Abstract
Background/Aims
It is important to determine the noninvasive parameters of histological features in nonalcoholic fatty liver disease (NAFLD). The aim of this study was to investigate the value of genetic variations as surrogate markers of histological features.
Methods
The parameters that affected the histological features of NAFLD were investigated in 211 Japanese patients with biopsy-proven NAFLD. The relationships between genetic variations in PNPLA3 rs738409 or TM6SF2 rs58542926 and histological features were analyzed. Furthermore, the impact of genetic variations that affected the pathological criteria for the diagnosis of nonalcoholic steatohepatitis (NASH) (Matteoni classification and NAFLD activity score) was evaluated.
Results
The fibrosis stage of PNPLA3 GG was significantly more progressive than that of CG by multiple comparisons. Multivariate analysis identified PNPLA3 genotypes as predictors of fibrosis of stage 2 or more, but the impact tended to decrease at stage 3 or greater. There were no significant differences among the histological features of the three genotypes of TM6SF2. PNPLA3 genotypes partly affected the definition of NASH by the NAFLD activity score, but TM6SF2 genotypes did not affect the definition of NASH.
Conclusions
In Japanese patients with biopsy-proven NAFLD, PNPLA3 genotypes may partly affect histological features, including stage of fibrosis, but the TM6SF2 genotype does not affect histological features.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Fibrosis stage, Patatin-like phospholipase domain containing 3, Transmembrane 6 superfamily member 2
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide across different ethnicities,1–6 and results in serious health-care issue. NAFLD includes a wide spectrum of liver pathologies ranging from nonalcoholic fatty liver, which is usually benign, to nonalcoholic steatohepatitis (NASH), which may lead to liver cirrhosis, hepatocellular carcinoma, and liver failure without excessive alcohol intake.7 Vitamin E8 and Farnesoid X nuclear receptor ligand obeticholic acid9 practically had improved the histological features. NASH can only be diagnosed by histological components, such as steatosis, lobular inflammation, ballooning, and fibrosis. Although histological diagnosis (such as steatosis, lobular inflammation, ballooning, and fibrosis) is currently the gold standard for diagnosing progressive NASH, liver biopsy has many drawbacks, such as cost, sampling error, and risk of complications.6 It is important to determine the noninvasive parameters as surrogate markers of histological features.
The severity and progression of NAFLD is influenced by a complex of multiple factors, including environmental factors and genetic variations.10 Especially, one of the most significant genetic risk factor for NAFLD is a variant located in the PNPLA3 (patatin-like phospholipase domain-containing 3) gene, the rs738409 encoding an amino acid substitution p.Ile148Met. PNPLA3 encodes a protein known as adiponutrin, and the rs738409 variant is associated with an increased hepatocyte fat content and the natural course of NAFLD, including the severity of disease.10–14 Furthermore, the recent report showed that the rs58542926 variant (encoding an amino acid substitution p.Glu167Lys) of TM6SF2 (transmembrane 6 superfamily member 2), affected hepatocytic triglyceride content (HTGC), significantly.15 The report indicated that the impact of TM6SF2 to HTGC had been independent of the impact of PNPLA3 variant, alcohol intake, insulin resistance, or obesity, and that the rs58542926 variant significantly affected the higher levels of serum alanine aminotransferase.15 However, the impact of TM6SF2 variant on the pathogenesis and genetic risk of NAFLD is still unclear.16–18
The present study included 211 patients with biopsy-proven NAFLD. The aims of the study were to investigate the relationships between genetic variations (PNPLA3 and TM6SF2 genotype) and histological features, and to analyze the impact of genetic variations as surrogate markers of histological features.
MATERIALS AND METHODS
1. Patients
A single-center retrospective cohort study was performed based on the patients of biopsy-proven NAFLD. Two hundred eleven Japanese patients were diagnosed with NAFLD by liver biopsy from 1980 to 2015 at Toranomon Hospital. NAFLD was diagnosed based on liver biopsy findings of steatosis in 5% or more of hepatocytes and the exclusion of other liver diseases (such as primary biliary cirrhosis, autoimmune hepatitis, drug induced liver disease, viral hepatitis, hemochromatosis, biliary obstruction, α-1-antitrypsin deficiency-associated liver disease, and Wilson disease). Patients consuming more than 20 g/day alcohol were excluded. The study protocol was in compliance with the Good Clinical Practice Guidelines and the 1975 Declaration of Helsinki, and was approved by the Institutional Review Board at Toranomon Hospital. All patients provided written informed consent at the time of liver biopsy.
2. Liver histology
Liver biopsy specimens were obtained using a 14-gauge modified Vim Silverman needle (Tohoku University style; Kakinuma Factory, Tokyo, Japan), a 16-gauge core tissue biopsy needle (Bard Peripheral Vascular Inc., Tempe, AZ, USA) or surgical resection. Tissue was fixed in 10% formalin, and sections were stained with hematoxylin and eosin, Masson trichrome, silver impregnation, and periodic acid-Schiff after diastase digestion. The specimens were evaluated by three pathologists (M.I., T.F., and T.F.) who were blinded to the clinical findings. An adequate liver biopsy sample was defined as a specimen of length of more than 1.5 cm and/or having more than 11 portal tracts. Specimen with steatosis of <5%, 5%–33%, 34%–66%, and >66% was scored as having steatosis grade of 0, 1, 2, and 3, respectively. Lobular inflammation of no foci, <2 foci, 2–4 foci, and >4 foci per 200× field was scored as 0, 1, 2, and 3, respectively. Hepatocyte ballooning of none, few cells, and many cells was scored as 0, 1, and 2, respectively. NAFLD activity score was the sum of steatosis, lobular inflammation, and hepatocyte ballooning scores (range, 0–8 points; 5–8 points as definition of NASH). Fibrosis stage of none, zone 3 perisinusoidal fibrosis (stage 1), zone 3 perisinusoidal fibrosis with portal fibrosis (stage 2), zone 3 perisinusoidal fibrosis and portal fibrosis with bridging fibrosis (stage 3), and cirrhosis (stage 4) was scored as 0, 1, 2, 3, and 4, respectively.19,20 Patients were also classified into four categories by histology according to the classification by Matteoni et al.21 as follows: type 1, fatty liver alone; type 2, fat accumulation and lobular inflammation; type 3, fat accumulation and ballooning degeneration; type 4, fat accumulation, ballooning degeneration, and either Mallory-Denk body or fibrosis (type 3 or 4 as definition of NASH).
3. Determination of PNPLA3 and TM6SF2 genotype
PNPLA3 rs738409 and TM6SF2 rs58542926 were genotyped by the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA).
4. Clinical parameters
Table 1 summarizes the patients’ characteristics at the biopsy of 211 patients, and these factors were investigated to determine the parameters that affected to histological features of NAFLD. Normal range of aspartate aminotransferase (AST) was evaluated as 13 to 33 IU/L. Normal range of alanine aminotransferase (ALT) was evaluated as 8 to 42 IU/L for male, and 6 to 27 IU/L for female. Obesity was defined as a body mass index (BMI) of more than 25.0 kg/m2. Cardiovascular disease (CVD) risk was investigated by exploring the total cholesterol (TC)/high-density lipoprotein cholesterol (HDL-C) ratio.22
Table 1.
Biopsy Characteristics of 211 Patients Diagnosed with Nonalcoholic Fatty Liver Disease
| Characteristic | Value |
|---|---|
| Demographic data | |
| No. of patients | 211 |
| Gender, male/female | 122/89 |
| Age, yr* | 52 (20–85) |
| Body mass index, kg/m2* | 25.9 (18.1–40.4) |
| Histological findings | |
| Steatosis, 5%–33%/33%–66%/>66% | 80/80/47 |
| Lobular inflammation, no foci/<2 foci/2–4 foci/>4 foci per 200× field | 25/113/58/11 |
| Ballooning, none/few cells/many cells | 28/113/66 |
| Stage, 0/1/2/3/4 | 30/82/25/56/18 |
| Matteoni classification, type 1/2/3/4 | 15/11/7/174 |
| NAFLD activity score, ≤2/3,4/≥5 | 31/81/99 |
| Genetic variation | |
| PNPLA3 rs738409, CC/CG/GG/not determined | 21/60/59/71 |
| TM6SF2 rs58542926, CC/CT/TT/not determined | 104/33/2/72 |
| Laboratory data* | |
| Serum aspartate aminotransferase, IU/L | 51 (12–312) |
| Serum alanine aminotransferase, IU/L | 78 (15–338) |
| γ-Glutamyl transpeptidase, IU/L | 68 (11–605) |
| Serum albumin, g/dL | 4.1 (2.8–5.8) |
| Platelet count, ×104/mm3 | 21.0 (4.5–38.9) |
| Fasting plasma glucose, mg/dL | 100 (65–273) |
| Uric acid, mg/dL | 5.9 (2.7–10.6) |
| Total cholesterol, mg/dL | 206 (101–370) |
| Triglycerides, mg/dL | 134 (31–610) |
| High-density lipoprotein cholesterol, mg/dL | 45 (14–82) |
| Low-density lipoprotein cholesterol, mg/dL | 125 (28–243) |
| Total cholesterol/high density lipoprotein cholesterol | 4.6 (1.7–10.3) |
| Serum ferritin, μg/L | 231 (10–1,474) |
| Hyaluronic acid, μg/L | 35 (1–814) |
| High sensitive C-reactive protein, mg/dL | 0.097 (0.006–2.240) |
| Type IV collagen 7S, ng/mL | 4.2 (2.0–11.0) |
The data displayed represent the number of patients, except those denoted by *, which represent the median (range) values.
NAFLD, nonalcoholic fatty liver disease.
5. Statistical analysis
Nonparametric tests (chi-squared test, Fisher exact probability test, and Mann-Whitney U test) were used to compare the characteristics of the groups. Multiple comparisons were examined by the Bonferroni test. Univariate and multivariate logistic regression analyses were used to determine those factors that significantly affected to histological features. The odds ratios (ORs) and 95% confidence intervals were also calculated. All p-values less than 0.05 by the two-tailed test were considered significant. Variables that achieved statistical significance (p<0.05) on univariate analysis were entered into multiple logistic regression analysis to identify significant independent predictive factors. Each variable was transformed into categorical data consisting of two simple ordinal numbers for univariate and multivariate analyses. Statistical analyses were performed using the SPSS software version 2 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Genetic variations and fibrosis stage
Among 211 patients, 140 could be evaluated PNPLA3 and TM6SF2 genotype. Table 2 indicated histological features, according to PNPLA3 and TM6SF2 genotype.
Table 2.
The Histological Features of Patients Diagnosed with Nonalcoholic Fatty Liver Disease according to PNPLA3 rs738409 and TM6SF2 rs58542926 Genotype
| PNPLA3 rs738409 | p-value* | |||
|---|---|---|---|---|
|
| ||||
| CC | CG | GG | ||
| Steatosis, 5%–33%/>33%–66%/>66% | 8/11/2 | 23/26/10 | 27/17/14 | 0.270 |
| Lobular inflammation, no foci/<2 foci/2–4 foci/>4 foci per 200× field | 1/14/6/0 | 7/33/18/1 | 6/24/20/8 | 0.074 |
| Ballooning, none/few cells/many cells | 3/11/7 | 8/38/13 | 5/25/28 | 0.060 |
| Stage, 0/1/2/3/4 | 2/12/0/3/4 | 10/27/7/14/2 | 5/11/11/24/8† | 0.001 |
| Matteoni classification, type 1/2/3/4 | 1/2/0/18 | 4/3/3/49 | 2/2/2/52 | 0.805 |
| NAFLD activity score, ≤2/3,4/≥5 | 2/12/7 | 9/26/25 | 6/17/36 | 0.100 |
|
| ||||
| TM6SF2 rs58542926 | p-value* | |||
|
|
||||
| CC | CT | TT | ||
|
| ||||
| Steatosis, 5%–33%/>33%–66%/>66% | 42/41/19 | 15/12/6 | 1/0/1 | 0.727 |
| Lobular inflammation, no foci/<2 foci/2–4 foci/>4 foci per 200× field | 9/53/35/5 | 5/17/8/3 | 0/1/1/0 | 0.803 |
| Ballooning, none/few cells/many cells | 11/57/34 | 5/15/13 | 0/2/0 | 0.574 |
| Stage, 0/1/2/3/4 | 13/38/12/31/10 | 4/11/5/9/4 | 0/1/0/1/0 | 0.990 |
| Matteoni classification, type 1/2/3/4 | 5/5/4/88 | 2/2/1/28 | 0/0/0/2 | 0.998 |
| NAFLD activity score, ≤2/3,4/≥5 | 13/40/51 | 4/14/15 | 0/1/1 | 0.976 |
NAFLD, nonalcoholic fatty liver disease.
Histological features were compared among the three genotypes of PNPLA3 and TM6SF2;
p=0.001, compared with the CG genotype by Bonferroni test.
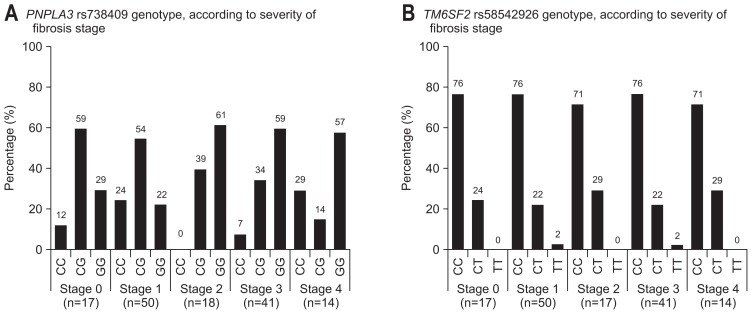
Fig. 1A shows the distribution of PNPLA3 rs738409 genotype, according to severity of fibrosis stage. PNPLA3 genotype partly affected to severity of fibrosis stage (stage 0 vs 1–4, p=0.362; stage 0–1 vs 2–4, p<0.001; stage 0–2 vs 3–4, p=0.007; stage 0–3 vs 4, p=0.058). There were significant differences in fibrosis stage among the three genotypes of PNPLA3 (p=0.001) (Table 2). Especially, fibrosis stage of genotype GG was significantly more progressive than that of genotype CG by multiple comparisons (p=0.001; Bonferroni test) (Table 2). Fig. 1B shows the distribution of TM6SF2 rs58542926 genotype, according to severity of fibrosis stage. TM6SF2 genotype did not affect to severity of fibrosis stage (stage 0 vs 1–4, p=0.867; stage 0–1 vs 2–4, p=0.936; stage 0–2 vs 3–4, p=0.955; Stage 0–3 vs 4, p=0.818). There were no significant differences in fibrosis stage among the three genotypes of TM6SF2 (p=0.990) (Table 2).
Fig. 1.
(A) The distribution of PNPLA3 rs738409 genotypes, according to severity of fibrosis stage, is shown. The PNPLA3 genotype partly affected the severity of fibrosis stage (stage 0 vs 1–4, p=0.362; stage 0–1 vs 2–4, p<0.001; stage 0–2 vs 3–4, p=0.007; stage 0–3 vs 4, p=0.058). (B) The distribution of TM6SF2 rs58542926 genotypes, according to severity of fibrosis stage, is shown. TM6SF2 genotypes did not affect the severity of fibrosis stage (stage 0 vs 1–4, p=0.867; stage 0–1 vs 2–4, p=0.936; stage 0–2 vs 3–4, p=0.955; stage 0–3 vs 4, p=0.818).
2. Factors associated with fibrosis stage, according to severity of fibrosis
Table 3 indicated the factors associated with fibrosis stage, according to severity of fibrosis. Multivariate analysis identified four parameters that independently influenced fibrosis stage 1 or more: AST (≥1.5×ULN; OR, 20.0; p=0.001), high-density lipoprotein cholesterol (<41 mg/dL; OR, 9.17; p=0.012), ferritin (≥191 μg/L; OR, 4.92; p=0.023), and age (≥55 years; OR, 4.35; p=0.030). Multivariate analysis identified six parameters that independently influenced fibrosis stage 2 or more: GGT (<219 IU/L; OR, 71.4; p=0.007), PNPLA3 rs738409 genotype (CG; OR, 18.8; p=0.008; and GG; OR, 38.2; p=0.001), low-density lipoprotein cholesterol (LDL-C) (<86 mg/dL; OR, 28.6; p=0.021), platelet count (<15.0×104/mm3; OR, 21.7; p=0.028), hyaluronic acid (≥51 μg/L; OR, 7.40; p=0.014), and AST (≥1.5×ULN; OR, 5.26; p=0.015). Multivariate analysis identified four parameters that independently influenced fibrosis stage 3 or more: AST (≥1.5×ULN; OR, 14.3; p=0.002), fasting plasma glucose (≥126 mg/dL; OR, 11.9; p=0.011), LDL-C (<86 mg/dL; OR, 11.9; p=0.040), and hyaluronic acid (≥51 μg/L; OR, 8.52; p=0.002). Multivariate analysis identified two parameters that independently influenced fibrosis stage 4: LDL-C (<86 mg/dL; OR, 41.7; p=0.003) and platelet count (<15.0×104/mm3; OR, 20.4; p=0.002).
Table 3.
Multivariate Analysis of Factors Associated with Fibrosis Stage, according to the Severity of Fibrosis in 211 Patients Diagnosed with Nonalcoholic Fatty Liver Disease
| Factor | Category | OR | 95% CI | p-value |
|---|---|---|---|---|
| Factors associated with stage 1 or more | ||||
| Serum aspartate aminotransferase, IU/L | <1.5×ULN | 1 | ||
| ≥1.5×ULN | 20.0 | 3.48–115 | 0.001 | |
| High-density lipoprotein cholesterol, mg/dL | ≥41 | 1 | ||
| <41 | 9.17 | 1.63–52.6 | 0.012 | |
| Serum ferritin, μg/L | <191 | 1 | ||
| ≥191 | 4.92 | 1.25–19.4 | 0.023 | |
| Age, yr | <55 | 1 | ||
| ≥55 | 4.35 | 1.15–16.4 | 0.030 | |
| Factors associated with stage 2 or more | ||||
| γ-Glutamyl transpeptidase, IU/L | ≥219 | 1 | ||
| <219 | 71.4 | 3.17–1,000 | 0.007 | |
| PNPLA3 rs738409 genotype | CC | 1 | ||
| CG | 18.8 | 2.11–166 | 0.008 | |
| GG | 38.2 | 4.29–341 | 0.001 | |
| Low-density lipoprotein cholesterol, mg/dL | ≥86 | 1 | ||
| <86 | 28.6 | 1.66–500 | 0.021 | |
| Platelet count, ×104/mm3 | ≥15.0 | 1 | ||
| <15.0 | 21.7 | 1.39–333 | 0.028 | |
| Hyaluronic acid, μg/L | <51 | 1 | ||
| ≥51 | 7.40 | 1.51–36.4 | 0.014 | |
| Serum aspartate aminotransferase, IU/L | <1.5×ULN | 1 | ||
| ≥1.5×ULN | 5.26 | 1.38–20.1 | 0.015 | |
| Factors associated with stage 3 or more | ||||
| Serum aspartate aminotransferase, IU/L | <1.5×ULN | 1 | ||
| ≥1.5×ULN | 14.3 | 2.64–77.6 | 0.002 | |
| Fasting plasma glucose, mg/dL | <126 | 1 | ||
| ≥126 | 11.9 | 1.77–80.1 | 0.011 | |
| Low-density lipoprotein cholesterol, mg/dL | ≥86 | 1 | ||
| <86 | 11.9 | 1.11–125 | 0.040 | |
| Hyaluronic acid, μg/L | <51 | 1 | ||
| ≥51 | 8.52 | 2.19–33.2 | 0.002 | |
| Factors associated with stage 4 | ||||
| Low-density lipoprotein cholesterol, mg/dL | ≥86 | 1 | ||
| <86 | 41.7 | 3.55–500 | 0.003 | |
| Platelet count, ×104/mm3 | ≥15.0 | 1 | ||
| <15.0 | 20.4 | 3.05–143 | 0.002 | |
OR, odds ratio; CI, confidence interval; ULN, the upper limit of normal.
Thus, PNPLA3 genotype was identified as the predictors of fibrosis stage 2 or more, but the impact tended to decrease on stage 3 or more.
3. Genetic variations and Matteoni classification, NAFLD activity score
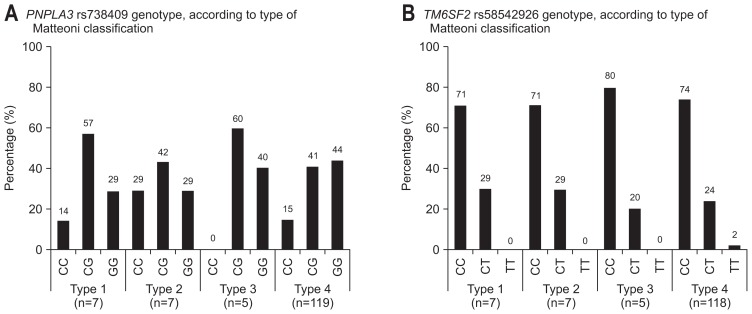
Fig. 2A shows the distribution of PNPLA3 rs738409 genotype, according to type of Matteoni classification. PNPLA3 genotype did not affect to type of Matteoni classification (type 1 vs 2–4, p=0.712; type 1–2 vs 3–4, p=0.533; type 1–3 vs 4, p=0.721). There were no significant differences in type of Matteoni classification among the three genotypes (p=0.805) (Table 2). Fig. 2B shows the distribution of TM6SF2 rs58542926 genotype, according to type of Matteoni classification. TM6SF2 genotype did not affect to type of Matteoni classification (type 1 vs 2–4, p=0.915; type 1–2 vs 3–4, p=0.828; type 1–3 vs 4, p=0.805). There were no significant differences in type of Matteoni classification among the three genotypes (p=0.998) (Table 2).
Fig. 2.
(A) The distribution of PNPLA3 rs738409 genotypes, according to type of Matteoni classification, is shown. PNPLA3 genotypes did not affect the type of Matteoni classification (type 1 vs 2–4, p=0.712; type 1–2 vs 3–4, p=0.533; type 1–3 vs 4, p=0.721). (B) The distribution of TM6SF2 rs58542926 genotypes, according to type of Matteoni classification, is shown. The TM6SF2 genotype did not affect the type of Matteoni classification (type 1 vs 2–4, p=0.915; type 1–2 vs 3–4, p=0.828; type 1–3 vs 4, p=0.805).
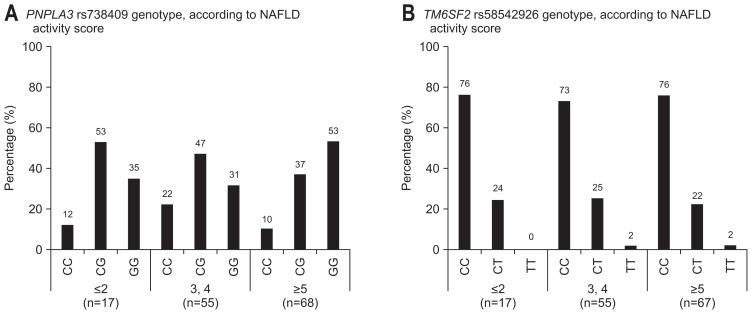
Fig. 3A shows the distribution of PNPLA3 rs738409 genotype, according to NAFLD activity score. PNPLA3 genotype partly affected to NAFLD activity score (≤2 vs ≥3, p=0.667; ≤4 vs ≥5, p=0.034). There were no significant differences in NAFLD activity score among the three genotypes (p=0.100) (Table 2). Fig. 3B shows the distribution of TM6SF2 rs58542926 genotype, according to NAFLD activity score. TM6SF2 genotype did not affect to NAFLD activity score (≤2 vs ≥3, p=0.867; ≤4 vs ≥5, p=0.936). There were no significant differences in NAFLD activity score among the three genotypes (p=0.976) (Table 2).
Fig. 3.
(A) The distribution of PNPLA3 rs738409 genotypes, according to nonalcoholic fatty liver disease (NAFLD) activity score, is shown. The PNPLA3 genotype partly affected the NAFLD activity score (≤2 vs ≥3, p=0.667; ≤4 vs ≥5, p=0.034). (B) The distribution of TM6SF2 rs58542926 genotypes, according to NAFLD activity score, is shown. The TM6SF2 genotype did not affect the NAFLD activity score (≤2 vs ≥3, p=0.867; ≤4 vs ≥5, p=0.936).
DISCUSSION
The impact of PNPLA3 genotype on the genetic risk and disease progression of NAFLD might appear to be consistent.11–14 Hotta and coworkers13 reported that the G-allele of rs738409 was significantly associated with increases in fibrosis stage in the patients with NAFLD, even after adjustment for age, gender, and BMI. Consistent with data previously reported,12 the present study indicated that fibrosis stage of genotype GG was significantly more progressive than that of genotype CG by multiple comparisons. Interestingly, PNPLA3 genotype was identified as the predictors of fibrosis stage 2 or more, but was not as the predictors of stage 3 or more. To our knowledge, this is the first report to investigate the different impact of PNPLA3 genotype, according to severity of fibrosis stage. PNPLA3 variant might be a strong modifier of the natural course of NAFLD, by modulating hepatocyte fat deposition and the severity of disease.11–14 The present study might suggest that the impact of PNPLA3 variant tended to decrease on patients with severe fibrosis stage (such as patients with burned-out NASH, in whom fatty changes and inflammatory cell infiltration resolving in fibrosis has progressed).6 One limitation of the present study based on NAFLD patients, not including control subjects (steatosis in <5% of hepatocytes), is that it could not be exactly evaluated whether PNPLA3 variant might affect to the presence of steatosis. Further large-scale studies based on the patients with NAFLD, including burned-out NASH, should be performed to explore the complicated relationships among PNPLA3 genotype, steatosis, and fibrosis stage.
The impact of TM6SF2 genotype on NAFLD is controversial.15–18 Liu and coworkers16 reported that TM6SF2 genotype affected necroinflammation score, but they could not replicate this result in their large-scale validation cohort, unfortunately. On the other hand, Liu et al.16 indicated that TM6SF2 genotype significantly affected liver fibrosis stage in the two explored cohorts by adopting an additive model. On the contrary, Wong and coworkers17 showed that TM6SF2 genotype might be not associated either with fibrosis stage or with hepatic fat accumulation in their large-scale study. This is the first report to investigate the impact of TM6SF2 genotype on NAFLD from Japan. TM6SF2 genotype was not associated with fibrosis stage, and pathological criteria for the diagnosis of NASH (Matteoni classification and NAFLD activity score). The present study, based on the small number of NAFLD patients, has some limitations. It was a hospital-based study and a retrospective cohort study, which might include the selection bias. All of participants were Japanese, and there is a possibility that the present results might not be applicable for NAFLD patients of the other races or ethnic groups.
Consistent with data previously reported,15 this preliminary study based on the small number of TM6SF2 rs58542926 genotype TT (only two patients) indicated that the T-allele was associated with decreases in serum levels of triglycerides in the patients with NAFLD (data not shown). Specifically, serum levels of triglyceride in genotype non CC (median, 112 mg/dL) tended to be lower than those of genotype CC (median, 138 mg/dL) (p=0.056, Mann-Whitney U test). Previous report indicated that the TM6SF2 variant was significantly associated with HTGC, and the impact of that was independent of the effect mediated by the PNPLA3 variant.15 The limitation of present study was that relationships between the TM6SF2 variant and HTGC could not be investigated. Further study should be performed to investigate the complicated relationships among TM6SF2 genotype, lipid metabolism, and histological features in NAFLD patients.
Although histological diagnosis is currently the gold standard for diagnosing progressive NASH, liver biopsy has many drawbacks, such as cost, sampling error, and risk of complications.6 It is important to determine the clinical parameters as surrogate markers of histological features. Furthermore, inter- and intraobserver variability, and pathological diagnosis also presents the serious problems for the histological diagnosis of NASH.6 To minimize these shortfalls, the impact of genetic variations that affected to pathological criteria for the diagnosis of NASH (Matteoni classification and NAFLD activity score) was evaluated. Kawaguchi and coworkers14 reported that Matteoni type 4 NAFLD was both a genetically and clinically different subset from the other spectrums of the disease and that the PNPLA3 gene was strongly associated with the progression of NASH in Japanese population. Consistent with data previously reported,14 the present study showed that PNPLA3 genotype partly affected to definition of NASH by NAFLD activity score (5 to 8 points), but TM6SF2 genotype did not affect to definition of NASH by Matteoni classification and NAFLD activity score. The limitation of the present study, based on the small number of patients, was that there were problems with pathological criteria for the diagnosis of NASH. Specifically, factor of inflammation was not included in the definition of NASH by Matteoni classification, and factor of fibrosis was not included in the definition of NASH by NAFLD activity score.6 As previously reported,23–26 further large-scale study should be performed to develop the simple clinical scoring system useful for noninvasive diagnosis of the differentiation between NASH and simple steatosis, the diagnosis of NASH with advanced fibrosis, and the prediction of hepatocarcinogenesis.
In conclusion, genetic variations might partly affect histological changes. PNPLA3 genotype might partly affect to histological features including fibrosis stage, but TM6SF2 genotype did not affect to histological features in Japanese patients with biopsy-proven NAFLD. Further comprehensive studies, based on the larger number of patients, should be performed to disclose the molecular mechanisms for the complicated relationships between the impact of PNPLA3, TM6SF2 variant on the genetic risk and pathogenesis of NAFLD.
ACKNOWLEDGEMENTS
Author contributions: N.A., Y.K., Y.A., F.S., H.S., T.H., M.K., M.K., S.S., Y.S., K.I., H.K. contributed to this work. N.A., Y.K. analyzed the data. N.A. wrote the paper. N.A., Y.K., Y.A., F.S., H.S., T.H., M.K., S.S., Y.S., K.I., H.K. provided the sample.
The authors thank the following individuals for assistance in pathological diagnosis: Masafumi Inoue, M.D., Department of Pathology, Toranomon Hospital; Toshio Fukusato, M.D., Department of Pathology, Teikyo University School of Medicine; and Takeshi Fujii, M.D., Department of Pathology, Toranomon Hospital.
Footnotes
CONFLICTS OF INTEREST
(1) Norio Akuta has received speakers’ bureau from MSD K.K., Mitsubishi Tanabe Pharma, Janssen Pharmaceutical K.K., Bristol-Myers Squibb, and holds a right to get some loyalty from SRL, Inc. (2) Hiromitsu Kumada has received speakers’ bureau from MSD K.K., Mitsubishi Tanabe Pharma, Dainippon Sumitomo Pharma, Bristol-Myers Squibb, Janssen Pharmaceutical K.K., GlaxoSmithKline K.K., and holds a right to get some loyalty from SRL., Inc. (3) Fumitaka Suzuki has received speakers’ bureau from Bristol-Myers Squibb. (4) Yoshiyuki Suzuki has received speakers’ bureau from Bristol-Myers Squibb. (5) Yasuji Arase has received speakers’ bureau from MSD K.K. (5) Kenji Ikeda has received speakers’ bureau from Dainippon Sumitomo Pharma, Eisai Co., Ltd., Olympus Co. The other authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 6.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sookoian S, Pirola CJ. The genetic epidemiology of nonalcoholic fatty liver disease: toward a personalized medicine. Clin Liver Dis. 2012;16:467–485. doi: 10.1016/j.cld.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50:2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotta K, Yoneda M, Hyogo H, et al. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi T, Sumida Y, Umemura A, et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One. 2012;7:e38322. doi: 10.1371/journal.pone.0038322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong VW, Wong GL, Tse CH, Chan HL. Prevalence of the TM6SF2 variant and non-alcoholic fatty liver disease in Chinese. J Hepatol. 2014;61:708–709. doi: 10.1016/j.jhep.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Sookoian S, Castaño GO, Scian R, et al. Genetic variation in trans-membrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61:515–525. doi: 10.1002/hep.27556. [DOI] [PubMed] [Google Scholar]
- 19.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, Mc-Cullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 22.Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.CIR.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 23.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 24.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 25.Sumida Y, Yoneda M, Hyogo H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 26.Kessoku T, Ogawa Y, Yoneda M, et al. Simple scoring system for predicting cirrhosis in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:10108–10114. doi: 10.3748/wjg.v20.i29.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]