Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive adult primary brain cancer, with <10% of patients surviving for more than 3 years. Demographic and clinical factors (e.g. age) and individual molecular biomarkers have been associated with prolonged survival in GBM patients. However, comprehensive systems-level analyses of molecular profiles associated with long-term survival (LTS) in GBM patients are still lacking. We present an integrative study of molecular data and clinical variables in these long-term survivors (LTSs, patients surviving >3 years) to identify biomarkers associated with prolonged survival, and to assess the possible similarity of molecular characteristics between LGG and LTS GBM. We analyzed the relationship between multivariable molecular data and LTS in GBM patients from the Cancer Genome Atlas (TCGA), including germline and somatic point mutation, gene expression, DNA methylation, copy number variation (CNV) and microRNA (miRNA) expression using logistic regression models. The molecular relationship between GBM LTS and LGG tumors was examined through cluster analysis. We identified 13, 94, 43, 29, and 1 significant predictors of LTS using Lasso logistic regression from the somatic point mutation, gene expression, DNA methylation, CNV, and miRNA expression data sets, respectively. Individually, DNA methylation provided the best prediction performance (AUC = 0.84). Combining multiple classes of molecular data into joint regression models did not improve prediction accuracy, but did identify additional genes that were not significantly predictive in individual models. PCA and clustering analyses showed that GBM LTS typically had gene expression profiles similar to non-LTS GBM. Furthermore, cluster analysis did not identify a close affinity between LTS GBM and LGG, nor did we find a significant association between LTS and secondary GBM. The absence of unique LTS profiles and the lack of similarity between LTS GBM and LGG, indicates that there are multiple genetic and epigenetic pathways to LTS in GBM patients.
Introduction
Glioblastoma multiforme (GBM) is the most frequent malignant form of primary brain cancer in adults. The median survival time for GBM patients is approximately 14 months with intensive multimodal therapy that includes surgical resection, chemotherapy, and radiotherapy [1] after initial diagnosis. GBM can develop both de novo or via progression from primary low grade glioma (LGG). A small proportion of patients survive for exceptionally long periods of time; for example, fewer than 10% of GBM patients survive more than 3 years [2–4]. Therefore, studying the clinical and molecular characteristics of these rare instances of long-term survival (LTS) among GBM patients may provide insights into both the molecular basis of GBM progression and identify potential new prognostic biomarkers.
Several patient characteristics like age, performance status and tumor localization have been identified as predictors of survival time [4–7]. However, many studies have often attributed the causes of LTS in GBM to erroneous histopathological diagnosis (i.e. misidentification of low-grade gliomas as GBM) or as statistical anomalies [8–10]. With advances in microarray and sequencing technologies, associations of molecular markers such as mutations, gene expression levels, DNA methylation states, and microRNAs with LTS tumors have been reported [2, 3, 11–21]. Using these techniques, MGMT hypermethylation and mutations in isocitrate dehydrogenase (IDH1) have been the most frequently identified genomic marker of improved patient response to chemotherapy and therefore longer patient survival [12, 22, 23]. Unfortunately, many of these studies have only independently considered a single class of molecular marker, so that integrative studies of multiple types of molecular marks specifically associated with LTS are still lacking. In [21], the transcriptional profiles of 7 LTS patients were compared to non-LTS, their study found no association between LTS and transcriptional subtype. The analysis in [21] was qualitative; there have been no model-based analyses integrating different classes of genomic data to systematically determine whether LTS cases simply represent extreme outliers of a distribution defined by what is effectively a single pathology, or, alternatively, if they represent a biologically distinct class of GBMs with unique genomic, epigenetic, and phenotypic characteristics. Similarly, other analyses (e.g. [24]) have identified correlations between genomic alterations and GBM patient survival times, the analysis and markers were not specific to LTS patients.
If LTS cases do have unique molecular characteristics among GBMs, there is also the potential for similarity between LTS GBM and LGG at the molecular level. There are two reasons to seriously investigate the hypothesis that LTS GBMs share molecular profiles with LGG. First, it is often suggested that many LTS GBMs are misdiagnosed instances of LGG. Second, the best-known genomic predictors of improved responses to temozolomide (TMZ) chemotherapy are mutations in IDH1 and methylation of the MGMT promoter, which are themselves frequently associated with secondary GBM (those that have progressed from LGG, as opposed to de novo GBM [25]). These and similar observations suggest that secondary GBMs may retain additional genomic traits and have clinical features that are more typical of LGG. We will determine whether this is indeed the case, and whether these secondary GBMs are also significantly characterized by LTS.
In this study, we leverage 6 types of molecular data from The Cancer Genome Atlas (TCGA) GBM samples, including germline and somatic point mutation, gene expression, DNA methylation, copy number variation (CNV) and microRNA (miRNA) expression data. We use machine learning to identify molecular markers characteristic of LTS in GBM, and construct integrative models that incorporate multiple sets of molecular profiles that are jointly predictive of LTS when combined with clinical and demographic data. We also explore whether LTS cases of GBM have molecular characteristics typical of LGG by comparing the similarity of LTS tumor genetic profiles to LGG. The results of our analyses have important implications for our understanding of the molecular pathology of GBM, as well as providing insight into the design of novel prognostic and therapeutics indicators.
Material and Methods
Classification of LTS Phenotypes
Clinical and demographic data describing patient age at initial diagnosis, gender, ethnicity/race, treatment history, vital status and follow-up/survival times were collected from TCGA [26] (Table 1). We used Kaplan-Meier survival analysis to build survival curves and identify LTS patients. In keeping with criteria for LTS used in the clinical literature (e.g. [2–4]), we used a three-year threshold survival time, regardless of vital status, in order to classify patients as LTS. Actual survival times were not used in the analysis, only the binary LTS/non-LTS classification. Only patients with documented survival times of less than 3 years were considered to be non-LTS patients in our study; patients with uncertain status beyond the 3 year point were excluded. For comparison, a more stringent cutoff of 1615 days (4.5 years, defining the upper 5% survival time in TCGA’s GBM data set) was also applied as an alternative criterion to classify patients as LTS and non-LTS. We also considered GBM cases with a prior history of LGG as a separate class of data, i.e. these secondary GBMs represent a set of patients who, in contrast to most GBM cases, have likely previously received chemotherapy and/or radiation therapy for the earlier cancer. Both treatment types have the potential to induce distinct genetic and epigenetic profiles.
Table 1. Summary of clinical and demographical information of the TCGA patient cohort used for this study.
| Total number of patients | 591 |
| Clinical outcomes | |
| Overall survival | 0–10.6 years |
| Median survival | 0.9 year |
| Event(Alive/Dead) | 146/443 |
| Classifications | |
| LTS (survival > 3 years) | 44 |
| nonLTS(survival < 3 years and dead) | 411 |
| Censored (survival < 3 years and alive) | 136 |
| Clinical Covariates | |
| Age at initial diagnosis | 10–89 (median 59) |
| Race (white/Asian/Black) | 503/13/50 |
| Gender (Female/Male) | 228/363 |
| History of LGG diagnosis (Yes/No) | 15/576 |
Genomic data processing
All genomic/molecular data, including exome sequences, germline mutations, probes for microarray gene expression, DNA methylation, CNV and miRNA expression were retrieved from TCGA for both GBM and LGG patients. The platforms and levels of data are summarized in Table 2. Somatic point mutations were called from the whole exome bam files using the pipeline described in [27], where SomaticSniper [28] was used to call mutations, the output was filtered for read quality with a custom Python script. Somatic point mutations were classified as missense, nonsense, silent, etc. using snpEff [29]. Non-silent somatic mutations that were identified in more than one tumor were retained as candidate predictors of survival time for subsequent inclusion in our models. Additionally, germline single nucleotide polymorphisms (SNPs) were identified from the Level II TCGA SNP data, these were processed to exclude low quality genotype calls and rare alleles, using a pipeline described in [30].
Table 2. Summary of the 6 types of molecular data and their platforms used for this study.
| Platform | Number of patients | LTS/non-LTS | Total features | ULR features | LLR features | |
|---|---|---|---|---|---|---|
| Germline Mutation | Affimetrix Genome Wide SNP6 array | 346 | 30/316 | 532954 | 0 | 0 |
| Somatic Mutation | Illumina Genome Analyzer DNA Sequencing | 187 | 18/169 | 1419 | 10 | 13 |
| Gene expression | Affymetrix Human Genome U133 Plus 2.0 Array | 415 | 39/376 | 22277 | 38 | 94 |
| DNA methylation | Illumina Infinium Human DNA Methylation 27/450 | 283 | 22/261 | 23233 | 38 | 43 |
| miRNA | Agilent 8X15 k human miRNA-specific microarray | 437 | 43/394 | 534 | 1 | 1 |
| CNV | Agilent Human Genome CGH Microarray 244A | 417 | 43/374 | 23169 | 11 | 29 |
Level 1 gene expression data were collected for GBM, LGG, and normal tissue samples from TCGA. The expression data was RMA adjusted [31] and transformed to a base-2 logarithmic scale. Level 3 DNA methylation data from two platforms (Illumina methylation arrays 27 and 450) were combined by intersecting the probe sets, excluding 10.1% of samples with more than 5% missing values. Missing values in the remaining probes were imputed using the median value across samples. The level 3 miRNA expression data was used without any further processing. For the Level 3 CNV data, a weighted average CNV score was computed if a gene spanned multiple segments of a CNV probe, with score weights proportional to the fraction of the gene spanned by each probe. Unless otherwise indicated, the data processing and all analyses were implemented in Python 2.7.5 and R 3.0.3.
Regression Analysis
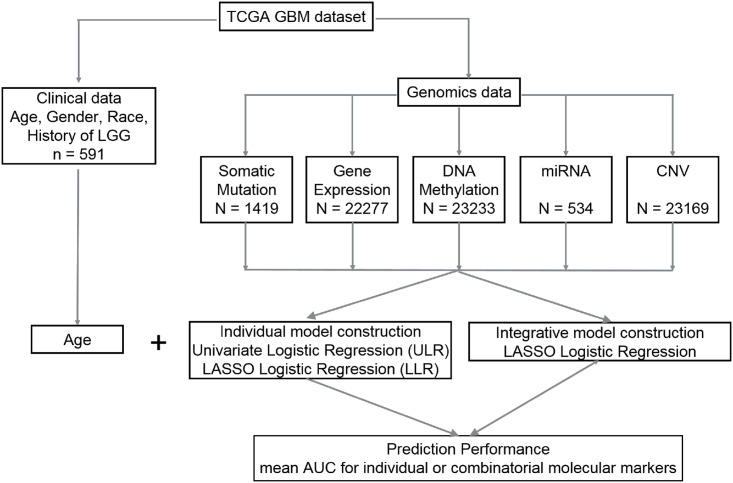
Prediction of LTS is a statistical binary classification problem. Models were individually constructed for each molecular data type using both False Discovery Rate (FDR)—adjusted univariate logistic regression (ULR) and Lasso logistic regression (LLR). Integrative models that combine clinical variables with one or more types of molecular profiles were constructed using LLR. The individual and integrative model construction procedures are schematically represented in Fig 1.
Fig 1. Flow chart with a schematic of the data analysis pipeline used in this study.
Univariate logistic regression (ULR). A univariate logistic regression model was fitted for each gene or probe in every class of genomic data, with the genotype at each variant site as a predictor Y = 0,1 (non-LTS vs. LTS) with p-values adjusted using a Bonferroni correction for each class of data (in cases with limited data or where no significant associations were found following Bonferonni correction, the less stringent Benjamini-Hochberg adjustment was applied). Significantly LTS-associated features were selected with Bonferroni-adjusted q < 0.05. On tests performed on individual traits, the unadjusted p < 0.05 was used for feature selection.
Lasso logistic regression (LLR). Least absolute shrinkage and selection operator (Lasso) is a penalized multivariable regression model whereby parameter shrinkage and feature selection are done simultaneously [32]. Lasso imposes a penalty on the regression coefficients β = (β1, …, βp) by restricting the sum of the absolute values (L1 norm) of the coefficients βj to values no greater than the shrinkage parameter λ. By selecting an appropriate λ, a Lasso model can be tuned to include any number of variables in the final regression model; smaller values of λ will set more coefficients βj to zero, effectively removing them from the model. We implemented Lasso logistic regression using the ‘glmnet’ library in R with the binomial distribution option, reflecting the binary response variable Y. λ was selected using 10-fold cross validation so that the model minimizes cross-validation error. Prediction performance was evaluated with Area Under the Receiver Operating Characteristic Curve (AUC) estimation, a commonly used evaluation metric for binary classification. A perfect model will score an AUC of 1, while at the other extreme an AUC near 0.5 reflects models with no predictive power that essentially select Y = 0,1 by a random guess.
Integrative Models. Integrative LASSO logistic regression models were constructed by using 4 classes of molecular data in combination as predictors of LTS, i.e. clinical information, gene expression levels, DNA methylation scores, CNV counts, and miRNA expression levels were used in combination to identify subsets of molecular markers that were jointly predictive of LTS. To prevent overfitting, initial feature selection was performed for each class of data by selecting all variables with unadjusted p < 0.05 in the ULR models; only those genes/probes above the threshold were pooled for multiple regression analysis. We identified a core set of n = 212 samples (including 23 LTSs) with all molecular data types represented except for point mutations. Both somatic and germline point mutations were excluded from the combined data sets because no point mutations were significant predictors of LTS in adjusted ULR models and their inclusion would have produced a much smaller sample set since so few tumors contained any particular somatic mutation. Consequently, separate regression analyses were performed with clinical information and point mutation genotypes as predictors of LTS.
A total of 69213 features representing the 4 classes of molecular data from 212 patients were jointly modeled using LASSO logistic regression. Prior to model fitting, each variable was z-transformed to zero mean and unit variance so that variables across different classes of data would be on the same scale. We also constructed regression models using specific combinations of data classes and excluding others (e.g. gene expression + methylation data used to predict LTS while excluding CNV and miRNA data etc), as this allows us to compare predictive performance of models and to determine the marginal effects of incorporating additional data classes on LTS prediction. To evaluate the prediction performance for individual models, we performed tenfold cross-validation and computed the mean AUC over 100 iterations. In every iteration, the data set was divided into 10 subsets, and the LLR was repeated 10 times. One of the 10 subsets was used as the test set and the other 9 subsets were pooled to form a training set in order to compute the average AUC across all 100 iterations. The advantage of this method is that it minimizes the bias from the division of data into training and test sets.
Imbalanced Sampling and Bootstrapping
Because LTS account for <10% of GBM samples, logistic and LASSO regression analyses of the entire data set by necessity use imbalanced data, which can potentially bias estimation and prediction in logistic regression and machine-learning models [33]. To determine the extent of artifacts introduced by imbalanced data, we performed a bootstrap analysis by downsampling with replacement the non-LTS set to equal the number of LTS samples over 100 replicates (random sampling with replacement of 90% of LTS, 10% of non-LTS). The sensitivity of regression models to downsampling is determined by computing the distributions of AUC values for LLR and logistic regression coefficients for ULR, and compared to the values obtained for models computed from the imbalanced complete data.
Principal Components Analysis and Hierarchical Clustering
To explore the relationship between LTS GBM and LGG tumors, we represent each sample in a coordinate space defined by the principal components of gene expression and methylation measures. Gene expression data from the AgilentGA4502A microarrays for both GBM and LGG samples was analyzed following Loess normalization and quantile normalization to correct for within and between-array bias, respectively. Significantly differentially expressed genes (DEGs) were identified as follows: a Student’s t-test with Benjamini-Hochberg FDR correction of the p-values was performed for each probe to compare mean expression levels between the sample sets. DEGs were identified as those in which the FDR adjusted q < 0.01 and the median log fold-change across probes was at least two fold (|log2FC| ≥ 2).
Principal Component Analysis (PCA) was performed on four sets of genes: 1. DEGs between LGG and GBM tumors. 2. DEGs between GBM and normal brain tissues. 3. genes whose expression levels were significant predictors of LTS in the ULR models. 4. genes selected for inclusion the LLR model. For each set of genes, the expression values were projected onto principal components 1 and 2, representing each sample’s coordinates in this PC space. Moran's I, a measure of autocorrelation, was used to measure the extent to which samples from a defined subset (e.g. LTS patients) cluster together due to similar expression values [34]. This measure was applied to determine the similarity of gene expression or methylation profiles among LTS samples in the coordinate spaces defined by the first two principal components. In the context of this study, a Moran’s I value near 0 indicates that LTS expression levels are randomly dispersed among the GBM samples, a value near 1 indicates that the LTS samples are closest to one another in PC space, while a value of -1 indicates a perfectly uniform spacing of LTS and non-LTS samples in PC space (i.e. negative autocorrelation, or a tendency of LTS and non-LTS samples to “alternate” in gene expression space). The same approach was used to study DNA methylation patterns between GBM and LGG with three different gene sets: 1. All genes with methylation probes; 2. ULR selected genes; 3. LLR selected genes.
Additionally, we used unsupervised hierarchical clustering of gene expression values to compare LTS with non-LTS GBM and LGG expression profiles. In this cluster analysis, each sample is represented as a vector of expression values and classified by pairwise Pearson Correlation Coefficient distance.
Results
Patient cohort
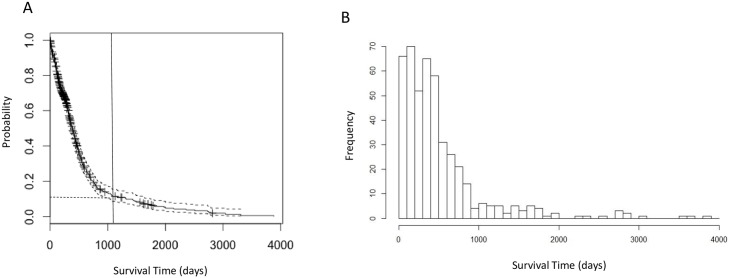
For the 591 patients in this study, the median age at initial diagnosis is 59 years; the patient cohort is 61.4% male (Table 1). We constructed a Kaplan-Meier survival curve (Fig 2A) and estimated the median survival time following initial resection to be 13.9 months (95% CI: 12.9–14.9 months). The survival curve resembles an exponential distribution, indicating a high probability of death within a short period after diagnosis and a comparatively low frequency of LTS. The distribution of survival times is essentially unimodal (Fig 2B), suggesting a homogeneous distribution, rather than a mixture of two or more distributions for LTS and the non-LTS samples. This observation was confirmed by applying Hartigan’s dip test [35](p = 0.9877), a distribution-free test for bimodality, which suggests that survival times follow a homogeneous, unimodal distribution.
Fig 2. Survival time analyses of GBM patients.
a. Kaplan-Meier plot of overall survival analysis of 591 GBM patients. The vertical line indicates the 3-year (1095 days) cutoff for LTS used in following analyses. The horizontal dashed line indicates 7.6% LTS patients corresponding to the cutoff. b. Histogram of survival time (in days) showing that the distribution of survival time is unimodal.
Among the 591 patients with known vital status and survival times, 44 (7.4%) survived longer than three years—a commonly-used survival milestone for GBM patients [5, 7, 9, 36]–and were classified as “long-term survival” (LTS) patients. Patients with shorter-than-three-year survival time were classified as non-LTS (411, 69.5%). There are a total of 29 patients surviving past the more stringent 4.5 years definition of LTS.
Regression Analyses of Clinical and Molecular Features
In a logistic regression model that included only clinical and demographical variables, younger age at initial diagnosis, Karnofsky performance score (KPS) and presence/absence of chemotherapy were the three significant predictive factors of LTS (Table 3). While post-operative KPS scores are stronger predictors of patient outcomes than pre-operative scores [37], the TCGA data contain only 76 samples with post-operative/post-adjuvant KPS values, so that pre- and post-operative scores were pooled. In the initial LLR analyses, patient age, KPS, and chemotherapy were considered jointly with the molecular biomarkers as independent variables in multiple regression analyses. Combining KPS and chemotherapy reduces the effective sizes of gene expression and DNA methylation datasets to 277 and 194 samples (due to 28% and 14% missing data in KPS and chemotherapy, respectively), while there was no missing age data.
Table 3. Partial regression coefficients for logistic regression model for LTS against clinical and demographical information.
| Estimate | Std error | z value | Pr(>|z|) | |
|---|---|---|---|---|
| Intercept | -2.300 | 1.9698 | -1.168 | 0.24299 |
| Age | -0.054 | 0.0135 | -4.008 | 6.12E-05* |
| Gender (male) | -0.025 | 0.4015 | -0.061 | 0.95128 |
| LGG history | 0.075 | 1.2110 | 0.062 | 0.95034 |
| Race (black) | -0.185 | 1.1205 | -0.165 | 0.86874 |
| Race (white) | -1.159 | 0.8813 | -1.316 | 0.18834 |
| Karnofsky score | 0.047 | 0.0170 | 2.790 | 0.00526* |
| Radiation | 2.011 | 1.2393 | 1.622 | 0.10473 |
| Chemotherapy | -1.555 | 0.7191 | -2.163 | 0.03054* |
Age at initial diagnosis, gender, ethnicity, presence/absence of prior LGG history and presence/absence of chemo and radiotherapy are used as independent variables in the model. * Independent covariates with statistically significant partial regression coefficients are indicated with ‘*’ (p < 0.05).
“Estimate” is the coefficient associated with the variable; “Std.Error” is the standard error associated with these estimates; “Pr (>|z|)” is the p-value associated with the z-value.
We evaluated the regression models with or without the inclusion of KPS and chemotherapy, and found that they converged to a set of predictors that included age but not KPS or chemotherapy (S1 Table), presumably reflecting the strong (r = -0.323, p = 1.93E-9) correlation between KPS and age, as well as the small fraction of patients (13.5%) who did not receive chemotherapy. Moreover, an identical set of predictor biomarkers and nearly identical coefficients were obtained from LLR that initially included KPS and chemotherapy as from models where only age is initially included on the same set of samples. Because of this and the reduction of sample size, age is the only clinical variable included in subsequent regression analyses.
ULR identified 10 somatic mutations as predictors of LTS with p < 0.05 (none of which are statistically significant after Bonferroni or Benjamini-Hochberg adjustment of p-values), among these are mutations in genes whose somatic variants are well-known to correlate with GBM survival time such as IDH1 and PRSS1 (S2 Table). LLR identified 13 somatic mutations as significant (Table 4 and S3 Table). Most of the significant LLR mutations are located in different genes from those identified from ULR, except for mutations in IDH1 and the mRNA splicing gene DHX16. We note that IDH1 somatic mutations are the most significant predictors of LTS in both cases, with unadjusted p = 3.2E-3 in ULR and β = 1.10 in LLR. This is consistent with the occurrence of non-synonymous mutations in IDH1 in 16.67% of the LTS patients versus 1.19% of the non-LTS patients in the TCGA sample set, corresponding to an odds ratio of 16.03 (p = 6.8E-3). There are 39592 germline mutations (SNPs) with unadjusted p < 0.05, although none are significant under either Bonferroni or Benjamini-Hochberg adjustments, even when the adjustment is restricted to the set of mutations in the exome. In LLR analysis, we identified 8 SNP genotypes with nonzero regression coefficients. The strongest associations are for mutations in the B3GALT5 (a Beta-galactosyltransferase gene) gene and the TGS1 (trimethyguanosine synthase), with β = -0.28, -0.18, respectively, indicating that the wild type genotypes at these loci are weakly predictive of LTS (AUC = 0.52, 95% CI: 0.44–0.60, Table 5).
Table 4. List of significant predictor genes in LLR using single classes of molecular data.
| Genes (Positive Association) | Genes (Negative Association) | |
|---|---|---|
| Germline Mutation | B3GALT5, TGS1 | None |
| Somatic Mutation | IDH1 | None |
| Gene Expression | MLNR, PI15, NOS3, NEUROG1, MFI2 | MST1L,CRLF2 |
| DNA Methylation | HS1BP3, CDKN1B, TMED10, PURB, RSPO3, LETMD1, STX17 | TNS4, C6orf48, SNORD48, LLGL1, VIM, NLRP4, CXorf23 |
| Copy Number Variation | AY289773,HPR | AL713660, DUSP28 |
| miRNA | None | hsa-miR-222 |
Only predictor genes with relative large beta are shown here (i.e. |β| > 1 for somatic mutation, gene expression, DNA methylation, CNV and miRNA; |β| > 0.1 for germline mutation). For a complete list, see S3 Table.
Table 5. Prediction performance of individual molecular type under LLR, as measured by AUC.
| Unbalanced | Balanced | |||
|---|---|---|---|---|
| Type of Variable | mean AUC | Std | mean AUC | Std |
| Age | 0.8034 | 0.0150 | 0.8070 | 0.0901 |
| Germline Mutation | 0.5169 | 0.0395 | 0.5490 | 0.0832 |
| Somatic Mutation | 0.6156 | 0.0354 | 0.6451 | 0.0724 |
| Gene Expression | 0.7385 | 0.0322 | 0.6665 | 0.0706 |
| DNA methylation | 0.8387 | 0.0341 | 0.6747 | 0.0962 |
| miRNA | 0.7350 | 0.0272 | 0.6577 | 0.0724 |
| CNV | 0.7002 | 0.0232 | 0.6785 | 0.0599 |
The last two columns are the mean and standard deviations of AUC under 100 bootstrap permutations (i.e. downsampling the ~10% of non-LTS cases and 90% of LTS so that the number of samples is equal to that in LTS).
For the gene expression data, there are 38 significant LTS predictors with FDR-adjusted ULR (S2 Table) vs 94 with LLR (Table 4 and S3 Table). Functional enrichment analysis of the 478 ULR significant predictor genes (q < 0.05) found a significant enrichment in phosphoproteins (1.32 fold enrichment, p = 1.6E-04) and genes in acetylation pathways (1.78 fold enrichment, p = 4.39E-06) (S4 Table). In contrast, the 94 significant predictor genes in LLR analysis did not identify enrichment with respect to any known KEGG pathway or structural/functional classes of genes. Among the genes whose expression levels are positively associated with LTS are NOS3 (nitrous oxide synthase, a known regulator of blood pressure and other vascular function) [38] and the neurogenin NEUROG1, a transcription factor regulating growth of neurons [39], indicating that the upregulation of these genes is linked to a higher probability of LTS.
Among the 4 classes of genomic data, DNA methylation is the strongest predictor of LTS with the highest mean AUC (AUC = 0.84, CI: 0.78–0.90) in LLR models (Table 5), which was confirmed through 100 replicates of 10-fold cross validation. Indeed, methylation is an even stronger predictor of LTS than age (i.e. AUC = 0.80, CI: 0.77–0.830). This is the case even though there are fewer samples in the methylation data set than for gene expression, miRNA, and CNVs. We found 38 methylation probes that are significant predictors of LTS in adjusted ULR models (S2 Table) vs. 43 in the LLR model (Table 4 and S3 Table). Genes with Lasso regression coefficients |β| > 10 include LETMD1, a known oncogene [35], the known tumor suppressor CDKN1B [36], as well as several other genes whose variants have been linked with other cancers, such as RSPO3 [37]. All of these genes are positively associated with LTS, indicating that their hypermethylation is predictive of improved patient outcomes. TNS4, whose oncogenic role has been documented for colorectal and other cancers[38], has the strongest negative association with LTS, suggesting that hypomethylation of this gene is predicts LTS (see Table 4 and S3 Table for a summary of genes that significantly predict LTS in LLR models). We remark that there is no significant association of MGMT promoter region methylation with LTS in LLR models, nor is methylation of this region a significant LTS predictor in a ULR model following FDR correction. However, the association between MGMT hypermethylation and LTS is significant in a ULR model (p = 0.036) without adjustment. There was no overlap between the set of genes that were differentially expressed between LTS and non-LTS and those that were differentially methylated.
A single microRNA was found to be significantly predictive of LTS with either the ULR or LLR analyses (Table 4, S2 and S3 Tables), namely hsa-miR-222. The regression coefficient of hsa-miR-222 expression levels on LTS is -0.169, indicating that downregulation of this miRNA is associated with LTS. There are 11 and 29 significant CNV probes prediction LTS with ULR and LLR, respectively (Table 4, S2 and S3 Tables). The strongest association of CNVs with LTS (|β| > 1) in the LLR data included the oncogene DUSP28 [40] (a negative association, indicating that deletion in this gene is predictive of LTS), as well as a positive association with HPR CNVs (i.e. duplication at this locus is correlated with LTS). Mutations in HPR have been documented in the literature as predictors of recurrent breast cancer [41]. STAM, encoding a signal transduction adapter molecule, was found to be a significant predictor in both gene expression and CNV analyses. Higher expression and amplification of this gene was associated with LTS (S3 Table), suggesting that the genomic amplification of STAM might lead to the upregulation of gene expression.
Imbalanced Sampling and Bootstrapping
The creation of balanced LTS vs. non-LTS data sets by downsampling did not substantially change the AUCs of the regression models. As can be seen in the last 2 columns in Table 5, the bootstrapped mean values of AUC are nearly identical for some data types (e.g. CNV and ULR using patient age), slightly higher for some data sets (e.g. somatic mutation and germline mutation) and somewhat lower for others (e.g. expression levels and methylation). These results indicate that the fit of models to data is not an artifact of imbalanced sampling.
Integrative Model Construction
In terms of AUC, combining one or more classes of genomic data with age in an LLR does not strongly enhance prediction of LTS when compared to age alone. It can be seen in Table 6 that combining age with methylation and microRNA expression data only marginally improves AUC, while AUC actually decreases when CNV counts or gene expression are combined with age, which is consistent with the relatively lower prediction performance of gene expression and CNV for individual models. The strongest improvement in prediction occurs when age is combined with the single significant miRNA hsa-miR-222, i.e. (AUC = 0.87, 95% CI: 0.83–0.91). The same is true when multiple classes of genomic data are combined in a single regression model, e.g. expression+methylation+microRNA data combined with age give virtually identical AUC values to age alone, indicating that pooled biomarker data does not outperform individual classes of biomarkers in LTS prediction, perhaps as a consequence of increased number of features. Applying a more stringent cutoff (4.5 years) for LTS classification does not change the results qualitatively in terms of either the significant predictors or the magnitude of AUC (prediction of miRNA expression and CNV is moderately enhanced, while diminishing for gene expression data, presumably due to fewer LTSs). This indicates that the results of the regression analyses are not strongly predicated on the choice of cutoff time used to define LTS.
Table 6. Prediction performance (area under curve, AUC) of integrative models.
| Combinations | cutoff = 3 y | cutoff = 4.5 y | ||
|---|---|---|---|---|
| mean AUC | Std | mean AUC | Std | |
| age | 0.8033 | 0.0170 | 0.8023 | 0.0150 |
| age+exp | 0.7246 | 0.0404 | 0.6350 | 0.0630 |
| age+met | 0.8067 | 0.0350 | 0.8095 | 0.0467 |
| age+mir | 0.8711 | 0.0202 | 0.9028 | 0.0262 |
| age+cnv | 0.7164 | 0.0547 | 0.7638 | 0.0638 |
| age+exp+met | 0.8095 | 0.0343 | 0.7980 | 0.0451 |
| age+exp+mir | 0.7470 | 0.0429 | 0.6761 | 0.0591 |
| age+exp+cnv | 0.6918 | 0.0475 | 0.6554 | 0.0596 |
| age+met+mir | 0.8126 | 0.0411 | 0.8012 | 0.0421 |
| age+met+cnv | 0.8034 | 0.0416 | 0.8128 | 0.0422 |
| age+mir+cnv | 0.7777 | 0.0481 | 0.8193 | 0.0619 |
| age+exp+met+mir+cnv | 0.8107 | 0.0379 | 0.8022 | 0.0408 |
These models combine age with one or more molecular types with LTS cutoff at 3 year (i.e. 7.6%) or 4.5 year (5%).
The principal value of constructing integrative LLR models lies in the fact they identify molecular markers that are jointly significant predictors of LTS which are not individually predictive in ULR, nor predictive in LLR when applied to a single class of data (see Table 7). For example, in LLR analysis of methylation probes alone, methylation of the oncogene BRAF does not appear as a significant predictor, whereas in the integrative model it has β = 4.62. While CAV1 (caveolin 1, a plasma membrane protein and tumor suppressor gene) appears in the LLR model, its β = 11.34 value is much larger in the joint regression model than in LLR on methylation data alone. Downregulation or loss of function in CAV1 has previously been documented as a determinant of aggressiveness in GBM tumor growth [42].
Table 7. List of significant predictor genes in integrative LLR models, with various combinations of data classes.
| Probe | Gene Symbol | Beta | In individual model |
|---|---|---|---|
| 205742_at | TNNI3 | 0.0987 | NO |
| 205923_at | RELN | 0.0024 | NO |
| 215443_at | TSHR | 0.0286 | YES |
| 216512_s_at | DCT | 0.2786 | NO |
| cg05790999 | HS1BP3 | 34.506 | YES |
| cg07964538 | CAV1 | 11.638 | YES |
| cg09307279 | GLT8D1;SPCS1 | 12.851 | NO |
| cg10141022 | BRAF | 4.6218 | NO |
| cg12927772 | C9orf64 | 2.8326 | YES |
| cg12989642 | PURB | 12.553 | YES |
| cg18672421 | TMED10 | 3.041 | YES |
| cg19133903 | AVPI1 | 0.553 | NO |
| cg21982518 | TMC7 | 1.3812 | NO |
| cg25913233 | SPARC | 2.1799 | NO |
| cg26864028 | EPOR | 0.086 | YES |
All but one of the gene expression probes that appear in the integrative LLR model are not significant LTS predictors for LLR on expression data alone. We remark, however, that most of these expression probes are only weakly predictive of LTS in the joint model, with β≤0.28 (the strongest association is with expression levels of DCT, dopachrome tautomerase). This result is consistent with methylation being the strongest individual predictor of LTS in both integrative models and in models that only incorporate a single class of data.
Relationship of LTS to LGG
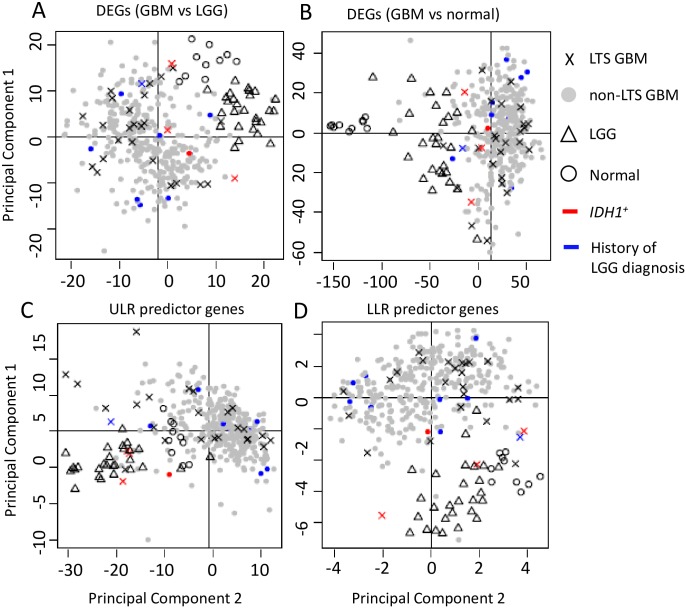
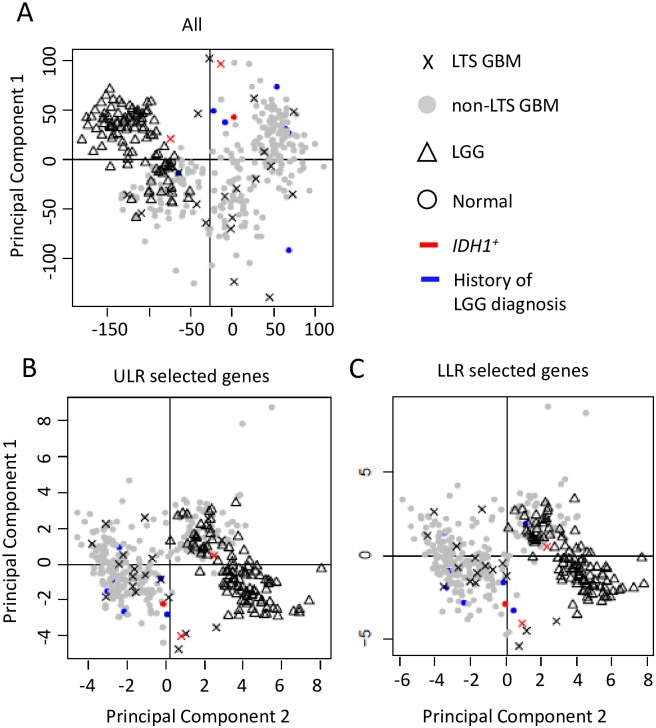
The PCA analyses reveal that the GBM, LGG, and normal samples have distinct profiles among their DEGs (Fig 3A and 3B). LGG and (all) GBM samples are distinct clusters in the PCA scatterplots (Moran’s I~0.1, p << 0.01 for LGG vs. GBM), and all LGG tumors share common nodes in the hierarchical clustering analysis discussed below (Fig 4). In contrast, LTS samples are interspersed among the non-LTS GBM samples (Moran’s I near 0, p > 0.1 in Table 8); methylation scores (Table 9) produces qualitatively similar results. Together, these results suggest that there is no “hallmark” gene expression profile for LTS GBM, consistent with the lack of association between expression profile subtype and LTS described in [21]. These observations all indicate weak mutual similarity between expression profiles of LTS GBMs, i.e. the majority of LTS GBMs have gene expression patterns that more closely resemble non-LTS GBM than they do the profiles of other LTS patients. Not surprisingly, if we consider the gene sets that are significant predictors of LTS in ULR and LLR models, we do find separation and autocorrelation among LTS samples, the Moran’s I values have p effectively 0 for genes identified ULR and LLR, respectively. This could also be seen by the greater “clumping” of LTS samples in PCA space and in the dendrograms (S1 and S2 Figs).
Fig 3. Scatterplot of the first two principal components of gene expression data.
PCA analyses were performed on a. DEGs between GBM and LGG (N = 491 genes). b. DEGs between GBM and normal controls (N = 4801). c. ULR predictor genes (N = 94). d. LLR predictor genes (N = 38).
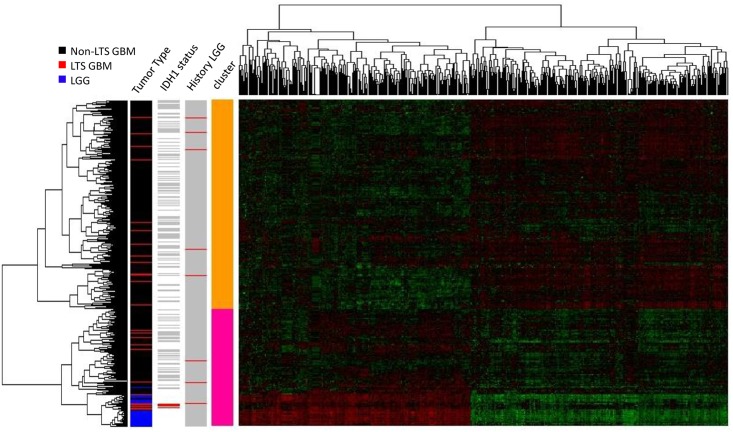
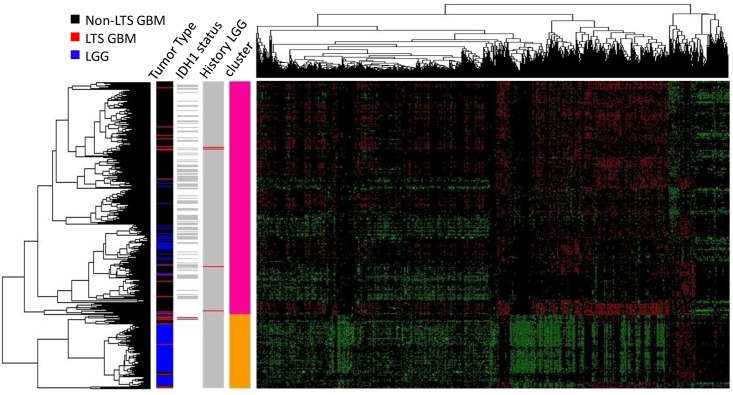
Fig 4. Heatmap of the gene expression levels in LTS GBM, non-LTS GBM and LGG samples (N = 383 genes).
Hierarchical clustering (HC) on the expression levels of DEGs between GBM and LGG (N = 491) was used to classify the samples, with a row dendrogram (clustering of samples) based on Pearson correlation coefficient, the column dendrogram on a Spearman correlation coefficient. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ or history of LGG diagnosis; grey indicates IDH1- and no history of LGG diagnosis, respectively; white indicates that no data is available for the sample.
Table 8. Moran's I for each binary category (e.g. LTS vs. non-LTS).
| DEGs (LGG vs GBM) | Observed | Expected | Std | p value |
|---|---|---|---|---|
| LGG vs GBM | 0.2991 | -0.0029 | 0.0055 | 0 |
| LTS vs nLTS | 0.0051 | -0.0029 | 0.0053 | 0.1340 |
| normal vs tumor | 0.1989 | -0.0026 | 0.0049 | 0 |
| DEGs (GBM vs normal) | ||||
| LGG vs GBM | 0.1238 | -0.0027 | 0.0052 | 0 |
| LTS vs nLTS | -0.0004 | -0.0029 | 0.0056 | 0.6525 |
| normal vs tumor | 0.2980 | -0.0026 | 0.0053 | 0 |
| ULR genes | ||||
| LGG vs GBM | 0.2863 | -0.0029 | 0.0066 | 0 |
| LTS vs nLTS | 0.0241 | -0.0029 | 0.0060 | 7.84E-06 |
| normal vs tumor | 0.1057 | -0.0026 | 0.0057 | 0 |
| LLR genes | ||||
| LGG vs GBM | 0.1047 | -0.0029 | 0.0068 | 0 |
| LTS vs nLTS | 0.0204 | -0.0029 | 0.0068 | 0.0007 |
| normal vs tumor | 0.1345 | -0.0026 | 0.0060 | 0 |
The statistics are computed over the coordinates of the first two principal components of gene expression.
Table 9. Moran's I for each binary category (e.g. LTS vs. non-LTS).
| All genes | Observed | Expected | Std | p value |
|---|---|---|---|---|
| LGG vs GBM | 0.3951 | -0.0026 | 0.0060 | 0 |
| LTS vs nLTS | 0.0027 | -0.0036 | 0.0068 | 0.3513 |
| normal vs tumor | -0.0030 | -0.0026 | 0.0005 | 0.4193 |
| ULR genes | ||||
| LGG vs GBM | 0.3373 | -0.0026 | 0.0059 | 0 |
| LTS vs nLTS | 0.0033 | -0.0036 | 0.0071 | 0.3356 |
| normal vs tumor | -0.0033 | -0.0026 | 0.0005 | 0.2393 |
| LLR genes | ||||
| LGG vs GBM | 0.3412 | -0.0026 | 0.0066 | 0 |
| LTS vs nLTS | 0.0014 | -0.0036 | 0.0086 | 0.5612 |
| normal vs tumor | -0.0033 | -0.0026 | 0.0005 | 0.2495 |
The statistics are computed over the coordinates of the first two principal components of DNA methylation.
In the hierarchical clustering analysis, selecting a twofold partition generates one subtree that consists solely of GBMs and another that contains both GBM and all LGG samples (i.e. all LGG samples are defined by a single node in this subtree, as shown in Fig 4). LTS samples occur in both subclusters, with a disproportionate representation of LTS tumors in the subtree containing the LGG. Specifically, 14 of the 28 LTS GBM samples occur in the subtree that also contains LGG, versus 93 of the 318 non-LTS GBM samples (OR = 1.72, p = 0). However, most of the neighbors of LTS in the dendrogram are non-LTS GBMs, even for those in the subtree that contains LGG (as in the scatterplot). The same is true of GBMs in patients who have a prior history of LGG, that is, known secondary GBMs occur throughout the dendrogram and show no specific association with LGG expression profiles. In fact, only a single LTS has a documented prior history of LGG (corresponding to an insignificant OR = 1.04 for LTS association with LGG). From both the hierarchical clustering and PCA analyses, we conclude there is no significant association between progression from LGG and subsequent LTS in GBM.
A closer examination of IDH1 mutation status in showed that all 5 IDH1+ GBMs (including 3 LTSs and 2 non-LTSs) cluster with LGGs when the dendrogram was partitioned into two clusters (Fig 4, row-side color bar on the right), indicating a similarity in gene expression and methylation profiles between IDH1+ genotypes and LGGs. However, while IDH1+ is a significant predictor of LTS, the majority of LTS cases in the TCGA data set are IDH1- wildtype. Furthermore, none of the IDH1+ genotypes was in a GBM with a prior LGG history.
Most of the LTS samples are similarly interspersed with non-LTS GBM’s in the PCA scatterplots, only a small subset of LTS samples cluster with LGGs. The lack of a strong overall LGG-like “signal” in LTS samples can be seen most clearly from the comparison of centroids for PCA 1+2 scores over the different gene sets (S5 Table), where the LTS distances to LGG were much greater than to non-LTS GBM. Indeed, except for the small subset of genetic markers that are predictive of LTS, the absence of significant autocorrelation among LTS samples further argues against LTS corresponding to a biologically unique and qualitative distinct class of GBM pathology, and against their proposed molecular affinity with LGG features.
Moreover, GBM samples from patients with prior LGG history do not cluster together in the dendrogram, nor do these known secondary GBMs cluster with LGGs, arguing against the hypothesis of that secondary GBMs retain LGG-like molecular profiles. Previous analyses of gene expression patterns have identified at least four subtypes of GBM [43–45], including secondary (proneural) GBM, as well as the mesenchymal, classical and neural GBM subtypes. However, we found no significant association between LTS and the proneural subtype (based on TCGA classification of samples), the OR = 1.21, p = 0.83. This lack of association of LTS with subtype is consistent with the observation that most LTS samples do not share a common node in the dendrogram nor a specific affinity with LGG.
Similar trends are observed with the methylation data (Figs 5 and 6, S6 Table), finding no association between LTS and LGG samples with respect to methylation scores. Furthermore, the tendency of LTS samples to co-occur in a single cluster is even weaker for the methylation profiles. The Moran’s I autocorrelation measures are statistically insignificant for LTS methylation scores with respect to non-LTS GBM (Table 9), even when the LLR subset of genes are considered separately. These results were unexpected in view of the fact that methylation is a stronger predictor of LTS in LLR models than expression profiles, which is probably a consequence a relatively small subset of the LTS samples with very similar expression profiles (high autocorrelation) in the LLR-selected genes.
Fig 5. Scatterplot of the first two principal components of the DNA methylation data.
PCA analyses were performed on a. All probes (N = 23233). b. ULR predictor genes (N = 38). c. LLR predictor genes (N = 43).
Fig 6. Heatmap of the DNA methylation levels in LTS GBM, non-LTS GBM and LGG samples (N = 383).
Hierarchical clustering (HC) on the DNA methylation levels of GBM and LGG (N = 23233) was used to classify the samples. The row dendrogram (clustering among samples) is based on Pearson correlation coefficients and the column dendrogram on Spearman correlation coefficients. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ or history of LGG diagnosis; grey indicates IDH1- or no history of LGG diagnosis; white indicates no data available for the sample.
Discussion
The principal goal of this study was to determine whether LTS GBM tumors have genomic features that distinguish them from those found in patients with more typical survival times, i.e. to evaluate whether they constitute a biologically distinct subclass of high-grade gliomas with unique molecular characteristics, and to evaluate the relationship between LTS GBMs and LGGs.
In spite of the limitations on statistical power due to comparatively small samples of LTS patients and incomplete clinical data, our analyses identified molecular biomarkers that significantly predict LTS, several of which have been documented in the literature as predictors of improved response to chemotherapy and overall improved patient prognosis. For example, among the somatic point mutations, non-synonymous mutations in IDH1 were the strongest predictors of LTS in ULR and LLR models (Table 4), consistent with the significantly higher proportion of IDH1 mutated patients observed in the LTS vs. non-LTS data sets. However, even the association of LTS with IDH1 mutations is weak, i.e. even though most of IDH1+ genotypes are LTS, most LTS cases are IDH1-. The low predictive performance of both somatic and germline mutations generally is in agreement with emerging clinical data suggesting that IDH1 is a only weak predictor of LTS in GBM, as survival beyond the fourth year can occur in patients without IDH1 mutations [12, 21]. Similarly, miR-222, the only differentially expressed miRNA identified as a predictor of LTS, has been previously documented in the literature [46] as predictor of GBM prognosis due to its role as a regulator of the PUMA [47], a P53 mediated regulator of apoptosis. Higher levels of PUMA protein are associated with increased apoptosis and, consequently, lower growth rates in GBM tumors. Upregulation of miR-222 is linked to repression of PUMA and higher tumor proliferation, consistent with LTS being negatively associated with miR-222 expression levels.
The scarcity of somatic mutation genotypes as predictors of LTS is largely the result of most somatic mutations occurring in few (2–3) samples in the data set. On the other hand, given the abundance of high frequency variant germline genotypes, the small number of germline mutation predictive of LTS is somewhat surprising, since genome wide association studies (GWAS) [48, 49] have identified inherited SNPs that predispose individuals to GBM. None of these GWAS-identified SNPs appeared as a significant predictor in our regression models. These results suggest that there are few if any inherited (familial) mutations that predict LTS in GBM patients, or that their effects are comparatively weak against the much stronger signal of variation among GBM types and the contribution of clinical variables such as age to patient survival.
Although mutational genotypic markers for LTS are limited, we did identify gene expression phenotypes, epigenetic markers, and copy number variant genotypes that are significantly predictive of LTS, with the exception of DNA methylation. The fact that DNA methylation has comparable or better predictive power than age is probably related to the coordinated regulation of aging and DNA methylation patterns [50]; the same is true for regression models of LTS combining age with methylation. While previous analyses have shown that combining clinical predictors with gene expression alone best predicts survival time [25], our results found the weakest prediction when age is combined solely with gene expression data, and strongest when combined with miRNAs (in spite of the limited number of miRNAs). This discrepancy is potentially due to different choices of algorithms (i.e. feature selection approach, shrinkage parameter optimization) and/or the nature of the model (i.e. response variable: hazard proportion ratio vs. binary response, as well as our use of integrative regression models) [51].
Apart from this comparatively small set of genomic markers, there is no strong genomic “signature” that unambiguously distinguishes LTSs from other GBM. No molecular markers unique to LTS were identified, and there wasn’t the large-scale statistical difference in either gene expression or methylation patterns such as those observed between GBM and LGG. This result is consistent with the observed unimodality of the survival time distribution for the GBM patients (Fig 2A and 2B). If LTS patients represented a biologically distinct subclass of cases, one would expect the distribution of survival times to resemble a bi or multimodal mixture, when in fact the distribution of time until death is basically monotonically decreasing for survival times greater than the mode.
Furthermore, while there are biomarkers that significantly predict LTS in logistic regression models, there are no molecular profiles that strongly define LTS as a distinct subclass in the way that LGGs are molecularly distinct from GBM. This is evident from the occurrence of LTS samples throughout PCA scatterplots, and the lack of a single node or subtree subtending most or all LTSs in dendrograms. Such results suggest that there are many patterns of gene expression and methylation that lead to LTS phenotypes. Similarly, the lack of a consistent molecular signature among secondary GBMs, or a shared signature between secondary GBM and LTS implies that secondary GBMs are not associated with improved patient outcomes in comparison to primary GBM.
Finally, this study found no tendency among LTS GBM cases to resemble the molecular profiles of LGG, which argues strongly against LTS cases being misdiagnosed LGGs. This observation, together with a lack of an association between LTS and secondary GBM, also suggests that LTS in GBM is not a consequence of the retention of LGG-like biological features in high-grade glioma tumor cells. Our finding that only IDH1+ GBMs have expression profiles resembling LGG may indicate that IDH1 mutated GBMs are either misidentified LGGs or represent a unique, LGG-like pathology among high-grade gliomas, this observation does not account the majority of LTS cases. The fact that the overall gene expression and methylation profiles of LTS tumors lack a unique molecular signature and don’t show a significant similarity to LGGs simply indicates that there are multiple genomic paths to similar clinical phenotypes and patient outcomes, just as there are multiple genetic and epigenetic paths to cancer. We anticipate that the molecular correlates of different categories of LTS will be further elucidated once larger data sets become available.
Supporting Information
Hierarchical clustering (HC) on the expression levels of ULR predictor genes (N = 38) was used to classify the samples. The row dendrogram, showing the relationship among samples, was based on Pearson correlation coefficients, thecolumn dendrogram on Spearman correlation coefficients. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ or history of LGG diagnosis; grey indicates IDH1- or no history of LGG diagnosis; white indicates no data available for the sample.
(TIF)
Hierarchical clustering (HC) on the expression levels of LLR predictor genes (n = 94) was used to classify the samples. The row dendrogram (clustering of samples) is based on Pearson correlation coefficient and column dendrogram on Spearman correlation coefficient. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ and a history of LGG diagnosis, respectively; grey indicates IDH1- or no history of LGG diagnosis, respectively; white indicates that no data is available for the sample.
(TIF)
The genes with non-zero penalized regression coefficients (β) in Lasso regression model are listed in the table.
(XLSX)
For somatic point mutation, genes with unadjusted p < 0.05 are shown. For the other classes of data, genes with adjusted q < 0.05 (Bonferroni correction, i.e. pbonf) were shown. “Estimate” is the coefficient associated with the variable; “Std.Error” is the standard error associated with these estimates; “Pr (>|z|)” is the p-value associated with the z-value.
(XLSX)
(XLSX)
Annotation clusters with adjusted p-value (Benjamini-Hochberg) q < 0.05 for related annotation terms are shown. Fold enrichment is the ratio of annotation terms of LTS predictors to those of all genes.
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors wish to thank Anurekha Ramakrishnan for bringing Moran’s I and several other statistical techniques to our attention. The authors were supported from a grant from the St. David’s Foundation Impact Fund.
Abbreviations
- GBM
Glioblastoma multiforme
- LTS
Long-term survival
- LGG
Low grade glioma
- CNV
Copy number variation
- TCGA
the Cancer Genome Atlas
- PCA
Principal component analysis
- LLR
Lasso logistic regression
- ULR
Univariate logistic regression
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by St. David’s Foundation Impact Fund.
References
- 1.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005; 109:93–108. [DOI] [PubMed] [Google Scholar]
- 2.Burton EC, Lamborn KR, Feuerstein BG, Prados M, Scott J, Forsyth P, et al. Genetic aberrations defined by comparative genomic hybridization distinguish long-term from typical survivors of glioblastoma. Cancer Res 2002; 62:6205–10. [PubMed] [Google Scholar]
- 3.Burton EC, Lamborn KR, Forsyth P, Scott J, O'Campo J, Uyehara-Lock J, et al. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin Cancer Res 2002; 8:180–7. [PubMed] [Google Scholar]
- 4.Das P, Puri T, Jha P, Pathak P, Joshi N, Suri V, et al. A clinicopathological and molecular analysis of glioblastoma multiforme with long-term survival. J Clin Neurosci 2011; 18:66–70. 10.1016/j.jocn.2010.04.050 [DOI] [PubMed] [Google Scholar]
- 5.Adeberg S, Bostel T, König L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol 2014; 9:95 10.1186/1748-717X-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babu R, Sharma R, Karikari IO, Owens TR, Friedman AH, Adamson C. Outcome and prognostic factors in adult cerebellar glioblastoma. J Clin Neurosci 2013; 20:1117–21. 10.1016/j.jocn.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 7.Sonoda Y, Kumabe T, Watanabe M, Nakazato Y, Inoue T, Kanamori M, et al. Long-term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurochir (Wien) 2009; 151:1349–58. [DOI] [PubMed] [Google Scholar]
- 8.Ullén H, Mattsson B, Collins VP: Long-term survival after malignant glioma. A clinical and histopathological study on the accuracy of the diagnosis in a population-based cancer register. Acta Oncol 1990;29:875–8. [DOI] [PubMed] [Google Scholar]
- 9.Kraus JA, Wenghoefer M, Schmidt MC, von Deimling A, Berweiler U, Roggendorf W, et al. Long-term survival of glioblastoma multiforme: importance of histopathological reevaluation. J Neurol 2000;247:455–60. [DOI] [PubMed] [Google Scholar]
- 10.Senger D, Cairncross JG, Forsyth PJ: Long-term survivors of glioblastoma: statistical aberration or important unrecognized molecular subtype? Cancer J 2003;9:214–221. [DOI] [PubMed] [Google Scholar]
- 11.Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013;155:462–477. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amelot A, De Cremoux P, Quillien V, Polivka M, Adle-Biassette H, Lehmann-Che J, et al. IDH-Mutation Is a Weak Predictor of Long-Term Survival in Glioblastoma Patients. PLoS One 2015; 10:e0130596 10.1371/journal.pone.0130596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erson-Omay EZ, Ca layan O, Schultz N, Weinhold N, Omay SB, Özduman K, et al. Somatic POLE mutations cause an ultramutated giant cell high-grade glioma subtype with better prognosis. Neuro Oncol 2015; 17:1356–1364. 10.1093/neuonc/nov027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraus JA, Glesmann N, Beck M, Krex D, Klockgether T, Schackert G, et al. Molecular analysis of the PTEN, TP53 and CDKN2A tumor suppressor genes in long-term survivors of glioblastoma multiforme. J Neurooncol 2000;48:89–94. [DOI] [PubMed] [Google Scholar]
- 15.Lai RK, Chen Y, Guan X, Nousome D, Sharma C, Canoll P, et al. Genome-wide methylation analyses in glioblastoma multiforme. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niyazi M, Zehentmayr F, Niemöller OM, Eigenbrod S, Kretzschmar H, Schulze-Osthoff K, et al. MiRNA expression patterns predict survival in glioblastoma. Radiat Oncol 2011;6:153 10.1186/1748-717X-6-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR. Molecular Heterogeneity in Glioblastoma: Potential Clinical Implications. Front Oncol 2015;5-:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel VN, Gokulrangan G, Chowdhury SA, Chen Y, Sloan AE, Koyutürk M, et al. Network signatures of survival in glioblastoma multiforme. PLoS Comput Biol 2013;9:e1003237 10.1371/journal.pcbi.1003237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AA, Huang Y-T, Eliot M, Houseman EA, Marsit CJ, Wiencke JK, et al. A novel approach to the discovery of survival biomarkers in glioblastoma using a joint analysis of DNA methylation and gene expression. Epigenetics 2014;9:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaak RGW, Hoadley K, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98–110. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber NK, Goenka A, Turcan S, Reyngold M, Makarov V, Kannan K, et al. Transcriptional diversity of long-term glioblastoma survivors. Neuro Oncol 2014;16:1186–95. 10.1093/neuonc/nou043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leu S, von Felten S, Frank S, Vassella E, Vajtai I, Taylor E, et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol 2013;15:469–79. 10.1093/neuonc/nos317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reifenberger G, Weber RG, Riehmer V, Kaulich K, Willscher E, Wirth H, et al. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int J Cancer 2014;135:1822–31. 10.1002/ijc.28836 [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Shi X, Xie Y, Huang J, Shia B, Ma S. Combining multidimensional genomic measurements for predicting cancer prognosis: observations from TCGA. Brief Bioinform 2015;16:291–303. 10.1093/bib/bbu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleihues P, Burger PC, Plate KH, Ohgaki H, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon: International Agency for Research on Cancer;1997. [Google Scholar]
- 26.The Cancer Genome Atlas: http://cancergenome.nih.gov/
- 27.Shpak M, Goldberg MM, Cowperthwaite MC: Rapid and convergent evolution in the Glioblastoma multiforme genome. Genomics 2015;105:159–167. 10.1016/j.ygeno.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 28.Larson DE, Harris CC, Chen K, Koboldt DC, Abbott TE, Dooling DJ, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 2012;28:311–7. 10.1093/bioinformatics/btr665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cingolani P, Platts A, Coon M, Nguyen T, Wang L, Land SJ, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012;6:80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shpak M, Hall AW, Goldberg MM, Derryberry DZ, Ni Y, Iyer VR, et al. An eQTL analysis of the human glioblastoma multiforme genome. Genomics 2014;103:252–263. 10.1016/j.ygeno.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 31.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–64. [DOI] [PubMed] [Google Scholar]
- 32.Efron B, Hastie T, Johnstone I, Tibshirani R. Least angle regression. Ann Stat 2015;32:407–499. [Google Scholar]
- 33.Chawla N: Data Mining for Imbalanced Datasets: An Overview In Data Mining and Knowledge Discovery Handbook SE—40. Edited by Maimon O, Rokach L. Springer; US;2005:875–886. [Google Scholar]
- 34.Getis A, Ord JK: The Analysis of Spatial Association by Use of Distance Statistics. Geogr Anal 2010;24:189–206. [Google Scholar]
- 35.Hartigan JA, Hartigan PM: The Dip test of unimodality. Ann Stat 1985;13:70–84. [Google Scholar]
- 36.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain 2007;130:2596–606. [DOI] [PubMed] [Google Scholar]
- 37.Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neuro Oncol 2015; 121:359–364. [DOI] [PubMed] [Google Scholar]
- 38.Stuehr DJ: Mammalian nitric oxide synthases. Biochim Biophys Acta 1999;1411:217–30. [DOI] [PubMed] [Google Scholar]
- 39.Dixit R, Wilkinson G, Cancino GI, Shaker T, Adnani L, Li S, et al. Neurog1 and Neurog2 control two waves of neuronal differentiation in the piriform cortex. J Neurosci 2014, 34:539–53. 10.1523/JNEUROSCI.0614-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Han S, Peng R, Jiao C, Wang X, Han Z, et al. DUSP28 contributes to human hepatocellular carcinoma via regulation of the p38 MAPK signaling. Int J Oncol 2014;45:2596–604. 10.3892/ijo.2014.2653 [DOI] [PubMed] [Google Scholar]
- 41.Kuhajda FP, Piantadosi S, Pasternack GR: Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med 1989;321:636–41. [DOI] [PubMed] [Google Scholar]
- 42.Martin S, Cosset EC, Terrand J, Maglott A, Takeda K, Dontenwill M. Caveolin-1 regulates glioblastoma aggressiveness through the control of alpha(5)beta(1) integrin expression and modulates glioblastoma responsiveness to SJ749, an alpha(5)beta(1) integrin antagonist. Biochim Biophys Acta 2009;1793:354–67. 10.1016/j.bbamcr.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 43.Huse JT, Phillips HS, Brennan CW: Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia 2011;59:1190–9. 10.1002/glia.21165 [DOI] [PubMed] [Google Scholar]
- 44.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157–73. [DOI] [PubMed] [Google Scholar]
- 45.Kleihues P, Ohgaki H: Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol 1999;1:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Zhang J, Hao, Shi Z, Wang Y, Han L, et al. High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J Transl Med 2012; 10:119 10.1186/1479-5876-10-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Zhang J, Zhang A, Shi ZD, Han L, Jia ZF, et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Caner 2010; 9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 2009;41:899–904. 10.1038/ng.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 2009;41:905–8. 10.1038/ng.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Q, Wagner W: Epigenetic Aging Signatures Are Coherently Modified in Cancer. PLoS Genet 2015;11:e1005334 10.1371/journal.pgen.1005334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan Y, Van Allen EM, Omberg L, Wagle N, Amin-Mansour A, Sokolov A, et al. Assessing the clinical utility of cancer genomic and proteomic data across tumor types. Nat Biotechnol 2014;32:644–652. 10.1038/nbt.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hierarchical clustering (HC) on the expression levels of ULR predictor genes (N = 38) was used to classify the samples. The row dendrogram, showing the relationship among samples, was based on Pearson correlation coefficients, thecolumn dendrogram on Spearman correlation coefficients. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ or history of LGG diagnosis; grey indicates IDH1- or no history of LGG diagnosis; white indicates no data available for the sample.
(TIF)
Hierarchical clustering (HC) on the expression levels of LLR predictor genes (n = 94) was used to classify the samples. The row dendrogram (clustering of samples) is based on Pearson correlation coefficient and column dendrogram on Spearman correlation coefficient. In the row-side color bars of IDH status and LGG history, red indicates IDH1+ and a history of LGG diagnosis, respectively; grey indicates IDH1- or no history of LGG diagnosis, respectively; white indicates that no data is available for the sample.
(TIF)
The genes with non-zero penalized regression coefficients (β) in Lasso regression model are listed in the table.
(XLSX)
For somatic point mutation, genes with unadjusted p < 0.05 are shown. For the other classes of data, genes with adjusted q < 0.05 (Bonferroni correction, i.e. pbonf) were shown. “Estimate” is the coefficient associated with the variable; “Std.Error” is the standard error associated with these estimates; “Pr (>|z|)” is the p-value associated with the z-value.
(XLSX)
(XLSX)
Annotation clusters with adjusted p-value (Benjamini-Hochberg) q < 0.05 for related annotation terms are shown. Fold enrichment is the ratio of annotation terms of LTS predictors to those of all genes.
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.