Abstract
We report molecular genetic analysis of 42 affected individuals referred with a diagnosis of aniridia who previously screened as negative for intragenic PAX6 mutations. Of these 42, the diagnoses were 31 individuals with aniridia and 11 individuals referred with a diagnosis of Gillespie syndrome (iris hypoplasia, ataxia and mild to moderate developmental delay). Array-based comparative genomic hybridization identified six whole gene deletions: four encompassing PAX6 and two encompassing FOXC1. Six deletions with plausible cis-regulatory effects were identified: five that were 3ʹ (telomeric) to PAX6 and one within a gene desert 5ʹ (telomeric) to PITX2. Sequence analysis of the FOXC1 and PITX2 coding regions identified two plausibly pathogenic de novo FOXC1 missense mutations (p.Pro79Thr and p.Leu101Pro). No intragenic mutations were detected in PITX2. FISH mapping in an individual with Gillespie-like syndrome with an apparently balanced X;11 reciprocal translocation revealed disruption of a gene at each breakpoint: ARHGAP6 on the X chromosome and PHF21A on chromosome 11. In the other individuals with Gillespie syndrome no mutations were identified in either of these genes, or in HCCS which lies close to the Xp breakpoint. Disruption of PHF21A has previously been implicated in the causation of intellectual disability (but not aniridia). Plausibly causative mutations were identified in 15 out of 42 individuals (12/32 aniridia; 3/11 Gillespie syndrome). Fourteen of these mutations presented in the known aniridia genes; PAX6, FOXC1 and PITX2. The large number of individuals in the cohort with no mutation identified suggests greater locus heterogeneity may exist in both isolated and syndromic aniridia than was previously appreciated.
Introduction
Abnormal development of the iris is a feature of a variety of congenital human ocular anomalies, of which, the best characterized is complete aniridia (MIM 106210), a dominantly inherited condition with an incidence of less than 1 in 50,000 [1]. Aniridia presents as congenital absence of the iris, although a visible partial rim or sector of iris tissue strand is often present [2]. Foveal hypoplasia, cataract, keratopathy and glaucoma sometimes develop in second or third decade contributing to visual morbidity [3]. Non-ocular anomalies including hyposmia and structural brain changes are sometimes observed in individuals with complete aniridia [4].
At least 90% of aniridia cases are caused by heterozygous loss-of-function mutations in PAX6 [5]. Almost all cases of classical aniridia associated with PAX6 haploinsufficiency present with foveal hypoplasia. Heterozygous, presumed hypomorphic, missense mutations in PAX6 have also been associated with other ocular diseases including anterior segment dysgenesis [6] and optic nerve malformations [7]. Rarely, isolated aniridia is caused by mutations in FOXC1 [8,9] or PITX2 [10]. Mutations in these genes are more commonly associated with juvenile-onset glaucoma [11] and anterior segment dysgenesis [12–14] presenting with syndromic features of rare cardiac anomalies for FOXC1 and hypodontia and umbilical anomalies for PITX2.
Several syndromic forms of iris developmental anomalies have been described. The best known is WAGR (Wilms’ tumour, aniridia, genital anomalies and mental retardation; MIM 194072), a contiguous deletion syndrome on 11p13 [15]. Gillespie syndrome (MIM 206700) is characterized by a pathognomonic iris anomaly; absence of the pars pupillaris of the iris and the pupillary border. Individuals with Gillespie syndrome are also distinguished from complete aniridia by having a normal fovea and no evidence of progressive opacification of the cornea and lens, nor development of glaucoma. The extraocular features are non-progressive cerebellar ataxia and psychomotor delay [16]. Several cases of Gillespie syndrome have been reported [17–39].
In the literature, Gillespie syndrome has been most commonly considered to be an autosomal recessive disorder [36–38]. Analysis of the PAX6 gene in six Gillespie syndrome patients revealed no intragenic mutations [20,26,40]. PAX6 mutations have been reported in two individuals [33,39] described as Gillespie syndrome but with significantly atypical features such as corectopia and ptosis (33). A single affected girl described as having a Gillespie syndrome-like phenotype has been reported with an apparently balanced X:autosome reciprocal translocation t(X;11)(p22.32;p12) [22] and atypical features of superior coloboma, foveal hypoplasia and vermis hypoplasia. This case is included in this study as individual 1371.
Here, we report genomic copy number and extended mutation analysis in 42 unrelated affected individuals all of whom had been scored as negative for intragenic PAX6 mutations. Eleven of these probands had been referred to us with a diagnosis of Gillespie syndrome and 31 with non-syndromic aniridia. One of the 11 Gillespie syndrome individuals was the case with the apparently balanced reciprocal translocation t(X:11)(p22.32;p12) [22]. In this case we used FISH to map both breakpoints. In total, 15 plausible disease-causing heterozygous loss-of-function mutations were identified: nine affecting PAX6, four affecting FOXC1, one affecting PITX2 and one affecting PHF21A. These data suggest that other disease loci or mutational mechanisms causing aniridia remain to be discovered.
Materials and Methods
Patient samples
All aspects of this study were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the participants and recorded. The study was approved by the UK Multicentre Regional Ethics Committee under the number 06/MRE00/76. All patients were phenotypically characterized by experienced ophthalmologists or geneticists. The study cohort consisted of 42 unrelated individuals with aniridia or Gillespie syndrome (S1 Table) each of whom had been previously screened for intragenic PAX6 mutations by single-strand conformation polymorphism (SSCP), denaturing high performance liquid chromatography (DHPLC) and direct sequencing (S2 Table).
DNA preparation and quality control
Genomic DNA was prepared from either lymphoblastoid cell lines (LCL) or saliva using a Nucleon DNA extraction kit (Tepnel Life Sciences, UK). DNA quality was checked by agarose gel electrophoresis and NanoDrop spectrophotometry (Thermo Scientific).
Array comparative genomic hybridization (aCGH)
Genome-wide analysis of DNA copy number was carried out using the Roche Nimblegen 12X135k whole-genome array (median probe spacing of approximately 12 kb) according to the manufacturer’s instructions with minor modifications, as described previously [41].
Targeted analysis of genomic deletions/duplications was performed using a customized oligonucleotide microarray (Agilent Technologies) consisting of 44,000 60-mer oligonucleotide probes (4X44k), designed using eArray (Agilent Technologies). The design consisted of a 3 Mb genomic region (chr11:30,262,916–33,296,085; hg18) containing the PAX6 gene with an average probe spacing of 76 bp. ‘Dye-swap’ experiments were performed followed by copy number analysis, as previously described [42].
Polymerase chain reaction (PCR) and mutation analysis
Primer sequences and PCR conditions used for amplification and sequencing of the FOXC1, PITX2, PHF21A and ARHGAP6 genes are provided in S2 Table. PCR reactions were performed in 12μl volumes containing 1μl of 1-in-20 diluted, whole-genome amplified DNA (Genomiphi, GE Healthcare), 6μl of 2 X ReddyMix PCR Mastermix (Abgene), 833 nM of each oligonucleotide primer and 2.4μl of 5 X GC-mix (where appropriate). PCR conditions generally consisted of an initial denaturation at 95°C for 5 minutes, followed by 32 cycles of 94°C for 60 seconds, primer annealing for 60 seconds, and 72°C for 60 seconds, and a final cycle of 72°C for 10 minutes. The products were visualized using agarose gel electrophoresis to ensure adequate yield and proper sizing of each exon fragment. Sequencing of PCR products was performed in both directions as described elsewhere [43]. Sequence traces were analyzed using Mutation Surveyor sequence analysis software version 3.30.
Fluorescence in situ hybridization (FISH)
Metaphase spreads for FISH were prepared from patient lymphocytes as described elsewhere [44]. BAC clones were selected from the Ensembl database (http://www.ensembl.org) or the UCSC Human Genome Browser (http://genome.ucsc.edu) and ordered from the BACPAC resources centre (Children’s Hospital Oakland Institute). For the initial mapping of the clones, DNA was isolated using a rapid alkaline lysis miniprep method (Qiagen mini/midi plasmid kit). Probes were labeled with biotin-16-dUTP or digoxigenin-11-dUTP (Roche) by nick translation. Probe labelling, DNA hybridization and antibody detection were carried out as described previously [45]. Following hybridization, slides were mounted with a drop of Vectorshield antifadent containing DAPI (Sigma). Antibody detection was carried out by fluorescent microscopy using a Zeiss Axioscop microscope. Images were collected using a cooled CCD (charged coupled device) camera and analyzed using SmartCapture software (Digital Scientific).
Results
Patient cohort
Our study cohort consisted of 42 unrelated individuals (14 male, 28 female) with iris developmental anomalies (Table 1, S1 Table). Eleven of these individuals (2 male, 9 female) had been referred to us with a diagnosis of Gillespie syndrome including individual 1371 who had been previously reported with an apparently balanced reciprocal translocation: t(X;11)(p22.32;p12) [22]. Each proband had been scored negative for intragenic PAX6 mutations by SSCP, DHPLC and/or direct sequencing in our lab.
Table 1. Details of the clinical diagnoses and genetic pathology identified in individuals in this study.
| Individual ID | DECIPHER ID | Clinical feature | Genetic pathology | Genomic coordinates (hg18) |
|---|---|---|---|---|
| 1851 (control) | 323119 | Aniridia | PAX6 deletion (previously identified by FISH) | chr11:21,254,000–32,564,000 |
| 2193 | 323118 | Aniridia | PAX6 whole-gene deletion | chr11:31,199,000–31,849,000 |
| 377 | 323104 | Aniridia | PAX6 whole-gene deletion | chr11:31,394,000–31,914,000 |
| 1510 | 323113 | Aniridia | PAX6 whole-gene deletion | chr11:31,779,000–31,933,000) |
| 1977 | 323116 | Aniridia | PAX6 whole-gene deletion | chr11:31,698,271–31,794,414 |
| 1514 | 323114 | Aniridia | PAX6 telomeric deletion | chr11:30,874,642–31,654,833 |
| 753 | 323108 | Aniridia | PAX6 telomeric deletion | chr11:30,967,000–31,704,000) |
| 555 | 323106 | Aniridia | PAX6 telomeric deletion | chr11:31,108,579–31,649,842) |
| 2014 | 323117 | Gillespie syndrome | PAX6 telomeric deletion | chr11:31,234,395–31,751,815 |
| 659 | 323107 | Aniridia | PAX6 telomeric deletion | chr11:31,379,000–31,708,000) |
| 1449 | 323112 | Gillespie syndrome | FOXC1 whole-gene deletion | chr6:1,543,591–1,675,085 |
| 1246 | 323110 | Aniridia | FOXC1 whole-gene deletion | chr6:1,543,591–1,675,085 |
| 1839 | Aniridia | FOXC1 c.235C>A p.(Pro79Thr) de novo | Not applicable | |
| 1634 | Aniridia | FOXC1 c.302T>C p.(Leu101Pro) de novo | Not applicable | |
| 1194 | 323109 | Aniridia | PITX2 telomeric deletion | chr4:111,994,000–115,504,000 |
| 1371 | n/a | Gillespie syndrome | Translocation t(X;11)(p22.32;p12) | See Fig 5 |
DNA copy number analysis of the PAX6 locus
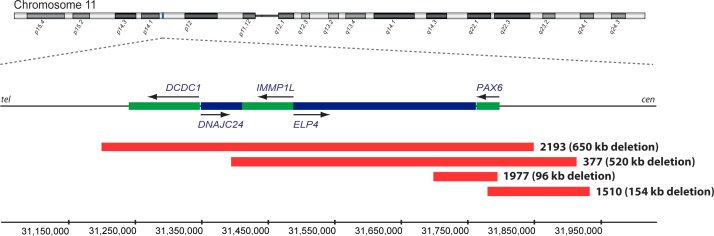
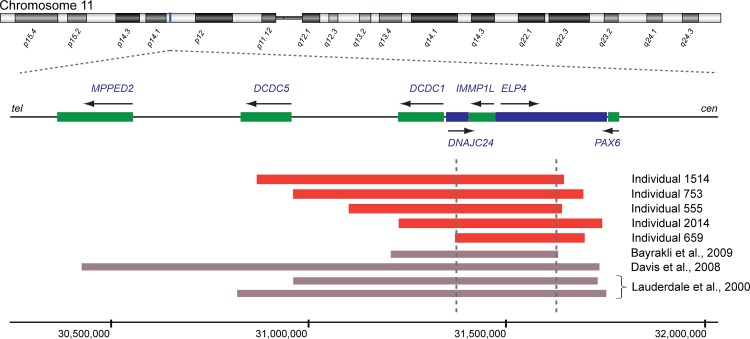
To identify causative segmental aneuploidy, two array-based comparative genomic hybridization (aCGH) approaches were used: a 135k whole-genome array and a custom-designed targeted array covering a contiguous 3 Mb genomic region (chr11:30,262,916–33,296,085; hg18) encompassing PAX6. This identified four individuals with heterozygous deletions, all encompassing PAX6 and ranging in size from 96 kb to 650 kb: individual 2193 (chr11:31,199,000–31,849,000; hg18), individual 377 (chr11:31,394,000–31,914,000; hg18), individual 1510 (chr11:31,779,000–31,933,000; hg18) and individual 1977 (chr11:31,698,271–31,794,414; hg18) (Table 1, Fig 1, S1 Fig). Five individuals had deletions with breakpoints immediately telomeric to PAX6: individual 1514 (chr11:30,874,642–31,654,833; hg18), individual 753 (chr11:30,967,000–31,704,000; hg18), individual 555 (chr11:31,108,579–31,649–842; hg18), individual 2014 (chr11:31,234,395–31,751,815; hg18) and individual 659 (chr11:31,379,000–31,708,000; hg18) (Table 1, Fig 2, S2 Fig). Combining these with published data, we suggested a 243.9 kb critical region for PAX6 transcriptional activation between chr11:31,379,000 (hg18) and chr11:31,622,916 (hg18) (Fig 2).
Fig 1. Identification of PAX6 whole-gene deletions.
Genome-wide array CGH analysis identified a 650 kb deletion in individual 2193 (chr11:31,199,000–31,849,000), a 520 kb deletion in individual 377 (chr11:31,394,000–31,914,000), a 154 kb deletion in individual 1510 (chr11:31,779,000–31,933,000) and a 96 kb deletion in individual 1977 (chr11:31,698,271–31,794,414), all involving PAX6. Red bars show the position of the deletions. Genes transcribed on the forward strand are in blue and those transcribed on the reverse strand are in green, also indicated by arrows. Genomic coordinates are based on the Human Genome Assembly hg18.
Fig 2. Identification of regulatory deletions telomeric to PAX6.
Regulatory deletions telomeric to PAX6 were identified in individual 1514 (chr11:30,874,642–31,654,833), individual 753 (chr11:30,967,000–31,704,000), individual 555 (chr11:31,108,579–31,649–842), individual 2014 (chr11:31,234,395–31,751,815) and individual 659 (chr11:31,379,000–31,708,000). The schematic diagram shows how the ‘critical region’ (delimited by grey dotted lines) required for PAX6 transcriptional activation was delineated by combining our data with published deletions with known coordinates [55,67,68]. PAX6 regulatory deletions from the present study are shown by red blocks. Genes transcribed on the forward strand are in blue and those transcribed on the reverse strand are in green, also indicated by arrows. Genomic coordinates are based on the Human Genome Assembly hg18.
Mutation analysis of the FOXC1 locus
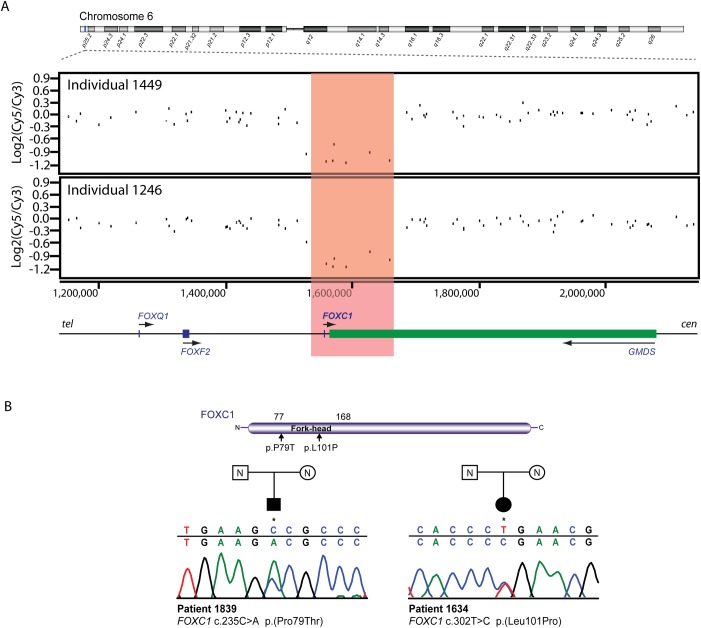
An apparently identical 131 kb deletion (chr6:1,543,591–1,675,085; hg18) encompassing FOXC1 was identified as a de novo occurrence in two unrelated individuals 1449 and 1246 (Table 1, Fig 3). Each of these deletions had been confirmed in an independent UK laboratory using an alternative method. Furthermore, the two individuals were shown to be distinct based on their aCGH profile of genome-wide copy number variants (data not shown). We then screened FOXC1 in our cohort by direct sequencing. Two individuals were found to carry missense mutations in the FOXC1 fork-head domain (Table 1, Fig 3). Individual 1839 had a C>A transversion in codon 79 (c.235C>A, p.(Pro79Thr)) and individual 1634 had a novel T>C transition in codon 101 (c.302T>C, p.(Leu101Pro)). In both individuals, the mutations were absent from the unaffected parents and had most likely occurred de novo (Fig 3). The amino acid substitution p.(Pro79Thr) has been reported previously in a family with classical Axenfeld-Rieger syndrome and the mutant protein has impaired nuclear localization and transactivation activity [46]. The novel p.(Leu101Pro) mutation is predicted to disrupt the second alpha helix of the fork-head domain.
Fig 3. Mutation analysis of the FOXC1 locus.
(A) Genome-wide array CGH identified two deletions encompassing the FOXC1 gene in individuals 1449 (chr6:1,543,591–1,675,085) and 1246 (chr6:1,543,591–1,675,085). (B) Direct sequencing of the FOXC1 coding region identified a heterozygous substitution in individual 1839 (c.235C>A, p.(Pro79Thr)) and another in individual 1634 (c.302T>C, p.(Leu101Pro)). FOXC1 mutation screening in unaffected parents of both patients showed that the mutations had occurred de novo. The locations of both mutations within the fork-head domain of the FOXC1 protein are indicated by vertical arrows. Genes transcribed on the forward strand are in blue and those transcribed on the reverse strand are in green, also indicated by arrows. Genomic coordinates are based on the Human Genome Assembly hg18. The genomic sequence identifier for FOXC1 is NG_009368.
Mutation analysis of the PITX2 locus
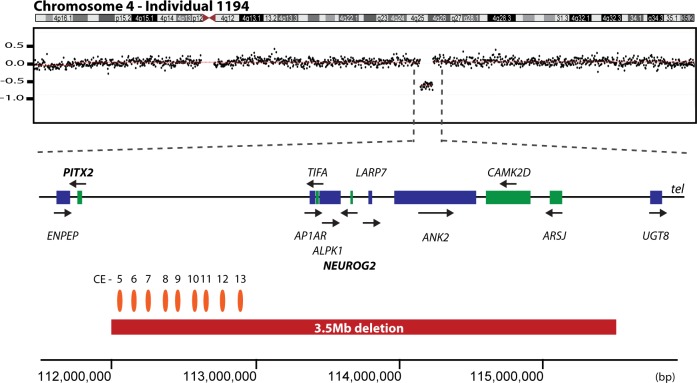
Array CGH identified a 3.5 Mb deletion of 4q25-q26 (chr4:111,994,000–115,504,000; hg18) in individual 1194 (Table 1, Fig 4). This deletion encompasses 8 genes (Fig 4). The centromeric breakpoint is located in a gene desert 230 kb telomeric (5ʹ) to PITX2 encompassing several conserved PITX2 enhancer elements [47]. Subsequent screening of the PITX2 coding sequence in our cohort revealed no plausible disease-causing mutations.
Fig 4. Identification of a potential PITX2 regulatory deletion.
Genome-wide array CGH identified a deletion of approximately 3.5 Mb in individual 1194 (chr4:111,994,000–115,504,000) (red bar). The deletion is located telomeric to the PITX2 gene on chromosome 4. The positions of conserved elements (CE) in the deleted region, as identified by Volkmann et al., 2011 [47] are marked by orange ellipses. Genes transcribed on the forward strand are in blue and those transcribed on the reverse strand are in green, also indicated by arrows. Genomic coordinates are shown on the x-axis and are based on the Human Genome Assembly hg18.
Breakpoint mapping of a translocation in an individual with Gillespie syndrome
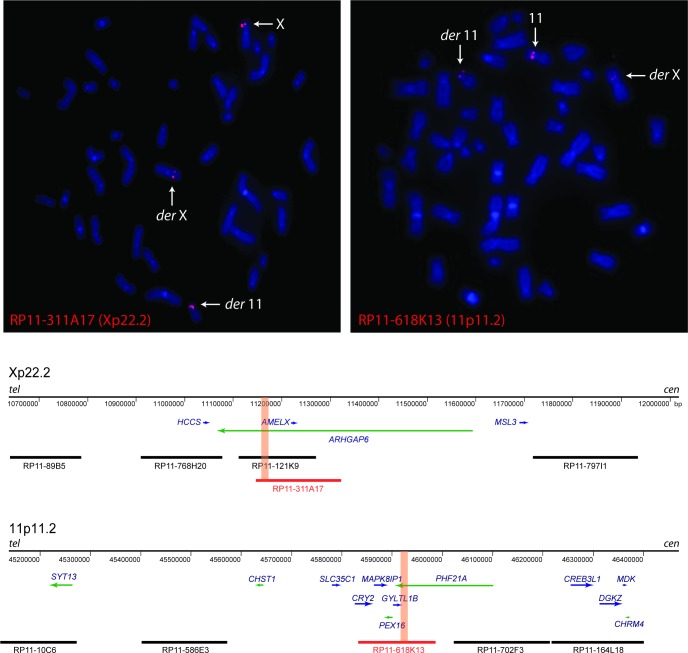
FISH was used to map the previously reported t(X;11)(p22.32;p12) reciprocal translocation in individual 1371 (Fig 5). The breakpoint on chromosome 11 (now 11p11.2) lay within a single BAC, RP11-618K13 [48], which contains 5 known genes, CRY2, MAPK8IP1, PEX16, GYLTL1B, and PHF21A (also known as BHC80), located approximately 14.1 Mb centromeric to PAX6. The breakpoint was shown to lie within PHF21A using probes generated by long-range PCR from exons 14–16 (telomeric to the breakpoint) and exons 4–11 (centromeric to the breakpoint) (data not shown). The X chromosome breakpoint (now Xp22.2) was spanned by two overlapping BACs (RP11-121K9 and RP11-311A17) [48] covering two genes, AMELX and ARHGAP6 (Fig 5). Using a probe generated by long-range PCR, the breakpoint was localized within a large intron of ARHGAP6 (Fig 5).
Fig 5. Fluorescence in situ hybridization (FISH) was used to map the translocation breakpoints on chromosomes 11 and X in individual 1371.
The breakpoint-spanning BAC clones RP11-311A17 (Xp22.2; left panel) and RP11-618K13 (11p11.2; right panel) show signals on both the derivative 11 and derivative X. The schematic diagram demonstrates the position of the BAC clones and the genes involved, to scale. Breakpoint-spanning BACs are coloured in red, with the approximate position of the breakpoints shown by orange bars, as determined by long-range PCR. Genes transcribed on the forward strand are in blue and those transcribed on the reverse strand are in green. Genomic coordinates are shown on the x-axis and are based on the Human Genome Assembly hg18.
Mutation analysis of breakpoint genes in Gillespie syndrome patients
Direct sequencing of the coding exons and essential splice sites of PHF21A and ARHGAP6 revealed only polymorphic variants in the 10 individuals with Gillespie syndrome who lacked a detectable chromosomal abnormality at these loci. HCCS is located approximately 150 kb telomeric to the X chromosome breakpoint in individual 1371. Mutations in this gene have been associated with microphthalmia with linear skin defects (MIM 309801). Direct sequencing of HCCS revealed no mutations in the 10 non-translocation Gillespie cases.
Discussion
A high proportion of cases of aniridia is caused by loss-of-function mutations in a single gene, PAX6. Here we studied individuals with aniridia and Gillespie syndrome, who had previously scored negative for intragenic PAX6 mutations, using a variety of molecular approaches to identify causative mutations. The rationale for the analysis was that we had a strong prior expectation that this cohort would be heavily enriched for causative structural chromosomal anomalies involving PAX6 itself, but also for possible new disease loci and/or novel mutational mechanisms. In the event, we identified deletions that result in PAX6 haploinsufficiency in only 9/42 probands: four encompassing PAX6 itself and five removing 3ʹ (telomeric) cis-regulatory elements that are essential for PAX6 function. A wealth of evidence exists from animal models [49–52] and human translocation breakpoint mapping [53,54] showing that genomic elements located in a region ~120kb 3ʹ to the transcription unit are essential for the transcriptional activation of PAX6. For chromosomal deletions the most convincing evidence is from somatic cell hybrid analysis of two deletions that were shown to abolish PAX6 transcription [55]. The deletions studied in this somatic cell hybrid analysis both overlap with the 3ʹ deletions identified here (Fig 2) and by combining our data with the published data we suggest a new 244 kb ‘critical region’ which contains essential cis-regulatory elements (Fig 2). The patient cohort in the present study is part of a larger cohort of iris developmental anomalies patients in which one individual with aniridia was recently found to have a plausibly causative de novo single nucleotide variant (SNV) in a conserved non-coding element within the ‘critical region’ [56]. While it is possible that similar mutations may exist in other cis-regulatory elements, it is significant that most of the individuals in the present study were included in the cohort of 60 individuals screened for PAX6 regulatory mutations by Bhatia et al. [56] and no further mutations were identified in the regions analyzed.
Four individuals had deletions or intragenic mutations which are likely to result in FOXC1 haploinsufficiency. One individual had a large deletion upstream of PITX2 that plausibly impairs developmental expression of this gene by removing known enhancer elements. Deletions of FOXC1 were previously shown to account for a considerable proportion of individuals with anterior segment dysgenesis, who also presented with extraocular features such as hearing defects and mental retardation [57]. FOXC1 and PITX2 encode transcriptional regulators that physically interact with each other and are co-expressed in a number of tissues during development including the periocular mesenchyme [58]. Mutations in these genes have most commonly been associated with Axenfeld-Rieger syndrome [59], but aniridia has been reported for both [8,9,60]. Of note, 3 of the 4 individuals reported here with FOXC1 haploinsufficiency, and the individual with the PITX2 cis-regulatory mutation have congenital glaucoma associated with their aniridia phenotype. However, none of the nine individuals with PAX6 mutations had congenital glaucoma. Digenic inheritance of FOXC1 and PITX2 mutations was reported in a severely affected individual in a family with several affected members presenting with variable ocular phenotypes associated with Axenfeld-Rieger syndrome [13]. The presence of both FOXC1 and PITX2 mutations impaired the transactivation activity of these proteins in vitro significantly more than when only one mutation was present. The cellular and developmental interactions between PAX6, FOXC1 and PITX2, and physical co-binding at regulatory elements in the developing iris are as yet poorly understood. This is presumably due to the difficulty in obtaining sufficient tissue, although the available human genetic data suggest that this would be an informative area of study.
We assessed the occurrence of particular descriptive phenotype terms (partial/variant aniridia, corneal anomalies, cataracts, glaucoma, microphthalmia/coloboma and extraocular features) in cases with and without a molecular diagnosis (S3 Table). The results showed an over-representation of individuals with partial/variant aniridia in whom no genetic defect was detected (approximately 60%) when compared to those with the same descriptive term but no genetic diagnosis (approximately 26%). This finding can be explained by the presence of 8/11 Gillespie syndrome patients in whom a genetic mutation is yet to be identified. The glaucoma feature appeared to be present in 26% of individuals with a molecular diagnosis (particularly in FOXC1 and PITX2 mutation-positive patients) compared to 7% of those without a diagnosis.
Finally, we report a more complex mutation associated with the breakpoints of a balanced X:autosome translocation in a single individual. On chromosome 11 the breakpoint disrupts PHF21A, which encodes a plant-homeodomain zinc finger protein and is highly expressed in brain tissue including the cerebellum [61]. The PHF12A protein is a component of the BRAF-histone deacetylase co-repressor complex, which mediates transcriptional repression of neuron-specific genes in non-neuronal cells [62]. Multiple translocation breakpoints disrupting PHF21A have been reported as causing intellectual disability [63] and alteration of PHF12A expression in the cerebellum might contribute to the ataxia seen in this case but we were unable to find any evidence that PHF21A could be causing the iris malformation. The breakpoint on the X chromosome disrupts ARHGAP6, which is highly expressed in kidney, heart, skeletal muscle, retina and fetal brain. ARHGAP6 encodes a guanine nucleotide exchange factor that activates Rho-GTPase to regulate signaling interactions within the actin cytoskeleton [64,65]. However, there is no human genetics evidence as yet that mutations in this gene are associated with any developmental disorder. We were also unable to find mutations in the neighbouring gene, HCCS, in the other Gillespie syndrome cases in our cohort. HCCS has been associated with syndromic microphthalmia [66]. It seems reasonable to consider individual 1371 as having a composite phenotype with PHF21A-disrupting breakpoint exacerbating the neurodevelopmental problems but the Gillespie syndrome being, as yet, unexplained.
Perhaps the most significant finding in this study is that we were unable to identify mutations in 27/42 individuals with aniridia and no detectable intragenic mutations in PAX6. Although there could be unidentified mechanisms for disrupting PAX6 function, our results also suggests that there may be as yet undiscovered genetic loci responsible for a considerable proportion of aniridia. Whole genome sequence analysis would be an attractive technique for the identification of novel causative mutations in the PAX6 region, and others involving new loci in PAX6-negative individuals with syndromic or isolated aniridia. The high frequency of cis-regulatory mutations that we have identified in this cohort highlight the importance of surveying the whole genome. This study has also confirmed that the majority of cases with Gillespie syndrome are not associated with detectable mutations at the PAX6 locus.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files. ArrayCGH data have been deposited in the Database of Genomic Variants (accession ID estd228) and the DECIPHER database (decipher.sanger.ac.uk).
Funding Statement
MA, JR, KAW, FS, LH, JF, VvH, and DRF are funded via programme grants to the MRC Human Genetics Unit. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boonstra N, Limburg H, Tijmes N, van Genderen M, Schuil J, van Nispen R (2012) Changes in causes of low vision between 1988 and 2009 in a Dutch population of children. Acta Ophthalmol 90: 277–286. 10.1111/j.1755-3768.2011.02205.x [DOI] [PubMed] [Google Scholar]
- 2.Gronskov K, Rosenberg T, Sand A, Brondum-Nielsen K (1999) Mutational analysis of PAX6: 16 novel mutations including 5 missense mutations with a mild aniridia phenotype. Eur J Hum Genet 7: 274–286. [DOI] [PubMed] [Google Scholar]
- 3.Dubey SK, Mahalaxmi N, Vijayalakshmi P, Sundaresan P (2015) Mutational analysis and genotype-phenotype correlations in southern Indian patients with sporadic and familial aniridia. Mol Vis 21: 88–97. [PMC free article] [PubMed] [Google Scholar]
- 4.Hever AM, Williamson KA, van Heyningen V (2006) Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin Genet 69: 459–470. [DOI] [PubMed] [Google Scholar]
- 5.Hingorani M, Hanson I, van Heyningen V (2012) Aniridia. Eur J Hum Genet 20: 1011–1017. 10.1038/ejhg.2012.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis LM, Semina EV (2011) Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol 22: 314–324. 10.1097/ICU.0b013e328349412b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azuma N, Yamaguchi Y, Handa H, Tadokoro K, Asaka A, Kawase E et al. (2003) Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. Am J Hum Genet 72: 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito YA, Footz TK, Berry FB, Mirzayans F, Yu M, Khan AO et al. (2009) Severe molecular defects of a novel FOXC1 W152G mutation result in aniridia. Invest Ophthalmol Vis Sci 50: 3573–3579. 10.1167/iovs.08-3032 [DOI] [PubMed] [Google Scholar]
- 9.Sadagopan KA, Liu GT, Capasso JE, Wuthisiri W, Keep RB, Levin AV (2015) Anirdia-like phenotype caused by 6p25 dosage aberrations. Am J Med Genet A 167A: 524–528. 10.1002/ajmg.a.36890 [DOI] [PubMed] [Google Scholar]
- 10.Khan AO, Aldahmesh MA, Alkuraya FS (2011) Genetic and genomic analysis of classic aniridia in Saudi Arabia. Mol Vis 17: 708–714. [PMC free article] [PubMed] [Google Scholar]
- 11.Khan AO, Aldahmesh MA, Al-Amri A (2008) Heterozygous FOXC1 mutation (M161K) associated with congenital glaucoma and aniridia in an infant and a milder phenotype in her mother. Ophthalmic Genet 29: 67–71. 10.1080/13816810801908152 [DOI] [PubMed] [Google Scholar]
- 12.Chang TC, Summers CG, Schimmenti LA, Grajewski AL (2012) Axenfeld-Rieger syndrome: new perspectives. Br J Ophthalmol 96: 318–322. 10.1136/bjophthalmol-2011-300801 [DOI] [PubMed] [Google Scholar]
- 13.Kelberman D, Islam L, Holder SE, Jacques TS, Calvas P, Hennekam RC et al. (2011) Digenic inheritance of mutations in FOXC1 and PITX2: correlating transcription factor function and Axenfeld-Rieger disease severity. Hum Mutat 32: 1144–1152. 10.1002/humu.21550 [DOI] [PubMed] [Google Scholar]
- 14.Law SK, Sami M, Piri N, Coleman AL, Caprioli J (2011) Asymmetric phenotype of Axenfeld-Rieger anomaly and aniridia associated with a novel PITX2 mutation. Mol Vis 17: 1231–1238. [PMC free article] [PubMed] [Google Scholar]
- 15.Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M (2005) WAGR syndrome: a clinical review of 54 cases. Pediatrics 116: 984–988. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie FD (1965) Aniridia, Cerebellar Ataxia, and Oligophrenia in Siblings. Arch Ophthalmol 73: 338–341. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal PK, Awan MA, Dutton GN, Strang N (2009) Gillespie syndrome with impaired accommodation. J Pediatr Ophthalmol Strabismus 46: 60 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal PK, Awan MA, Strang N, Dutton GN (2009) Gillespie syndrome with impaired accommodation. J Pediatr Ophthalmol Strabismus 46: 317 10.3928/01913913-20090903-12 [DOI] [PubMed] [Google Scholar]
- 19.Boughamoura L, Yacoub M, Abroug M, Chabchoub I, Bouguezzi R, Charfeddine L et al. (2006) [Gillespie syndrome: 2 familial cases]. Arch Pediatr 13: 1323–1325. [DOI] [PubMed] [Google Scholar]
- 20.Defreyn A, Maugery J, Chabrier S, Coullet J (2007) [Gillespie syndrome: an uncommon presentation of congenital aniridia]. J Fr Ophtalmol 30: e1 [DOI] [PubMed] [Google Scholar]
- 21.Dell'acqua Cassao B, de Rezende DT, Silva LC, Herbella FA (2013) Esophageal dysmotility in gillespie syndrome. J Neurogastroenterol Motil 19: 538–539. 10.5056/jnm.2013.19.4.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dollfus H, Joanny-Flinois O, Doco-Fenzy M, Veyre L, Joanny-Flinois L, Khoury M et al. (1998) Gillespie syndrome phenotype with a t(X;11)(p22.32;p12) de novo translocation. Am J Ophthalmol 125: 397–399. [DOI] [PubMed] [Google Scholar]
- 23.Donald KA, Grotte R, Crutchley AC, Wilmshurst JM (2006) Gillespie syndrome: two further cases. J Child Neurol 21: 337–340. [DOI] [PubMed] [Google Scholar]
- 24.Eden U, Beijar C, Riise R, Tornqvist K (2008) Aniridia among children and teenagers in Sweden and Norway. Acta Ophthalmol 86: 730–734. 10.1111/j.1755-3768.2008.01310.x [DOI] [PubMed] [Google Scholar]
- 25.Francois J, Lentini F (1984) [Gillespie syndrome (incomplete aniridia, cerebellar ataxia and oligophrenia)]. Klin Monbl Augenheilkd 184: 313–315. [PubMed] [Google Scholar]
- 26.Glaser T, Ton CC, Mueller R, Petzl-Erler ML, Oliver C, Nevin NC et al. (1994) Absence of PAX6 gene mutations in Gillespie syndrome (partial aniridia, cerebellar ataxia, and mental retardation). Genomics 19: 145–148. [DOI] [PubMed] [Google Scholar]
- 27.Kieslich M, Vanselow K, Wildhardt G, Gebhardt B, Weis R, Bohles H (2001) [Present limitations of molecular biological diagnostics in Gillespie syndrome]. Klin Padiatr 213: 47–49. [DOI] [PubMed] [Google Scholar]
- 28.Luquetti DV, Oliveira-Sobrinho RP, Gil-da-Silva-Lopes VL (2007) Gillespie syndrome: additional findings and parental consanguinity. Ophthalmic Genet 28: 89–93. [DOI] [PubMed] [Google Scholar]
- 29.Marien P, Brouns R, Engelborghs S, Wackenier P, Verhoeven J, Ceulemans B et al. (2008) Cerebellar cognitive affective syndrome without global mental retardation in two relatives with Gillespie syndrome. Cortex 44: 54–67. 10.1016/j.cortex.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 30.Nelson J, Flaherty M, Grattan-Smith P (1997) Gillespie syndrome: a report of two further cases. Am J Med Genet 71: 134–138. [PubMed] [Google Scholar]
- 31.Nevin NC, Lim JH (1990) Syndrome of partial aniridia, cerebellar ataxia, and mental retardation—Gillespie syndrome. Am J Med Genet 35: 468–469. [DOI] [PubMed] [Google Scholar]
- 32.Quarrell O (1993) Gillespie syndrome reported as bilateral congenital mydriasis. Br J Ophthalmol 77: 827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ticho BH, Hilchie-Schmidt C, Egel RT, Traboulsi EI, Howarth RJ, Robinson D (2006) Ocular findings in Gillespie-like syndrome: association with a new PAX6 mutation. Ophthalmic Genet 27: 145–149. [DOI] [PubMed] [Google Scholar]
- 34.Verhulst S, Smet H, Ceulemans B, Geerts Y, Tassignon MJ (1993) Gillespie syndrome, partial aniridia, cerebellar ataxia and mental retardation in mother and daughter. Bull Soc Belge Ophtalmol 250: 37–42. [PubMed] [Google Scholar]
- 35.Wittig EO, Moreira CA, Freire-Maia N, Vianna-Morgante AM (1988) Partial aniridia, cerebellar ataxia, and mental deficiency (Gillespie syndrome) in two brothers. Am J Med Genet 30: 703–708. [DOI] [PubMed] [Google Scholar]
- 36.Crawfurd MD, Harcourt RB, Shaw PA (1979) Non-progressive cerebellar ataxia, aplasia of pupillary zone of iris, and mental subnormality (Gillespie's syndrome) affecting 3 members of a non-consanguineous family in 2 generations. J Med Genet 16: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dilling-Ostrowska E, Zuchowicz M (1973) [Case of Gillespie's syndrome]. Neurol Neurochir Pol 7: 97–99. [PubMed] [Google Scholar]
- 38.Francois J, Lentini F, de Rouck F (1984) Gillespie's syndrome (incomplete aniridia, cerebellar ataxia and oligophrenia). Ophthalmic Paediatr Genet 4: 29–32. [DOI] [PubMed] [Google Scholar]
- 39.Graziano C, D'Elia AV, Mazzanti L, Moscano F, Guidelli Guidi S, Scarano E et al. (2007) A de novo nonsense mutation of PAX6 gene in a patient with aniridia, ataxia, and mental retardation. Am J Med Genet A 143A: 1802–1805. [DOI] [PubMed] [Google Scholar]
- 40.Hanson I, Churchill A, Love J, Axton R, Moore T, Clarke M et al. (1999) Missense mutations in the most ancient residues of the PAX6 paired domain underlie a spectrum of human congenital eye malformations. Hum Mol Genet 8: 165–172. [DOI] [PubMed] [Google Scholar]
- 41.Gerth-Kahlert C, Williamson K, Ansari M, Rainger JK, Hingst V, Zimmermann T et al. (2013) Clinical and mutation analysis of 51 probands with anophthalmia and/or severe microphthalmia from a single center. Mol Genet Genomic Med 1: 15–31. 10.1002/mgg3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansari M, Rainger JK, Murray JE, Hanson I, Firth HV, Mehendale F et al. (2014) A syndromic form of Pierre Robin sequence is caused by 5q23 deletions encompassing FBN2 and PHAX. Eur J Med Genet 57: 587–595. 10.1016/j.ejmg.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 43.Aijaz S, Clark BJ, Williamson K, van Heyningen V, Morrison D, FitzPatrick D et al. (2004) Absence of SIX6 mutations in microphthalmia, anophthalmia, and coloboma. Invest Ophthalmol Vis Sci 45: 3871–3876. [DOI] [PubMed] [Google Scholar]
- 44.Weier HU, Zitzelsberger HF, Gray JW (1991) Non-isotopical labeling of murine heterochromatin in situ by hybridization with in vitro-synthesized biotinylated gamma (major) satellite DNA. BioTechniques 10: 498–502, 504–5 [PubMed] [Google Scholar]
- 45.Chong SS, Pack SD, Roschke AV, Tanigami A, Carrozzo R, Smith AC et al. (1997) A revision of the lissencephaly and Miller-Dieker syndrome critical regions in chromosome 17p13.3. Hum Mol Genet 6: 147–155. [DOI] [PubMed] [Google Scholar]
- 46.Saleem RA, Banerjee-Basu S, Berry FB, Baxevanis AD, Walter MA (2003) Structural and functional analyses of disease-causing missense mutations in the forkhead domain of FOXC1. Hum Mol Genet 12: 2993–3005. [DOI] [PubMed] [Google Scholar]
- 47.Volkmann BA, Zinkevich NS, Mustonen A, Schilter KF, Bosenko DV, Reis LM et al. (2011) Potential novel mechanism for Axenfeld-Rieger syndrome: deletion of a distant region containing regulatory elements of PITX2. Invest Ophthalmol Vis Sci 52: 1450–1459. 10.1167/iovs.10-6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fantes JA, Boland E, Ramsay J, Donnai D, Splitt M, Goodship JA et al. (2008) FISH mapping of de novo apparently balanced chromosome rearrangements identifies characteristics associated with phenotypic abnormality. Am J Hum Genet 82: 916–926. 10.1016/j.ajhg.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatia S, Gordon CT, Foster RG, Melin L, Abadie V, Baujat G et al. (2015) Functional assessment of disease-associated regulatory variants in vivo using a versatile dual colour transgenesis strategy in zebrafish. PLoS Genet 11: e1005193 10.1371/journal.pgen.1005193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleinjan DA, Bancewicz RM, Gautier P, Dahm R, Schonthaler HB, Damante G et al. (2008) Subfunctionalization of duplicated zebrafish pax6 genes by cis-regulatory divergence. PLoS Genet 4: e29 10.1371/journal.pgen.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravi V, Bhatia S, Gautier P, Loosli F, Tay BH, Tay A et al. (2013) Sequencing of Pax6 loci from the elephant shark reveals a family of Pax6 genes in vertebrate genomes, forged by ancient duplications and divergences. PLoS Genet 9: e1003177 10.1371/journal.pgen.1003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowan S, Siggers T, Lachke SA, Yue Y, Bulyk ML, Maas RL (2010) Precise temporal control of the eye regulatory gene Pax6 via enhancer-binding site affinity. Genes Dev 24: 980–985. 10.1101/gad.1890410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fantes J, Redeker B, Breen M, Boyle S, Brown J, Fletcher J et al. (1995) Aniridia-associated cytogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum Mol Genet 4: 415–422. [DOI] [PubMed] [Google Scholar]
- 54.Kleinjan DA, Seawright A, Schedl A, Quinlan RA, Danes S, van Heyningen (2001) Aniridia-associated translocations, DNase hypersensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum Mol Genet 10: 2049–2059. [DOI] [PubMed] [Google Scholar]
- 55.Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T (2000) 3' deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci U S A 97: 13755–13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhatia S, Bengani H, Fish M, Brown A, Divizia MT, de Marco R et al. (2013) Disruption of autoregulatory feedback by a mutation in a remote, ultraconserved PAX6 enhancer causes aniridia. Am J Hum Genet 93: 1126–1134. 10.1016/j.ajhg.2013.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'haene B, Meire F, Claerhout I, Kroes HY, Plomp A, Arens YH et al. (2011) Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Invest Ophthalmol Vis Sci 52: 324–333. 10.1167/iovs.10-5309 [DOI] [PubMed] [Google Scholar]
- 58.Acharya M, Huang L, Fleisch VC, Allison WT, Walter MA (2011) A complex regulatory network of transcription factors critical for ocular development and disease. Hum Mol Genet 20: 1610–1624. 10.1093/hmg/ddr038 [DOI] [PubMed] [Google Scholar]
- 59.Tumer Z, Bach-Holm D (2009) Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet 17: 1527–1539. 10.1038/ejhg.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perveen R, Lloyd IC, Clayton-Smith J, Churchill A, van Heyningen V, Hanson I et al. (2000) Phenotypic variability and asymmetry of Rieger syndrome associated with PITX2 mutations. Invest Ophthalmol Vis Sci 41: 2456–2460. [PubMed] [Google Scholar]
- 61.Iwase S, Shono N, Honda A, Nakanishi T, Kashiwabara S, Takahashi S et al. (2006) A component of BRAF-HDAC complex, BHC80, is required for neonatal survival in mice. FEBS Lett 580: 3129–3135. [DOI] [PubMed] [Google Scholar]
- 62.Klajn A, Ferrai C, Stucchi L, Prada I, Podini P, Baba T et al. (2009) The rest repression of the neurosecretory phenotype is negatively modulated by BHC80, a protein of the BRAF/HDAC complex. J Neurosci 29: 6296–6307. 10.1523/JNEUROSCI.5943-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HG, Kim HT, Leach NT, Lan F, Ullmann R, Silahtaroglu A et al. (2012) Translocations disrupting PHF21A in the Potocki-Shaffer-syndrome region are associated with intellectual disability and craniofacial anomalies. Am J Hum Genet 91: 56–72. 10.1016/j.ajhg.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prakash SK, Paylor R, Jenna S, Lamarche-Vane N, Armstrong DL, Xu B et al. (2000) Functional analysis of ARHGAP6, a novel GTPase-activating protein for RhoA. Hum Mol Genet 9: 477–488. [DOI] [PubMed] [Google Scholar]
- 65.Schaefer L, Prakash S, Zoghbi HY (1997) Cloning and characterization of a novel rho-type GTPase-activating protein gene (ARHGAP6) from the critical region for microphthalmia with linear skin defects. Genomics 46: 268–277. [DOI] [PubMed] [Google Scholar]
- 66.Wimplinger I, Morleo M, Rosenberger G, Iaconis D, Orth U, Meinecke P et al. (2006) Mutations of the mitochondrial holocytochrome c-type synthase in X-linked dominant microphthalmia with linear skin defects syndrome. Am J Hum Genet 79: 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayrakli F, Guney I, Bayri Y, Ercan-Sencicek AG, Ceyhan D, Cankaya T et al. (2009) A novel heterozygous deletion within the 3' region of the PAX6 gene causing isolated aniridia in a large family group. J Clin Neurosci 16: 1610–1614. 10.1016/j.jocn.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 68.Davis LK, Meyer KJ, Rudd DS, Librant AL, Epping EA, Sheffield VC et al. (2008) Pax6 3' deletion results in aniridia, autism and mental retardation. Hum Genet 123: 371–378. 10.1007/s00439-008-0484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. ArrayCGH data have been deposited in the Database of Genomic Variants (accession ID estd228) and the DECIPHER database (decipher.sanger.ac.uk).