Abstract
Observations in experimental murine myeloperoxidase (MPO)-ANCA-associated vasculitis (AAV) show mast cells degranulate, thus enhancing injury as well as producing immunomodulatory IL-10. Here we report that, compared with biopsy specimens from control patients, renal biopsy specimens from 44 patients with acute AAV had more mast cells in the interstitium, which correlated with the severity of tubulointerstitial injury. Furthermore, most of the mast cells were degranulated and spindle-shaped in patients with acute AAV, indicating an activated phenotype. We hypothesized that the mast cell stabilizer disodium cromoglycate would attenuate mast cell degranulation without affecting IL-10 production. We induced anti-MPO GN by immunizing mice with MPO and a low dose of anti-glomerular basement membrane antibody. When administered before or after induction of MPO autoimmunity in these mice, disodium cromoglycate attenuated mast cell degranulation, development of autoimmunity, and development of GN, without diminishing IL-10 production. In contrast, administration of disodium cromoglycate to mast cell-deficient mice had no effect on the development of MPO autoimmunity or GN. MPO-specific CD4+ effector T cell proliferation was enhanced by co-culture with mast cells, but in the presence of disodium cromoglycate, proliferation was inhibited and IL-10 production was enhanced. These results indicate that disodium cromoglycate blocks injurious mast cell degranulation specifically without affecting the immunomodulatory role of these cells. Thus as a therapeutic, disodium cromoglycate may substantially enhance the regulatory role of mast cells in MPO-AAV.
Mast cells (MCs) are best characterized in pathology by their effector roles in IgE-dependent degranulation and by their release of pro-inflammatory mediators in allergy and anaphylaxis.1 However, it is now recognized that MCs also play important roles in host defense and also in non-allergic inflammatory diseases, particularly those initiated by autoimmunity. The functional diversity of MC phenotypes allows for their participation in the generation of adaptive immune responses, playing either injurious or modulatory roles in many chronic human diseases and animal models of these diseases.2
A functional role for MCs in a particular human disease can be suspected by confirming MC presence in diseased target organs and demonstrating a correlation between MC activation status and disease outcome. This potential cause and effect association can be strengthened by studies in relevant murine models of the particular diseases comparing disease patterns and outcomes between MC-deficient (KitWsh/Wsh) mice and KitWsh/Wsh mice reconstituted with MCs.2–5 The mechanistic basis of MC-enhanced injury is by MC degranulation, which promotes injurious inflammation and enhances the capacity of dendritic cells (DCs) to drive autoimmunity.6 Using these techniques, MCs have been demonstrated to be pathogenic in many diseases, including experimental autoimmune encephalomyelitis,7 collagen induced arthritis,8 type 1 diabetes mellitus (in non-obese diabetic mice),9 bullous pemphigus10 and systemic sclerosis.11
The somewhat simplistic concept that MCs are only pro-inflammatory has been complicated by evidence demonstrating an essential role for MCs in the induction and maintenance of tolerance. The list of diseases in which the net effect of MCs is immunomodulatory is growing and includes studies in ultraviolet-B light12 or chemical induced suppression of contact hypersensitivity,13 mosquito bite induced suppression of delayed type hypersensitivity (DTH),14 induced peripheral tolerance to skin allograft transplants,15 protection from anti-glomerular basement membrane (GBM),16,17 and anti-myeloperoxidase glomerulonephritis (anti-MPO GN).18 The mechanistic basis of these effects is also becoming better understood and includes MC synthesis of anti-inflammatory molecules (TGF-β and IL-10), the expression of surface molecules (OX40L and PD-L1) that may facilitate immunoregulation following direct contact with regulatory T cells (Tregs)19 and reciprocally, Treg-derived IL-9 to enhance MC immunomodulation.17
In this current study, we investigated possible associations between infiltrating renal MCs and kidney function in patients with GN, a key feature of MPO-ANCA-associated vasculitis (MPO-AAV). This is an autoimmune disease that, despite current best practice, has a 5-year mortality of 30% and for which current treatments are non-specific and have considerable toxicities.20 The disease is characterized by its strong association with circulating autoantibodies (ANCA) that recognize auto-antigens21 found in neutrophil lysosomal azurophilic granules,22 typically proteinase-3 and MPO. The renal lesion of MPO-AAV has a unique pathology characterized by focal and segmental necrotizing crescentic GN with little or no immunoglobulin deposition in glomeruli (thereby being designated as ‘pauci-immune’). While immunoglobulin deposits are absent or rare in active ANCA-associated crescentic GN, kidney biopsies demonstrate DTH effectors; CD4+ T cells, macrophages, and fibrin.23 Several studies have shown that MCs are present in renal lesions in this disease but the functional role of these cells remains to be defined.24,25 In this current study, we show that MCs are prominent in MPO-AAV GN, displaying an activated degranulating phenotype and greater numbers in patients with the most severe tubulointerstitial injury.
We have established an experimental autoimmune murine model of anti-MPO GN that exhibits the pathognomonic features observed in patients with MPO-AAV and found that MCs are immunomodulatory via MC IL-10 production enhancing immunosuppressive functions of Tregs.18 Other studies in skin transplantation have shown that MCs closely interact with Tregs in the transplanted skin to maintain tolerance. However, induced degranulation of MCs leads to acute inflammation and graft rejection.15 We hypothesize that in the autoimmune anti-MPO GN model, MC degranulation would similarly be pro-inflammatory and injurious in the induction of MPO autoimmunity by promoting the loss of tolerance to MPO. Therefore MCs could play opposing roles in MPO-AAV. Within the lymph nodes (LNs), IL-10 secreted by MCs is immunomodulatory and favors tolerance, while degranulating MCs may be pro-inflammatory in the induction of autoimmunity and also enhance effector responses in the major target organ, the kidney. Several MC stabilizing drugs have been developed. Among the best known is disodium cromoglycate (DSCG), a calcium channel targeting drug26 that blocks MC degranulation and has been used in the treatment of anaphylaxis and allergic diseases including asthma and mastocytosis for over 30 years.27,28 Its predominant effect is on preventing degranulation, while de novo synthesis and release of cytokines is not significantly affected.29 Given that both degranulation and synthesis of immunomodulatory IL-10 may occur in MPO-AAV, selective enhancement of MC modulatory function by DSCG administration may represent a new therapeutic strategy.
Results
Human Studies of MC Phenotype and Prominence in MPO-AAV and GN
The study population comprised 44 patients who satisfied study inclusion requirements (first presentation with renal biopsy with proven focal segmental, immune negative, necrotizing crescentic GN together with circulating MPO-ANCA, the absence of granulomata and a renal biopsy with at least six glomeruli). The mean age of the patients was 67±2 years, 68% were male and 34% had extra renal disease (one or more of respiratory, joint or skin involvement). The patients had evidence of systemic inflammation with elevated C-reactive protein (CRP) (70±13.9 mmol/l) and positive enzyme-linked immunosorbent assay (ELISA) for MPO-ANCA (mean 134±16 U/ml). The cohort had significant renal functional impairment (eGFR mean 21.6±2.4 ml/min per 1.73 m2), serum creatinine (mean 395±53 µmol/l), proteinuria (mean 1.8±0.4 g/24 hrs), and hematuria (547±682 red cells/l) (Table 1).
Table 1.
Clinical, demographic and immunologic data and indices of renal disease features among MPO-AAV patients
| Demographic data | ||
|---|---|---|
| Patient number | 44 | |
| Sex (f/m) | 14/30 | |
| Agea (years) | 67±2 | |
| Renal involvement | ||
| Serum creatininea (µmol/l) | 395±53 | |
| eGFRa (ml/min per 1.73 m2) | 21.6±2.4 | |
| Proteinuriaa,b (g/day) | 1.8±0.4 | |
| RBCa,c (urine cells/hpf) | 547±682 | |
| Extra renal involvement | ||
| Lung or upper respiratory tract or skin or arthralgia presenting creatinine | 15/44 | |
| Immunologic data | ||
| ANCA (MPO) titera (U/ml) | 134±16 | |
| CRPd (mmol/l) | 70±13.9 |
Reported as mean±SEM.
Urinary total protein over 24 hours.
Red blood cell excretion.
CRP on admission.
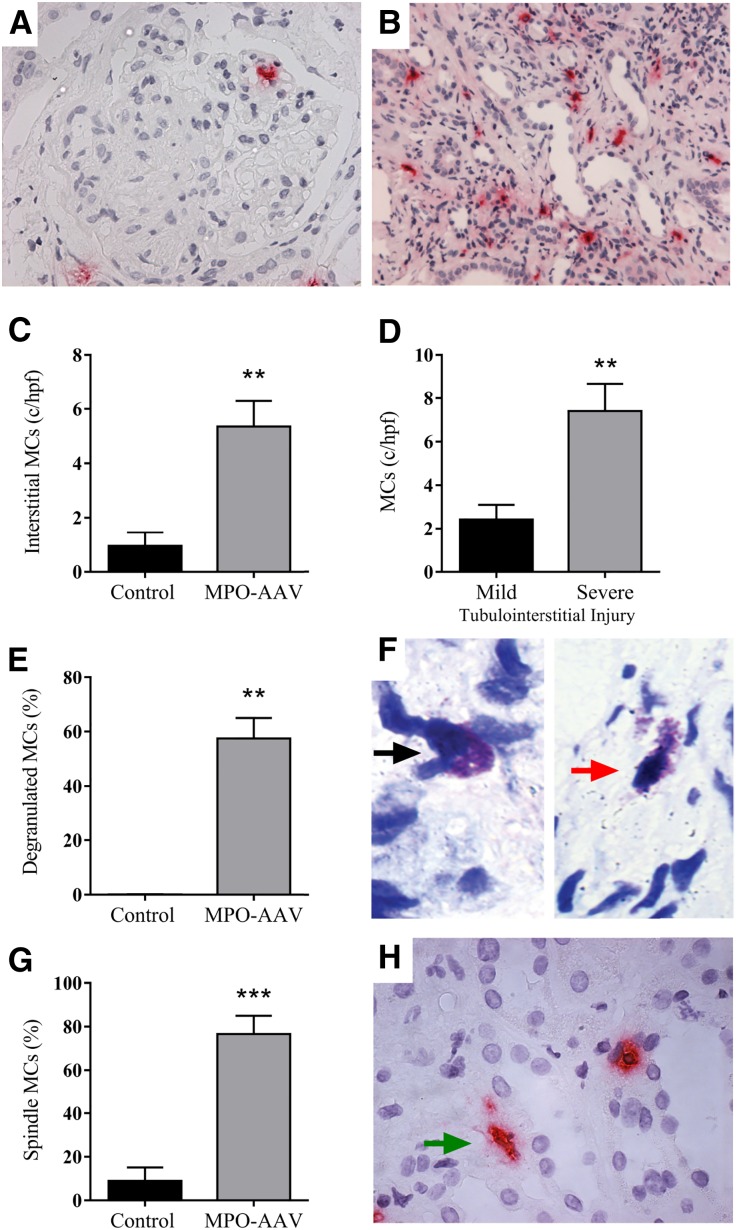
To assess the participation and significance of MCs in the GN of AAV, we analyzed the extent of renal MC infiltration in kidney biopsies from patients with MPO-AAV compared with biopsies from control patients with minimal glomerular damage, the non-inflammatory conditions; minimal change disease and thin basement membrane diseases (n=9). MCs were seen throughout the kidneys of patients with MPO-AAV. Although glomerular MC presence was rarely observed in control patients, MCs were detected in the glomeruli of MPO-AAV patients at low levels (0.03±0.01 MCs/gcs, P<0.05) (Figure 1A). MCs were predominantly found in the interstitium (Figure 1, B and C) and their frequency correlated with severe tubulointerstitial injury (Figure 1D). There was no significant correlation between the numbers of interstitial MCs and eGFR at presentation in patients with MPO-AAV (Spearman’s rank-order correlation; r= −0.27, P=0.07). Furthermore, we assessed MC phenotypic markers indicative of an activated phenotype and more than 50% of MCs found in MPO-AAV patients were degranulated (Figure 1, E and F; red arrow and intact MC [black arrow]) and >70% were spindle shaped significantly higher than among controls (Figure 1G and H; green arrow). Therefore, MCs are found in kidney biopsies of patients with MPO-AAV and GN; showing an activated phenotype suggesting they play a pro-inflammatory, pathogenic role in disease.
Figure 1.
MC presence and activation status in renal biopsies from patients with MPO-AAV. Frequency of kidney MCs was determined by immunohistochemical staining for MC tryptase (A and B). Prominent interstitial MC accumulation was observed in patients with MPO-AAV (n=44 patients biopsies) compared with patients with minimal change GN (n=9 patient biopsies) (C). Patients with severe tubulointerstitial injury had greater numbers of infiltrating MCs (D). Most MCs in MPO-AAV biopsies (n=25) were degranulated (E and F; intact MC, black arrow and degranulated MC, red arrow) and spindle shaped (G and H; green arrow). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. ***P<0.001, **P<0.01.
DSCG Prevents the Development of Systemic Anti-MPO T Cell Autoimmunity
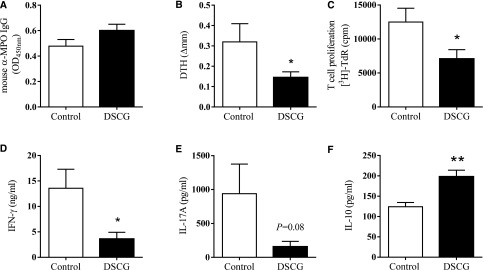
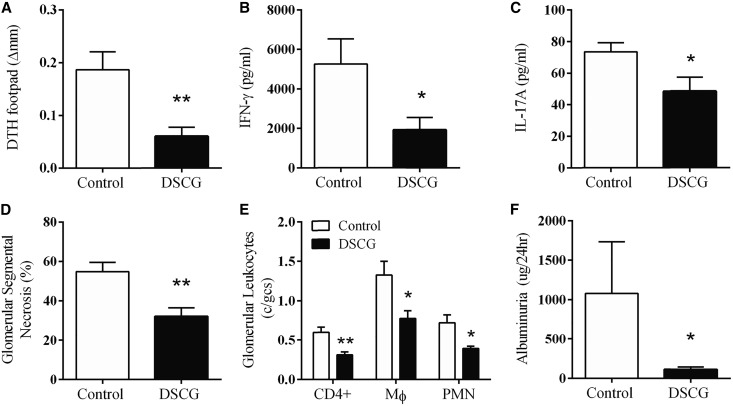
To assess the effects of DSCG in experimental autoimmune anti-MPO GN, C57BL/6 mice received DSCG (intraperitoneally, daily) while controls received saline. We induced a T cell mediated model of autoimmune anti-MPO GN by immunizing mice with native mouse MPO on day 0, then boosting the response on day 7. On day 16, disease was triggered by the passive transfer of low-dose anti-GBM globulin that recruits neutrophils to glomeruli and deposits MPO, the autoantigen, in the kidney.30,31 Autoimmunity and renal injury were assessed at the end of the experiment after a further 4 days. DSCG did not alter anti-MPO antibody titers (Figure 2A) but did attenuate systemic anti-MPO T cell responses. MPO-specific dermal footpad DTH responses were reduced compared with saline-treated controls (Figure 2B). Effects on the anti-MPO autoimmune response were determined by isolating draining LNs from MPO immunization sites and re-stimulating with MPO ex vivo. T cell proliferation was reduced in mice treated with DSCG, associated with diminished IFN-γ and IL-17A production (Figure 2, C–E). However, the anti-inflammatory cytokine, IL-10, was significantly elevated in DSCG-treated mice (Figure 2F). These results highlight that DSCG is an immunomodulatory therapeutic capable of attenuating the development of anti-MPO autoimmunity.
Figure 2.
DSCG prevents the development of anti-MPO autoimmunity. ANCA levels were unaffected (A), MPO induced T cell recall responses assessed by DTH (B), proliferation (C), IFN-γ (D), and IL-17A (E) were all reduced while IL-10 responses were increased (F) by DSCG treatment (DSCG n=8, controls n=7). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. **P<0.01, *P<0.05.
Preventative Treatment of DSCG Protects from the Development of GN
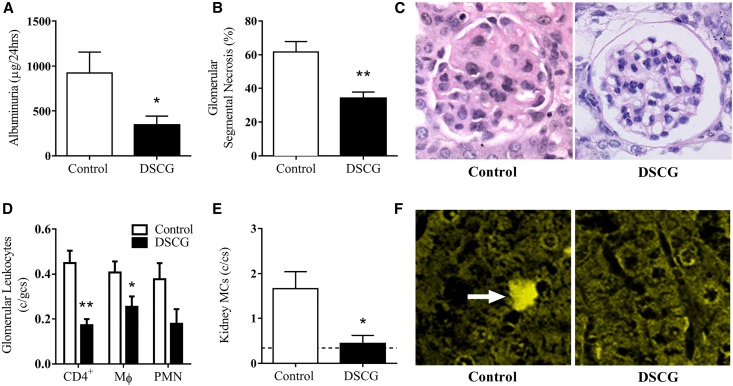
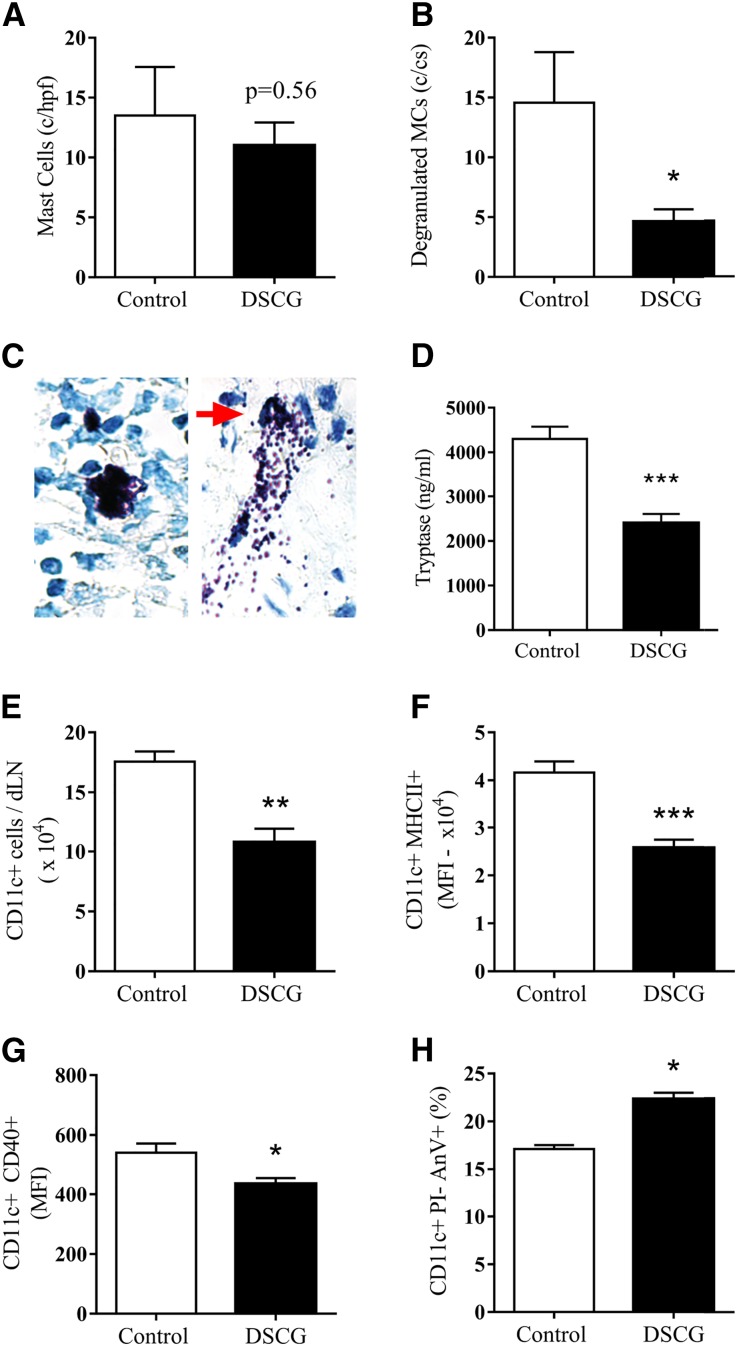
DSCG administered prior to establishment of anti-MPO autoimmunity attenuated functional kidney injury as measured by albuminuria (Figure 3A) and the proportion of glomeruli with segmental necrosis (Figure 3, B and C) compared with controls. Reduction in functional kidney injury was associated with decreased numbers of glomerular leukocytes, including CD4+ T cells and macrophages, while no difference in glomerular neutrophils was observed (Figure 3D). Furthermore, DSCG as a preventative treatment significantly reduces kidney MC infiltration to a similar level observed in naïve C57BL/6 mice (dotted line) (Figure 3, E and F).
Figure 3.
DSCG administration prior to MPO immunization, as a preventative, protected from the development of GN. Renal injury assessed by albuminuria (A), glomerular segmental necrosis (B and C) and glomerular leukocyte accumulation (D) was attenuated in mice that received DSCG (n=8), compared with saline treated controls (n=7). Kidney MCs were identifiable in controls and significantly reduced by DSCG to numbers similar to those observed in naïve C57BL/6 mice (dotted line; n=5) (E). Kidney MCs were identified and quantified following methyl Carnoy’s fixation and Berberine sulfate staining, white arrow (F). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. **P<0.01, *P<0.05.
DSCG Effects are Mast Cell Specific
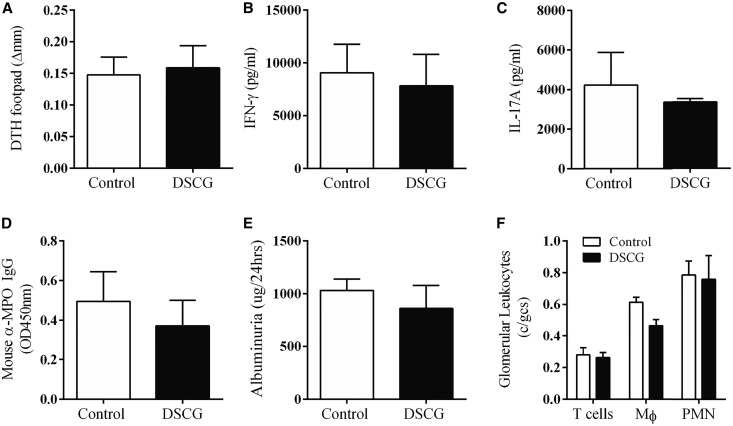
The selectivity of DSCG as an MC stabilizer is controversial as any generalized mechanisms of action, independent of MCs, have not been well defined. DSCG may inhibit the activation of human neutrophils, eosinophils and monocytes, and stimulate activated B cells to produce immunoglobulin in vitro.32,33 However, in over 30 years of safe clinical use, major effects on immunity have not been evident. To verify that the protective effects of DSCG in the development of experimental autoimmune anti-MPO GN were due only to its effects on MCs, DSCG was administered to MC-deficient (KitWsh/Wsh) mice prior to induction of autoimmune anti-MPO GN (control KitWsh/Wsh mice received saline). KitWsh/Wsh mice treated with DSCG exhibited similar auto-reactivity and disease to control treated KitWsh/Wsh mice, with no significant differences in anti-MPO autoimmunity (dermal DTH, T cell cytokine production and anti-MPO antibodies; Figure 4A–D). Renal injury was similarly (albuminuria and glomerular leukocyte accumulation; Figure 4, E and F) unaffected. These results demonstrate that in experimental autoimmune anti-MPO GN, DSCG acts specifically through MCs.
Figure 4.
DSCG effects are mast cell specific. MPO immunized KitWsh/Wsh mice treated with DSCG (n=9) in a preventative protocol developed similar systemic MPO T cell (DTH, IFN-γ and IL-17A; A–C) and humoral responses (mouse anti-MPO IgG, D) compared with saline treated MPO immunized KitWsh/Wsh mice (n=9). No difference in renal injury (albuminuria and glomerular leukocyte accumulation; E and F) was observed between groups. Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test.
DSCG Effects on MC–Dendritic Cell Interactions
We hypothesized that the major effects of DSCG in this disease model would be mediated by preventing MC degranulation. This was assessed in the LN draining the sites of MPO immunization, the maneuver that provokes loss of tolerance and development of nephritogenic autoimmunity. While MC numbers in draining LNs were similar (Figure 5A), we observed significant attenuation of MC degranulation as determined by quantitating the number of degranulating MCs (Figure 5B; red arrow and Figure 5C) and by measuring extracellular tryptase released from MCs in LNs draining sites of MPO immunization (Figure 5D) following DSCG administration. This was associated with fewer DCs (Figure 5E) that were less activated (CD11c+MHCII+ and CD11c+CD40+; Figure 5, F and G), and more apoptotic (Figure 5H) compared with saline treated controls. These data support our hypothesis that DSCG acts by reducing MC degranulation, mitigating against the development and maintenance of injurious anti-MPO autoimmunity.
Figure 5.
Blocking MC degranulation limits dendritic cell activation. In draining LNs 18 hours post-MPO immunization, DSCG treatment (n=5) does not affect MC numbers (A) but reduces the proportion of degranulated MCs (B) as indicated by the red arrow (C) compared with controls (n=5). Extracellular tryptase protein obtained from LNs draining MPO immunization sites was significantly reduced in mice that received DSCG compared with saline treated controls (D; DSCG n=8, control n=8). DSCG treatment decreased DC numbers (E), activation markers, CD11c+MHC-II+, CD11c+CD40+ expression (F and G) and enhanced DC apoptosis (H). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. ***P<0.001, **P<0.01, *P<0.05.
Therapeutic Potential of DSCG to Dampen Anti-MPO Autoimmunity and GN
To determine whether DSCG is therapeutic in mice with established anti-MPO autoimmunity, daily DSCG administration was commenced on day 10 (after the completion of the MPO immunization schedule). Compared with saline-treated controls, DSCG treatment attenuated systemic anti-MPO T cell responses measured by dermal DTH (Figure 6A) as well as diminished IFN-γ and IL-17A by lymph node cells (Figure 6, B and C). The diminished anti-MPO autoimmunity in DSCG-treated mice was associated with less glomerular segmental necrosis (Figure 6D), fewer glomerular CD4+ T cells, macrophages, neutrophils (Figure 6E), and less albuminuria (Figure 6F). Collectively, DSCG, a low clinical toxicity MC stabilizer, has the capacity to limit established anti-MPO autoimmunity as well as preventing the development of GN.
Figure 6.
DSCG treatment of mice with established MPO autoimmunity (day 10) diminishes the extent of effector T cell responses assessed by MPO T cell recall responses including skin DTH (A) and LN IFN-γ and IL-17A responses (B and C). Renal injury, segmental glomerular necrosis (D), glomerular leukocyte accumulation (E), and functional injury (albuminuria, F) were also reduced by DSCG treatment (DSCG n=6, control n=6). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. **P<0.01, *P<0.05.
Effects of DSCG on Mast Cells to Inhibit T Cell Proliferation Ex Vivo
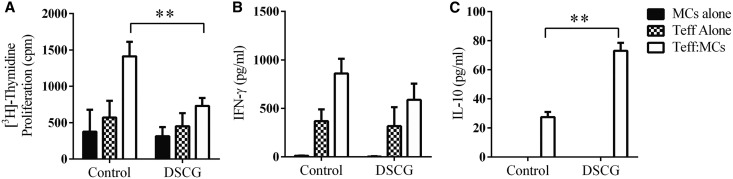
CD4+Foxp3− T effector cells (anti-MPO CD4+ Teff) from MPO-immunized Foxp3-GFP mice were stimulated ex vivo with MPO and compared with anti-MPO Teffs stimulated with MPO, but co-cultured with bone marrow derived mast cells (BMMCs) in the presence or absence of DSCG. The MPO triggered anti-MPO Teff proliferation increased 3-fold in the presence of BMMCs. The addition of DSCG to co-cultures significantly restricted Teff proliferation (Figure 7A), while no difference was observed in IFN-γ concentration in cultured supernatants (Figure 7B). Interestingly, DSCG was able to significantly enhance IL-10 production by co-cultured BMMCs and Teffs compared with untreated BMMCs and Teffs (Figure 7C). No IL-10 was detectable when Teff or MCs were cultured alone. These studies demonstrate a direct immunomodulatory effect of DSCG on BMMCs and Teffs co-cultures.
Figure 7.
DSCG attenuates MC enhancement of MPO induced recall responses of anti-MPO Teffs. Controls demonstrate that DSCG does not alter the proliferative response and IFN-γ production of either Teff alone or MCs alone (A and B). Co-culture of Teff and MCs induced IL-10 production which was significantly enhanced in the presence of DSCG (C) while the addition of DSCG did not induce IL-10 production by Teff or MCs alone in this assay. Error bars represent mean±SEM with statistical analysis by two-tailed paired t-test. **P<0.01, *P<0.05.
The Protection Afforded by DSCG is IL-10 Dependent
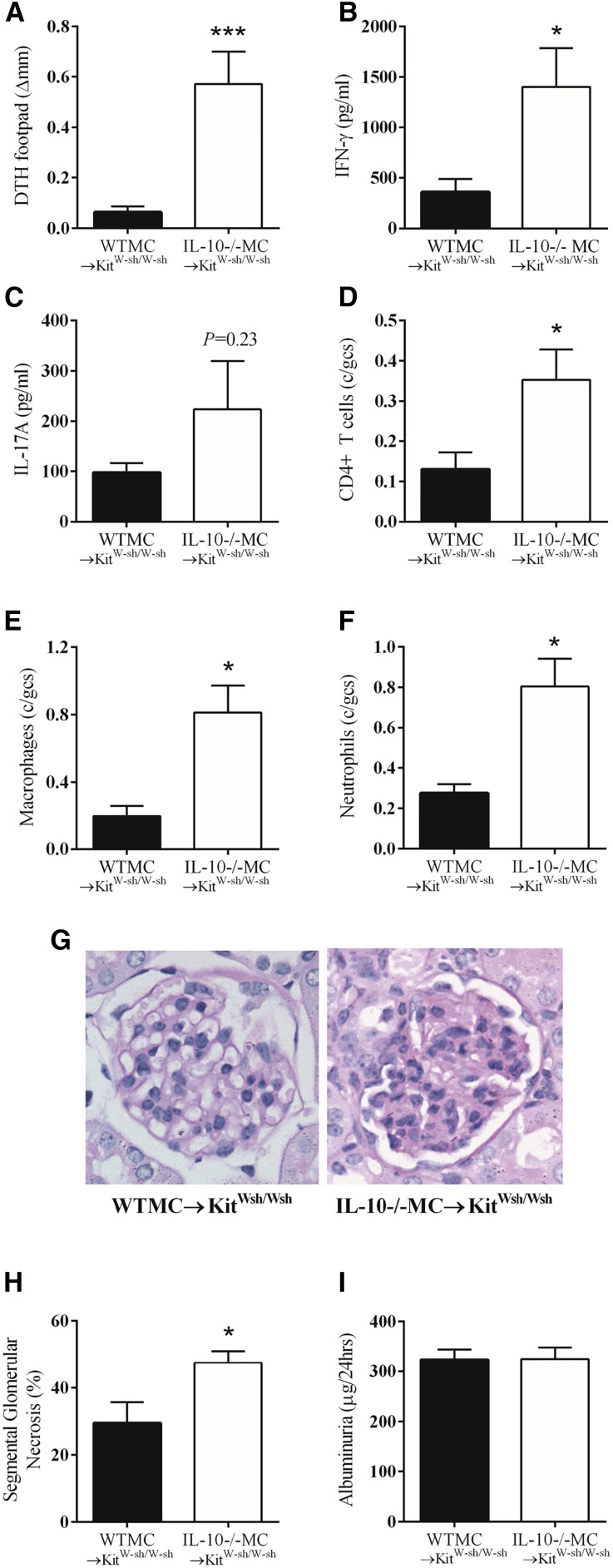
MCs are immunomodulatory by their capacity to synthesize and secrete IL-10. To determine whether DSCG’s protective effects are IL-10 dependent, anti-MPO GN was induced in KitWsh/Wsh mice that were either reconstituted with WT or IL-10 deficient (−/−) BMMCs. Both groups were then treated with DSCG. DSCG treated IL-10−/−MC→KitWsh/Wsh mice developed significant MPO specific dermal DTH footpad swelling that was markedly reduced in WTMC→KitWsh/Wsh mice treated with DSCG (Figure 8A). Comparing MPO-specific T cell production of pro-inflammatory cytokines by ex vivo MPO restimulation of cells from LN draining MPO immunization sites between WT and IL-10−/−MC reconstituted KitWsh/Wsh mice receiving DSCG shows that IFN-γ production was significantly higher in IL-10−/−MC→KitWsh/Wsh mice (Figure 8B). No difference in IL-17A production was observed between groups (Figure 8C). Additionally, DSCG treatment did not limit numbers of infiltrating glomerular leukocytes in IL-10−/−MC→KitWsh/Wsh mice, compared with WTMC→KitWsh/Wsh mice (Figure 8, D–F). Glomerular segmental necrosis was assessed histologically on PAS-stained kidney sections, demonstrating significantly more structural glomerular injury in IL-10−/−MC→KitWsh/Wsh mice (Figure 8, G and H). There was no difference in 24-hour albuminuria (Figure 8I). These results reiterate the importance of MC IL-10 to modulate the extent of MPO autoimmunity and GN, demonstrating that daily DSCG administration works by blocking MC degranulation while requiring IL-10 production.
Figure 8.
MC IL-10 attenuates anti-MPO GN and is not inhibited by DSCG. DSCG treated IL-10−/−MC→KitWsh/Wsh mice (n=7) did not attenuate MPO specific DTH footpad swelling (A), anti-MPO T cells, IFN-γ (B) and IL-17A production (C). Accumulation of glomerular T cells, macrophages, neutrophils and segmental necrosis was greater in DSCG treated IL-10−/−MC→KitWsh/Wsh versus WTMC→KitWsh/Wsh mice (n=7) (D–H). Albuminuria was NS between groups (I). Error bars represent mean±SEM with statistical analysis by Mann–Whitney t-test. ***P<0.001, *P<0.05.
Discussion
The current studies, using observations in human MPO-AAV and intervention studies in an experimental model of MPO-ANCA associated GN, suggest MC degranulation enhances the development of renal vasculitis and that MC stabilizers such as DSCG might be employed in limiting autoimmunity and disease. MCs have emerged as potential key players in the induction, amplification, and paradoxically immunomodulation of autoimmunity in general and rapidly progressive forms of GN in particular.2,17,34,35 In MPO-AAV, MCs are a major leukocyte population seen in infiltrating damaged kidneys. Although the functional role of MCs in patients with MPO-AAV is uncertain, the patients with the highest MC density had the most severe tubulointerstitial damage. Furthermore, little is known about the phenotype of renal MCs in human MPO-AAV. The current study confirms that MCs in the kidney of patients with this disease degranulate and adopt a spindle shape, thus demonstrating a pro-inflammatory phenotype. One recent paper reports that MCs are prominent producers of IL-17 in ANCA-associated GN.36 These findings are consistent with MCs having an acute injurious role.
In addition, our current experimental studies suggest that MCs can enhance the development of autoimmunity by interacting with DCs in LNs. We have previously shown in this model that MCs have a net protective effect, resulting from their interactions with Tregs which enhances immunomodulation.18 MC-deficient mice develop more severe disease than WT mice and this enhanced autoimmunity and glomerular injury can be attenuated by MC reconstitution. Those results together with the current studies confirm that MCs play two distinct roles – pro-inflammatory and immunomodulatory. MC degranulation occurs within seconds of MC activation and preformed mediators are released within hours. While independent of degranulation, MCs can synthesize and release an array of cytokines de novo, depending on the activating stimulus. MC activation through TLR4 induces synthesis and release of TNF-α, IL-6, IL-1, and IL-13,37 which can potentially activate DCs. However, MC activation through vitamin D receptors results in the release of IL-10, without inducing MC degranulation.38 In experimental anti-MPO GN, MCs were observed to be degranulating in LNs, where tolerance to MPO was being lost. We therefore used DSCG, a drug whose major MC effect was to prevent degranulation. DSCG attenuated MC degranulation and this was associated with reduced DC activation and increased apoptosis. At the same time, DSCG administration was also associated with enhanced IL-10 generation. These events were accompanied by significant reduction in anti-MPO autoimmunity and glomerular injury.
Cromolyns have been used for over 30 years as standard treatment for stabilizing (i.e., preventing degranulation) MCs in allergy and mastocytosis. These drugs have also been widely used in experimental animal models. There is some controversy as to whether DSCG can effectively attenuate MC degranulation in mice.39 However, there are a number of reports showing that this drug successfully stabilizes MCs by attenuating MC degranulation in a number of murine models.40–42 We also demonstrated that DSCG could significantly attenuate MC degranulation in a model of passive cutaneous anaphylaxis in C57BL/6 mice (Supplemental Figure 1). To demonstrate that DSCG’s mechanism of action did not extend beyond stabilizing MCs in experimental anti-MPO GN, the same experiments were performed in MC-deficient (KitWsh/Wsh) mice. In the absence of MCs, DSCG administration had no effect on the generation of anti-MPO autoimmunity (DTH responses to MPO, MPO-specific cytokine production of IFN-γ or IL-17A and serum anti-MPO titers), glomerular leukocyte accumulation or glomerular histologic and functional injury.
While the therapeutic potential of DSCG as a preventative therapy in the development of experimental anti-MPO GN is interesting, clinically, MPO-AAV patients undergo treatment only after autoimmunity is established. Therefore, we sought to determine whether DSCG treatment after established anti-MPO autoimmunity will attenuate GN. DSCG treatment diminished the severity of GN. This attenuation was associated with reduced DTH and MPO specific Th1 and Th17 cell directed responses. Together, these studies strongly support consideration of a clinical trial of DSCG as a potentially safe and effective treatment for anti-MPO GN.
To further explore the capacity of DSCG to block MC enhancement of anti-MPO CD4+ effector T cell MPO recall responses, ex vivo T cell/MC co-cultures were studied. Recall responses of CD4+ T cells to MPO were enhanced by co-culture with MCs. However this enhancement was significantly less when effector cells were co-cultured with DSCG treated MCs. In previous studies of ex vivo co-culture of MCs, anti-MPO Teff and Tregs, we were able to show that MC IL-10 augments Treg immunosuppression.18 These current experiments show that DSCG could stimulate IL-10 production from co-culture of MCs and Teff cells in the absence of Foxp3+ Tregs. These results support an interpretation that DSCG’s effects on preventing MC degranulation result in the modulation of MC interactions on Teff cells, with the resultant attenuation of anti-MPO autoimmunity and GN.
Taking the results from the human biopsy observations and the animal experiments, we believe MCs play both injurious and protective roles. MC degranulation is directly injurious in the major affected organ, the kidney and in the immune system, degranulating MCs drive the induction and severity of anti-MPO autoimmunity. However, MCs also play a protective role in modulating the intensity of effector anti-MPO T cell responses via IL-10 production in the immune system. DSCG acts only on the injurious contribution of MCs (degranulation) without effecting MC mediated immunomodulation. These findings suggest a new therapeutic use of this drug in treating autoimmune anti-MPO GN.
Concise Methods
Patient Cohort
Forty-four patients admitted to Monash Medical Centre (MMC) were categorized as having MPO-AAV by clinical, serological and histologic features. Patients with first presentation of vasculitis, a positive MPO-ANCA result by both direct immunofluorescence and antigen-specific ELISA was obtained along with clinical evidence of significant renal impairment (serum creatinine >200 µmol/L) and active urinary sediment with the presence of a systemic inflammation (raised CRP and erythrocyte sedimentation rate), together with focal and segmental necrotizing crescentic GN with little or no immunostaining for immunoglobulin or complement were retrospectively sequentially collected. Biopsies were assessed if they had a minimum of six glomeruli (range 6–37 glomeruli). Tubulointerstitial disease was graded by the extent of interstitial fibrosis and atrophy as Grade 1 (mild) or Grade 2 (severe).43 Renal function was assessed by eGFR (ml/min per 1.73 m2) assessed at the time of renal biopsy. Nine patients with thin basement membrane disease or adult minimal change GN were used as ‘normal’ controls. Studies were approved by the Monash University Human Research Ethics Committee.
Mast Cell Staining in Human Biopsies
Mast Cell Tryptase
Formalin fixed paraffin embedded 2 µm tissue specimens (n=44 MPO-AAV, n=9 Control patients), were mounted, dewaxed, and rehydrated in graded alcohols, and pretreated with antigen retrieval solution tris-EDTA (pH 9) in a pressure cooker for 10 minutes, blocked (30 minutes) in 5% bovine serum albumin (BSA)/phosphate-buffered saline (PBS) (immunofluorescence) with 10% horse serum (immunohistochemistry) and probed with mouse anti-human mast cell tryptase 1/1000 (DAKO, Glostrup, Denmark) in 1% BSA/PBS for 16 hours (4°C). A universal VECTORSTAIN ABC-AP kit (Vector, Peterborough, UK) was used and visualized with a VECTOR red alkaline phosphatase substrate kit (Vector) and counterstained with hematoxylin. Sections were assessed for the total numbers of spindle MCs and expressed as a percentage of the total MC population, within the glomerulus (cells/gcs), peri-glomerular regions (cells/gcs), and the interstitium (expressed as the average of 10 hpf).
Toluidine Blue
The kidney biopsies of 25 MPO-AAV patients with adequate, available sample material were used to analyze MC degranulation. Their clinical, demographic, and immunologic data and indices of renal disease characteristic were representative of the total MPO-ANCA patient population studied (Supplemental Table 1). Formalin-fixed paraffin embedded 2 µm sections of tissue specimens were mounted on Superfrost Plus slides (Menzel, Braunschweig, Germany), dewaxed, rehydrated in graded alcohols, and stained with 0.5% potassium permanganate (Sigma-Aldrich, St Louis, MO) for 2 minutes, washed and stained for 1 minute with a 2% aqueous solution of potassium metabisulphite (Sigma-Aldrich), washed and stained with acidified 0.1% toluidine blue O (Sigma-Aldrich) for 5 minutes, washed, dehydrated, cleared and cover slipped. MCs were identified by characteristic metachromatic granules. Degranulated MCs were identified as those with metachromatic granules close to the cell membrane or staining less than half of the cytoplasm as previously described.44 MCs were quantified in 10 random non-overlapping high-powered fields (100×) of view and expressed as percentage of degranulating MCs/mm2 per total mast cells per mm2 for human biopsies, and the same procedure was repeated for mouse LN.
Mice
C57BL/6 (wild-type), KitWsh/Wsh and Foxp3-GFP male mice (n=6–10 per group) were bred at Monash Medical Centre Animal Facilities, Monash University, Australia. IL-10−/− C57BL/6 male mice purchased from the University of Adelaide (South Australia, Australia) and originally from Jackson Laboratories, were used as donors to obtain BMMCs. All mice were housed in specific pathogen-free conditions at Monash Medical Centre, and studies were approved by Monash University Animal Ethics Committee in accordance with the Australian National Health and Medical Research Council animal experimentation guidelines.
Experimental Design
WT mice were immunized intraperitoneally with 20 µg murine MPO in Freund’s complete adjuvant (FCA; Sigma-Aldrich) and boosted subcutaneously with 10 µg murine MPO in Freund’s incomplete adjuvant (FIA; Sigma-Aldrich) on day 7. Murine MPO was purified from differentiated 32Dcl3 cells as described previously.45 Disease was initiated (‘triggered’) by intravenous injection of 1.5 mg anti-GBM globulin consecutively on days 16 and 17. Anti-MPO autoimmunity and GN was assessed 3 days later (day 20).
Mast Cell Reconstitution
KitWsh/Wsh was reconstituted with 5×106 MCs (intravenously) from 6- to 8-week-old wild-type or IL-10−/− mice using a standard in vitro differentiation of MC technique.5,18 Following MC reconstitution (WTMC→KitWsh/Wsh and IL-10−/−MC→KitWsh/Wsh), mice were administered daily with DSCG beginning a day prior to the induction of MPO autoimmunity.
Disodium Cromoglycate (DSCG) Treatment
We chose a protocol of administration that has been shown to attenuate MC degranulation in several mouse models of disease in vivo.40–42 In all experiments, to stabilize MC degranulation, 10 mg/kg DSCG (Sigma-Aldrich) was reconstituted in saline and injected intraperitoneally.
Passive Cutaneous Anaphylaxis
To assess the capacity of DSCG to attenuate MC degranulation in C57BL/6 WT mice, we confirmed in an established model of MC mediated injury (passive cutaneous anaphylaxis) that DSCG (10 mg/kg) could attenuate this well studied model of MC degranulation. WT mice were passively sensitized with α-DNP IgE (10 ng subcutaneous footpad; Sigma-Aldrich) and challenged 24 hours later with DNP-BSA (100 µg intravenously, Life Technologies). Two hours prior to challenge, WT mice received either DSCG (n=10) or saline (n=10) intraperitoneally. Six hours post-challenge, footpads were harvested, fixed in methyl Carnoy’s and toluidine blue stained for degranulated MCs (as described above). Results are expressed as percentage degranulated MCs.
DSCG Administration in Anti-MPO Autoimmunity and GN
DSCG was administered daily intraperitoneally beginning a day prior to MPO immunization (day −1). When DSCG was used to treat mice with established anti-MPO autoimmunity, DSCG was administered daily intraperitoneally post-MPO immunization schedule (day 10). Non-treated control mice received the same volume of saline daily.
Assessment of Renal Disease and Immune Cell Infiltration
Histologic assessment of renal injury was performed on 3 µm thick, formalin-fixed, paraffin-embedded, periodic acid-Schiff-stained kidney sections. A minimum of 30 consecutive glomeruli/mouse were examined and results expressed as percentage of segmental glomerular necrosis per glomerular cross-section (gcs). Glomerular CD4+ T cells, macrophages, and neutrophils were assessed by an immunoperoxidase-staining technique on 6-µm thick, periodate lysine paraformaldehyde fixed, OCT frozen kidney sections. The primary antibodies used were GK1.5 for CD4+ T cells (anti-mouse CD4+; American Type Culture Collection, Manassas, VA), FA/11 for macrophages (anti-mouse CD68 from Dr. Gordon L. Koch, Cambridge, England), and RB6–8CS for neutrophils (anti-GR-1; DNAX, Palo Alto, CA). A minimum of 30 glomeruli were assessed and results expressed as cells/gcs (c/gcs).
Kidney MCs were detected by staining with MC heparin. Acidified toluidine blue, while it is effective for the detection of LN MCs, fails to detect kidney MCs. Mouse kidneys were removed, halved, and fixed in Methyl Carnoy’s fixative (60% methanol, 30% chloroform, and 10% glacial acetic acid) for 4 hours at room temperature, subjected to routine tissue processing and embedded in wax. Non-serial 3 µm cross-sections were cut and every 4th kidney section was mounted on glass slides, cleared, immersed in ethanol/acetic acid (3:1) for 15 minutes, rinsed in 100% ethanol for a further 15 minutes, washed in distilled water for 10 minutes and stained with 0.02% (w/v) berberine sulfate (Sigma-Aldrich Pty Ltd) diluted in acidic distilled water (pH 4, with 1% citric acid), washed in acidic distilled water (pH 4), air dried and mounted with glycerol. Slides were examined under a fluorescent microscope using the FITC/488 filter set (Leica-Microsystems, North Ryde, Australia). A minimum of six kidney cross-sections were examined for berberine sulfate positive cells as determined by bright yellow fluorescence counted using 400× magnification. Results are expressed as MCs per kidney cross-section (c/cs).
Urine was collected by housing mice in individual metabolic cages over the final 24 hours of experiment. Albuminuria was assessed by ELISA (Bethyl Laboratories, Montgomery, TX) and expressed as µg/24 hr.
Systemic Immune Responses to MPO
ELISA was used to detect circulating serum anti-MPO IgG titers using 100 µl/well, 1 µg/ml murine MPO and horseradish peroxidase conjugated sheep anti-mouse IgG (1:1000; Amersham Biosciences, Rydalmere, Australia).
To assess MPO-specific dermal DTH, mice were challenged by intradermal injection of 10 µg murine MPO in 30 µl saline in the right hind footpad (the contralateral footpad received saline). DTH was quantified 24 hours later by measuring the difference between footpad thickness (∆mm) using a micrometer.
For assessment of cytokine production, lymphocytes were cultured for 72 hours (37°C, 5% CO2) at 4×106 cells/ml per well with or without MPO (10 µg/ml) in supplemented RPMI (10% FCS, 2 mM L-glutamine, 50 µM 2-ME, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; Sigma). IFN-γ, IL-17A, and IL-10 were measured by ELISA.46 For IFN-γ, the mAbs were rat anti-mouse IFN-γ (R4–6A2; BD Pharmingen) and biotinylated rat anti-mouse IFN-γ (XMG1.2; BD Pharmingen). IL-17A was measured by using paired antibodies (DuoSet; eBioscience, San Diego, CA), IL-10 by using rat anti-mouse IL-10 capture antibody (18141D; BD Pharmingen), and biotinylated rat anti-mouse IL-10 detection antibody (BD Pharmingen).
MPO-specific cell proliferation was measured by culturing lymphocytes at 5×105 cells/well in 96-well flat-bottom plates (Sarstedt, Newton, NC), re-stimulated with or without 10 µg/ml MPO and incubated for 72 hours. During the last 16 hours of culture, 0.5 µCi of [3H]-thymidine (PerkinElmer, Waltham, MA) was added. [3H]-Thymidine incorporation was measured as previously described.18
Assessment of MC Degranulation and DC Status in Experimental Anti-MPO GN
DSCG was administered intraperitoneally to WT mice a day before 15 µg MPO/FCA immunization (subcutaneously). Control MPO/FCA immunized WT mice were administered with saline. Eighteen hours post MP/FCA immunization, LNs draining MPO immunization sites were harvested. Histologic assessment of MC prominence and MC degranulation was determined by staining LN sections using the toluidine blue method as described above.
A standard method of quantifying MC degranulation is by measuring extracellular tryptase. A MC degranulation ELISA kit determines the amount of tryptase protein by measuring tryptase activity (IMM001; EMD Millipore, Billerica, MA). The assay was performed according to the manufacturer’s recommendations. Briefly, draining LNs were harvested (controls, n=8 and DSCG, n=8), placed in 1× assay buffer (IMM001; Part No. 90570), and pushed through a 35 µm cell strainer cap (Falcon, Mexico). Cell suspensions were centrifuged at 700 g and supernatant collected, discarding the cell pellet. Tryptase positive control standards (IMM001; Part No. 90572) and experimental samples were incubated at 37°C for 90 minutes with Tryptase Substrate (IMM001; Part No. 90569). The absorbance values were read at 405 nm using a microplate reader (Tecan, Infinite M1000 PRO, Austria) and results expressed as ng/ml.
DC activation (identified as CD11chi cells) on isolated LNs was measured by flow cytometry. LN cells were stained for 20 minutes at 4°C with the following directly conjugated antibodies: hamster anti-mouse CD11c PE (HL3; BD Pharmingen), rat anti-mouse CD40 FITC (3/32; BD Pharmingen), and anti-mouse MHC-II PE (M5/114, gift from K. Shortman, Walter and Eliza Hall Institute, Parkville, Australia). For the detection of apoptotic cells, isolated LN cells were resuspended in 100 µl of Annexin-V labelling solution (Roche, Mannheim, Germany), which contained 10 µg/ml of propidium iodine and incubated for 15 minutes at room temperature. Cells were analyzed on the FACSCanto II machine using FACSDiva software (BD Biosciences) and data analyzed using FlowJo (TreeStar, Palo Alto, CA).
Assessment of DSCG to Inhibit in vitro MPO CD4 T Effector Cell Recall Response
To obtain MPO-CD4+ Teff cells, Foxp3-GFP mice (n=6) were immunized with MPO and FCA subcutaneously. Ten days later, draining LNs from MPO immunization sites were harvested, and CD4+ T cells isolated by magnetic separation using mouse CD4 (L3T4) microbeads (Miltenyi Biotec, Bergisch, Gladbach) and then sorted based on GFP expression on the Mo-Flo XDP cell sorter (Beckman Coulter, Lane Cove, NSW, Australia). CD4+Foxp3− MPO Teff cells (1×105) were co-cultured with WT BMMCs (1×105) together with erythrocyte-lysed, MACS CD4 depleted, mitomycin C-treated (50 µg/ml for 30 mins at 37°C) KitWsh/Wsh splenocytes (2×105 cells). Cells were added in a 96-well round-bottom plate with 10 µg/ml MPO for 72 hours, and proliferation was measured by adding [3H]-thymidine for the last 16 hours of culture. Results were expressed as percentage anti-MPO Teff proliferation. IFN-γ in cultured supernatants was measured using BD Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences) and IL-10 was measured using a BD Cytometric Bead Array Mouse IL-10 Enhanced Sensitivity Flex Set with Enhanced Sensitivity Master Buffer Kit (BD Biosciences) as per manufacturer’s instructions.
Statistical Methods
Results are expressed as the mean±SEM. Mann–Whitney t-test was used for non-parametric data and paired t-test for comparison in ex vivo co-culture experiments. All data were analyzed with Graph Pad Version 6 (GraphPad Prism; GraphPad Software Inc., San Diego, CA). Differences were considered to be statistically significant if P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported by Grant No. 1011724 from the National Health and Medical Research Council of Australia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090906/-/DCSupplemental.
References
- 1.Kalesnikoff J, Galli SJ: New developments in mast cell biology. Nat Immunol 9: 1215–1223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker ME, Hatfield JK, Brown MA: New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta 1822: 57–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MA, Hatfield JK: Mast cells are important modifiers of autoimmune disease: With so much evidence, why is there still controversy? Front Immunol 3: 147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli SJ, Kitamura Y: Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol 127: 191–198, 1987 [PMC free article] [PubMed] [Google Scholar]
- 5.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ: Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN: Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 6: 331–342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secor VH, Secor WE, Gutekunst CA, Brown MA: Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med 191: 813–822, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimentel TA, Sampaio AL, D’Acquisto F, Perretti M, Oliani SM: An essential role for mast cells as modulators of neutrophils influx in collagen-induced arthritis in the mouse. Lab Invest 91: 33–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hübner MP, Larson D, Torrero MN, Mueller E, Shi Y, Killoran KE, Mitre E: Anti-FcεR1 antibody injections activate basophils and mast cells and delay Type 1 diabetes onset in NOD mice. Clin Immunol 141: 205–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbach L, Li Z, Berkowitz P, Zhao M, Li N, Rubenstein DS, Diaz LA, Liu Z: The C5a receptor on mast cells is critical for the autoimmune skin-blistering disease bullous pemphigoid. J Biol Chem 286: 15003–15009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seibold JR, Giorno RC, Claman HN: Dermal mast cell degranulation in systemic sclerosis. Arthritis Rheum 33: 1702–1709, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ: Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 8: 1095–1104, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Limón-Flores AY, Chacón-Salinas R, Ramos G, Ullrich SE: Mast cells mediate the immune suppression induced by dermal exposure to JP-8 jet fuel. Toxicol Sci 112: 144–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depinay N, Hacini F, Beghdadi W, Peronet R, Mécheri S: Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol 176: 4141–4146, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ: Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442: 997–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, Maurer M, Rosenkranz AR: Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol 35: 3074–3082, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM: IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 186: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan PY, Summers SA, Ooi JD, O’Sullivan KM, Tan DS, Muljadi RC, Odobasic D, Kitching AR, Holdsworth SR: Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol 23: 1955–1966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frossi B, Gri G, Tripodo C, Pucillo C: Exploring a regulatory role for mast cells: ‘MCregs’? Trends Immunol 31: 97–102, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Little MA, Pusey CD: Glomerulonephritis due to antineutrophil cytoplasm antibody-associated vasculitis: an update on approaches to management. Nephrology (Carlton) 10: 368–376, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Davies DJ, Moran JE, Niall JF, Ryan GB: Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed) 285: 606, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR: Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol 10: 499–506, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Otsubo S, Nitta K, Uchida K, Yumura W, Nihei H: Mast cells and tubulointerstitial fibrosis in patients with ANCA-associated glomerulonephritis. Clin Exp Nephrol 7: 41–47, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 19: 2254–2261, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mazurek N, Berger G, Pecht I: A binding site on mast cells and basophils for the anti-allergic drug cromolyn. Nature 286: 722–723, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Kuzemko, JA: Twenty years of sodium cromoglycate treatment: a short review. Respir Med 83 Suppl A: 11–14; discussion 15–16, 1989 [DOI] [PubMed]
- 28.Horan RF, Sheffer AL, Austen KF: Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol 85: 852–855, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D: Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev 217: 65–78, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Kay AB, Walsh GM, Moqbel R, MacDonald AJ, Nagakura T, Carroll MP, Richerson HB: Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol 80: 1–8, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Kimata H, Yoshida A, Ishioka C, Mikawa H: Disodium cromoglycate enhances ongoing immunoglobulin production in vitro in human B cells. Immunology 73: 31–35, 1991 [PMC free article] [PubMed] [Google Scholar]
- 34.Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR: A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol 17: 150–159, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Scandiuzzi L, Beghdadi W, Daugas E, Abrink M, Tiwari N, Brochetta C, Claver J, Arouche N, Zang X, Pretolani M, Monteiro RC, Pejler G, Blank U: Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol 185: 624–633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velden J, Paust HJ, Hoxha E, Turner JE, Steinmetz OM, Wolf G, Jabs WJ, Özcan F, Beige J, Heering PJ, Schröder S, Kneißler U, Disteldorf E, Mittrücker HW, Stahl RA, Helmchen U, Panzer U: Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am J Physiol Renal Physiol 302: F1663–F1673, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H: Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest 109: 1351–1359, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA: Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med 207: 455–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ: Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Invest 92: 1472–1482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ: Mast cell degranulation breaks peripheral tolerance. Am J Transplant 9: 2270–2280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summers SA, Gan PY, Dewage L, Ma FT, Ooi JD, O’Sullivan KM, Nikolic-Paterson DJ, Kitching AR, Holdsworth SR: Mast cell activation and degranulation promotes renal fibrosis in experimental unilateral ureteric obstruction. Kidney Int 82: 676–685, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Summers SA, Chan J, Gan PY, Dewage L, Nozaki Y, Steinmetz OM, Nikolic-Paterson DJ, Kitching AR, Holdsworth SR: Mast cells mediate acute kidney injury through the production of TNF. J Am Soc Nephrol 22: 2226–2236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford SL, Polkinghorne KR, Longano A, Dowling J, Dayan S, Kerr PG, Holdsworth SR, Kitching AR, Summers SA: Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 63: 227–235, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Huang M, Pang X, Letourneau R, Boucher W, Theoharides TC: Acute stress induces cardiac mast cell activation and histamine release, effects that are increased in Apolipoprotein E knockout mice. Cardiovasc Res 55: 150–160, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR: The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods 308: 167–178, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O’Sullivan KM, Tipping PG, Takeda K, Akira S, Holdsworth SR: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.