Case Presentation
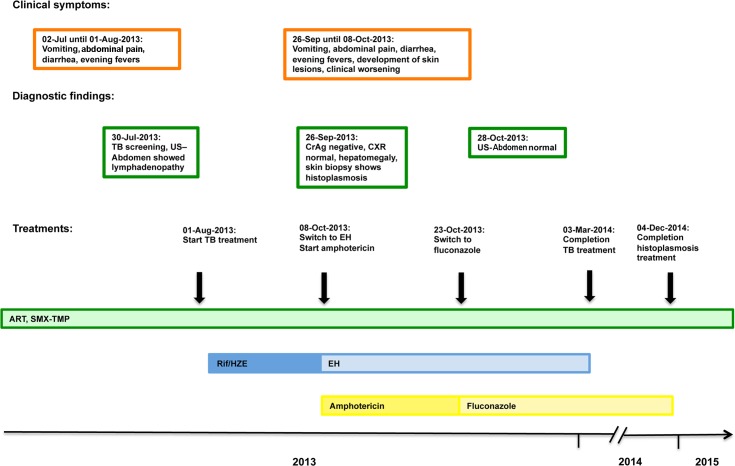
On 2nd July 2013, a 29-year-old HIV-positive woman presented herself to the outpatient clinic at the Infectious Diseases Institute in Kampala, Uganda. Her weight had decreased from 46 kg to 42 kg in the past few weeks. In addition, she complained about abdominal pain, diarrhea, vomiting, and evening fevers during the week leading up to her visit (see Table 1 and Fig 1 for patient characteristics). Her CD4 T cell count in June 2013 was 34 cells/μl, and she had documented second-line antiretroviral treatment (tenofovir disoproxil fumarate, emtricitabine, and lopinavir-ritonavir) failure. She admitted to taking her medications irregularly and was on trimethoprim-sulfamethoxazole prophylaxis. Her last HIV-1 RNA viral load in June 2013 was 199,994 copies/ml. Based on her immunosuppression and symptoms, we screened her for tuberculosis (TB). At the time of screening, she could not produce sputum, but an abdominal ultrasound in late June 2013 showed a lymphadenopathy. A chest X-ray was not carried out at baseline, as it would not have changed the clinical decision to treat the presumptive diagnosis of extrapulmonary TB. Her glomerular filtration rate (GFR) was 55 mL/min, the liver enzyme alanine aminotransferase was 33 IU/L (normal range 0–35 IU/L), and her albumin level was slightly decreased (35.5 g/L; normal range 38–47 g/L).
Table 1. Patient characteristics.
| Date | Weight (kg) | Medication | Symptoms | Laboratory findings | Remarks |
|---|---|---|---|---|---|
| 04-Jun-2013 | 46 | ART, TMP-SMX | Routine follow-up | ||
| 02-Jul-2013 | 42 | ART, TMP-SMX | Vomiting, abdominal pain, diarrhea, evening fevers | CD4: 34 c/μLVL: 199,994 c/ml | |
| 30-Jul-2013 | 42 | ART, TMP-SMX | Vomiting, abdominal pain, diarrhea, evening fevers | TB screening, US; abdomen showed lymphadenopathy | |
| 01-Aug-2013 | n.d. | ART, TMP-SMX, Rif/HZE | Vomiting, abdominal pain, diarrhea, evening fevers | ALT: 33 IU/LAlbumin: 35.5 g/LGFR: 55 ml/min | Start TB treatment |
| 12-Aug-2013 | 41 | ART, TMP-SMX, Rif/HZE | Clinical improvement | ||
| 26-Sep-2013 | 36 | ART, TMP-SMX, Rif/HZE | Vomiting, abdominal pain, diarrhea, development of skin lesions | Serum CrAg negative | Hepatomegaly, CXR normal, skin biopsy shows histoplasmosis |
| 08-Oct-2013 | n.d. | ART, TMP-SMX, EH, Ampho | Clinical worsening | ALT: 25 IU/LGFR: 57 ml/min | Switch to EH, start histoplasmosis treatment |
| 28-Oct-2013 | n.d. | ART, TMP-SMX, EH, Fluconazole | Generalized body weakness | GFR: 46 ml/min | US; abdomen normal |
| 22-Feb-2014 | 46 | ART, TMP-SMX, EH, Fluconazole | Skin lesions healed | GFR: 50 ml/min | Routine follow-up |
| 03-Mar-2014 | 48 | ART, TMP-SMX, Fluconazole | Completion TB treatment | ||
| 04-Dec-2014 | n.d. | ART, TMP-SMX | Fluconazole stopped | ||
| 16-Jun-2015 | 49 | ART, TMP-SMX | CD4: 19 c/μLVL: 252,000 c/ml | Routine follow-up |
kg, kilogram; ART, antiretroviral treatment; TMP-SMX, trimethoprim-sulphamethoxazole; CD4, CD-4 T cell count; c/μL, cells per microliter; VL, HIV-1 RNA plasma viral load; c/ml, copies per milliliter; TB, tuberculosis; US, ultrasound; n.d., not done; Rif/HZE, rifabutin, isoniazid, pyrazinamide, ethambutol; CXR, chest X-ray; ALT, alanine aminotransferase; IU/L, International Unit per liter; g/L, gram per liter; GFR, glomerular filtration rate; ml/min, milliliter per minute; CrAG, cryptococcal antigen; EH, ethambutol, isoniazid; Ampho, amphotericin B deoxcholeate.
Fig 1. Schematic representation of the patient’s 18-month follow-up with the clinical symptoms, most important diagnostic findings, and treatment timeline.
Please note that the scheme is not to scale.
Progress
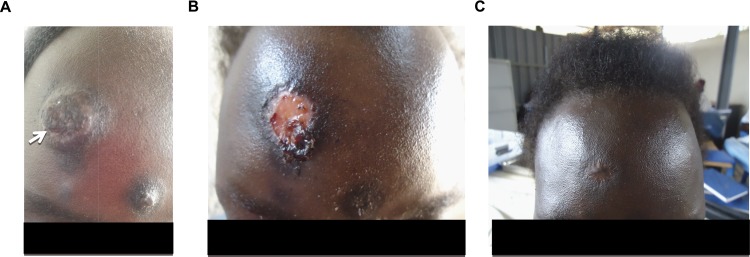
She was started on antituberculosis treatment consisting of rifabutin, isoniazid, ethambutol, and pyrazinamide on 1st August 2013. During follow-up, the patient claimed to adhere to her treatments and reported that the abdominal pain, vomiting, diarrhea, and evening fever stopped; nevertheless, she continued to lose weight in the weeks that followed. At the follow-up visit on 26th September 2013, the patient reported the development of hyperpigmented, progressive nodular, dome-shaped, non-itchy, and well-demarked facial skin lesions that had increased in number and size during the previous four weeks (see Fig 2A). She did not have prior lesions and complained again about abdominal pain, diarrhea, vomiting, and evening fevers. Despite adherence to treatment, she had lost about 15% of her body weight since treatment began. Apart from generalized weakness and a hepatomegaly (approximately 5 centimeters under the right costal margin), no abnormalities were found during physical examination, especially neither palpable lymph nodes nor oral or eye symptoms.
Fig 2. The patient’s skin lesions before and during antifungal treatment.
A) Patient presented with skin lesions on 26th September 2013. A biopsy was taken from the large lesion and led to a diagnosis of Histoplasma capsulatum (see arrow). B) Clinical response after a 14-day course of amphotericin B deoxycholate (0.7 mg/kg body weight), followed by six days of fluconazole (400 mg twice daily) maintenance therapy. C) Four months into maintenance treatment, the lesions had resolved.
What Is Your Differential Diagnosis?
Despite the possible explanation of (multi-) drug resistant TB, the continued weight loss and the appearance of new lesions warranted a revision of the initial diagnosis and additional investigations. In this woman, the differential diagnoses included but were not limited to TB of the skin, Kaposi sarcoma, atypical mycobacteria infection, disseminated fungal diseases, and neoplastic mimics of Kaposi sarcoma–like cutaneous T cell lymphoma. The situation was complicated by the fact that the patient had a high HIV-1 viral load at the beginning of TB treatment, and restarting her antiviral treatment together with her TB treatment could have led to clinical deterioration because of immune reconstitution inflammatory syndrome occurrence.
What Investigations Would You Ask For?
In our resource-limited setting, we opted for a stepwise approach. A chest X-ray was unremarkable (not shown); the serum antigen test for Cryptococcus neoformans was negative. A skin biopsy showed Histoplasmosis capsulatum infection. We did not have access to H. capsulatum detection tests. For a review of molecular epidemiology, clinical findings, diagnosis, and treatment of histoplasmosis in HIV-infected patients, please refer to the review of Adenis [1].
How Would You Manage This Case? Would You Continue TB Treatment?
The woman was hospitalized for treatment of disseminated histoplasmosis. Treatment for severe disseminated histoplasmosis ideally consists of liposomal amphotericin B 3 mg/kg/d for seven to 14 days or until clinical improvement, followed by itraconazole 200 mg three times daily for three days, then 200 mg twice daily for at least 12 months where available [2]. In our setting, only amphotericin B deoxycholate and fluconazole were available. We treated the patient with 0.7 mg/kg amphotericin B deoxycholate for 14 days, then switched to fluconazole 400 mg twice daily for two weeks and afterwards to 400 mg once daily for a maintenance phase.
Because of the initial clinical response to TB treatment, the TB treatment was continued. Based on the Ugandan national guidelines, and to avoid rifampicin-lopinavir/ritonavir interaction, we choose ethambutol/isoniazid for maintenance treatment, although this regime has been shown to be inferior to rifampicin/isoniazid [3].
Follow-up
The lesions responded very well to amphotericin B deoxycholate treatment, and the patient’s weight began to increase (see Table 1 and Fig 1). A follow-up abdominal ultrasound on 28th October 2013 showed that the lymphadenopathy had been resolved. During the amphotericin B deoxycholate therapy, we regularly monitored serum electrolytes and the GFR. The GFR remained stable over time. Four months into antifungal treatment, the patient reached her baseline body weight of 46 kg and the lesions had healed (see Fig 2B and 2C).
The patient was seen regularly, and the fluconazole was stopped on 4th December 2014. She did not develop any new skin lesions and was last seen on 16th June 2015. On that day, her CD4 T cell count was 19 cells/microliter, and her HIV-1 RNA viral load was 252,000 copies/milliliter. Her body weight was stable at 49 kg, and the patient did not have any clinical complaints. The patient is still on failing second-line antiretroviral treatment and has been enrolled in the control arm of the AIDS Clinical Trial Group Study A5288 (Management Using the Latest Technologies in Resource-Limited Settings to Optimize Combination Therapy After Viral Failure).
Case Discussion
This case illustrates the challenges of diagnosing (disseminated) histoplasmosis in our particular setting. Without skin involvement, the diagnosis would have been difficult to ascertain, as H. capsulatum serum antigen tests were not available. The overlap of histoplasmosis and TB clinical findings is shown in Table 2.
Table 2. Common clinical symptoms of disseminated histoplasmosis and tuberculosis.
| Fever |
| Weight loss |
| Lung disease |
| Hepatosplenomegaly |
| CNS complaint and/or meningitis |
| Lymphadenopathy |
Data on drug-resistant, extrapulmonary TB in Uganda is only anecdotal, but the first national survey published in 2013 showed that the 1.4% of previously untreated sputum smear–positive TB patients were multidrug-resistant [4]. Because the patient was critically ill and response to TB treatment can take several weeks or even months, we decided to continue TB treatment. In view of the response to the antifungal treatment, continuation of TB treatment should be seen as critical in retrospect.
Because of financial constraints, state-of-the-art maintenance treatment with itraconazole could not be provided. In addition, use of itraconazole warrants pharmacological monitoring, which was not available in our setting. Nevertheless, the patient responded well to fluconazole.
Key Learning Points
Diagnosis of TB can be challenging in resource-limited settings. No “antibiotic trial” for TB diagnosis should be given.
The clinical symptoms of histoplasmosis and TB overlap, which results in underdiagnosis of histoplasmosis in Africa, but H. capsulatum polysaccharide can be detected with high sensitivity and specificity in serum and bronchioloalveolar lavages.
Fluconazole has been shown to be less effective in the maintenance phase than itraconazole in clinical trials but has been used on an ad-hoc basis. A maintenance phase should be carried out for at least 12 months, and lifelong suppressive therapy may be required if immunosuppression cannot be reversed.
Although consolidation phase treatment with rifampicin/isoniazid has been proven to be superior to ethambutol/isoniazid, this treatment circumvented the hepatotoxic interaction between the protease inhibitor and rifampicin, and eight months of ethambutol/isoniazid continues to be the standard in Uganda.
Acknowledgments
We would like to thank the patient for providing informed consent and support for sharing her case. We are very grateful to the clinical team at the Infectious Diseases Institute for providing clinical care and follow-up.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Adenis AA, Aznar C, Couppie P. Histoplasmosis in HIV-Infected Patients: A Review of New Developments and Remaining Gaps. Curr Trop Med Rep. 2014;1:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–25. [DOI] [PubMed] [Google Scholar]
- 3.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364(9441):1244–51. [DOI] [PubMed] [Google Scholar]
- 4.Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS ONE. 2013;8(8):e70763 doi: 10.1371/journal.pone.0070763 [DOI] [PMC free article] [PubMed] [Google Scholar]