assumed that photoassimilates from spring mainly remain in the shoots to support shoot growth, whereas photoassimilates from summer and autumn are allocated in larger portions to the fine roots (compare also [38]). Our findings correspond with reports by Ericsson et al. [39] and by Hansen et al. [40], using a 14CO2-label, who observed that spring assimilates were used mainly for aboveground growth, whereas autumn assimilates were allocated mainly to belowground parts as storage. Keel et al. [41] found similarly that 13C-labels from June were transported to the roots to a lesser amount compared to August. Moreover, Kuptz et al. [42] stated upon their labelling results that 'during spring, only negligible amounts of new photosynthates enter the transfer pool', but 'during early summer, new photosynthates enter the transfer pool, directly supplying growth and being further transported to roots and soils, and during late summer, new photosynthates enter both the transfer and storage pools, supplying both growth and maintenance' [42]. Our isotopic data fully support these findings of Kuptz et al. [42]. Subsequently, highest 13C-enrichments in the starch in the fine roots were recorded in our study in autumn after autumn labelling.
As proposed in our second hypothesis, the incorporation of the 13C-label into the roots was strongly dependent on the root type and the root diameter. Labelling in summer and autumn showed that relatively high amounts of the 13C-label (1–5%) were allocated to the fine roots, with 3–5% in newly formed fine roots, 1–3% in the ≤0.5 mm root class, and 1–1.5% in the >0.5–1.0 mm root class. Comparably, the absolute highest 13C-values in the starch were measured in the newly formed fine roots after the autumn labelling. Keel et al. [41], recording 13C-labels in starch likewise, observed highest values in the finest root class.
In accordance to our third hypothesis, clipping strongly influenced the amount of allocated 13C-label as well as the site of incorporation and the form of the incorporated 13C-label. Clipping had a significantly positive effects on the 13C-allocation to fine roots after spring labelling, with significantly higher relative 13C-contents observed in the ≤0.5 mm and the >0.5–1.0 mm fine-root classes of clipped trees than those of control trees. Similarly, significantly higher relative 13C-contents of starch were observed in the ≤0.5 mm fine-root class of clipped trees after spring labelling. After summer and autumn labelling, however, mainly no effects in the 13C-allocation to the fine roots were observed, assuming that clipping had not a strong effect on the 13C-allocation pattern.
Our study showed that clipping resulted in a reduction of the belowground biomass but not in an increase of the starch concentration in the roots. Thus, we conclude that no trade-off between growth and storage occurred. A trade-off would occur only if a C limitation would be present [43–45]. A sustained severe defoliation would lead to such a C limitation, with increased storage at the expense of growth enhancing the survival chance [45]. In general, tree growth can be limited by the availability of C within the plant (C limitation) or by the tree’s ability to use the available C it has because of e.g. nutrient shortage or environmental conditions (sink limitation) [44]. A growing body of literature suggests that current tree growth is sink limited under most conditions, with much of the evidence deducted from trees’ high amounts of nonstructural carbon (NSC) [44]. These conclusions rely on the assumption that C storage is passive and that NSC does not accumulate when growth is C limited. However, storage may be an active process, occurring at the expense of growth [44].
The processes behind the source-to-sink transport within plants are complex. Plants respond to herbivore feeding by increasing the production of the phytohormone jasmonic acid [46]. A systemic jasmonic acid induction of defense responses also occurs from roots to shoots and vice versa, thereby affecting the performance of herbivores. However, jasmonic acid also causes the re-allocation of primary metabolites between roots and shoots, and it controls several developmental processes such as root growth or senescence [46]. The molecular processes behind this source-to-sink transport most likely are phloem sucrose transport and invertases in sink organs, as the latter increase sucrose unloading by converting sucrose to hexoses [47,48].
In conclusion, our data imply that season of C assimilation and, thus, the seasonal phenology of trees is a main driver of C allocation from shoots to roots, and, as seen in our experiment, cleary more decisive than simulated browsing. However, within the fine-root system, the C allocation pattern varied greatly. Fine roots which are highly physiologically active, e.g. newly grown fine roots, received the highest amounts of newly assimilated C, but only after summer and autumn labelling. This indicates that newly formed fine roots are highly active only in the second half of the vegetation period in exploring the soil and storing C.
Supporting Information
Number of harvested trees with the varying origins of new leader shoots for two clipping treatments and for four harvesting seasons. Treatments are 'Control' = unclipped trees, and 'Clipped' = clipped trees.
(DOCX)
Means of starch concentrations (mg g-1 dry weight, ±SE) in new fine roots and in fine roots of class ≤0.5 mm for two clipping treatments and for three harvesting seasons. Treatments are 'Control' = unclipped trees, and 'Clipped' = clipped trees.
(DOCX)
Acknowledgments
We thank Anton Burkart and his team of the WSL tree nursery for providing plant material and helping with the shading of the plants. Thanks to Helene Häsler and Josef Senn who helped with the browsing treatment, and to Ursula Graf for help with the starch 13C measurements. We also thank Nicole Regier and Nadine Grisel who helped with the labelling treatment. We further thank Silvia Dingwall for correcting the English text.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Swiss ‘State Secretariat for Education, Research and Innovation’ SERI, additional support was received from European Cooperation in Science and Technology (COST) Action E38 'Woody Root Processes'. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Köstler JN, Brückner E, Bibelriether H. Die Wurzeln der Waldbäume: Untersuchungen zur Morphologie der Waldbäume in Mitteleuropa. Hamburg: Paul Parey; 1968. [Google Scholar]
- 2.Robakowski P, Tomasz W, Samardakiewicz S, Kierzkowski D. Growth, photosynthesis, and needle structure of silver fir (Abies alba Mill.) seedlings under different canopies. For Ecol Manag. 2004;201: 211–227. [Google Scholar]
- 3.Motta R. Impact of wild ungulates on forest regeneration and tree composition of mountain forests in the Western Italian Alps. For Ecol Manag. 1996;88: 93–98. [Google Scholar]
- 4.Hester AJ, Bergman M, Iason GR, Moen J. Impacts of large herbivores on plant community structure and dynamics In: Danell K, Bergström R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge: Cambridge University Press;2006. pp. 97–141. [Google Scholar]
- 5.Eiberle K. Ergebnisse einer Simulation des Wildverbisses durch den Triebschnitt. Schweiz Z Forstwes. 1975;126: 821–839. [Google Scholar]
- 6.Gill RMA. A review of damage by mammals in North temperate forests: 1. Deer. Forestry. 1992;65: 145–169. [Google Scholar]
- 7.Honkanen T, Haukioja E, Suomela J. Effects of simulated defoliation and debudding on needle and shoot growth in Scots pine (Pinus sylvestris): implications of plant source/sink relationships for plant-herbivore studies. Funct Ecol. 1994;8: 631–639. [Google Scholar]
- 8.Duncan AJ, Hartley SE, Iason GR. The effect of previous browsing damage on the morphology and chemical composition of Sitka spruce (Picea sitchensis) saplings and on their subsequent susceptibility to browsing by red deer (Cervus elaphus). For Ecol Manag. 1998;103: 57–67. [Google Scholar]
- 9.Hester AJ, Millard P, Baillie GJ, Wendler R. How does timing of browsing affect above- and below-ground growth of Betula pendula, Pinus sylvestris and Sorbus aucuparia? Oikos. 2004;105: 536–550. [Google Scholar]
- 10.Häsler H, Senn J, Edwards PJ. Light-dependent growth responses of young Abies alba to simulated ungulate browsing. Funct Ecol. 2008;22: 48–57. [Google Scholar]
- 11.Bardgett RD, Wardle DA. Herviore-mediated linkages between aboveground and belowground communities. Ecology. 2003;84: 2258–2268. [Google Scholar]
- 12.Bezemer TM, van Dam NM. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20: 617–624. [DOI] [PubMed] [Google Scholar]
- 13.Väre H, Ohtonen R, Mikkola K. The effect and extent of heavy grazing by reindeer in oligotrophic pine heaths in northeastern Fennoscandia. Ecography. 1996;19: 245–253. [Google Scholar]
- 14.Ruess RW, Hendrick RL, Bryant JP. Regulation of fine root dynamics by mammalian browsers in early successional Alaskan taiga forests. Ecology. 1998;79: 2706–2720. [Google Scholar]
- 15.Palacio S, Hester AJ, Maestro M, Millard P. Browsed Betula pubescens trees are not carbon-limited. Funct Ecol. 2008;22: 808–815. [Google Scholar]
- 16.Huttunen L, Saravesi K, Markkola A, Niemelä P. Do elevations in temperature, CO2, and nutrient availability modify belowground carbon gain and root morphology in artificially defoliated silver birch seedlings? Ecol Evol. 2013;3: 2783–2794. 10.1002/ece3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquet JS, Bosc A, O’Grady A, Jactel H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiol. 2014;34: 367–376. 10.1093/treephys/tpu018 [DOI] [PubMed] [Google Scholar]
- 18.Piper FI, Fajardo A. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J Ecol. 2014;102: 1101–1111. [Google Scholar]
- 19.Puri E, Hoch G, Körner C. Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees. 2015;29: 1187–1196. [Google Scholar]
- 20.Wiley E, Huepenbecker S, Casper BB, Helliker BR. The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013;33: 1216–1228. 10.1093/treephys/tpt093 [DOI] [PubMed] [Google Scholar]
- 21.Palacio S, Paterson E, Sim A, Hester AJ, Millard P. Browsing affects intra-ring carbon allocation in species with contrasting wood anatomy. Tree Physiol. 2011;31: 150–159. 10.1093/treephys/tpq110 [DOI] [PubMed] [Google Scholar]
- 22.Endrulat T, Saurer M, Buchmann N, Brunner I. Incorporation and remobilisation of 13C within the fine-root systems of individual Abies alba trees in a temperate coniferous stand. Tree Physiol. 2010;30: 1515–1527. 10.1093/treephys/tpq090 [DOI] [PubMed] [Google Scholar]
- 23.Welch D, Staines BW, Scott D, French DD, Catt DC. Leader browsing by red and roe deer on young Sitka spruce trees in western Scotland. I. Damage rates and the influence of habitat factors. Forestry. 1991;64: 61–82. [Google Scholar]
- 24.Kagawa A, Sugimoto A, Maximov TC. Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol. 2006;171: 793–804. [DOI] [PubMed] [Google Scholar]
- 25.Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nature Protocols. 2006;1: 1342–1345. [DOI] [PubMed] [Google Scholar]
- 26.Regier N, Streb S, Cocozza C, Schaub M, Cherubini P, Zeeman SC, et al. Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defense. Plant Cell Environ. 2009;32: 1724–1736. 10.1111/j.1365-3040.2009.02030.x [DOI] [PubMed] [Google Scholar]
- 27.Göttlicher S, Knohl A, Wanek W, Buchmann N, Richter A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Commun Mass Spectrom. 2006;20: 653–660. [DOI] [PubMed] [Google Scholar]
- 28.Philip LJ, Simard SW. Minimum pulses of stable and radioactive carbon isotopes to detect belowground carbon transfer between plants. Plant Soil. 2008;308: 23–35. [Google Scholar]
- 29.Keel SG, Siegwolf RTW, Jäggi M, Körner C. Rapid mixing between old and new C pools in the canopy of mature forest trees. Plant Cell Environ. 2007;30: 963–972 [DOI] [PubMed] [Google Scholar]
- 30.Edenius L, Danell K, Bergström R. Impact of herbivory and competition on compensatory growth in woody plants: winter browsing by moose on Scots pine. Oikos. 1993;66: 286–292. [Google Scholar]
- 31.Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, et al. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol. 2005;167: 63–72. [DOI] [PubMed] [Google Scholar]
- 32.Frost CJ, Hunter MD. Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol. 2008;178: 835–845. 10.1111/j.1469-8137.2008.02420.x [DOI] [PubMed] [Google Scholar]
- 33.Ayres E, Heath J, Possell M, Black HIJ, Kersiens G, Bardgett RD. Three physiological responses to above-ground herbivory directly modify below-ground processes of soil carbon and nitrogen cycling. Ecol Lett. 2004;7: 469–479. [Google Scholar]
- 34.Kosola KR, Dickmann DI, Paul EA, Parry D. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplar. Oecologia. 2001;129: 65–74. [DOI] [PubMed] [Google Scholar]
- 35.Kosola KR, Dickmann DI, Parry D. Carbohydrates in individual poplar fine roots: effects of root age and defoliation. Tree Physiol. 2002;22: 741–746. [DOI] [PubMed] [Google Scholar]
- 36.Blessing CH, Werner RA, Siegwolf R, Buchmann N. Allocation dynamics of recently fixed carbon in beech saplings in response to increased temperatures and drought. Tree Physiol. 2015;35: 585–598. 10.1093/treephys/tpv024 [DOI] [PubMed] [Google Scholar]
- 37.Epron D, Bahn M, Derrien D, Lattanzi FA, Pumpanen J, Gessler A, et al. Pulse-labelling trees to study carbon allocation dynamics: a review of methods, current knowledge and future prospects. Tree Physiol. 2012;32: 776–798. 10.1093/treephys/tps057 [DOI] [PubMed] [Google Scholar]
- 38.Abramoff RZ, Finzi AC. Are above- and below-ground phenology in sync? New Phytol. 2015;205: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 39.Ericsson T, Rytter L, Vapaavuori E. Physiology of carbon allocation in trees. Biomass Bioenerg. 1996;11: 115–127. [Google Scholar]
- 40.Hansen J, Vogg G, Beck E. Assimilation, allocation and utilization of carbon by 3-year-old Scots pine (Pinus sylvestris L.) trees during winter and early spring. Trees. 1996;11: 83–90. [Google Scholar]
- 41.Keel SG, Campbell CD, Högberg MN, Richter A, Wild B, Zhou X, et al. Allocation of carbon to fine root compounds and their residence times in a boreal forest depend on root size class and season. New Phytol. 2012;194: 972–981. 10.1111/j.1469-8137.2012.04120.x [DOI] [PubMed] [Google Scholar]
- 42.Kuptz D, Fleischmann F, Matyssek R, Grams TEE. Seasonal pat- terns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011;191: 160–172. 10.1111/j.1469-8137.2011.03676.x [DOI] [PubMed] [Google Scholar]
- 43.Millar P, Way DA. Tree competition and defense against herbivores: currency matters when counting the cost. Tree Physiol. 2011;31: 579–581. 10.1093/treephys/tpr053 [DOI] [PubMed] [Google Scholar]
- 44.Wiley E, Helliker B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012;195: 285–289. 10.1111/j.1469-8137.2012.04180.x [DOI] [PubMed] [Google Scholar]
- 45.Palacio S, Hoch G, Sala A, Körner C, Millard P. Does carbon storage limit tree growth? New Phytol. 2014;201: 1096–1100. 10.1111/nph.12602 [DOI] [PubMed] [Google Scholar]
- 46.Tytgat TOG, Verhoeven KJF, Jansen JJ, Raaijmakers CE, Bakx-Schotman T, Lauren M, et al. Plants know where it hurts: Root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS ONE. 2013;8: e65502 10.1371/journal.pone.0065502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klopotek Y, Franken P, Klaering HP, Fischer K, Hause B, Hajirezaei MR, et al. A higher sink competitiveness of the rooting zone and invertases are involved in dark stimulation of adventitious root formation in Petunia hybrida cuttings. Plant Sci. 2016;243: 10–22. 10.1016/j.plantsci.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 48.Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013;4: 272 10.3389/fpls.2013.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of harvested trees with the varying origins of new leader shoots for two clipping treatments and for four harvesting seasons. Treatments are 'Control' = unclipped trees, and 'Clipped' = clipped trees.
(DOCX)
Means of starch concentrations (mg g-1 dry weight, ±SE) in new fine roots and in fine roots of class ≤0.5 mm for two clipping treatments and for three harvesting seasons. Treatments are 'Control' = unclipped trees, and 'Clipped' = clipped trees.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.