Abstract
During atherogenesis, LDL is oxidized, generating various oxidation-specific neoepitopes, such as malondialdehyde-modified (MDA-modified) LDL (MDA-LDL) or the phosphorylcholine (PC) headgroup of oxidized phospholipids (OxPLs). These epitopes are recognized by both adaptive T cell–dependent (TD) and innate T cell–independent type 2 (TI-2) immune responses. We previously showed that immunization of mice with MDA-LDL induces a TD response and atheroprotection. In addition, a PC-based immunization strategy that leads to a TI-2 expansion of innate B-1 cells and secretion of T15/EO6 clonotype natural IgM antibodies, which bind the PC of OxPLs within oxidized LDL (OxLDL), also reduces atherogenesis. T15/EO6 antibodies inhibit OxLDL uptake by macrophages. We now report that immunization with MDA-LDL, which does not contain OxPL, unexpectedly led to the expansion of T15/EO6 antibodies. MDA-LDL immunization caused a preferential expansion of MDA-LDL–specific Th2 cells that prominently secreted IL-5. In turn, IL-5 provided noncognate stimulation to innate B-1 cells, leading to increased secretion of T15/EO6 IgM. Using a bone marrow transplant model, we also demonstrated that IL-5 deficiency led to decreased titers of T15/EO6 and accelerated atherosclerosis. Thus, IL-5 links adaptive and natural immunity specific to epitopes of OxLDL and protects from atherosclerosis, in part by stimulating the expansion of atheroprotective natural IgM specific for OxLDL.
Introduction
Atherosclerosis is a chronic inflammatory disease (1, 2) whose pathogenesis involves disturbed lipoprotein metabolism, the formation of proinflammatory lipid peroxidation products, and the host’s immune responses (3, 4). Oxidized LDL (OxLDL) is present in atherosclerotic lesions and contains a wide variety of lipid peroxidation products, which in turn can form neo-self determinants recognized by specific innate and adaptive immune responses (3, 4).
Typically, peroxidation of the abundant phospholipid phosphatidylcholine is initiated at the oxidation-prone sn-2 polyunsaturated fatty acid. Its subsequent decomposition generates a wide spectrum of reactive molecular species, such as malondialdehyde (MDA) and the residual core-aldehydes of the oxidized phospholipids (OxPLs) that contain the phosphorylcholine (PC)-headgroup, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC). These reactive aldehydes can modify autologous molecules, including the LDL particle, generating multiple immunogenic oxidation–specific epitopes.
Specific humoral responses to different oxidation-specific epitopes of OxLDL have been described (5), but their role in health and disease as well as their immunobiological orchestration are largely unknown. IgM and IgG autoantibodies to OxLDL develop in humans and in animal models of atherosclerosis, and their titers often correlate with the extent of disease (5). We have extensively characterized IgM monoclonal anti-OxLDL autoantibodies that were derived from the spleens of atherosclerotic ApoE-deficient mice, as these autoantibodies partially reflect the disease-associated immune responses (6–9). In vitro, most of these IgMs bound to OxLDL, which is made by copper-catalyzed oxidation of LDL and therefore contains many different oxidation products, including oxidized phospholipids, such as POVPC. Genetic analysis indicated that EO6, the prototypic IgM anti-OxLDL antibody, was a natural antibody secreted by innate B-1 cells in a T cell–independent (TI) manner and possessed the germline-encoded T15 clonotype (8). T15 antibodies have previously been shown to recognize the PC moiety (10), which is an immunodominant component of the cell wall of many microbes, such as Streptococcus pneumoniae (11). Our previous studies show that T15/EO6 also recognizes the PC moiety of oxidized phospholipids, as present on OxLDL and apoptotic cells, but does not recognize native PC-containing unoxidized phospholipids, as found on native LDL or viable cells (8). Thus, oxidation of phosphatidylcholine “exposes” the PC moiety, making it an epitope for T15/EO6 or a ligand for scavenger receptors, such as CD36 (3). Indeed, the IgM EO6 is able to block the uptake of OxLDL by macrophages in vitro, preventing foam cell formation (7, 8). Furthermore, immunization of LDL receptor–deficient (LDLR–/–) mice with S. pneumoniae, which results in the near monoclonal expansion of IgM T15/EO6 antibodies, exerts a protective effect on lesion formation (12).
IgG antibodies to OxLDL epitopes could potentially be detrimental by promoting macrophage foam-cell formation via Fcγ receptors. However, we (13, 14) and others (15, 16) have shown that active immunization of hypercholesterolemic rabbit and murine models of atherosclerosis with an oxidation-specific epitope — homologous MDA-modified LDL (MDA-LDL) — induces robust T cell–dependent (TD) IgG titers and reduces the progression of atherogenesis, suggesting that components of the adaptive immune response to these antigens can also be protective. MDA-LDL is prepared by chemical derivatization of native LDL, and though it contains many MDA epitopes, the LDL itself is not oxidized and therefore does not contain other oxidation-specific epitopes, such as oxidized phospholipids recognized by EO6 (6, 7).
There is strong evidence that adaptive immunity is involved in the natural course of atherogenesis (3, 17), and substantial evidence supports a disease-promoting role for Th1 responses and Th1 cytokines in particular (17). Specifically, adaptive responses to OxLDL have been shown to increase during disease progression, but their exact role in atherogenesis is uncertain (18, 19). Notably, in cholesterol-fed apoE–/– mice, advanced stages of atherosclerosis are associated with increased accumulation of OxLDL (20), and in this setting there is an immune deviation of OxLDL-specific Th1 responses toward Th2 (21, 22). The Th2 cytokine IL-4 has been reported to have either pro- or anti-atherogenic effects (23–25), whereas IL-10 secretion by T cells decreases atherogenesis (26, 27). Thus, it is not known what effect a general Th2 immune deviation may have on disease progression.
Immunization with MDA-LDL induces a specific TD response (14, 16). In the course of studies attempting to define the mechanisms of the protective effect of MDA-LDL immunization, we found a marked Th2 bias of the induced MDA-specific TD responses that were characterized by prominent secretion of the Th2 cytokine IL-5. Surprisingly, there was a parallel induction of TI anti–PC T15/EO6 antibodies, which do not recognize MDA modifications. We further established that IL-5 could stimulate these natural TI humoral responses to oxidized phospholipid epitopes in vivo and in vitro in a noncognate manner, and we demonstrated the importance of this by showing that IL-5 deficiency accelerated atherosclerosis. These data support a paradigm in which an antigen-driven specific Th2 response not only leads to classic TD responses, but in turn enhances innate humoral responses to other oxidation-specific epitopes of OxLDL, which in aggregate provide protection from atherosclerosis.
Results
Immunization with MDA-LDL induces a specific Th2-biased response.
We first immunized normocholesterolemic C57BL/6 mice with homologous MDA-LDL in Freund’s adjuvant and examined the antigen-specific proliferation in splenic cultures. Splenocytes from immunized but not naive mice exhibited dose-dependent proliferation in response to MDA-LDL, but not to native LDL (Figure 1A). We next quantified titers of TD antibody isotypes to MDA-LDL in plasma. Measurements from three independent studies revealed more than an eight-fold greater induction of MDA-LDL-specific IgG1 titers over IgG2a titers (P < 0.01), demonstrating a strong Th2 bias of the induced response (Figure 1B), which occurred despite the use of CFA in the C57BL/6 genetic background that typically results in Th1 responses (28). Studies in which MHC class II–/– and T-cell receptor β–/– mice were injected with MDA-LDL indicated that the IgG responses to MDA-LDL were dependent on MHC II class antigen presenting cells and αβT-cell receptor–expressing T cells (data not shown). In parallel studies, we also immunized C57BL/6 mice with MDA-LDL, this time without adjuvant. Although fewer than half of these animals developed an antigen-specific titer, again IgG1 was the dominant isotype in the responding mice, and even at plasma dilutions as low as 1:50, no IgG2a binding was detected (data not shown).
Figure 1.
Immunization with MDA-LDL induces a specific Th2 response. C57BL/6 mice were immunized with homologous MDA-LDL in Freund’s adjuvant or remained naive. One week after the third injection, cellular and humoral immune responses were assessed. Three independent immunization studies were performed. (A) Splenocyte proliferation assay. Splenocytes of immunized (n = 6) or naive (n = 6) mice were cultured with titrated amounts of murine native LDL (open circles, immunized; open squares, naive) or murine MDA-LDL (filled circles, immunized; filled squares, naive), and antigen-specific proliferation was measured by 3[H]-thymidine uptake. (B) Increased IgG1 titers in plasma of immunized mice. Data represent titers from all mice studied (n = 14; P < 0.01, Student’s paired t test). (C) Increased frequency of MDA-LDL–specific IL-5 secreting cells in spleens of immunized mice as assessed by ELISpot assay. Splenocytes of immunized (n = 8; filled circles) and naive (n = 8; open circles) mice were incubated overnight with and without murine MDA-LDL, and the frequencies of MDA-LDL–specific IFN-γ or IL-5 spot-forming cells (SFCs) were assessed. Shown are the mean SFCs per 2 × 106 cells for IFN-γ and IL-5 of individual mice from two experiments (P < 0.01, Student’s paired t test; IFN-γ vs. IL-5 SFC of immunized mice). (D) MDA-LDL–specific Th2 cytokine secretion in cultures of splenocytes from four mice incubated for 72 hours with 25 μg/ml murine native LDL (white bars) or murine MDA-LDL (black bars). Control cultures stimulated with anti-CD3 and anti-CD28 produced 11.7 ± 2.1 ng/ml IFN-γ, 156 ± 39 pg/ml IL-5, 470 ± 43 pg/ml IL-10, and 1.2 ± 0.4 ng/ml IL-13. *P < 0.05; MANOVA followed by Newman-Keuls test, comparing the stimulation with increasing amounts (0.25 [not shown], 2.5 [not shown], and 25 μg/ml) of native and MDA-LDL. All values shown in Figure 1 are mean ± SEM.
To demonstrate the dominant Th2 responses, we quantified the relative frequencies of MDA-LDL–specific Th1 and Th2 cells in splenocyte cultures by enzyme-linked immunospot (ELISpot) assay using IFN-γ and IL-5 as surrogate Th1 and Th2 cytokines, respectively. After overnight incubation with MDA-LDL, an increased and significantly higher frequency of IL-5 secreting cells specific for MDA-LDL could be detected (P < 0.01) compared with an only minimal induction of specific IFN-γ–secreting cells in the spleens of the same mice (Figure 1C). Splenocytes from naive mice showed no response.
In addition, measurement of cytokines present in the supernatants of splenocytes from immunized mice cultured with MDA-LDL revealed an MDA-LDL–specific secretion of Th2 cytokines IL-5, IL-10, and IL-13, but not of the Th1 cytokine IFN-γ (Figure 1D). Supernatants from splenocytes cultured with native LDL did not secrete appreciable levels of these cytokines, with the exception of IL-10, which was not consistent in every experiment (Figure 1D). Splenocytes from naive control mice, cultured with either MDA-LDL or native LDL, did not exhibit specific cytokine secretion (data not shown).
Th2 responses persist in MDA-LDL–immunized mice with atherosclerosis.
To determine whether such a Th2 bias in response to MDA-LDL immunization would also occur in mice with developing atherosclerosis, which has been associated with increased Th1 responses (17), we immunized cholesterol-fed LDLR–/– mice with MDA-LDL. Mice were initially immunized twice with either MDA-LDL (n = 10) or PBS (n = 11) emulsified in Freund’s adjuvant and were then fed a high-cholesterol diet for 13 weeks, during which time three additional boosts were given. At the end of this intervention, mice in the MDA-LDL and PBS groups gained equal weight (27.5 ± 0.9 g vs. 27.5 ± 0.7 g) and had similar total plasma cholesterol (1,119 ± 17 mg/dl vs. 1,114 ± 34 mg/dl) and triglyceride levels (224 ± 12 mg/dl vs. 251 ± 28 mg/dl). Both groups of mice had identical lipoprotein profiles, as measured by fast-performance liquid chromatography (FPLC; Figure 2A). Despite the marked hypercholesterolemia, consistent with earlier studies (13, 14), MDA-LDL immunization led to significantly reduced (P < 0.02) atherosclerosis in the aortic origin (Figure 2B), the site where atherosclerotic lesions first develop in mice. However, the percentage of surface area of the arch covered by lesions was not significantly different at this site between the two groups (10.3 ± 1.0% vs. 10.9 ± 1.0%).
Figure 2.
Decreased atherosclerosis and dominant Th2 response in immunized LDLR–/– mice. Mice were immunized with homologous MDA-LDL (n = 10) or PBS (n = 11) in Freund’s adjuvant, and then fed a high-cholesterol diet for 13 weeks, during which they received further booster immunizations. (A) Lipoprotein profiles at time of death in pooled plasma of all mice immunized with MDA-LDL (filled circles) or PBS (open circles) as determined by FPLC. (B) Decreased atherosclerotic lesion size in cross-sections through the aortic origin in mice immunized with MDA-LDL. Values are mean ± SEM in mm2/section. (C and D) Plasma IgG1 (filled circles) and IgG2a (open circles) dilution curves of binding to MDA-LDL of mice immunized with (C) MDA-LDL or (D) PBS. Values are the mean ± SEM of all final plasma samples for each group measured in duplicate. (E and F) ELISpot assay of frequencies of MDA-LDL–specific cytokine-secreting cells in the spleens of mice immunized with (E) MDA-LDL or (F) PBS. Splenocytes were incubated overnight in the absence or presence of murine MDA-LDL with and without anti-CD28, and the frequencies of MDA-LDL–specific IFN-γ (white bars) or IL-5 (black bars) SFCs were assessed. Bars represent the mean SFCs ± SEM of 2 × 106 cells of all mice for each group. P < 0.01, Student’s paired t test.
Similarly to the normocholesterolemic C57BL/6 mice (Figure 1B), immunized cholesterol-fed LDLR–/– mice developed IgG1 titers to MDA-LDL that were more than 2 logs higher than the IgG2a titers (Figure 2C). In contrast, LDLR–/– mice injected with PBS in Freund’s adjuvant developed minimal IgG2a titers to MDA-LDL as a result of the atherogenic diet, but no specific IgG1 titers were induced (Figure 2D). This Th2 bias was further confirmed by the study of MDA-LDL–specific T cells in the spleens of these mice. ELISpot analysis for cells secreting IFN-γ or IL-5 revealed an increased frequency of IL-5–secreting cells specific for MDA-LDL in the spleens of MDA-LDL–immunized mice (Figure 2E), but not in the spleens of LDLR–/– mice injected with PBS in Freund’s adjuvant (Figure 2F). This pattern of response was further enhanced by the addition of anti-CD28 for in vitro costimulation of these MDA-LDL–specific T cells previously committed in vivo (Figure 2E). Splenocytes from LDLR–/– control mice displayed a low frequency of MDA-LDL–specific IFN-γ–secreting cells, even when cultures were costimulated with anti-CD28 (Figure 2F), consistent with the moderate IgG2a titers to MDA-LDL (Figure 2D). Thus, even during the development of atherosclerosis, which is accompanied by many proinflammatory stimuli associated with Th1 responses (17), MDA-LDL–specific Th2 responses remained dominant in immunized mice.
IL-5 is prominently secreted by MDA-LDL–specific T cells.
To further define the Th2 response in the MDA-LDL–immunized LDLR–/– mice, we quantified the cytokines secreted by splenocytes from immunized mice cultured with MDA-LDL or native LDL, in the presence of anti-CD28 to provide sufficient costimulation. To identify the dominant cytokine secreted by MDA-specific T cells, we stimulated parallel cultures with anti-CD3 and anti-CD28 (anti-CD3/anti-CD28) to achieve maximal nonspecific cytokine release and compared the results with the cytokine release obtained after stimulation with MDA-LDL (Figure 3, left panels). Cytokine production in response to anti-CD3/anti-CD28 did not differ between the two groups (data not shown). In cultures with native LDL, no substantial cytokine secretion was observed. Splenocyte cultures from LDLR–/– mice that received PBS in Freund’s adjuvant exhibited no specific cytokine secretion (Figure 3, left panels). Strikingly, MDA-LDL–specific secretion of IL-5 by T cells of MDA-immunized mice was greater than 75% of the amount achieved with maximal nonspecific stimulation in parallel cultures. By comparison, MDA-LDL–specific secretion of other Th2 cytokines (IL-4, IL-10, IL-13) was only 20–40% of that induced by anti-CD3/anti-CD28. In contrast, MDA-LDL–specific IFN-γ secretion was negligible. This pattern of response was also seen in cultures where dose-dependent cytokine secretion was assessed in response to stimulation with MDA-LDL alone (i.e., without additional costimulation; Figure 3, right panels).
Figure 3.
Antigen-specific cytokine secretion of splenocytes from cholesterol-fed LDLR–/– mice immunized with MDA-LDL (n = 10; black bars) or PBS (n = 11; white bars). Left column: Splenocytes were cultured for 72 hours in the presence of anti-CD28 and either anti-CD3, murine native LDL, or murine MDA-LDL, and supernatants were analyzed for cytokines. Data are presented as percentage of the cytokine secretion in parallel cultures maximally stimulated with anti-CD3/CD28 (=100%). Right column: splenocytes were stimulated either alone or with indicated amounts of murine MDA-LDL (without anti-CD28). Values are mean ± SEM of splenocyte cultures of all mice from each group.
Plasma levels of IL-5 are increased by MDA-LDL immunization.
To assess whether IL-5 was also increased in vivo, we measured cytokine levels in the plasma of all mice at the end of the intervention study. MDA-LDL–immunized mice had significantly higher plasma levels of IL-5 when compared to PBS–immunized mice (Figure 4A). Plasma IFN-γ levels were much lower and close to the quantification limit (50 pg/ml), with no measured differences between the two groups (data not shown).
Figure 4.
Increased levels of IL-5 and EO6 in the plasma of cholesterol-fed LDLR–/– mice immunized with MDA-LDL. At time of death, plasma was obtained from mice immunized with MDA-LDL (n = 10) and PBS (n = 11). (A) IL-5 levels in plasma of MDA-LDL immunized mice are increased. (B) EO6 antibody levels in plasma of MDA-LDL–immunized mice are increased. The amount of EO6 present was determined using an ELISA based capture assay with anti–idiotypic AB1-2 as described in Methods and was calculated based on an EO6 standard curve. (C) Comparison of T15/EO6 antibody titers in LDLR–/– mice immunized with MDA-LDL (current study) versus mice immunized with S. pneumoniae (R36a; n = 9) from a previous study (12). EO6 antibody titers were determined at a plasma dilution of 1:500 in the same assay. Purified EO6 was used as a positive control. Biot., biotinylated. (D) Circulating IgM/apoB ICs are increased in MDA-LDL–immunized mice. Results are expressed as IgM/apoB. All bars represent the mean ± SEM values of all mice from each intervention group.
MDA-LDL immunization increases T15/EO6 natural IgM antibodies to OxLDL.
Consistent with a previous study (14), the MDA-LDL immunization led to significant increases in IgM titers to MDA-LDL (data not shown), but surprisingly, we also found increased titers of IgM to OxLDL, and increased titers of IgM specific to the PC of OxPLs (data not shown). As noted, such epitopes are not present in the model MDA-LDL used as an immunogen (6, 7). To determine the absolute plasma levels of T15/EO6 IgM antibodies, we used a capture assay with the T15-specific anti-idiotypic antibody AB1-2 (29). MDA-LDL–immunized mice had significantly higher T15/EO6 plasma levels compared with PBS–immunized mice (806 ± 157 vs. 277 ± 68 ng/ml, P < 0.005; Figure 4B). To put these increases in perspective, we compared in the same assay the plasma titers of T15/EO6 induced by MDA-LDL to those induced in a prior study by immunization with S. pneumoniae, a potent PC antigen that induced a robust increase in T15/EO6 sufficient to inhibit the progression of atherosclerosis (12). These data indicate that immunization with MDA-LDL led to T15/EO6 levels that were on average 35% of those achieved by immunization with pneumococci (Figure 4C).
IgMs form immune complexes in plasma of MDA-LDL–immunized mice.
The increased T15/EO6 titers in MDA-LDL–immunized LDLR–/– mice demonstrated the additional engagement of innate immunity and suggested that EO6 could have contributed to the protective effect of this immunization. Therefore, we tested the possibility that there were higher levels of IgM/apoB immune complexes (ICs) in the plasma of MDA-LDL–immunized mice than in the plasma of PBS-immunized LDLR–/– mice. Indeed, mice immunized with MDA-LDL had higher plasma levels of IgM/apoB ICs than the PBS group did (Figure 4D, P < 0.01). There were only low levels of circulating IgG/apoB ICs, and these did not differ between the two groups (0.11 ± 0.02 vs. 0.10 ± 0.02 relative light units [RLU]/RLU for the MDA and PBS groups, respectively).
IL-5 stimulates B-1 cells to secrete natural antibody T15/EO6.
The expansion of T15/EO6 IgM in the MDA-LDL–immunized mice was unexpected, given that MDA-LDL does not bind to T15/EO6 antibodies and thus could not have directly ligated the B-cell receptor of PC-specific B-1 cells, which would lead to the secretion of T15/EO6. Although the secretion of B-1 cell–dependent IgM antibodies is known to be T cell independent, T cells are thought to provide noncognate help for B-1 cells (30), and IL-5 has been described as an important factor in B-1 cell biology (31, 32).
To directly test the hypothesis that IL-5 derived from the MDA-LDL–specific T-cell responses provided noncognate T cell help for B-1 cells to secrete T15/EO6, we isolated B-cell subsets from the peritoneal cavity and the spleens of naive C57BL/6 mice by FACS, based on the surface expression of CD19 and CD23 (33). Peritoneal B-1 cells were separated from conventional B-2 cells, and splenic B cells were separated into marginal-zone B cells and follicular B cells. These individual subsets of B cells were cultured in growth media alone or with IFN-γ, IL-4, or IL-5. After 7 days, culture supernatants were harvested and antibody binding to OxLDL was determined by ELISA. Only IL-5 strongly stimulated B-1 cells to secrete IgM antibodies to OxLDL (Figure 5A), and addition of an IL-5 neutralizing antibody abolished this response. None of the other B cell subsets showed equivalent anti-OxLDL IgM secretion following IL-5 stimulation. There was no comparable IgG binding of these culture supernatants (data not shown), which is consistent with the fact that B-1 cells predominantly secrete IgM. In addition, using the anti-idiotypic antibody AB1-2, we definitively demonstrated that the B-1 cells secreted T15/EO6 following IL-5 stimulation (Figure 5B).
Figure 5.
Role of IL-5 in the production of T15/EO6 natural antibodies. (A and B) IL-5 stimulates antibody secretion in vitro. Peritoneal B-1 cells (black bars) and B-2 cells (white bars), and splenic marginal zone (MZ) B cells (dark gray bars) and follicular B cells (light gray bars) were cultured for 7 days in either medium alone, or with IFN-γ, IL-4, or IL-5 with and without anti–IL-5 mAb. Polymyxin B was added to all cultures to neutralize contaminating LPS effects. (A) IgM binding to OxLDL in culture supernatants. (B) T15/EO6 antibodies in culture supernatants, measured with the anti-idiotypic antibody AB1-2. Values are mean RLU ± SEM from duplicate determinations of triplicate cultures. This experiment was repeated three times. (C) IL-5 stimulates the production of T15/EO6 antibodies in vivo. C57BL/6 mice received daily intraperitoneal injections with recombinant mouse IL-5 (n = 6) or vehicle only (BSA; n = 4) for 7 days, and the amount of T15/EO6 antibodies was determined in the plasma. Shown is the fold increase over the baseline levels at a 1:100 plasma dilution. Bars represent mean ± SEM of triplicate determinations of individual mice. P < 0.05, Student’s unpaired t test; Welch corrected. (D) Naive IL-5–/– mice have decreased T15/EO6 antibody levels. In IL-5+/+ (open circles) and IL-5–/– C57BL/6 mice (filled circles), 15–16 weeks of age (both n = 3), T15/EO6 antibody titers were determined. Shown is the binding of T15/EO6 antibodies of individual plasma samples diluted 1:100. Values are the mean RLU of triplicate determinations. (E–G) Impaired induction of T15/EO6 antibodies in IL-5–/– mice immunized with MDA-LDL. IL-5+/+ (open circles) and IL-5–/– (filled circles) C57BL/6 mice were immunized with MDA-LDL and plasma antibody titers were determined. (E) IgG1 binding to MDA-LDL (F), IgM binding to OxLDL, and (G) T15/EO6 antibodies of individual plasmas at 1:250 dilution before and after immunization. Values are the mean RLU of triplicate determinations.
We next tested the capacity of IL-5 to induce T15/EO6 antibodies in vivo by daily intraperitoneal injections of naive C57BL/6 mice with murine recombinant IL-5 for 7 days. On day 8, there was more than a four-fold increase in plasma T15/EO6 titers in mice injected with IL-5 (P < 0.05), compared to vehicle (BSA; Figure 5C).
We analyzed plasma levels of T15/EO6 antibodies in IL-5–/– mice, which have been reported to have transiently decreased numbers of B-1 cells at a young age (<8 weeks). Surprisingly, the baseline levels of T15/EO6 antibodies were strongly affected by the IL-5 deficiency even in adult mice. While 16 weeks old, naive IL-5+/+ C57BL/6 mice had measurable plasma levels of T15/EO6 antibodies at dilutions tested; age-matched IL-5–/– mice had no detectable T15/EO6 antibodies (Figure 5D). Interestingly, neither basal IgM levels to OxLDL, nor those to MDA-LDL, differed between the two groups (data not shown), suggesting the existence of other OxLDL-specific IgM antibodies that are not derived from B-1 cells.
Next, we examined whether IL-5 is essential for the induction of T15/EO6 as a consequence of MDA-LDL immunization. IL-5–/– and IL-5+/+ mice (both on a C57BL/6 background) were immunized with murine MDA-LDL. After one primary and two booster immunizations, IL-5–/– and IL-5+/+ mice displayed similar IgG1 (Figure 5E) and IgG2a titers (data not shown) to MDA-LDL, demonstrating that IL-5 deficiency did not impair TD responses. In contrast, the development of IgM titers to MDA-LDL was lower in IL-5–/– mice than in the IL-5+/+ control group (data not shown). Most striking, however, was the fact that the induction of IgMs to OxLDL (Figure 5F), and specifically the induction of T15/EO6 (Figure 5G), was greatly impaired in IL-5–/– mice, despite three injections (Figure 5, E–G), suggesting a pivotal role for IL-5 in the induction of T15/EO6 as a consequence of immunization with MDA-LDL.
IL-5 deficiency accelerates atherosclerosis.
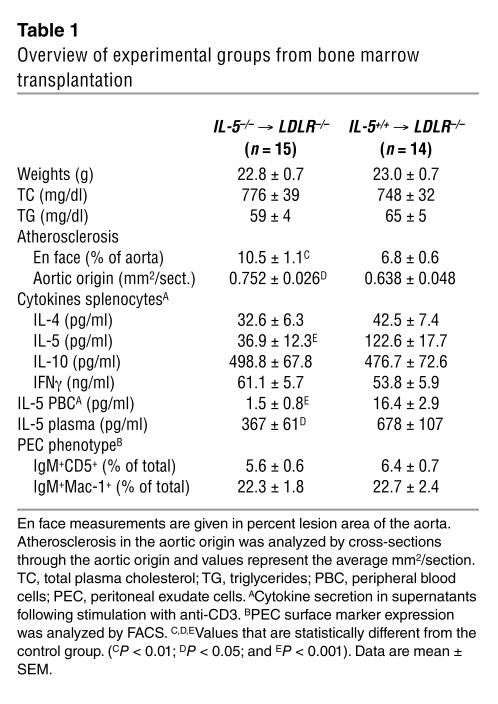
We asked whether IL-5 itself could play a functional role in atherogenesis, even in the absence of exogenous immunizations. To generate LDLR–/– mice with deficient production of IL-5, we transplanted irradiated LDLR–/– mice with bone marrow from IL-5–/– or IL-5+/+ mice. Four weeks after bone marrow transplantation (BMT), mice were switched to an atherogenic diet for the subsequent 16 weeks to induce lesion formation. At the time of sacrifice, splenocytes of the LDLR–/– mice with IL-5–/– bone marrow showed significantly less production of IL-5 following anti-CD3 stimulation compared with those that received IL-5+/+ bone marrow (Table 1). The production of the cytokines IL-4, IL-10, and IFN-γ, however, did not differ between the two groups (Table 1). Moreover, production of IL-5 by anti-CD3–stimulated peripheral blood cells was virtually absent in recipients of IL-5–/– bone marrow (Table 1). Consequently, plasma IL-5 levels were significantly decreased in these mice (Table 1). Thus, the IL-5–/– LDLR–/– bone marrow chimeras had a selective insufficiency, but not absence, of IL-5 production compared to the control mice that received IL-5+/+ bone marrow. En face analyses of the extent of atherosclerosis in the entire aorta revealed a significantly increased degree of lesion formation as a result of transplantation of IL-5–/– bone marrow, supporting the hypothesized protective role of IL-5 in atherogenesis (Table 1 and Figure 6A). In addition, atherosclerosis in the aortic origin of recipients of IL-5–/– bone marrow was also significantly greater than that in controls, despite the very advanced extent of atherosclerotic lesion formation (Table 1). To determine whether the defective IL-5 secretion resulted in impaired stimulation of B-1 cells to secrete atheroprotective antibodies, we evaluated the titers of T15/EO6 antibodies in the plasma of these mice. Indeed, at the time of sacrifice, IL-5–/– LDLR–/– bone marrow chimeras had significantly lower T15/EO6 plasma titers than the IL-5+/+ controls (Figure 6B). This was not due to an absolute deficiency of B-1 cells, as both recipient groups had similar numbers of peritoneal B-1 cells at the time of death, as determined by FACS (Table 1). Analyses of autoantibody titers to MDA-LDL and OxLDL revealed decreased levels of TI IgG3 titers but not of TD IgG1 or IgG2a titers (data not shown). T15/EO6 IgM antibodies can bind OxLDL and prevent its uptake by macrophages (7). To determine whether the decreased levels of T15/EO6 antibodies also led to an impaired formation of ICs with minimally oxidized LDL in vivo, we measured the levels of circulating LDL ICs that contained T15/EO6 antibodies. Recipients of IL-5–/– bone marrow had significantly lower levels of plasma T15/EO6 antibody–apoB ICs during the period of cholesterol feeding (Figure 6C). IgG-apoB ICs were very low and did not differ between the two recipient groups (data not shown).
Table 1.
Overview of experimental groups from bone marrow transplantation
Figure 6.
Increased atherosclerosis in IL-5–deficient LDLR–/– mice. LDLR–/– mice were reconstituted with bone marrow from either IL-5–/– mice (IL-5–/–; n = 15) or IL-5+/+ mice (IL-5+/+; n = 14) and fed an atherogenic diet for 16 weeks. (A) Increased extent of atherosclerosis in aortas of recipients of IL-5–/– bone marrow (n = 15) compared to recipients of IL-5+/+ bone marrow (n = 14; P < 0.01). Horizontal bars indicate means of each group. (B) Decreased titers of T15/EO6 antibodies in plasma of LDLR–/– mice reconstituted with IL-5–/– bone marrow. Data are mean ± SEM titers of all mice of each group (P < 0.05). (C) Reduced formation of circulating T15/EO6-apoB ICs in recipients of IL-5–/– bone marrow. Results are expressed as T15/EO6 antibodies per apoB. Data are the mean ± SEM values of all mice from each intervention group at 4, 8, and 16 weeks after initiation of the atherogenic diet. P < 0.05, repeated-measures ANOVA.
Discussion
Here we report four main findings that are relevant to the impact of adaptive and innate immunity in atherogenesis. First, we show that immunization of mice with homologous MDA-LDL results in a strongly biased TD activation of Th2 cells with specificity for the MDA modification; this bias is intrinsic to the immunogen and independent of hypercholesterolemia (34). Consistent with our previous results (13, 14), this immunization ameliorates the progression of atherosclerosis. Second, we show that immunization with MDA-LDL surprisingly also results in a significant expansion of innate T15/EO6 clonotypic antibodies that bind POVPC-like OxPLs, which are typically not present in MDA-LDL. These natural antibodies can block the uptake of OxLDL by macrophages. Their near-monoclonal expansion in mice, which was achieved by immunization with a potent PC-containing pneumococcal preparation, significantly reduced atherosclerosis (12). Remarkably, the MDA-LDL immunization increased titers of T15/EO6 to levels 35% as high as those induced by pneumococcal immunization. Third, we define the mechanism by which immunization with MDA-LDL led to expansion of T15/EO6 antibodies. Because the expansion of the B-1 cell–derived T15/EO6 is independent of classic cognate T-cell help (30), it was unclear how the TD response to MDA-LDL led to increased T15/EO6 titers. In these studies, we demonstrate that the induced Th2 cells produced large amounts of IL-5, which in turn provided noncognate stimulation of B-1 cells to secrete the innate T15/EO6 IgM antibodies (7, 12). Fourth, using BMT in which LDLR–/– mice were reconstituted with wild-type or IL-5–/– bone marrow, we establish that IL-5 had an overall atheroprotective role. Thus, our data demonstrate a mechanism whereby immunization with an oxidation-specific epitope leads to a classic adaptive Th2 response, which in turn leads to the recruitment of natural innate immunity to OxLDL. In aggregate, these events provided atheroprotection. In particular, our data demonstrate that IL-5 links adaptive and natural immunity specific for epitopes of OxLDL and is atheroprotective. These data may explain in part the postulated atheroprotective role of Th2-mediated immunity.
Th1 adaptive responses, and Th1 cytokines in particular have been shown to play a key role in promoting atherogenesis (17, 35–37). In contrast, we now demonstrate that immunization with MDA-LDL directly triggers an antigen-specific Th2 response that is associated with an atheroprotective effect. There are a number of possible mechanisms by which such a biased Th2 response could be atheroprotective in general, as discussed in several recent reviews (3, 17). We now specifically demonstrate that MDA-LDL immunization induces a decidedly biased Th2 response characterized by the prominent secretion of IL-5, sufficient to cause increased systemic levels of IL-5 in immunized mice. Moreover, IL-5–/– LDLR–/– bone marrow chimeras, which showed decreased production of IL-5, developed significantly more atherosclerosis than recipients of wild-type bone marrow. These mice did not show decreases in their anti-OxLDL IgG1 and IgG2a titers compared with wild-type mice. Thus, the protective effect of IL-5 seems to be independent of TD IgG responses to OxLDL.
IL-5 has been detected in human (38, 39) and murine (our unpublished results) atherosclerotic lesions, although it is irregularly expressed. In our present study, we did not find increased expression of IL-5 mRNA in lesions of MDA-LDL–immunized mice when determined by quantitative PCR analysis (data not shown). Nevertheless, for immunoregulatory functions that affect atherogenesis, IL-5 need not be expressed in lesions. IL-5 is mainly produced by Th2 cells and mast cells. As an eosinophil differentiation and maturation factor (40, 41), it is widely studied for its role in asthma and allergies, as well as in host defense from intestinal parasites. Interestingly, patients with asthma have been reported to have decreased atherosclerotic disease (42). Eosinophilic infiltrations are rarely found in atherosclerotic lesions (38), and we did not observe differences in peripheral blood eosinophil counts in the recipients of IL-5–/– and IL-5+/+ bone marrow (data not shown).
Importantly, IL-5 also induces proliferation and differentiation of B cells, particularly B-1 cells, which constitutively express the IL-5 receptor (31, 43–45). Accordingly, IL-5 transgenic mice have (in addition to eosinophilia) increased numbers of B-1 cells in the peritoneal cavity and elevated levels of serum IgM and IgA (46), while IL-5Rα–/– mice do not show IL-5–induced enhancement of B cell responses to TI type 2 (TI-2) antigens (47). Immune responses to PC are examples of classic TI-2 responses. In the current studies, we demonstrate that IL-5 stimulated the production of the natural IgM T15/EO6 in vitro and in vivo, whereas mice deficient in IL-5 had virtually no T15/EO6 antibodies in their plasma. Therefore, IL-5 seems to be pivotally involved in the expansion of atheroprotective T15/EO6 natural antibodies derived from B-1 cells (8, 12). Indeed, LDLR–/– mice that received bone marrow from IL-5–/– mice exhibited significantly lower titers of B-1 cell–derived T15/EO6 antibodies and decreased IgG3 titers to OxLDL — another TI-2 response. Consequently, there was also a reduced formation of ICs with minimally oxidized LDL in the plasma of these mice.
Interestingly, we observed a proatherogenic phenotype in the IL-5–/– LDLR–/– bone marrow chimeras despite the fact that these mice still had the capacity to secrete low levels of IL-5. Thus, this BMT model represents an IL-5 hypomorphic phenotype. We are currently cross-breeding IL-5–/– mice with LDLR–/– mice, which then can be used to assess the full impact of IL-5 deficiency on atherogenesis, as well as the quantitative role that IL-5 plays in mediating the atheroprotective effect of immunization with MDA-LDL. Nevertheless, the fact that even reduced IL-5 production led to accelerated lesion formation argues for an important role of IL-5 during atherogenesis. While our results do not unequivocally establish that the atheroprotective effect of IL-5 is mediated by the expansion of atheroprotective T15/EO6 antibodies, our data clearly demonstrate a strong relationship between IL-5 and the secretion of T15/EO6 antibodies.
B-1 cells are the major source of natural IgM antibodies and are prominently involved in TI responses (48). Because multivalent cross-linking of the B cell receptor is not sufficient to drive optimal antibody production, it has been suggested that noncognate interactions with T cells, NK cells and other cells enhance these responses (30). The exact mechanisms underlying such noncognate T cell help are still unknown, but T cells, once activated, could either directly or indirectly costimulate B1 cells. Although many stimuli, including antigen, LPS and cytokines, have been demonstrated to activate B-1 cells, the exact physiological triggers during an orchestrated immune response are not clear. IL-5 specifically plays a role in stimulating IgM secretion by B-1 cells (43, 49). Moreover, IL-10 seems to be involved in B-1 cell activation as well (44, 50), although partially in an autocrine manner (51), whereas IL-12 has been shown to inhibit B-1 cell proliferation (52). Thus, it seems that specific Th2 subsets could positively affect B-1 cell activity. Since immunization of wild-type mice with MDA-LDL prominently induced IL-5–secreting Th2 cells and T15/EO6 antibodies, whereas immunization of IL-5–/– mice with MDA-LDL failed to induce T15/EO6 antibodies, MDA-specific Th2 cells secreting IL-5 could represent such a specific Th subset.
Although the stimulation of B-1 cells by IL-5 does not exclusively result in the secretion of T15/EO6, the general concept that B-1 cells have specificity for stress-induced self-structures (53) would allow the recruitment of other natural antibodies that have related specificities, such as to other oxidation-specific epitopes. Consistent with this notion, we found that in vitro incubation of IL-5 with B-1 cells derived from normal mice also stimulated anti–MDA-LDL IgM antibodies (unpublished observations), suggesting that MDA epitopes are yet another set of stress-induced self-structures that are also recognized by B-1 cells (54). Importantly, we have previously shown that many monoclonal IgM antibodies derived from the spleens of atherosclerotic apoE–/– mice bind to MDA-LDL (6). Thus, during atherogenesis, a setting in which the generation of oxidation-specific epitopes of OxLDL is greatly increased, oxidation-specific B-1 cells would be preferentially activated through their B cell receptors. In turn, this activation would be helped by the augmented IL-5. Thus, de facto, generalized expansion of B-1 cells in this setting would be expected to preferentially enhance secretion of IgM directed at oxidation-specific epitopes.
As recently demonstrated, without the natural immunoprotection afforded by B cells, atherogenesis can be dramatically accelerated (55). In particular, B-1 cells are primitive tiers of innate immunity and arise spontaneously in mice early in ontogeny (56). T15/EO6 antibodies secreted by B-1 cells are present at low titers even in normal mice but are expanded during atherogenesis, presumably as a result of increased antigenic pressure (6, 8, 57). These antibodies could provide significant protection against atherosclerosis by inhibition of OxLDL uptake by macrophages, removal of pro-atherogenic particles away from the artery wall, or neutralization of proinflammatory effects of OxPLs. Indeed, mice that have undergone splenectomy, which removes the anatomical site from which B-1 cells secrete antibodies, have accelerated atherogenesis (58).
In conclusion, our findings demonstrate that the adaptive immune response against MDA can in turn efficiently boost the expansion of innate natural T15/EO6 IgM antibodies, which recognize PC, a different oxidation-specific epitope. In this paper we provide direct evidence that this positive modulation of protective natural immunity to OxLDL is mediated by IL-5 and that IL-5 deficiency accelerates atherogenesis. Because strategies are currently being developed to inhibit IL-5 actions in patients with asthma and other allergic diseases (59, 60), a detailed understanding of the role of IL-5 in atherogenesis is needed.
Methods
Immunogens and antigens.
Murine LDL, murine MDA-LDL, human MDA-LDL, and human OxLDL were prepared as described (7, 14). All murine preparations used for immunization and cell culture were tested for endotoxin levels by chromogenic Limulus amoebocyte assay (QCL-1000; BioWhittaker Inc, Wakersville, Maryland, USA) and contained less than 1.5 ng lipopolysaccharides/mg protein (ApoB).
Mice, immunizations, and diets.
Female C57BL/6 mice, MHCII–/– and T cell receptor β–/– mice were from The Jackson Laboratories (Bar Harbor, Maine, USA), and IL-5–/– mice were a kind gift of Manfred Kopf (ETH Zürich, Zürich, Switzerland) (32). All mice were on a C57BL/6 background and were bred and maintained in our colony. All mice used were more than 8 weeks of age and fed nonatherogenic rodent chow (TD8604; Harlan Teklad, Madison, Wisconsin, USA). Primary immunizations consisted of 50 μg murine MDA-LDL in 100 μl sterile PBS emulsified in an equal volume of complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, Missouri, USA) and were injected subcutaneously into both inguinal regions. Booster immunizations consisted of 25 μg antigen in 100 μl sterile PBS emulsified in incomplete Freund’s adjuvant (Sigma-Aldrich) and were given intraperitoneally every 2–3 weeks. If no adjuvant was used, the same amounts of antigen were given in 200 μL sterile PBS.
For the atherosclerosis intervention study, 24 female LDLR–/– mice (tenth generation C57BL/6), 11–13 weeks of age, were divided into equal groups matched for body weight, age, and plasma cholesterol. Mice in the MDA group (n = 12) were immunized with murine MDA-LDL in Freund’s adjuvant, and mice in the PBS group (n = 12) received PBS in Freund’s adjuvant. Mice were initially fed nonatherogenic chow and received the primary and one booster immunization on day 14. On day 17, mice were switched to an atherogenic diet containing 21.2% milk fat and 1.25% cholesterol (TD96121; Harlan Teklad) for the 13 remaining weeks during which they received three more booster immunizations every 3–4 weeks. Three mice (one in the PBS group and two in the MDA group) were a priori excluded from the final analysis because they developed skin lesions, loss of weight and/or dropping plasma cholesterol levels.
BMT studies were performed as previously described (61). Thirty 8-week-old female LDLR–/– mice on a C57BL/6 background, bred at the Scripps Research Institute Animal Facility, were given 10-Gy lethal total body-irradiation to eliminate endogenous bone marrow stem cells and bone marrow–derived cells. Bone marrow cells used for repopulation were extracted from the femur and tibia of three female IL-5–/– and three female IL-5+/+ mice (8 weeks old, C57BL/6 background). Irradiated mice were injected intravenously with 2 × 106 bone marrow cells harvested from either IL-5–/– (n = 15) or IL-5+/+ mice (n = 15). Mice were fed regular chow for 4 weeks after BMT to allow for bone marrow reconstitution and then switched to an atherogenic diet containing 15.8% fat and 1.25% cholesterol (TD94059; Harlan Teklad) for an additional 16 weeks to induce lesion formation. One mouse from the IL-5+/+ control group was excluded from the formal analysis because plasma cholesterol levels were more than 2.5 standard deviations above the mean of all mice. Differences in the extent of atherosclerosis between groups would remain significant even if this mouse were included. All experimental protocols were approved by the Animal Subjects Committee at University of California, San Diego, and by the Institutional Animal Care and Use Committee (IACUC) of The Scripps Research Institute.
Plasma cholesterol and triglyceride levels were determined using automated enzymatic assays (Roche Diagnostics, Indianapolis, Indiana, USA; Equal Diagnostics, Exton, Pennsylvania, USA) and are presented as time averages, that is, the area under the cholesterol curve over time divided by days of cholesterol feeding. Plasma lipoprotein profiles were determined by FPLC as described previously (62).
Immunoassays.
Specific antibody titers were determined by chemi-luminescent enzyme immunoassays as previously described (7). To determine the plasma titers of specific antibody binding to given antigens, plasmas were serially diluted and antibody binding measured by chemiluminescent ELISA. A titer was defined as the reciprocal of the maximal dilution at which binding of the secondary antibody was two times above the background binding. Several secondary antibodies were used: alkaline phosphatase–labeled (AP-labeled) goat anti-mouse IgM (μ-chain specific) and IgG (γ-chain specific) (Sigma-Aldrich); AP-labeled rat anti-mouse IgG1 (LO-MG1-2) (Zymed Laboratories Inc., South San Francisco, California, USA); and AP-labeled rat anti-mouse IgG2a (R19-15) and rat anti-mouse IgG3 (clone R40-82) (BD Biosciences — Pharmingen, San Diego, California, USA).
Flow cytometry.
At time of death, peritoneal exudate cells (PEC) were harvested by peritoneal lavage using ice-cold PBS supplemented with 1% BSA. After blocking with a specific anti-Fcγ receptor monoclonal antibody (clone 2.4G2, BD Biosciences — Pharmingen) for 10 minutes at 4°C, 106 cells were stained with the fluorescently labeled monoclonal antibodies (FITC-labeled anti-IgM [II/41], phycoerythrin-labeled [PE-labeled] anti-CD5 [53-7.3], and APC-labeled anti-CD11b/Mac-1 [M1/70]; all BD Biosciences — Pharmingen) in 100 μL volumes of staining buffer for 30 minutes at 4°C in darkness, followed by two washes of large volumes of staining buffer. Cell populations were analyzed by multiparameter flow cytometry using a BD FACSCalibur or a FACScan instrument (BD, San Jose, California, USA). More than 105 cells were analyzed per sample, with dead cells excluded by forward and side scatter properties. Surface marker analysis was performed using CellQuest software (BD).
Splenocyte proliferation assay.
Splenocytes were suspended in culture media (RPMI 1640 media containing 10% heat-inactivated fetal calf serum, 10 mM Hepes buffer, 2 mM L-glutamine, 0.1 mM nonessential amino acid solution, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.05 mM 2-mercaptoethanol; all Invitrogen, Carlsbad, California, USA) and seeded at 2 × 105 cells per well in 96-well flat-bottom plates in final culture volumes of 200 μl. Triplicate cultures of splenocytes from individual mice were incubated in the absence or presence of titrated amounts of antigen for 72 hours at 37°C/5% CO2 and then pulsed with 1 μCi [3H]-thymidine (ICN Biomedicals, Irvine, California, USA) per well for 18 hours, after which cells were harvested onto filter paper using a cell harvester (Wallac; PerkinElmer, Gaithersburg, Maryland, USA). Thymidine incorporation was determined by liquid scintillation counting. Values are presented as cellular [3H]-thymidine uptake in cpm per well from which values for wells with media alone were subtracted.
Enzyme-linked immunospot (ELISpot) assay for single-cell IFN-γ and IL-5 secretion.
Splenocytes from individual mice were cultured in 100 μl culture media with and without 25 μg/ml of murine MDA-LDL in duplicate at 2 × 106 and 1 × 106 cells/well in washed and blocked 96-well MultiScreen-HA sterile nitrocellulose plates (Millipore, Bedford, Massachusetts, USA), which had been coated overnight with capture antibodies for IFN-γ (R4-6A2, BD Biosciences — Pharmingen) or IL-5 (TRFK5, BD Biosciences — Pharmingen). In some assays, parallel incubations were performed in the presence of 10 μg/ml anti-CD28 (37.51, BD Biosciences — Pharmingen). After 22 hours incubation at 37°C/5% CO2 cells were removed by washing and antigen-specific cytokine secreting spot-forming cells (SFC) were detected using matched biotinylated antibodies against IFN-γ (XMG1.2, BD Biosciences — Pharmingen) or IL-5 (TRFK4, BD Biosciences — Pharmingen), followed by HRP-Streptavidin (Zymed Inc.). Plates were developed using a tetramethylbenzidine membrane substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA), and spots were quantified by examination either under a dissecting microscope or using an automated ImmunoSpot Image Analyzer (Cellular Technology, Cleveland, Ohio, USA).
Cytokine release assay and ELISA.
Splenocytes (5 × 105) were seeded into 96-well round-bottom plates in culture media in a final volume of 200 μl. Triplicate cultures of splenocytes from individual mice were incubated for 72 hours at 37°C/5% CO2 in culture media with and without titrated amounts of antigen (unless indicated otherwise, results represent incubations with 25 μg/ml antigen). In some assays, parallel incubations were performed in the presence of 10 μg/ml anti-CD28. In separate wells, cells were cultured in the presence of 10 μg/ml plate-bound anti-CD3 (145-2C11, BD Biosciences — Pharmingen) and 10 μg/ml soluble anti-CD28 (37.51, BD Biosciences — Pharmingen). For the stimulation of peripheral blood lymphocytes, blood was obtained in heparinized tubes, and after red blood cell lysis, 2.5 × 105 nucleated cells were cultured for 48 hours with and without plate bound anti-CD3. The amounts of specific cytokines in supernatants were determined by a chemiluminescent-based sandwich ELISA using matched pairs of specific antibodies for capture and detection, followed by incubation steps with AP-labeled NeutrAvidin (Pierce Biotechnology Inc., Rockford, Illinois, USA) and LumiPhos 530 (Lumigen Inc., Southfield, Michigan, USA). The following antibodies were paired with appropriate biotinylated detecting antibodies (R&D Systems, Minneapolis, Minnesota, USA): anti–IFN-γ 37801.11/37875.11, anti–IL-4 30340.11, anti–IL-5 TRFK5, anti–IL-10 JES052A5, and anti–IL-13 38213.11. The recombinant cytokines used for standardization were purchased from R&D Systems. The detection limit for all cytokines was approximately 10 pg/ml, and plasma was used at dilutions of 1:5 to 1:25.
Evaluation of atherosclerosis.
The extent of atherosclerosis was determined in a blinded fashion in en face preparations of the arch or the entire aorta, as well as in cross sections through the aortic origin, by computer-assisted image analysis as previously described (61, 63).
Measurement of T15 clonotypic antibodies.
For the detection of T15 clonotypic antibodies (e.g., EO6) in the plasma of mice, a chemiluminescent-based double antibody capture assay was developed and validated, using the monoclonal anti-T15–idiotypic antibody AB1-2, a mouse IgG1, which is absolutely specific for both the canonical T15 VH and the T15 VL regions (29) (a kind gift of J.F. Kearney, University of Alabama at Birmingham, Birmingham, Alabama). For the assay, 5 μg/ml of AB1-2 or an IgG1 isotype control MOPC31c (Sigma-Aldrich) in PBS were coated onto microtitration plates overnight at 4°C. After washing and blocking steps, 50 μl of murine plasma diluted stepwise or at a given dilution in BSA-PBS were incubated overnight at 4°C. After further washing, captured T15-clonotypic antibodies were detected using 0.1 μg/ml biotinylated AB1-2 diluted in BSA-PBS, followed by AP-labeled NeutrAvidin (Pierce Biotechnology Inc.) diluted in BSA-TBS, and a 50% aqueous solution of LumiPhos 530. Biotinylated antibodies were prepared using the EZ-Link Biotin Hydrazide method (Pierce Biotechnology Inc.) according to the manufacturer’s protocol. Purified EO6 and EO14, which is an IgM monoclonal autoantibody specific for MDA-LDL and not T15 clonotypic (8), were used as positive and negative controls, respectively (6). In some experiments a standard curve was constructed with EO6.
ApoB-100 IC measurement.
ApoB-100 particles were captured on microtiter wells using LF3, a monoclonal antibody specific for murine apoB-100 (64) (kindly provided by S.G. Young, Gladstone Institute of Cardiovascular Disease, San Francisco, California, USA), which was coated on microtiter wells at 5 μg/ml in PBS. After washing and blocking steps, plasmas (1:100 in BSA-PBS) were incubated in wells for 1 hour at room temperature and after further washing, bound IgM or IgG was detected using AP-conjugated goat anti-mouse IgM or anti-IgG by chemiluminescent ELISA. For detection of T15/EO6 antibodies bound to the captured apoB-containing particles, biotinylated AB1-2 was used, followed by incubation with AP-labeled NeutrAvidin and LumiPhos 530. In parallel wells, the relative amount of apoB captured in each sample was determined using biotinylated LF5, another monoclonal antibody specific for mouse apoB-100 (64), followed by incubation with AP-labeled NeutrAvidin and LumiPhos 530. Because LF5 binds to only one epitope of apoB-100, the amount of each antibody used in this assay bound to the captured LDL was then normalized for the amount of captured apoB, and expressed as a ratio of antibody counts (RLU/100ms) to apoB-100 counts (RLU/100ms).
B-cell isolation and stimulation assay.
Splenocytes and peritoneal exudate cells were isolated from adult naive C57BL/6 mice. After red blood cell lysis and a 2-hour incubation at 37°C/5% CO2 in culture medium, nonadherent cells were incubated with an anti-FcγR antibody (2.4G2, BD Biosciences — Pharmingen), followed by staining with PE-labeled anti-CD19 (1D3, BD Biosciences — Pharmingen) and FITC-labeled anti-CD23 (B3B4, BD Biosciences — Pharmingen). Washed cells were sorted at the Flow Cytometry Core Facility at UCSD using a FACS Vantage SE (BD, San Jose, California, USA). Post-sort analysis revealed a purity of greater than 93% for B-1 cells (CD19+/CD23–), greater than 94% for B-2 cells (CD19+/CD23+), greater than 74% for marginal-zone (MZ) B cells (CD19+/CD23–), and greater than 99% for follicular B cells (CD19+/CD23+). For stimulation assays of sort-purified B cells, 4 × 104 cells were incubated in 96-well flat-bottom plates with indicated concentrations of recombinant mouse cytokines (BD Biosciences — Pharmingen) or an equivalent volume of vehicle (BSA) in triplicate cultures in culture media in final culture volumes of 200 μl for 7 days at 37°C/5% CO2. Culture supernatants were harvested, diluted 1:1 in BSA-PBS, and antibody binding was determined by ELISA. In indicated cultures, cells were also incubated in the presence of 0.5 μg/ml anti–IL-5 (TRFK5, BD Biosciences — Pharmingen). Because B cells are particularly sensitive to endotoxins (65) and low amounts of endotoxin were measured in the BSA used, parallel assays were performed in the presence of 0.1 mg/ml Polymyxin B (Sigma-Aldrich) to neutralize potential endotoxin activity. This concentration was effective but not toxic to either of the cell types tested, as determined in pilot experiments.
IL-5 injections.
Sixteen-week-old female C57BL/6 mice received daily intraperitoneal injections of 200 ng recombinant mouse IL-5 (BD Biosciences — Pharmingen) or equal volumes of vehicle (BSA) alone in 200 μl PBS for 7 days. Plasma was obtained at baseline and one day after the last injection.
Statistical analysis.
Data are presented as mean ± SEM. Results were analyzed by one-way ANOVA and Student’s unpaired t test, unless indicated differently. P < 0.05 was considered significant.
Acknowledgments
We thank Gregg Silverman for helpful discussions. This work was supported by NIH grants HL56989 (SCOR in Molecular Medicine and Atherosclerosis), HL69464 and HL57505 (to J.L. Witztum), and HL35297 and HL43815 (to L.K. Curtiss). C.J. Binder was supported by a Ph.D. scholarship from the Boehringer Ingelheim Fonds, a scholarship from the Austrian Academy of Science, and a fellowship from the American Heart Association (AHA, Western Affiliates). K. Hartvigsen was supported by grants from the Novo-Nordisk/Danish-American Foundation, the Reinholdt W. Jorck & Hustrus Foundation, the Otto Monsteds Foundation, the Arvid Nilssons Foundation, the Villum Kann Rasmussen Foundation, and a fellowship from the AHA (Western Affiliates). M.-K. Chang was supported by a scientist development grant from the AHA. We are grateful to Manfred Kopf for provision of the IL-5–/– mice. We thank Gary Bradshaw, Joshua Bulgrien, Susan Butler, Florencia Casanada, Brian Crain, Joseph Juliano, Elizabeth Miller, Jennifer Pattison, and Mercedes Silvestre for excellent technical assistance; and Dennis J. Young in the UCSD Cancer Center Flow Cytometry Shared Resource for flow cytometer expertise.
Footnotes
See the related Commentary beginning on page 317.
Nonstandard abbreviations used: alkaline phosphatase (AP); bone marrow transplantation (BMT); fast-performance liquid chromatography (FPLC); immune complex (IC); LDL receptor (LDLR); malondialdehyde (MDA); MDA-modified LDL (MDA-LDL); oxidized LDL (OxLDL); oxidized phospholipid (OxPL); 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC); phosphorylcholine (PC); relative light units (RLU); T cell–dependent (TD); T cell–independent (TI); T cell–independent type 2 (TI-2).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Binder CJ, et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 5.Hörkkö S, et al. Immunological responses to oxidized LDL. Free Radic. Biol. Med. 2000;28:1771–1779. doi: 10.1016/s0891-5849(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 6.Palinski W, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hörkkö S, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman P, Hörkkö S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol concentration. J. Biol. Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 10.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harnett W, Harnett MM. Phos-phorylcholine: friend or foe of the immune system? Immunol. Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 12.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 13.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialde-hyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. U. S. A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler. Thromb. Vasc. Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 15.George J, et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 17.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 18.Palinski W, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. U. S. A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1995;15:1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 20.Palinski W, et al. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 23.Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 24.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor–/– mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 25.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallat Z, et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85:E17–E24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 27.Pinderski LJ, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 28.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- 29.Kearney JF, Barletta R, Quan ZS, Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur. J. Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- 30.Snapper CM, et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 2001;22:308–311. doi: 10.1016/s1471-4906(01)01926-3. [DOI] [PubMed] [Google Scholar]
- 31.Takatsu K. Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 1998;9:25–35. doi: 10.1016/s1359-6101(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 32.Kopf M, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 33.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J. Immunol. 2001;166:1531–1539. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand. J. Immunol. 2004;59:285–293. doi: 10.1111/j.0300-9475.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E–/– mice. J. Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 37.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J. Clin. Invest. 2001;108:251–259. doi:10.1172/JCI200111380. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frostegard J, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 39.Schönbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am. J. Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi Y, et al. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J. Exp. Med. 1988;167:1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J. Exp. Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasser EC, Berry C, Kortman K. Diminished atherosclerotic arterial calcifications in asthma. A possible role for elevated endogenous heparin-like material. Allergy. 1987;42:549–552. doi: 10.1111/j.1398-9995.1987.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 43.Wetzel GD. Interleukin 5 regulation of peritoneal Ly-1 B lymphocyte proliferation, differentiation and autoantibody secretion. Eur. J. Immunol. 1989;19:1701–1707. doi: 10.1002/eji.1830190926. [DOI] [PubMed] [Google Scholar]
- 44.Nisitani S, Tsubata T, Murakami M, Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur. J. Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]
- 45.Sakiyama T, Ikuta K, Nisitani S, Takatsu K, Honjo T. Requirement of IL-5 for induction of autoimmune hemolytic anemia in anti-red blood cell autoantibody transgenic mice. Int. Immunol. 1999;11:995–1000. doi: 10.1093/intimm/11.6.995. [DOI] [PubMed] [Google Scholar]
- 46.Tominaga A, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J. Exp. Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida T, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 48.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 49.Tominaga A, et al. Establishment of IL-5-dependent early B cell lines by long-term bone marrow cultures. Growth Factors. 1989;1:135–146. doi: 10.3109/08977198909029123. [DOI] [PubMed] [Google Scholar]
- 50.Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J. Exp. Med. 1992;175:1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Garra A, et al. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 52.Vogel LA, Lester TL, Van Cleave VH, Metzger DW. Inhibition of murine B1 lymphocytes by interleukin-12. Eur. J. Immunol. 1996;26:219–223. doi: 10.1002/eji.1830260134. [DOI] [PubMed] [Google Scholar]
- 53.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 54.Chang MK, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 56.Stall AM, Wells SM, Lam KP. B-1 cells: unique origins and functions. Semin. Immunol. 1996;8:45–59. doi: 10.1006/smim.1996.0007. [DOI] [PubMed] [Google Scholar]
- 57.Kearney JF. Immune recognition of OxLDL in atherosclerosis [comment] J. Clin. Invest. 2000;105:1683–1685. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 2002;109:745–753. doi:10.1172/JCI200207272. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leckie MJ. Anti-interleukin-5 monoclonal antibodies: preclinical and clinical evidence in asthma models. Am. J. Respir. Med. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
- 60.Danzig M, Cuss F. Inhibition of interleukin-5 with a monoclonal antibody attenuates allergic inflammation. Allergy. 1997;52:787–794. doi: 10.1111/j.1398-9995.1997.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 61.Schiller NK, Kubo N, Boisvert WA, Curtiss LK. Effect of gamma-irradiation and bone marrow transplantation on atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:1674–1680. doi: 10.1161/hq1001.096724. [DOI] [PubMed] [Google Scholar]
- 62.Li AC, et al. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid. Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 64.Zlot CH, et al. Generation of monoclonal antibodies specific for mouse apolipoprotein B-100 in apolipoprotein B-48-only mice. J. Lipid. Res. 1999;40:76–84. [PubMed] [Google Scholar]
- 65.Wetzel GD. Induction of interleukin-5 responsiveness in resting B cells by engagement of the antigen receptor and perception of a second polyclonal activation signal. Cell Immunol. 1991;137:358–366. doi: 10.1016/0008-8749(91)90085-p. [DOI] [PubMed] [Google Scholar]