Abstract
Urea has a critical role in urinary concentration. Mice lacking the inner medullary collecting duct (IMCD) urea transporter A1 (UT-A1) and urea transporter A3 (UT-A3) have very low levels of urea permeability and are unable to concentrate urine. To investigate the role of UT-A1 in the concentration of urine, we transgenically expressed UT-A1 in knockout mice lacking UT-A1 and UT-A3 using a construct with a UT-A1 gene that cannot be spliced to produce UT-A3. This construct was inserted behind the original UT-A promoter to yield a mouse expressing only UT-A1 (UT-A1+/+/UT-A3−/−). Western blot analysis demonstrated UT-A1 in the inner medulla of UT-A1+/+/UT-A3−/− and wild-type mice, but not in UT-A1/UT-A3 knockout mice, and an absence of UT-A3 in UT-A1+/+/UT-A3−/− and UT-A1/UT-A3 knockout mice. Immunohistochemistry in UT-A1+/+/UT-A3−/− mice also showed negative UT-A3 staining in kidney and other tissues and positive UT-A1 staining only in the IMCD. Urea permeability in isolated perfused IMCDs showed basal permeability in the UT-A1+/+/UT-A3−/− mice was similar to levels in wild-type mice, but vasopressin stimulation of urea permeability in wild-type mice was significantly greater (100% increase) than in UT-A1+/+/UT-A3−/− mice (8% increase). Notably, basal urine osmolalities in both wild-type and UT-A1+/+/UT-A3−/− mice increased upon overnight water restriction. We conclude that transgenic expression of UT-A1 restores basal urea permeability to the level in wild-type mice but does not restore vasopressin-stimulated levels of urea permeability. This information suggests that transgenic expression of UT-A1 alone in mice lacking UT-A1 and UT-A3 is sufficient to restore urine-concentrating ability.
Keywords: vasopressin, urea transporter, IMCD, transgenic mice, aquaporin, urine, concentrating mechanism
Urea plays a critical role in the urinary concentrating mechanism and in the regulation of water balance.1,2 Maximal urine-concentrating ability is decreased by protein deprivation and restored by urea infusion (reviewed in Klein et al.3). Urea transporter A1/urea transporter A3 (UT-A1/UT-A3),4–7 urea transporter A2 (UT-A2),8 urea transporter B1 (UT-B1),9–11 and UT-A2/UT-B112 knockout mice each have urine-concentrating defects. The UT-A1/UT-A3 knockout mouse has a severe urine-concentrating defect. Thus, any hypothesis regarding the mechanism by which the kidney concentrates urine needs to include some effect derived from urea and urea transporters, especially UT-A1 and/or UT-A3.
The mechanism by which urine concentration occurs in the inner medulla remains controversial. The most widely accepted mechanism remains the passive reabsorption of NaCl, in excess of solute secretion, from thin ascending limbs of the loops of Henle.13,14 However, the passive mechanism hypothesis is not universally accepted. Studies of the UT-A1/UT-A3 knockout mouse have been interpreted both to refute and support the passive mechanism.15–17 Regardless, knockout of UT-A1 and UT-A3 results in a severe urine-concentrating defect.4,5
UT-A1 is expressed in the inner medullary collecting duct (IMCD) apical plasma membrane.18 UT-A3 is expressed in the IMCD basolateral plasma membrane, but is also detected in the apical plasma membrane following stimulation by vasopressin.19 Vasopressin increases both the phosphorylation and the apical plasma membrane accumulation of UT-A1 and UT-A3 in freshly isolated suspensions of rat IMCDs,19,20 and increases facilitated urea transport in perfused terminal IMCDs.21 Because both UT-A1 and UT-A3 are present, it is not possible to determine the relative contribution of each isoform to urea transport.
Both UT-A1 and UT-A3 are under the control of UT-A promoter I; there is a second, internal promoter, UT-A promoter II, which controls UT-A2.22–24 UT-A3 is essentially the N-terminal half of UT-A1, with the sole difference being that UT-A3 has a unique amino acid at its C-terminus and a unique 3′-untranslated region.25 UT-A2 is essentially the C-terminal half of UT-A1, with a unique exon that encodes the N-terminus of UT-A2.22–24,26 Thus, the UT-A gene structure precludes making a UT-A3 knockout mouse without also knocking out UT-A1. The goal of this study was to use an innovative approach to make a mouse expressing UT-A1 but lacking UT-A3 by genetically engineering UT-A1 back into a UT-A1/UT-A3 knockout mouse. The transgenic expression of UT-A1 in knockout mice lacking UT-A1 and UT-A3 allows us to examine the role of UT-A1 in producing a concentrated urine without the influence of UT-A3.
Results
Generation of UT-A1+/+/UT-A3−/− Mice
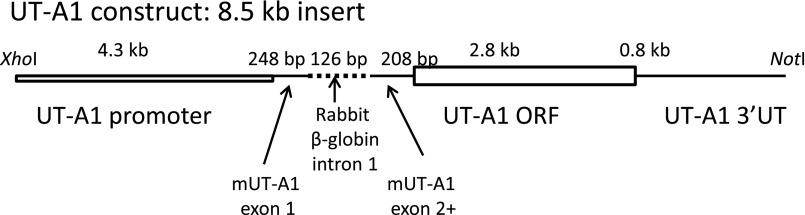
Our colony of UT-A1/UT-A3 knockout mice were rederived from breeding stock kindly provided by Dr. Knepper (National Institutes of Health [NIH]).4,7 A UT-A1 DNA construct was prepared with the full-length exon sequence for UT-A1 behind the native UT-A1 promoter, and was designed to eliminate possible cleavage sites so that the shorter UT-A3 version of this protein is not made (Figure 1). The final UT-A1 construct has the following structure: the 5′-end consists of a 4.3-kb stretch of the mouse UT-A1 promoter I and is continued beyond the transcription initiation site until the end of the first exon. This 4.3-kb promoter length has been shown to guide expression of a reporter gene to principal cells in the terminal IMCD.27 Following the first exon, an intron was included to increase the expression of the transgene28; we used intron 1 of the rabbit β-globin gene instead of intron 1 of UT-A1, which would be much too long (25 kb). This was followed by the cDNA of UT-A1 starting at the sequence corresponding to exon 2 until the end of the 3′-untranslated region, including the polyadenylation sites.
Figure 1.
Schematic diagram of the UT-A1 construct introduced to produce the UT-A1+/+/UT-A3−/− mice. The construct has the native UT-A1 promoter, spliced to mouse Urea Transporter (mUT-A1) exon 1, the rabbit beta-globin intron 1, mUT-A1 exon 2, followed by the open reading frame (ORF) for UT-A1 , and then the 3′ untranslated region (3′UT) for UT-A1.
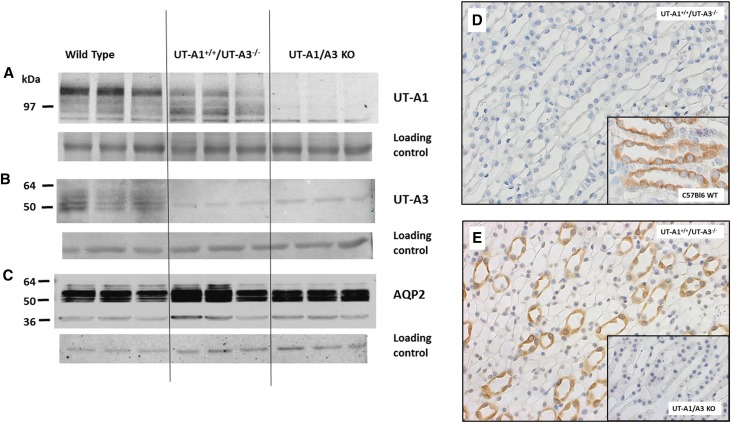
This construct was introduced into wild-type (WT) C57Bl6 mice. Over 15 generations, the mice were back-bred to UT-A1/UT-A3 knockout mice, selecting for the transgene and absence of the native UT-A gene to achieve a line of mice that are homozygous for the UT-A1 gene without the UT-A3 gene and produce the proteins as shown in Figure 2. WT C57Bl6 mice show the 97- and 117-kDa UT-A1 protein (panel A, left lanes) as well as the UT-A3 glycoproteins (panel B, left lanes). These are absent in the UT-A1/UT-A3 knockout mice (panels A and B, right lanes). The UT-A1+/+/UT-A3−/− mice show UT-A1 protein (panel A, center) with no evidence of UT-A3 (panel B, center). Aquaporin-2 (AQP2) protein was present at comparable levels in WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice (Figure 2C). Immunohistochemistry confirmed the absence of UT-A3 in UT-A1+/+/UT-A3−/− mice (Figure 2D). The positive control for the presence of UT-A3 in the WT mice is provided in the inset micrograph (Figure 2D, inset). Conversely, while UT-A1 is present in the UT-A1+/+/UT-A3−/− mice (Figure 2E), it is absent in the UT-A1/UT-A3 knockout mice (Figure 2E inset). Staining for UT-A1 or UT-A3 in other kidney sections and extrarenal tissues were negative (data not shown), confirming that the targeting construct led to UT-A1 expression only in the IMCD.
Figure 2.
The UT-A1+/+/UT-A3−/− mice express UT-A1 and lack UT-A3. (A, B) Western blot of WT (left), UT-A1+/+/UT-A3−/− (center), and UT-A1/UT-A3 knockout ([KO] right) mice probed with the C-terminal UT-A1 antibody (panel A identifies UT-A1) or MQ2 antibody (panel B identifies UT-A3). (C) AQP2 in the same samples. Each blot was also stained with Ponceau S for general protein content as a loading control, shown immediately below each Western blot. (D and E) Immunohistochemical verification of the urea transporter protein expression in the UT-A1+/+/UT-A3−/− mice. (D) Lack of staining of UT-A3 in the UT-A1+/+/UT-A3−/− mice. The positive control staining of UT-A3 in the WT mice is provided in the inset micrograph. (E) Positive staining of UT-A1 (brown) in the inner medulla of the UT-A1+/+/UT-A3−/− mice. The negative staining of the knockout mice is provided in the inset picture. Images were acquired at 400× using an Olympus inverted microscope fitted with a 40× objective.
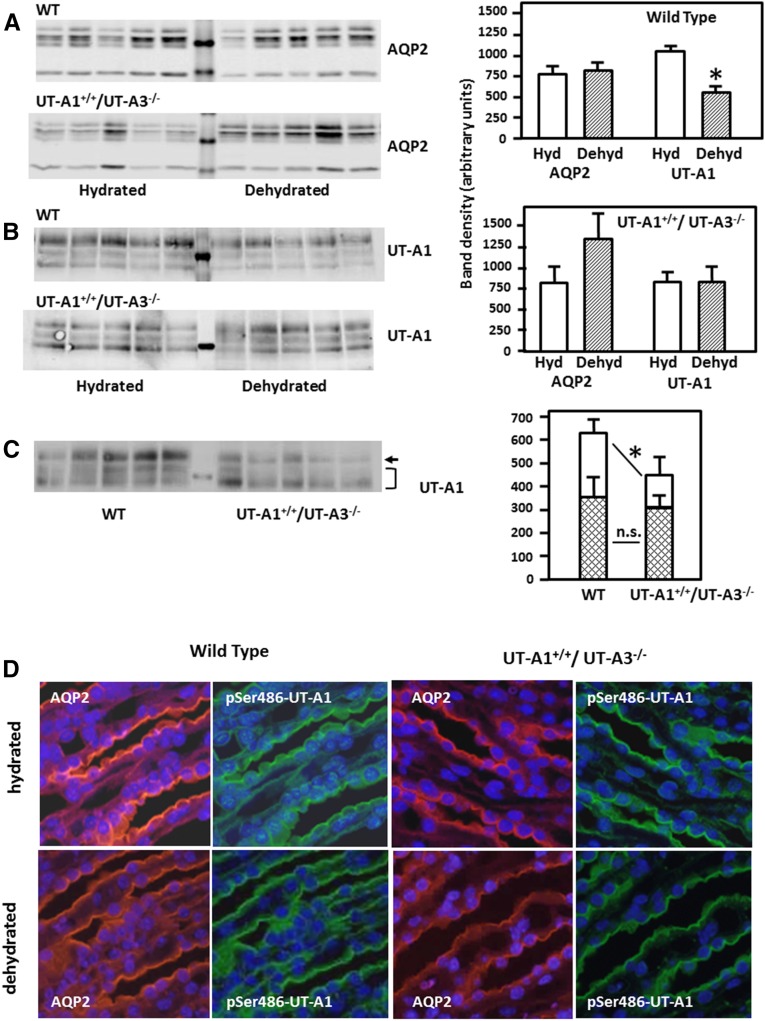
Comparison of AQP2 and UT-A1 in hydrated and dehydrated mice was performed by Western blot analysis (Figure 3). The abundance of AQP2 was not altered by 24 hours of dehydration in either WT or UT-A1+/+/UT-A3−/− mice (Figure 3A). UT-A1 also showed no statistically significant difference upon dehydration in UT-A1+/+/UT-A3−/− mice, but was decreased by dehydration in WT mice (Figure 3B). The decrease in UT-A1 in the WT mice is similar to the response in rats.29 Under basal conditions, there was no significant difference between total UT-A1 abundance in WT (1457±73) and UT-A1+/+/UT-A3−/− mice (1156±89). However, there was a significant decrease in the abundance of the 117-kDa glycoprotein band and a nonsignificant increase in the abundance of the 97-kDa glycoprotein band in the UT-A1+/+/UT-A3−/− mice compared with WT mice (Figure 3C). Immunofluorescence staining of kidneys from WT and UT-A1+/+/UT-A3−/− mice with antibodies against AQP2 and p486-UT-A1 was used to show the uniformity of UT-A1 distribution in the inner medulla. The data are presented in pairs of micrographs showing AQP2 staining on the left and p486-UT-A1 staining on the right (Figure 3D). From top to bottom, the pairs show WT mice under basal conditions, kidneys from WT mice that were dehydrated for 24 hours, UT-A1+/+/UT-A3−/− mice under basal conditions, and kidneys from dehydrated UT-A1+/+/UT-A3−/− mice. Comparison of AQP2, used as a marker for IMCDs, with p486-UT-A1 shows that all IMCDs in the UT-A1+/+/UT-A3−/− mice express UT-A1 in a distribution that is indistinguishable from WT mice. The staining also confirms that the orientation of p486-UT-A1 is at the apical plasma membrane of the IMCDs.
Figure 3.
UT-A1 levels in UT-A1+/+/UT-A3−/− and WT mice are comparable and unchanged with dehydration. WT and UT-A1+/+/UT-A3−/− mice were dehydrated for 24 hours, then kidneys from hydrated (Hyd) and dehydrated (Dehyd) mice were collected and dissected. Inner medullas were analyzed for AQP2 and UT-A1 content. Shown are representative Western blots and summary densitometries in bar graphs. (A) AQP2 in WT and UT-A1+/+/UT-A3−/− mouse inner medullas from normal and dehydrated mice (n=6/condition per group). (B) UT-A1 in the same mice. Representative Western blots are shown with average band densities provided in the bar graph; *P<0.05. (C) Western blot comparing levels of the 117- and 97-kDa glycoforms of UT-A1 in WT and UT-A1+/+/UT-A3−/− mice. Bar graphs show average band densities (±SEM), n=8 WT and 9 UT-A1+/+/UT-A3−/− mice. *P<0.05 for the 117-kDa band. The differences in density were not statistically significant (NS) for the 97-kDa band or for total UT-A1 abundance (not shown in figure). (D) Immunofluorescence staining of AQP2 (left micrographs, red) and p486-UT-A1 (right micrographs, green) in kidney slices from WT and UT-A1+/+/UT-A3−/− mice under normal hydrated and 24-hour dehydrated conditions. Slides were double-stained and images were collected using wavelength-specific filters. Nuclei were costained with 4',6-diamidino-2-phenylindole. Imaged with a 40× objective as described in Concise Methods.
Urea Permeability in the IMCD
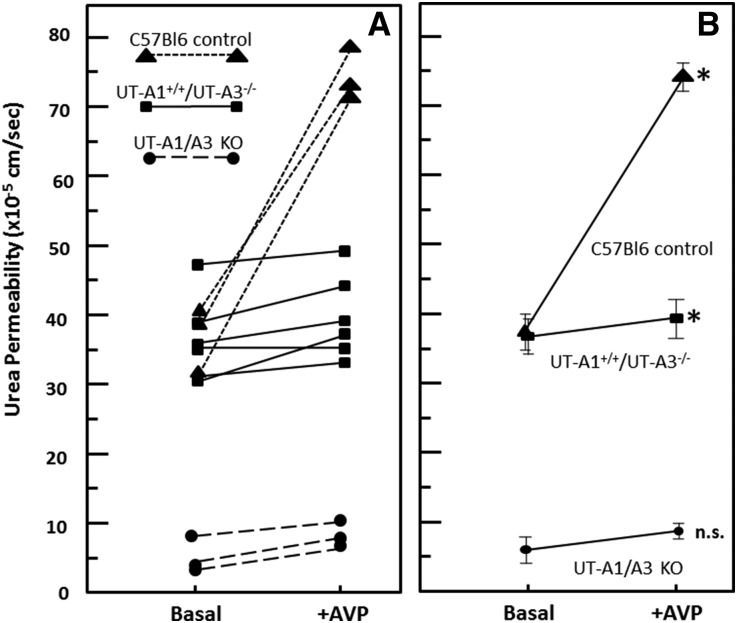
The urea permeability of IMCDs from WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice was measured in microdissected, isolated, perfused tubules. Figure 4A provides individual tubule responses and Figure 4B shows the average urea permeabilities with SEMs. In the WT mice (Figure 4, triangles), where both UT-A1 and UT-A3 are present, vasopressin (10−10 M) increased basal urea permeability by 100% (n=3, P<0.01). UT-A1/UT-A3 knockout mice had a very low basal urea permeability with no significant effect of vasopressin (n=3, Figure 4, circles). These values are consistent with those measured in the original line of UT-A1/UT-A3 knockout mice.4 The UT-A1+/+/UT-A3−/− mice had a significantly higher basal urea permeability (36.8±2.5×10−5 cm/s, n=6, Figure 4, squares) than the UT-A1/UT-A3 knockout mice (6.0±1.9×10−5 cm/s), and a similar basal urea permeability as WT mice (37.1±2.6×10−5 cm/s). The urea permeability of the UT-A1+/+/UT-A3−/− mice was slightly increased by vasopressin to 39.7±2.3×10−5 cm/s, although to a significantly lesser extent (8%) than the increase measured in WT mice with both UT-A1 and UT-A3 present (74.3±2.1×10−5 cm/s; 100% increase). Thus, the transgenic expression of UT-A1 in knockout mice lacking UT-A1 and UT-A3 restored basal urea permeability, but vasopressin was unable to stimulate urea permeability.
Figure 4.
UT-A1 alone supports basal but not vasopressin (AVP)-stimulated urea permeability. Shown is the urea permeability in isolated perfused tubules from WT (triangles), UT-A1+/+/UT-A3−/− (squares), and UT-A1/A3 knockout ([KO] circles) mice. (A) Each line represents the permeability results from a single animal. Control n=3; UT-A1+/+/UT-A3−/− n=6, and UT-A1/A3 KO n=3. Urea permeability was measured at basal levels, then 1×10−10 M AVP was added to the bath for 15 minutes and urea permeability was again measured. (B) Mean urea permeability (±SEM) in each genotype at basal and AVP-stimulated conditions. *P<0.05 by paired t test.
Urine-Concentrating Ability
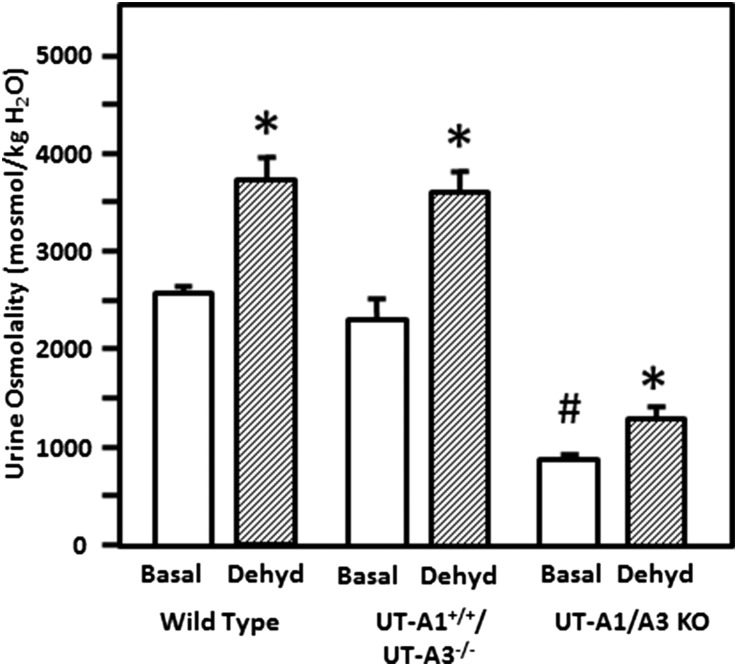
WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice were placed in metabolic cages and their urine was collected for 24 hours. Water was withheld for an additional 24 hours while urine was again collected. These determinations were performed many times over a period of 3 years, and urine osmolalities were stable in all three lines of mice over time. The bar graph in Figure 5 provides averaged values for osmolality with and without dehydration collected over this time period. In WT mice, urine osmolality was increased 47% by dehydration (n=12, P<0.01). UT-A1/UT-A3 knockout mice were unable to concentrate their urine above the basal (hydrated) level (n=19, p=NS), consistent with the findings in the original line of UT-A1/UT-A3 knockout mice.4 The UT-A1+/+/UT-A3−/− mice showed a 56% increase in urine osmolality in response to dehydration (n=19 hydrated, 45 dehydrated, P<0.001).
Figure 5.
Mice without the IMCD urea transporters (UT-A1/UT-A3 knockout [KO] mice) cannot concentrate their urine, but mice with transgenic expression of UT-A1 can concentrate urine (UT-A1+/+/UT-A3−/− mice). Urine was collected over 24 hours from mice following acclimatizing them in metabolic cages to determine basal urine osmolality (basal, open bars). Water was withheld and urine collected for an additional 24 hours to determine urine osmolality under maximal concentration conditions (dehydrated [dehyd], patterned bars) after which mice were returned to normal housing. Determinations were performed in mice over a period of 4 years and averaged in the bar graph. Osmolality is expressed as mosmoles/kg H2O. Data: mean±SEM, *P<0.05 for hydrated versus dehydrated in each group; #P<0.05 for KO hydrated versus WT hydrated; WT, n=12 hydrated, 19 dehydrated; UT-A1+/+/UT-A3−/−, n=12 hydrated, 45 dehydrated; UT-A1/UT-A3 knockout, n=21 hydrated and 19 dehydrated.
Urea excretion for a single experiment comparing eight animals per genotype per treatment is provided in Table 1. Dehydration for 24 hours caused urine volumes to decrease in all groups. The mmol of urea excreted per day per 100 g body wt was not statistically different between WT and UT-A1+/+/UT-A3−/− mice. UT-A1/A3 KO mice showed higher levels of urea excretion compared with WT or UT-A1+/+/UT-A3−/− mice under basal conditions (P<0.01).
Table 1.
Urine parameters for urea transporter–deficient mice
| Control | UT-A1+/+/UT-A3−/− | UT-A1−/−/UT-A3−/− | ||||
|---|---|---|---|---|---|---|
| Hydrated | Dehydrated | Hydrated | Dehydrated | Hydrated | Dehydrated | |
| 24-hour volume (ml) | 1.7±0.2 | 0.65±0.1 | 1.8±0.2 | 0.6±0.1 | 5.0±0.6 | 2.1±0.4 |
| Urine urea (mmol/L) | 1543±52 | 2229±209 | 1286±64 | 1968±188 | 799±124 | 1037±164 |
| Urea excretion/24-hour (mmoles) | 2.6±0.3 | 1.3±0.1a | 2.3±0.2 | 1.1±0.2a | 3.6±0.4 | 1.9±0.1a |
| Urea excretion (mmol/day/100 g body wt) | 8.9±1.0 | 5.2±0.5a | 7.6±0.7 | 4.2±0.7a | 11.6±1.2b | 6.7±0.3a |
Values are mean±SEM, n=8 mice per genotype per condition.
Significantly different from hydrated of the same genotype, P<0.05.
Significantly different from hydrated WT, P<0.05.
Effect of Chronic Vasopressin Administration
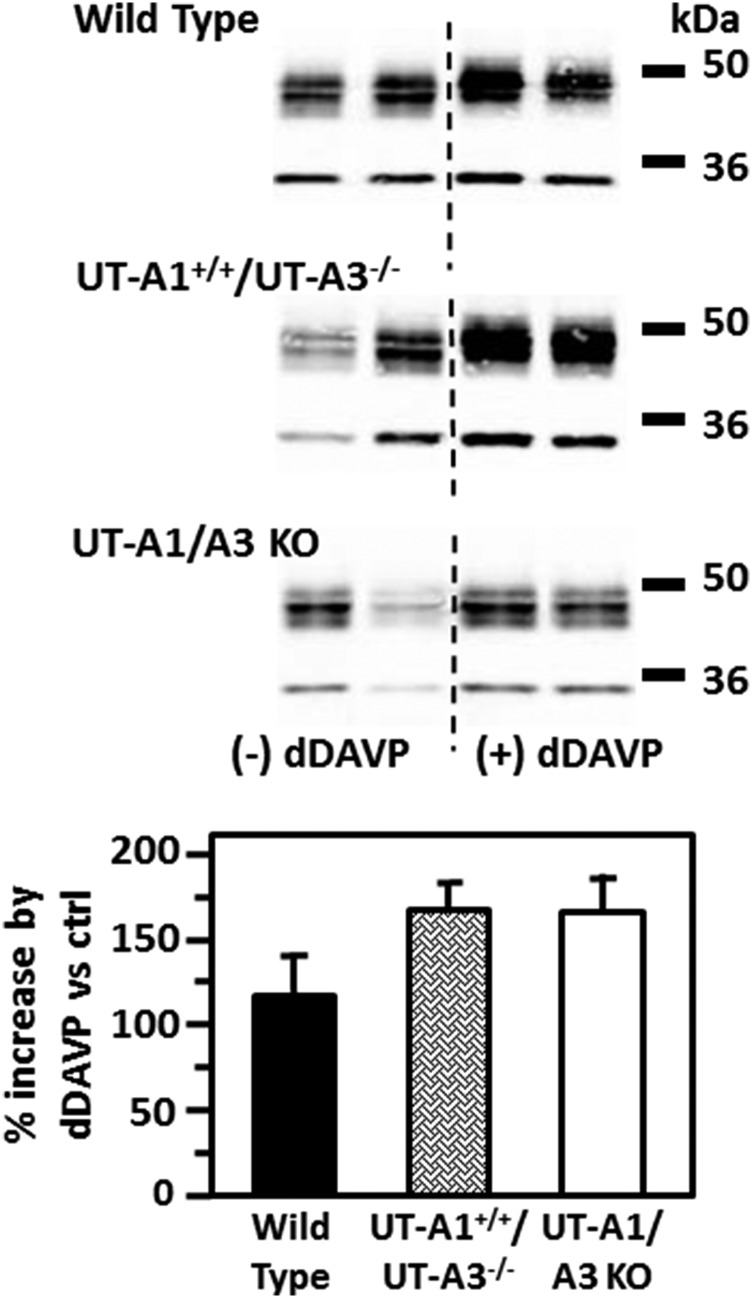
We previously showed that 7 days of vasopressin increased AQP2 water channel protein abundance to a similar degree in WT and UT-A1/UT-A3 knockout mice.7 To determine if the transgenic expression of UT-A1 in mice lacking UT-A1 and UT-A3 would alter the characteristic vasopressin-stimulated increase in AQP2 protein abundance, WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice were injected twice daily with desmopressin (dDAVP) for 3 days. AQP2 protein abundance was significantly increased to 174±22% in the inner medulla of UT-A1+/+/UT-A3−/− mice (P<0.01, n=8). The increase in AQP2 protein abundance following dDAVP was similar in WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice (Figure 6).
Figure 6.
AQP2 response to dDAVP is unaffected by the lack of UT-A3. (A) The representative Western blots show AQP2 in the inner medullas of WT (top), UT-A1+/+/UT-A3−/− (middle), or UT-A1/UT-A3 knockout ([KO] bottom) mice under basal (left lanes) and after 4 days of dDAVP injections (right lanes). The bar graphs show the percent increase in AQP2 protein levels resulting from dDAVP treatment. Data: mean±SEM, n=8.
Discussion
Urea transport across the IMCD is mediated by the two urea transporters expressed in this portion of the collecting duct, UT-A1 and UT-A3. Genetic knockout of both UT-A1 and UT-A3 results in a mouse with a very low urea permeability in the IMCD and a profound urine-concentrating defect.4–7 Because both UT-A1 and UT-A3 are knocked out, it is not possible to determine the contribution of each urea transporter to the reduction in urea permeability or urine-concentrating ability. One approach would be to genetically engineer mice in which only one of these urea transporters was knocked out. However, the structure of the UT-A gene precludes the selective knockout of only UT-A1 or UT-A3 using conventional approaches. In this study, we used a novel approach of engineering UT-A1 into a UT-A1/UT-A3 knockout mouse to generate a mouse expressing only UT-A1. We used a promoter, UT-A promoter 1, which has been shown to target specifically the IMCD.27 Using this approach, we were able to create a mouse that has transgenic expression of UT-A1 in knockout mice lacking UT-A1 and UT-A3 only in the IMCD, where it is normally expressed, but lacks UT-A3.
The UT-A1+/+/UT-A3−/− mouse has a basal urea permeability that is similar to WT mice. This suggests that transgenic expression of UT-A1 alone in mice lacking UT-A1 and UT-A3 is sufficient to restore the basal level of urea transport across the IMCD. We did not detect any difference in the qualitative localization of p486-UT-A1 between WT and UT-A1+/+/UT-A3−/− mice, nor was there any obvious heterogeneity of p486-UT-A1 expression between different IMCDs. Although there was a trend toward decreased UT-A1 abundance in the UT-A1+/+/UT-A3−/− mice, the average band density for UT-A1 in WT mice was not statistically different from the UT-A1 abundance in the UT-A1+/+/UT-A3−/− mice. However, there was a decrease in the abundance of the 117-kDa glycoform of UT-A1 (discussed below). Thus, the transgenic expression of UT-A1 in UT-A1/UT-A3 knockout mice does not recapitulate the native pattern of UT-A1 glycoprotein expression observed in WT mice. There was no difference in basal urea permeability or urine-concentrating ability between WT and UT-A1+/+/UT-A3−/− mice. These findings suggest that restoration of a normal level of basal urea transport by transgenic expression of UT-A1 in UT-A1/UT-A3 knockout mice is sufficient to produce concentrated urine.
In contrast, the UT-A1+/+/UT-A3−/− mouse has a markedly reduced level of vasopressin-stimulated urea permeability compared with WT mice. Vasopressin increased urea permeability by only 8%, as compared with doubling urea permeability in WT mice. The reason for the lack of an increase in urea permeability in response to vasopressin is not due to an inability of the kidney to respond to vasopressin, because AQP2 protein abundance and urine osmolality both increased following chronic vasopressin administration. One possible explanation is that the absence of UT-A3, which is the primary urea transporter in the basolateral membrane, changes the rate-limiting membrane from apical to basolateral. R. Star showed that phloretin-inhibitable urea transport is present in both the apical and basolateral membranes, but that the apical membrane was rate-limiting due to the smaller surface area.30 The transgenic expression of UT-A1 in the apical membrane, generating a highly permeable apical membrane, without UT-A3 in the basolateral membrane, may change the rate-limiting barrier to the basolateral membrane. If so, then it may not be possible for vasopressin to stimulate urea permeability above the basal level.
While UT-A3 is detected only in the basolateral membrane under basal conditions, it is detected in the apical membrane in vasopressin-treated animals.19 Thus, another possible explanation for the lack of a vasopressin-mediated increase in urea permeability is that UT-A3 in the apical membrane makes a key contribution to the vasopressin-stimulated increase in urea permeability. X-ray crystallographic studies of urea transporters from the bacterium Desulfovibrio vulgaris suggest that the functional unit is a homotrimer.31 By analogy, it has been suggested that the functional unit in the IMCD is a heterodimer of UT-A1 and UT-A3.31 We previously showed that UT-A1 and UT-A3 do not coimmunoprecipitate.19 The present findings also go against this hypothesis because basal urea permeability is similar in WT and UT-A1+/+/UT-A3−/− mice. We cannot rule out that UT-A1 and UT-A3 form a heterodimer only in the presence of vasopressin. If a heterodimer formed only in response to vasopressin, this would imply that UT-A1 alone is the functional unit under basal conditions, and that the functional structural unit for urea transport is different under basal compared with post vasopressin-stimulated conditions.
We previously suggested an association between the abundance of the 117-kDa glycoform of UT-A1 and the ability of vasopressin to stimulate urea transport.32 Thus, a third possible explanation for the lack of vasopressin stimulation of urea transport is the decrease in the abundance of the 117-kDa glycoform of UT-A1 in UT-A1+/+/UT-A3−/− mice. Current studies have been unable to determine the reason for the decrease in the 117-kDa glycoform, or whether it is related to the absence of UT-A3 or to the observation that transgenic expression of UT-A1 in knockout mice lacking UT-A1 and UT-A3 does not recapitulate the native pattern of UT-A1 glycoprotein expression observed in WT mice.
The restoration of nearly normal urine-concentrating ability in the UT-A1+/+/UT-A3−/− mouse, despite the lack of vasopressin-stimulation of urea transport, was surprising. One possible interpretation is that transgenic expression of UT-A1 alone results in sufficient urea reabsorption to facilitate nearly normal urine-concentrating ability. In other words, restoring basal urea permeability may be sufficient to restore nearly normal urine-concentrating ability following 24 hours of dehydration. It is possible that a more profound stress, such as a longer period of dehydration, may elicit a difference between WT and UT-A1+/+/UT-A3−/− mice. However, such prolonged studies were not permitted by our Institutional Animal Care and Use Committee.
In summary, we used a novel approach to engineer a mouse with transgenic expression of UT-A1 alone in knockout mice lacking UT-A1 and UT-A3 in the IMCD. Transgenic expression of UT-A1 restores basal urea permeability to the level measured in WT mice but does not restore vasopressin-stimulated levels of urea permeability. This suggests that transgenic expression of UT-A1 alone in mice lacking UT-A1 and UT-A3 is sufficient to restore urea-concentrating ability.
Concise Methods
Animals
All animal protocols and procedures were approved by the Emory University Animal Care and Use Committee. Mice were environmentally acclimated in metabolic cages for 24 hours, then urine was collected for another 24 hours. Finally, water was withheld for an additional 24 hours and urine was collected to determine urine-concentrating ability. Urine osmolality was determined using a Wescor vapor pressure osmometer.
Sample Preparation
In some cases, mice were treated with dDAVP, a V2-selective vasopressin agonist. WT, UT-A1+/+/UT-A3−/−, and UT-A1−/−/UT-A3−/− (UT-A1/A3 knockout) mice were injected subcutaneously, twice daily, for 3 days with dDAVP 8 μg/kg per day. Following euthanasia, organs were removed and homogenized in ice-cold isolation buffer containing triethanolamine 10 mM, sucrose 250 mM, pH 7.6, leupeptin 1 μg/ml, and PMSF 2 mg/ml, sheared through a 26G insulin syringe, and SDS added to a final concentration of 1% SDS for Western blot analysis of total cell lysate. The kidneys were removed and dissected into cortex, outer medulla, and inner medulla, then the tissue was prepared as described above. Total protein in each sample was measured by a modified Lowry method (Bio-Rad DC protein assay reagent, Bio-Rad, Richmond, CA).
Western blotting was performed as previously described.33 Proteins (20 μg/lane) were size-separated by SDS-PAGE, then electroblotted to polyvinylidene difluoride membranes as described previously.33 Blots were blocked with 5% nonfat dry milk in Tris-buffered saline ([TBS] 20 mM Tris HCl, 0.5 M NaCl, pH 7.5) at room temperature for 1 hour, then incubated with our polyclonal antibody to the C-terminus of UT-A1 overnight at 4°C.33 Blots were washed three times in TBS with 0.5% Tween-20 (TBS/Tween) and then incubated with Alexa Fluor 680-linked anti-rabbit IgG (Molecular Probes, Eugene, OR). Blots were washed two times with TBS/Tween, and then the bound secondary antibody was visualized using infrared detection with the Licor Odyssey protein analysis system.
Tubule Perfusion
Terminal IMCDs from WT, UT-A1+/+/UT-A3−/−, and UT-A1/UT-A3 knockout mice were collected, mounted, and perfused as described previously.21,34 To measure basal urea permeability, three collections were made 45 minutes after warming the tubules to 37°C. Next, vasopressin (10−10 M) or vehicle was added to the bath solution. After a 20-minute equilibration period, three collections were made. The collected solutions were assayed for urea content by ultramicrofluorometry. Urea flux was calculated as described previously.21
Immunohistochemistry
Mice were injected subcutaneously with vasopressin 45 minutes prior to perfusion and paraformaldehyde fixation, and paraffin embedding as described previously.7 Paraffin sections of 4-μm thickness were sliced. Sections were dewaxed and hydrated in preparation for immunostaining as previously described.35 Sections were incubated overnight at 4°C with the following primary antibodies: AQP2, c-terminal UT-A1, and MQ2 anti–UT-A3. Slides were washed free of primary antibody and then incubated for 2 hours in peroxidase-conjugated secondary antibody (donkey anti-rabbit IgG). Diaminobenzidine and 35% H2O2 were added to detect peroxidase activity identifying the primary antibodies. Slides were also stained with Mayers hematoxylin to visualize nuclei. Stained sections were visualized using a bright field on an Olympus IX71 inverted microscope. For immunofluorescence staining, the primary antibodies were goat anti-AQP2 (Santa Cruz Biotechnology) and rabbit anti–phospho-486-UT-A1 (prepared for our laboratory by Phosphosolutions). The fluorescent secondary antibodies were Alexa 546 donkey anti-goat IgG (Life Technologies) for AQP2 identification and Alexa 488 goat anti-rabbit IgG to identify anti–UT-A1. Visualization of immunofluorescence staining was accomplished with appropriate filter selections on the Olympus IX71 microscope. IMCDs were imaged with a 40× objective. Slices were double-stained for both AQP2 and UT-A1. All slides were costained with 4′,6-diamidino-2-phenylindole to identify nuclei.
Statistical Analyses
All data are presented as mean±SEM. A t test was used to test two groups. ANOVA was used to test more than two groups, followed by Fisher least significant difference (protected t test)36 to determine which groups were significantly different. The criterion for statistical significance was set as P<0.05.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health grants R21-DK91147 and R01-DK41707.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Fenton RA, Chou CL, Sowersby H, Smith CP, Knepper MA: Gamble’s “economy of water” revisited: studies in urea transporter knockout mice. Am J Physiol Renal Physiol 291: F148–F154, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Gamble JL, McKhann CF, Butler AM, Tuthill E: An economy of water in renal function referable to urea. Am J Physiol 109: 139–154, 1934 [Google Scholar]
- 3.Klein JD, Blount MA, Sands JM: Urea transport in the kidney. Compr Physiol 1: 699–729, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Fenton RA, Chou C-L, Stewart GS, Smith CP, Knepper MA: Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci U S A 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA: Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob VA, Harbaugh CM, Dietz JR, Fenton RA, Kim SM, Castrop H, Schnermann J, Knepper MA, Chou CL, Anderson SA: Magnetic resonance imaging of urea transporter knockout mice shows renal pelvic abnormalities. Kidney Int 74: 1202–1208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilori TO, Blount MA, Martin CF, Sands JM, Klein JD: Urine concentration in the diabetic mouse requires both urea and water transporters. Am J Physiol Renal Physiol 304: F103–F111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S: Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol 25: 7357–7363, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS: Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem 277: 10633–10637, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Verkman AS: Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B. Evidence for UT-B-facilitated water transport in erythrocytes. J Biol Chem 277: 36782–36786, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Klein JD, Sands JM, Qian L, Wang X, Yang B: Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol 15: 1161–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Lei T, Zhou L, Layton AT, Zhou H, Zhao X, Bankir L, Yang B: Role of thin descending limb urea transport in renal urea handling and the urine concentrating mechanism. Am J Physiol Renal Physiol 301: F1251–F1259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokko JP, Rector FC Jr: Countercurrent multiplication system without active transport in inner medulla. Kidney Int 2: 214–223, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Stephenson JL: Concentration of urine in a central core model of the renal counterflow system. Kidney Int 2: 85–94, 1972 [DOI] [PubMed] [Google Scholar]
- 15.Fenton RA, Knepper MA: Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 18: 679–688, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Pannabecker TL, Dantzler WH, Layton HE, Layton AT: Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sands JM, Layton HE: The urine concentrating mechanism and urea transporters. In: Seldin and Giebisch's The Kidney: Physiology and Pathophysiology, 5th Ed., edited by Alpern RJ, Caplan MJ, Moe OW, San Diego, CA, Academic Press, 2013, pp 1463–1510 [Google Scholar]
- 18.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW: Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM: Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Sands JM, Klein JD: Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Sands JM, Nonoguchi H, Knepper MA: Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol 253: F823–F832, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Bagnasco SM, Peng T, Janech MG, Karakashian A, Sands JM: Cloning and characterization of the human urea transporter UT-A1 and mapping of the human Slc14a2 gene. Am J Physiol Renal Physiol 281: F400–F406, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Fenton RA, Cottingham CA, Stewart GS, Howorth A, Hewitt JA, Smith CP: Structure and characterization of the mouse UT-A gene (Slc14a2). Am J Physiol Renal Physiol 282: F630–F638, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Nakayama Y, Naruse M, Karakashian A, Peng T, Sands JM, Bagnasco SM: Cloning of the rat Slc14a2 gene and genomic organization of the UT-A urea transporter. Biochim Biophys Acta 1518: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM: Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol 10: 230–237, 1999 [DOI] [PubMed] [Google Scholar]
- 26.You G, Smith CP, Kanai Y, Lee W-S, Stelzner M, Hediger MA: Cloning and characterization of the vasopressin-regulated urea transporter. Nature 365: 844–847, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Fenton RA, Shodeinde A, Knepper MA: UT-A urea transporter promoter, UT-Aalpha, targets principal cells of the renal inner medullary collecting duct. Am J Physiol Renal Physiol 290: F188–F195, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi T, Huang M, Gorman C, Jaenisch R: A generic intron increases gene expression in transgenic mice. Mol Cell Biol 11: 3070–3074, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terris J, Ecelbarger CA, Sands JM, Knepper MA: Long-term regulation of renal urea transporter protein expression in rat. J Am Soc Nephrol 9: 729–736, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Star RA: Apical membrane limits urea permeation across the rat inner medullary collecting duct. J Clin Invest 86: 1172–1178, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin EJ, Quick M, Zhou M: Crystal structure of a bacterial homologue of the kidney urea transporter. Nature 462: 757–761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pech V, Klein JD, Kozlowski SD, Wall SM, Sands JM: Vasopressin increases urea permeability in the initial IMCD from diabetic rats. Am J Physiol Renal Physiol 289: F531–F535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM: Glucocorticoids mediate a decrease in AVP-regulated urea transporter in diabetic rat inner medulla. Am J Physiol 273: F949–F953, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Klein JD, Fröhlich O, Sands JM: Role of protein kinase C alpha in hypertonicity-stimulated urea permeability in mouse inner medullary collecting ducts. Am J Physiol Renal Physiol 304: F233–F238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein JD, Murrell BP, Tucker S, Kim Y-H, Sands JM: Urea transporter UT-A1 and aquaporin-2 proteins decrease in response to angiotensin II or norepinephrine-induced acute hypertension. Am J Physiol Renal Physiol 291: F952–F959, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Snedecor GW, Cochran WG: Statistical Methods, Ames, IA, Iowa State University Press, 1980 [Google Scholar]