Abstract
Obesity and diabetes mellitus are the leading causes of renal disease. In this study, we determined the regulation and role of the G protein-coupled bile acid receptor TGR5, previously shown to be regulated by high glucose and/or fatty acids, in obesity-related glomerulopathy (ORG) and diabetic nephropathy (DN). Treatment of diabetic db/db mice with the selective TGR5 agonist INT-777 decreased proteinuria, podocyte injury, mesangial expansion, fibrosis, and CD68 macrophage infiltration in the kidney. INT-777 also induced renal expression of master regulators of mitochondrial biogenesis, inhibitors of oxidative stress, and inducers of fatty acid β-oxidation, including sirtuin 1 (SIRT1), sirtuin 3 (SIRT3), and Nrf-1. Increased activity of SIRT3 was evidenced by normalization of the increased acetylation of mitochondrial superoxide dismutase 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2) observed in untreated db/db mice. Accordingly, INT-777 decreased mitochondrial H2O2 generation and increased the activity of SOD2, which associated with decreased urinary levels of H2O2 and thiobarbituric acid reactive substances. Furthermore, INT-777 decreased renal lipid accumulation. INT-777 also prevented kidney disease in mice with diet-induced obesity. In human podocytes cultured with high glucose, INT-777 induced mitochondrial biogenesis, decreased oxidative stress, and increased fatty acid β-oxidation. Compared with normal kidney biopsy specimens, kidney specimens from patients with established ORG or DN expressed significantly less TGR5 mRNA, and levels inversely correlated with disease progression. Our results indicate that TGR5 activation induces mitochondrial biogenesis and prevents renal oxidative stress and lipid accumulation, establishing a role for TGR5 in inhibiting kidney disease in obesity and diabetes.
Keywords: diabetic nephropathy, obesity, metabolism, mitochondria, oxidative stress
Obesity and diabetes mellitus are the leading causes of renal disease.1–5 The pathogenesis of obesity and diabetes associated renal disease is multifactorial.6–14 In spite of all the beneficial interventions implemented in diabetic patients, including tight glucose and blood pressure control, renal disease still progresses in most patients.15,16 Additional treatments able to control pathogenic pathways involved in obesity and diabetic nephropathy (DN) are required.
Bile acids (BAs) act as signaling molecules that activate BA receptors which regulate BA homeostasis, as well as glucose homeostasis, lipid homeostasis, and energy expenditure. Two major receptors for BA have been identified: the nuclear receptor farnesoid X receptor (FXR), and the membrane-bound, G protein-coupled receptor TGR5. Expression and function of TGR5 are distinct from expression and function of FXR, although in some cases they are complementary.17,18 While the effects of TGR5 in the muscle, brown adipose tissue, immune cells and enteroendocrine cells have been studied,19–26 the role of TGR5 in the kidney is not known.
TGR5 (GPBAR1 or GPR131) has been identified as a membrane BA-activated G protein-coupled receptor (GPCR, class A).19,20 TGR5 is a member of the rhodopsin-like subfamily of GPCRs (Class A). TGR5 mRNA is expressed in the gall bladder, kidney, brown adipose tissue, liver, intestine, and selected areas of the central nervous system. TGR5 is activated by several BAs, lithocholic acid (LCA) being the most potent natural agonist with an EC50 of 530 nM.17,18
Upon ligand binding, TGR5 activation leads to the release of the Gαs subunit and activation of adenylate cyclase. The increase in intracellular concentration of cyclic AMP activates protein kinase A (PKA), which phosphorylates cAMP-response element binding protein (CREB). Activated CREB transactivates its target genes by binding to cAMP-response elements (CREs) contained in their promoter.17,18
In brown adipose tissue and muscle, BA-induced increases in cAMP lead to the activation of type 2 iodothyronine deiodinase (D2), which results in increased intracellular production of thyroid hormone. Activation of the thyroid receptor (TR) increases mitochondrial oxidative phosphorylation in muscle and uncoupling in brown adipose tissue, resulting in enhanced energy expenditure.24,27 TGR5 also increases PGC-1α expression, which is a master regulator of mitochondrial biogenesis and metabolism.24 In enteroendocrine cells, activation of TGR5 results in enhanced glucagon-like peptide 1 (GLP1) release, which induces insulin secretion and improves glucose homeostasis.17,22,23
TGR5 activation also has immunomodulatory effects, in particular on macrophage function. TGR5 agonists inhibit NF-κB, suppressing LPS-induced increases in IL-1α, IL-1β, IL-6, and TNF-α production.19,25 By reducing the inflammatory response and lipid loading in macrophages, TGR5 activation inhibits atherosclerosis.26,28
The purpose of the present study was to determine the expression and protective role of TGR5 in obesity-related glomerulopathy (ORG) and DN. Our studies indicate a novel role for TGR5 activation in inducing energy metabolism, mitochondrial biogenesis, and fatty acid oxidation in the kidney by activating AMPK, SIRT1, PGC-1α, ERRα, and SIRT3, which lead to prevention of oxidative stress and lipid accumulation, thus firmly establishing an important role for TGR5 in preventing kidney disease in obesity and diabetes.
Results
Treatment of Diabetic Mice with theTGR5-Specific Agonist INT-777 Prevents DN
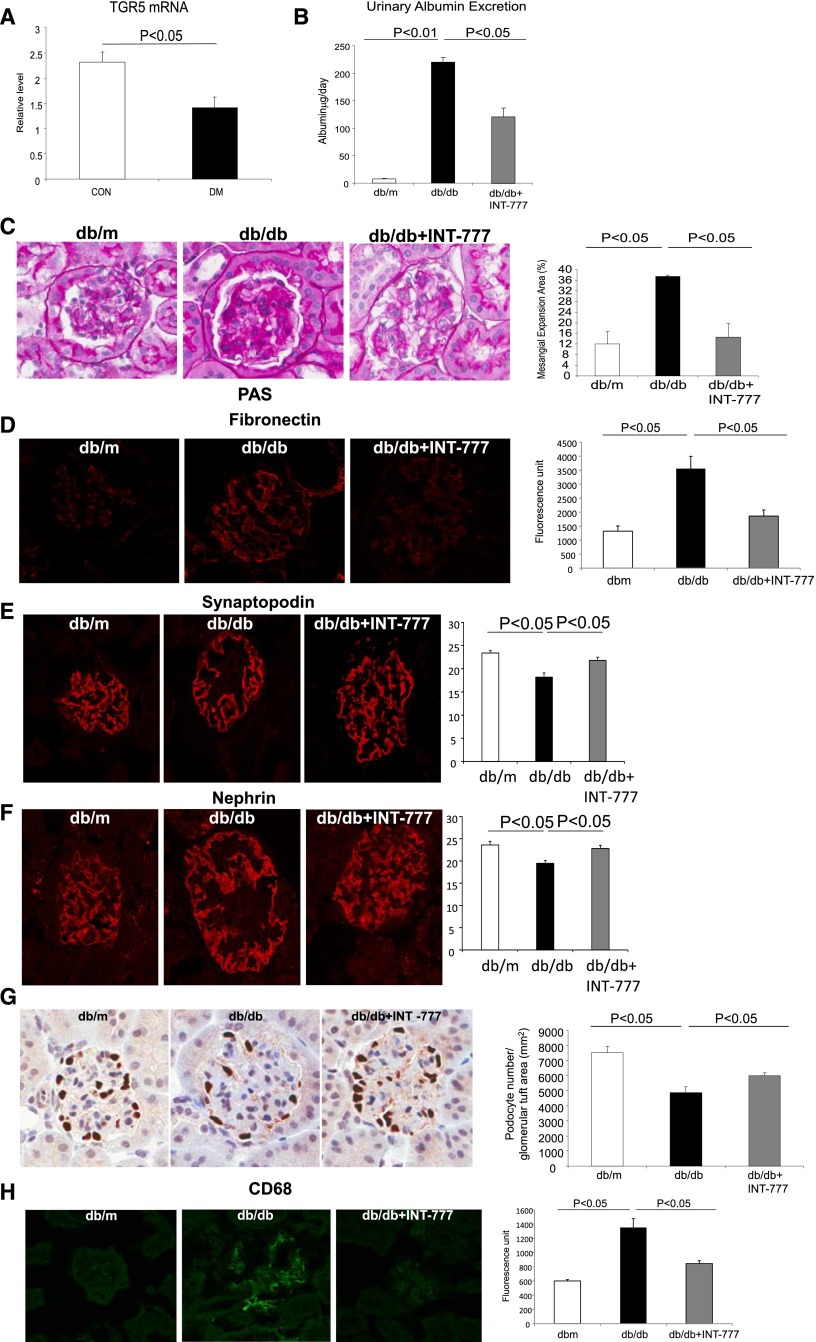
TGR5 mRNA expression is decreased in diabetic mice (Figure 1A). To explore the role of TGR5 signaling in diabetic kidney disease, we treated db/db mice with TGR5 agonist INT-777. INT-777 decreased urinary albumin excretion (Figure 1B), glomerular mesangial expansion (Figure 1C), accumulation of extracellular matrix proteins (Figure 1D), prevented glomerular podocyte injury and loss (Figure 1, E–G), and decreased macrophage accumulation in the kidney (Figure 1H). Thus, INT-777 prevented all of the major phenotypic characteristics of DN. These beneficial effects are independent of any alterations in plasma glucose, cholesterol, or arterial systolic blood pressure, but were associated with a marked decrease and normalization of plasma triglycerides (Table 1).
Figure 1.
TGR5 specific agonist INT-777 prevents DN in db/db mice. (A) TGR5 mRNA expression is decreased in the kidneys of mice with diabetes. (B) Urine albumin excretion is decreased with the treatment of INT-777 in db/db mice. (C) Periodic acid Schiff staining indicates marked mesangial expansion in db/db mice and treatment with INT-777 results in a significant decrease in mesangial expansion. (D) Fibronectin immunofluorescence microscopy indicates marked accumulation of glomerular matrix in db/db mice and treatment with INT-777 results in a significant decrease in fibronectin immunostaining. (E) Synaptopodin immunofluorescence microscopy indicates significant decrease in the percentage of immunostaining area in glomerular tufts in db/db mice, which is indicative of decreased podocyte density, and treatment with INT-777 results in preservation of synaptopodin positive area. (F) Nephrin immunofluorescence microscopy shows that the treatment with INT-777 restores the decreased percentage of podocyte marker nephrin positive area in glomerular tuft in db/db kidneys. (G) p57 immunohistochemistry indicates significant decrease in podocyte number in db/db mice, and treatment with INT-777 prevents the loss of podocyte number. (H) CD68 immunofluorescence microscopy indicates the decreased macrophage accumulation by the treatment with INT-777 in db/db kidneys.
Table 1.
Metabolic parameters in diabetic mice
| db/m | db/m + INT-777 | db/db | db/db + INT-777 | |
|---|---|---|---|---|
| Final body weight (g) | 33.4±0.43 | 31.1±0.83 | 38.6±1.76a | 36.3±3.09 |
| Percentage of body weight change | 13.4±1.32 | 10.6±3.69 | −12.8±1.58a | −14.8±5.5 |
| Kidney weight (g) | 0.16±0.004 | 0.14±0.005 | 0.19±0.005a | 0.18±0.008 |
| Plasma glucose (mg/dl) | 98±9 | 125±19 | 337±36a | 341±45 |
| Plasma TG (mg/dl) | 48.8±3.96 | 53.5±5.14 | 150.5±32.3a | 70.1±6.77b |
| Plasma TC (mg/dl) | 74.4±2.91 | 70.2±3.48 | 93.5±1.89a | 87.9±5.90 |
| Systolic blood pressure (mmHg) | 116±12 | 110±9 | 111±5 | 109±3 |
Data are means±SEM (n=6 mice in each group).
P<0.05 versus db/m.
P<0.05 versus db/db.
Treatment of Diabetic Mice with INT-777 Increases Renal Mitochondrial Biogenesis, Decreases Oxidative Stress, and Increases Fatty Acid β-Oxidation
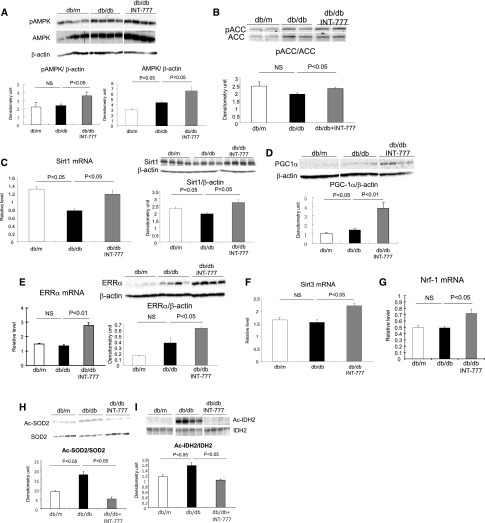
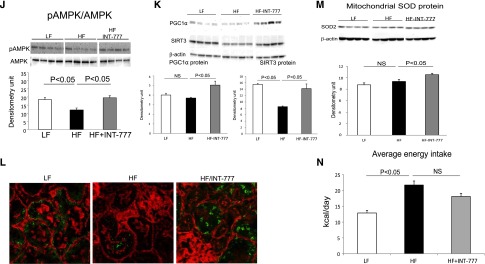
INT-777 induces significant increases in mRNA and/or protein abundance of (1) phospho-AMP kinase (p-AMPK) and phospho-ACC (Figure 2, A and B), (2) SIRT1 (Figure 2C), (3) PPARγ coactivator-1α (PGC-1α) (Figure 2D), (4) estrogen related receptor-α (ERRα) (Figure 2E), (5) SIRT3 (Figure 2F), and (6) nuclear respiratory factor 1 (Nrf-1) (Figure 2G). These are important mediators of mitochondrial biogenesis, oxidative stress, and fatty acid β-oxidation. SIRT3 activity is increased following treatment with INT-777, as manifested by the decreased acetylation of SIRT3 targets SOD2 (Figure 2H) or IDH2 (Figure 2I).
Figure 2.
Treatment of diabetic mice with INT-777 increases mediators of renal mitochondrial biogenesis. Representative QPCR or Western blot results show that the treatment with INT-777 induces significant increases in (A) phospho-AMPK and AMPK protein, (B) phospho-ACC protein, (C) SIRT1 mRNA and protein, (D) PGC-1α protein, (E) ERRα mRNA and protein, (F) SIRT3 mRNA, (G) Nrf1 mRNA, and SIRT3 activity as shown by the decreased acetylation of SIRT3 targets (H) SOD2 and (I) IDH2.
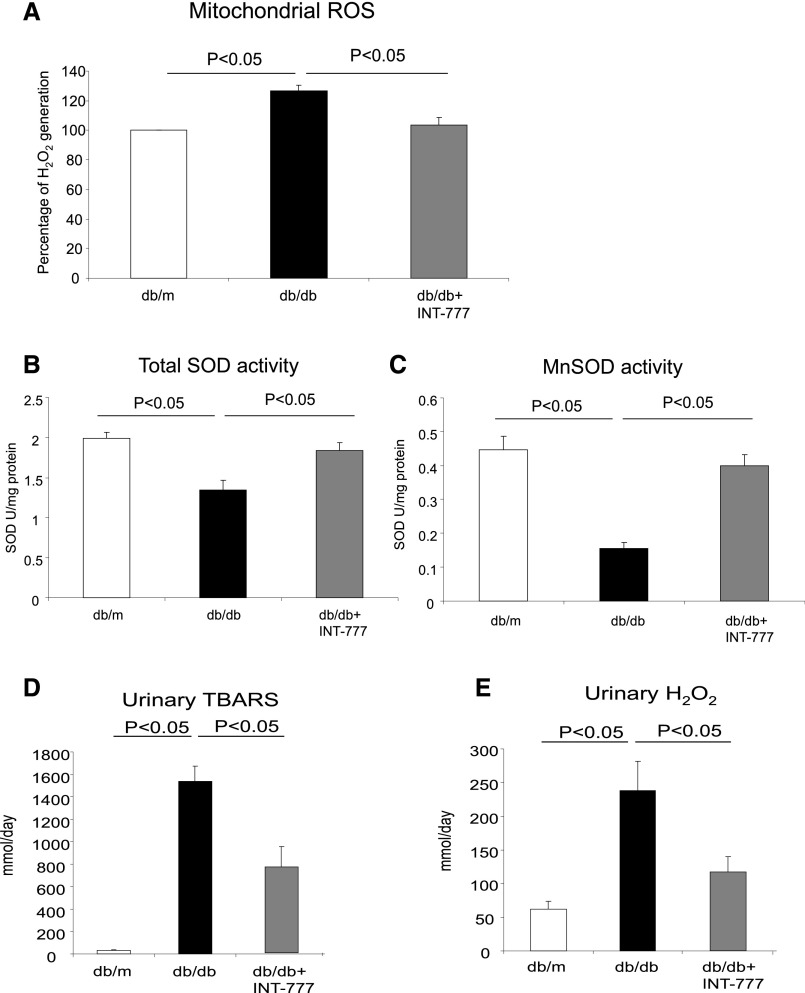
INT-777 (1) decreases mitochondrial ROS production (Figure 3A), and (2) increases total superoxide dismutase (SOD) (Figure 3B) and mitochondrial SOD2 (Figure 3C) activity. This is associated with significant decreases in urinary thiobarbituric acid reactive substances (TBARS) (Figure 3D) and urinary H2O2 excretion (Figure 3E), which are indicative of a decrease in overall renal oxidative stress.
Figure 3.
Treatment of diabetic mice with INT-777 decreases oxidative stress. (A) Mitochondrial ROS generation is increased in mitochondria isolated from db/db mice and treatment with INT-777 decreased mitochondrial ROS generation. (B) Total SOD activity is decreased in the kidneys of db/db mice and treatment with INT-777 increases total SOD activity. (C) Mitochondrial SOD activity is decreased in mitochondria isolated from db/db mice and treatment with INT-777 increases mitochondrial SOD activity. (D) Urinary TBARS excretion, a marker of oxidative stress, is increased in db/db mice and treatment with INT-777 decreases urinary TBARS excretion. (E) Urinary H2O2 excretion, a marker of oxidative stress, is increased in db/db mice and treatment with INT-777 decreases urinary H2O2 excretion.
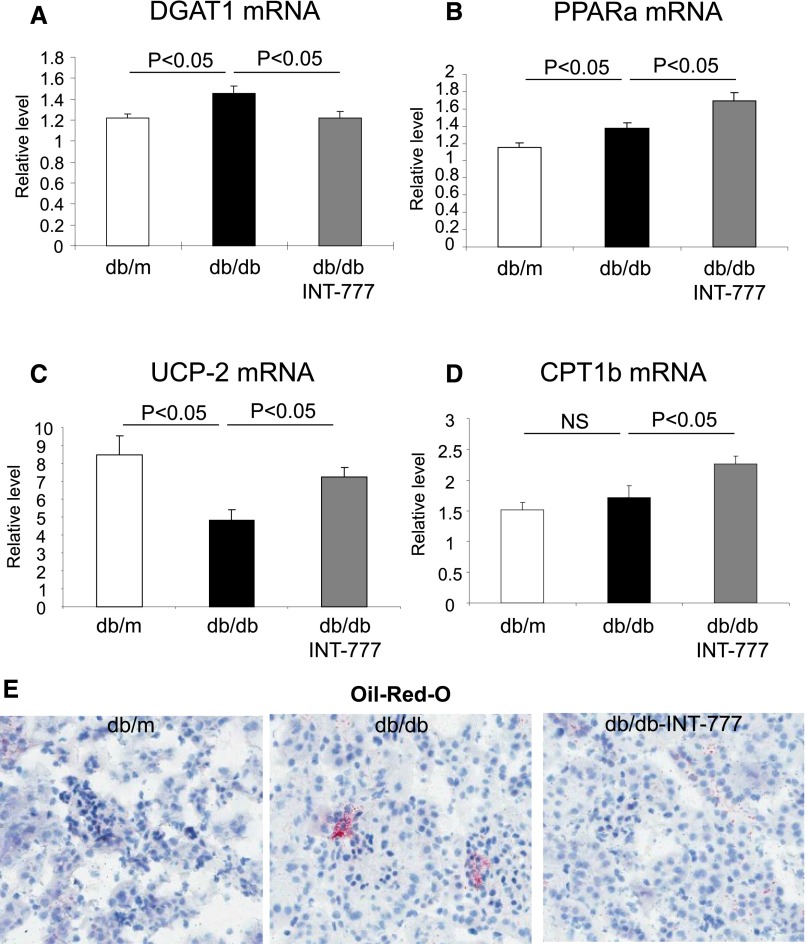
INT-777 also (1) decreases DGAT1 (Figure 4A), an enzyme critical for triglyceride synthesis, (2) increases PPAR-α (Figure 4B), a nuclear receptor critical for fatty acid oxidation, (3) increases uncoupling protein-2 (Figure 4C), and (4) increases carnitine palmitoyltransferase Ib (Figure 4D), which mediates fatty acid β-oxidation, resulting in decreased (5) renal neutral lipid accumulation (Figure 4E). These results are consistent with the capacity of INT-777 to reduce plasma triglycerides in diabetic mice (Table 1).
Figure 4.
Treatment of diabetic mice with INT-777 decreases lipid accumulation. (A) Representative images of oil red O staining, which stains for neutral lipids, show that neutral lipid accumulation in kidneys of db/db mice is prevented by the treatment with INT-777. The decrease in neutral lipid accumulation is associated with decrease in (B) DGAT1, mediator of triglyceride synthesis, and increases in (C) PPAR-α, (D) UCP-2, and (E) CPT1B, mediators of fatty acid synthesis.
Treatment of Mice with Diet-Induced Obesity with INT-777 Prevents Obesity-Associated Nephropathy
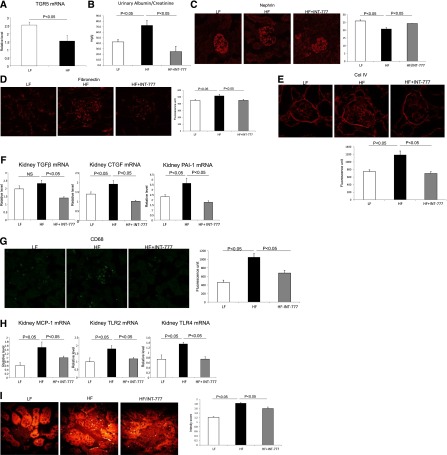
TGR5 mRNA expression is also decreased in the kidneys of DIO mice (Figure 5A). INT-777 treatment of DIO mice decreased urinary albumin (Figure 5B), podocyte injury (Figure 5C), extracellular matrix proteins fibronectin and type IV collagen accumulation (Figure 5, D and E), and profibrotic growth factors TGF-β, CTGF, and PAI-1 (Figure 5F), CD68 macrophages (Figure 5G), proinflammatory cytokine and receptors MCP-1, TLR2, and TLR4 (Figure 5H). INT-777 also decreased neutral lipid accumulation as determined by label-free coherent anti-Stokes Raman (CARS) microscopy (Figure 5I). INT-777 induced significant increases in p-AMPK (Figure 5J), PGC-1α (Figure 5K), and SIRT3 protein abundance as determined both by Western blotting (Figure 5K) and immunofluorescence microscopy (Figure 5L). Increased SIRT3 activity resulted in increased MnSOD protein abundance (Figure 5M).Therefore, similar to the actions of INT-777 in diabetic mice, INT-777 prevents the phenotypic manifestations of obesity-related renal disease in mice with DIO.
Figure 5.
TGR5 specific agonist INT-777 prevents nephropathy in diet-induced obesity mice. (A) TGR5 mRNA expression is decreased in the kidneys of mice with diet-induced obesity. (B) Urinary albumin excretion is increased in mice fed a high fat (HF) diet and treatment with INT-777 decreases albuminuria. (C) Nephrin immunofluorescence microscopy shows that the percentage of podocyte marker nephrin positive area in glomerular tuft, indicative of podocyte density, is restored by the treatment with INT-777 in db/db kidneys. (D) Fibronectin immunofluorescence microscopy indicates marked accumulation of glomerular matrix in HF mice and treatment with INT-777 results in a significant decrease in fibronectin immunostaining. (E) Type IV collagen immunofluorescence microscopy indicates that the marked accumulation of glomerular matrix in HF mice is significantly decreased by the treatment with INT-777. (F) Profibrotic growth factors TGF-β, CTGF, and PAI-1 expression are increased in HF diet and treatment with INT-777 results in marked decreases in the expression of these profibrotic growth factors. (G) CD68 immunofluorescence microscopy indicates significant increase in immunostaining in HF mice, which is indicative of increased macrophage accumulation, and treatment with INT-777 results in decrease of CD68 immunostaining. (H) Proinflammatory cytokines MCP-1, TLR2, and TLR4 expression are increased in HF diet and treatment with INT-777 results in marked decreases in the expression of these proinflammatory cytokines. (I) Coherent anti-stokes Raman scattering (CARS) microscopy imaging indicates increased lipid accumulation in the kidneys of HF mice and treatment with INT-777 results in decreased lipid accumulation. (J and K) Representative Western blots show that INT-777 treatment increases expression of mediators of mitochondrial biogenesis, including p-AMPK (J), PGC-1α and SIRT3 (K). Increased SIRT3 expression with the INT-777 treatment is further manifested by immunofluorescence (L, green SIRT3, red F-actin). (M) Treatment of HF mice with INT-777 induced increased mitochondrial SOD protein. (N) Average caloric intake shows no difference in HF mice with INT-777 treatment from HF mice without treatment.
In spite of similar caloric intake, average daily caloric intake low fat 12.8±0.8 versus low fat + INT-777 12.6±0.1, P=NS, high fat 21.7±1.3 versus high fat + INT-777 18.1±1.0, P=NS (Figure 5N), INT-777 prevented weight gain in DIO mice (Table 2). INT-777 also induced significant decreases in plasma triglyceride and cholesterol concentration (Table 2). The prevention of weight gain has been seen in previous studies as well21 and may be related to increased mitochondrial biogenesis in muscle and browning of adipose tissue.
Table 2.
Metabolic parameters in diet-induced obesity mice
| LF | LF + INT-777 | HF | HF + INT-777 | |
|---|---|---|---|---|
| Final body weight (g) | 29.5±0.29 | 28.6±0.48 | 51.9±0.54a | 40.7±1.43b |
| Percentage of body weight change | 23.0±1.31 | 22.2±3.14 | 121.6±4.54a | 62.0±5.64b |
| Kidney weight (g) | 0.17±0.007 | 0.16±0.005 | 0.22±0.007a | 0.20±0.010 |
| Plasma glucose (mg/dl) | 226±7 | 190±11a | 234±13 | 203±17 |
| Plasma TG (mg/dl) | 33.8±2.60 | 39.6±1.6 | 76.2±5.10a | 48.8±4.38b |
| Plasma TC (mg/dl) | 151.5±3.99 | 163.6±5.30 | 202.5±5.21a | 153.0±7.61b |
| Systolic blood pressure (mmHg) | 114±6 | 112±5 | 110±3 | 112±6 |
Data are means±SEM (n=6 mice in each group). LF, low-fat diet; HF, high-fat diet.
P<0.05 versus LF.
P<0.05 versus HF.
Endogenous TGR5 Modulates Renal Expression of PGC-1α, ERRα, and SIRT3
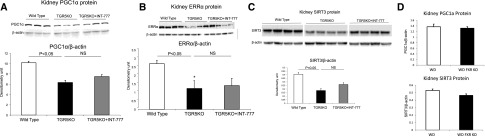
To determine if TGR5 per se modulates renal expression of PGC-1α, ERRα, and SIRT3, we analyzed the kidneys of TGR5 knockout (KO) mice. TGR5 KO mice have significant decreases in PGC-1α, ERRα, and SIRT3 protein expression (Figure 6, A–C). INT-777 did not induce upregulation of PGC-1α, ERRα, or SIRT3 in TGR5 KO mice (Figure 6, A–C). These results are consistent with specific TGR5-mediated signaling induced by INT-777. In addition, the regulation of these signaling processes in the kidney is specific to the bile acid activated G protein-coupled receptor TGR5 as the BA-activated nuclear receptor FXR KO mice have no alterations in PGC-1α or SIRT3 protein (Figure 6D).
Figure 6.
Endogenous TGR5 modulates renal expression of mediators in mitochondrial biogenesis and anti-oxidative stress. (A–C) Kidneys from TGR5 deficient mice express less PGC-1α protein (A), ERRα protein (B), and SIRT3 protein (C), compared with wild type mice. Treatment with INT-777 does not reverse the decreased expression in kidneys of TGR5 knockout mice. (D) In contrast, PGC-1α and SIRT3 protein are not altered in the kidneys of FXR knockout mice.
TGR5 Signaling Acts on Both Glomerular and Tubular Fractions Isolated from Diabetic Mice
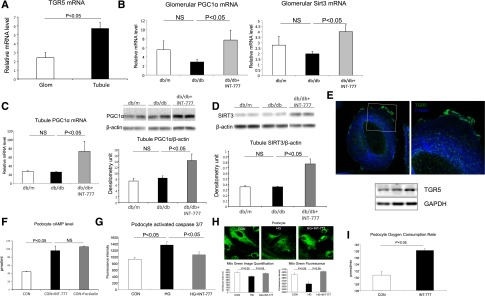
TGR5 mRNA is expressed in isolated glomeruli and tubules with a significantly higher level in the tubules (Figure 7A). To determine whether TGR5 acts in glomeruli versus tubules, we further checked the regulation of TGR5 targets in glomerular and tubular fractions isolated from db/db mice treated with INT-777. In glomeruli, we observed increased mRNA expression of PGC1α and SIRT3 (Figure 7B). A similar increase in PGC1α mRNA and protein (Figure 7C), as well as SIRT3 protein level, was found in the isolated tubule fraction (Figure 7D).
Figure 7.
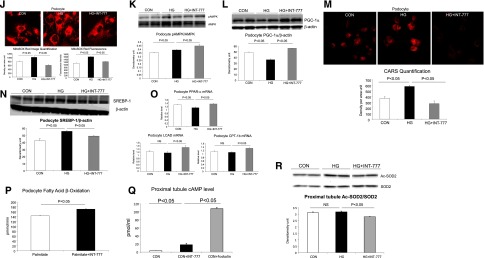
TGR5 signaling acts on both glomerular and tubular fractions. (A) mRNA level of TGR5 in isolated mouse glomeruli and tubules. (B) Treatment with INT-777 increases PGC1α and SIRT3 mRNA in isolated glomeruli from db/db mice. (C and D) Treatment with INT-777 increases PGC1α mRNA and protein level (C) and SIRT3 protein level (D) in the tubule fraction of db/db mice. (E) Both immunofluorescence microscopy and Western blot indicate the expression of TGR5 in cultured human podocytes. (F) INT-777 induces cAMP induction in cultured human podocytes. (G) Treatment with INT-777 prevents apoptosis, indicated by activated caspase 3/7, in human podocytes cultured with high glucose (HG) condition. (H) HG induces decrease in mitochondrial number as determined by Mito Green fluorescence staining, which is prevented by treatment with INT-777. (I) Treatment of HG podocytes with INT-777 increases oxygen consumption rate. (J) HG induces podocyte oxidative stress and ROS production as determined by MitoSox Red fluorescence staining, which is prevented by treatment with INT-777. (K) Treatment of podocytes with INT-777 increases p-AMPK protein. (L) HG induces decrease in PGC-1α protein, which is prevented by treatment with INT-777. (M) INT-777 prevents HG induced podocyte lipid accumulation as determined by CARS microscopy imaging. (N) HG induces increase in nuclear SREBP-1 protein, a transcriptional factor which is a master regulator of fatty acid and triglyceride synthesis, which is prevented by treatment with INT-777. (O) Treatment with INT-777 prevents HG induced decrease in PPAR-α, LCAD and CPT-1B, mediators of fatty acid β-oxidation. (P) Treatment of HG podocytes increases fatty acid β-oxidation rate. (Q) INT-777 induces cAMP induction in cultured human proximal tubule cells. (R) INT-777 increases SIRT3 activity in cultured human proximal tubule cells as shown by the reduction of acetylated SOD2.
TGR5 Signaling Modulates Response of Podocytes and Proximal Tubular Cells to High Glucose
To further confirm the glomerular and tubular actions of TGR5 signaling and to determine a direct effect of TGR5 activation in the kidney independent of any systemic actions, we studied cultured human podocytes and human proximal tubule cells with the INT-777 treatment, respectively. TGR5 protein is expressed in human podocytes (Figure 7E) and INT-777 induced a rapid increase of cAMP at levels comparable to those observed when using forskolin stimulation (Figure 7F). INT-777 prevented high glucose-induced mitochondrial apoptosis as determined by a decrease in activated caspase 3/7 (Figure 7G) and mitochondrial damage as determined by Mito Green staining (Figure 7H). INT-777 increased mitochondrial oxygen consumption rate (Figure 7I) and suppressed mitochondrial ROS production as determined by Mito Sox staining (Figure 7J). INT-777 also induced increases in mitochondrial p-AMPK (Figure 7K) and PGC-1α protein (Figure 7L). INT-777 prevented podocyte lipid accumulation as determined by label-free CARS imaging (Figure 7M), which was mediated by a significant decrease in sterol regulatory element-binding protein 1 (SREBP-1) (Figure 7N) that would decrease fatty acid synthesis, and an increase in PPAR-α, long-chain acyl-CoA dehydrogenase (LCAD), and carnitine palmitoyl transferase-1 (CPT-1) that would increase fatty acid oxidation (Figure 7O). Indeed, INT-777 treatment induced an increase in the oxygen consumption rate when bovine serum albumin-palmitate was used as a substrate (Figure 7P), which is indicative of increased fatty acid oxidation activity.
In cultured human proximal tubule cells, there is also rapid cAMP response to INT-777 (Figure 7Q). Increased TGR5 signaling led to the increased SIRT3 activity as shown by the reduction of acetylated SOD2 (Figure 7R).
TGR5 Signaling in Human Kidney Disease in Obesity and Diabetes
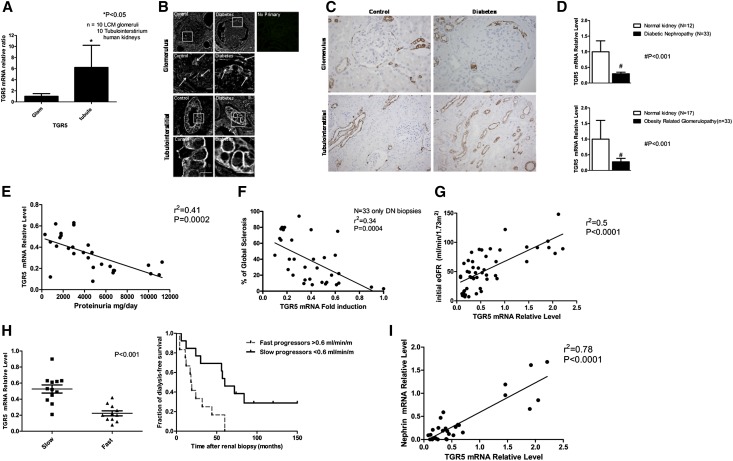
TGR5 is also expressed in human kidney, with much higher TGR5 mRNA expression in the tubules compared with glomeruli (Figure 8A). TGR5 protein is also expressed in the glomeruli and tubules as determined by immunohistochemistry (Figure 8B) and immunofluorescence microscopy (Figure 8C), using two different anti-human TGR5 antibodies. In spite of a significant decrease in TGR5 mRNA in the kidney biopsies of DN and ORG subjects (Figure 8D), we were unable to determine major and consistent differences in TGR5 protein level in glomeruli or tubules. There are several possible explanations for the discordant results in human diabetic tissue, with TGR5 mRNA being reduced while TGR5 protein appears unaffected by diabetes. These reasons include a biologic difference (e.g., prolonged protein half-life in diabetes compared with normal) and a methodologic difference (e.g., reduced dynamic range of immunohistochemistry compared with RT-PCR). Further, pathologic processes in humans are complex, and protein expression patterns may reflect underlying diseases, aging, co-morbidities, and the effects of medications. In addition, our inability to detect a change in glomerular or tubular TGR5 protein in spite of a decrease in TGR5 mRNA may be due to the specificity of the currently available antibodies.
Figure 8.
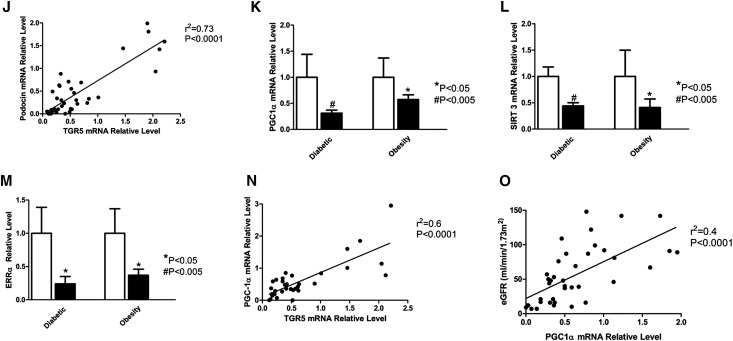
TGR5 signaling in human kidney disease in obesity and diabetes. (A) TGR5 mRNA expression in LCM acquired glomeruli and tubule-interstitium fraction from human kidney biopsies. (B) Immunofluorescence microscopy shows the TGR5 expression in human kidneys from both non-diabetic controls and diabetic subjects. The localization of TGR5 expression is found in both glomeruli and tubule-interstitium. Blow-up images show the staining in the boxed areas. Scale bar: 10 µm. (C) TGR5 immunohistochemistry using human kidney biopsies. (D) Human TGR5 mRNA is decreased in kidney biopsies from subjects with DN or ORG compared with subjects with normal kidneys. (E) TGR5 mRNA expression in the kidney biopsies is inversely correlated with proteinuria. (F) TGR5 mRNA expression in the kidney biopsies is inversely correlated with global sclerosis as determined by histology. (G) TGR5 mRNA expression in the kidney biopsies is directly correlated with initial eGFR. (H) TGR5 mRNA expression in the kidney biopsies correlates with rate of decline of eGFR, an indicator of renal disease progression. (I) TGR5 mRNA expression in the kidney biopsies is directly correlated with nephrin mRNA expression. (J) TGR5 mRNA expression in the kidney biopsies is directly correlated with podocin mRNA expression. (K–M) TGR5 signaling related molecules, PGC1α (K), SIRT3 (L), and ERRα (M) mRNA level is decreased in kidney biopsies from subjects with DN or ORG compared with subjects with normal kidneys. (N) TGR5 mRNA expression in the kidney biopsies is directly correlated with PGC1α mRNA expression. (O) PGC1α mRNA expression in the kidney biopsies is directly correlated with eGFR.
However, the decrease in TGR5 mRNA is significantly correlated with (1) level of proteinuria (Figure 8E), (2) glomerulosclerosis (Figure 8F), (3) initial eGFR (Figure 8G), and (4) follow-up eGFR and progression of kidney disease (Figure 8H). Definition of slow versus rapid progression of eGFR decline in relationship of initial TGR5 mRNA level is based on initial and follow-up eGFR. In the whole patient cohort, the median rate of GFR decline was 0.6 ml/min/month (Supplemental Table 1). To study the association of TGR5 mRNA with longitudinal eGFR decline, we analyzed TGR5 expression after classifying the patients into two groups: (A) slow progressors: eGFR decline below 0.6 ml/min/month versus (B) fast progressors: eGFR decline above 0.6 ml/min/month. Median time for starting dialysis was 18 months in the fast progressors group versus 60 months for the slow progressors group. On biopsy day the group’s demographic data were different only in HbA1c levels and age. The faster eGFR decline group showed significantly lower expression of TGR5 (Figure 8H). The decrease in TGR5 mRNA also correlated with decreases in (5) the glomerular podocyte markers nephrin and podocin (Figure 8, I and J).
In addition, the TGR5 targets PGC-1α, SIRT3, and ERRα that we identified in the mouse kidney, as well as human podocytes and proximal tubular cells, are also regulated in the human diabetic kidney, with significant decreases in PGC-1α (Figure 8K), SIRT3 (Figure 8L), and ERRα mRNA (Figure 8M). In fact the PGC-1α mRNA levels both correlate with TGR5 mRNA expression (Figure 8N) and initial eGFR (Figure 8O).
Discussion
Our data demonstrate decreased TGR5 mRNA expression, with an inverse correlation between TGR5 expression level and progression of kidney disease defined by decrease in eGFR, glomerulosclerosis, proteinuria, and direct correlation with expression of the podocyte markers nephrin and podocin. Studies in cultured human podocytes and glomerular endothelial cells indicate that high glucose and/or fatty acids, two metabolic perturbations common in diabetes and obesity, induce downregulation of TGR5, thus providing a potential cause and mechanism for decreased TGR5 expression in obesity and in diabetes.
While these studies indicate the relevance of TGR5 to human disease, they are only correlative and do not provide a direct cause and effect relationship. We therefore utilized two well-studied mouse models of obesity and diabetes to determine whether TGR5 agonists would have any utility in prevention of DN and ORG. As in human disease, we found that TGR5 mRNA expression is also decreased in mouse models of diabetes and obesity. To determine the role of TGR5 in these conditions, we used the specific TGR5 agonist INT-777 (6a-ethyl-23(S)-methyl-cholic acid), a semisynthetic cholic acid derivative with systemic bioavailability.29 INT-777, due to its relatively low intestinal absorption, can effectively activate TGR5 in enteroendocrine cells, triggering GLP-1 secretion, and because of its systemic biodistribution can activate TGR5 in different districts, including the kidney.30
INT-777 treatment in mice with DIO leads to enhanced mitochondrial function in muscle, brown adipose tissue, and enteroendocrine cells, resulting in increased energy expenditure and incretin secretion, and inducing a range of beneficial metabolic effects that include resistance to weight gain and hepatic steatosis, preservation of liver and pancreatic function, and maintenance of glucose homeostasis and insulin sensitivity.21 In addition, INT-777 exerts, via TGR5 activation, immune modulatory and anti-inflammatory effects leading to inhibition of macrophage NF-κB signaling and to prevention of atherosclerotic lesion formation.26,28
In db/db mice, treatment with INT-777 decreases albuminuria, mesangial expansion, extracellular matrix protein accumulation, podocyte loss, and macrophage infiltration. TGR5 activates AMPK, SIRT1, PGC-1α, ERRα, SIRT3, and Nrf-1, indicating activation of mitochondrial biogenesis.31–45 This is consistent with several observations in animal models and humans linking reduced mitochondrial function and oxidative phosphorylation to the development of type 2 diabetes.46 INT-777 also activates the TGR5-D2 signaling pathway, and robustly increases mitochondrial activity and oxidative phosphorylation.21 We found that INT-777 prevents diabetes-induced mitochondrial ROS generation, while it reverses the diabetes-induced decrease in superoxide dismutase activity. The net result is that INT-777 prevents diabetes-induced increases in urinary H2O2 and TBARS, well-established markers of oxidative stress. In addition, INT-777 also prevents lipid accumulation in the kidney by decreasing DGAT1, an enzyme involved in triglyceride synthesis, and increasing PPAR-α, an uncoupling protein-2, and CPT1β, an inducer of fatty acid oxidation and energy uncoupling.
Treatment of DIO mice with INT-777 decreased urinary albumin excretion, podocyte loss, accumulation of extracellular matrix proteins, expression of profibrotic growth factors TGFβ, CTGF, and PAI-1, accumulation of CD68 positive macrophages, and expression of proinflammatory cytokines MCP-1, TLR2, and TLR4. INT-777 also decreased accumulation of lipids and induced AMPK, PGC-1α, and SIRT3 expression. As AMPK activation has been found to markedly reduce glomerular TGF-β, collagen, and fibronectin accumulation in several mouse models of diabetic kidney disease,32 our findings suggest an important role for the anti-inflammatory and anti-fibrotic effects induced by INT-777 treatment in chronic kidney disease.
The role of TGR5 in regulating PGC-1α, ERRα, and SIRT3, important elements in the control of mitochondrial biogenesis, antioxidant generation, and fatty acid β-oxidation, is illustrated by the observation that PGC-1α, ERRα, and SIRT3 mRNA expression are decreased in the kidneys of patients with ORG and DN. We have found that PGC-1α, ERRα, and SIRT3 protein expression are also reduced in the kidneys of TGR5 KO mice. Additionally, treatment with INT-777 in TGR5 KO mice does not induce PGC-1α, ERRα, and SIRT3, further indicating the key role of TGR5 in their upregulation and the specificity of INT-777 as a TGR5 agonist.
In previous studies we had demonstrated that agonists of FXR, the BA-activated nuclear receptor, can prevent diabetes and obesity-related kidney disease.47–49 In contrast, the effects of streptozotocin-induced hyperglycemia in the kidney were markedly accentuated in FXR KO mice.49 The effects of TGR5 in the kidney, however, are distinct from FXR, as in FXR KO mice, unlike in TGR5 KO mice, we did not see regulation of PGC-1α or SIRT3, thereby differentiating TGR5 from FXR actions in the kidney.
Because beneficial effects of TGR5 agonism could be mediated by its multiple extrarenal effects to regulate metabolism,19–26 we also performed studies in human podocytes cultured in the presence of high glucose and treated with INT-777. INT-777 reproduced most of the effects seen in vivo. INT-777 increased mitochondrial biogenesis and podocyte mitochondrial oxygen consumption rate and decreased mitochondrial ROS production. INT-777 activated AMPK and PGC-1α, and prevented high glucose induced podocyte lipid accumulation. The prevention of lipid accumulation was mediated by inhibition of fatty acid synthesis mediated by SREBP-1 and activation of fatty acid oxidation mediated PPAR-α, LCAD, and CPT-1β. Accordingly, in podocytes INT-777 induced fatty acid oxidation as determined by increased oxygen consumption rate, when cultured in the presence of palmitate.
Mitochondrial dysfunction has been proposed to play a critical role in the pathogenesis and complications of type 2 diabetes mellitus and obesity.50–53 A recent urine metabolomics-based study revealed evidence for suppression of mitochondrial activity in diabetic kidney disease, as well as decreased expression of PGC-1α mRNA.51 Our results are in agreement with these findings and furthermore indicate that TGR5 is an important regulator of PGC-1α in the kidney. It needs to be determined whether TGR5 activates PGC-1α via a PKA and p-CREB signaling pathway, via activation of AMPK which phosphorylates and activates PGC-1α, or via activation of SIRT1 which deacetylates and activates PGC-1α.35
Previous studies have shown reduced PGC-1α levels in the diabetic kidney associated with reduced AMPK, reduced mitochondrial content, and reduced mitochondrial complex activity, and have demonstrated a beneficial effect of AMPK activation in preventing kidney disease in mouse models of obesity and diabetes.32,54 It is likely that these beneficial effects were mediated, at least in part, via activation of PGC-1α. PGC-1α is a master regulator of mitochondrial biogenesis. Our results in mice with diabetes and obesity and cultured human podocytes indicate that TGR5 stimulates the AMPK-SIRT1-PGC-1α axis, resulting in increased and even restored mitochondrial biogenesis.
TGR5 is also associated with activation of ERRα and SIRT3, a downstream target gene of PGC-1α, which have important effects in enhancing mitochondrial biogenesis, decreasing mitochondrial ROS generation and inducing fatty acid β-oxidation.39,45,55 Indeed, TGR5 activation in the kidney leads to decreased oxidative stress and lipid accumulation, effects also observed in cultured podocytes treated with INT-777.
In agreement with earlier studies, which have shown that TGR5 regulates energy metabolism and energy expenditure in the muscle,21,24 our studies also indicate that in spite of similar food intake, mice with high fat diet-induced obesity treated with INT-777 gain significantly less weight than their untreated counterparts. Our studies were not designed to measure energy expenditure in the kidney. However, the regulation of mitochondrial biogenesis in cultured podocytes, with increased mitochondrial respiration and fatty acid β-oxidation, suggests that TGR5 may also increase energy expenditure in the kidney.
In summary, our results indicate that TGR5 expression and activity is impaired in the kidneys of humans and rodents with obesity and diabetes. TGR5 activation induces mitochondrial biogenesis, while preventing renal oxidative stress and lipid accumulation, firmly establishing an important role for TGR5 activation in inhibiting kidney disease in obesity and diabetes.
Concise Methods
Animal Models: Diabetic Mice
Eight-week-old male db/m and db/db mice (BLKS/J genetic background) were obtained from Jackson Laboratories (Bar Harbor, ME). They were maintained on a 12-h light/12-h dark cycle. They were fed for 12 weeks a regular chow diet or the TGR5 agonist 6α-ethyl-23(S)-methylcholic acid (6-EMCA, INT-777), 30 mg/kg body weight/day, admixed with chow.21 One week prior to the end of the study the mice were placed in metabolic cages for a 24-h urine collection. At the end of the study period, following anesthesia, blood was drawn from the aorta for chemistry and the kidneys were rapidly removed and processed for histology and immunofluorescence microscopy, RNA extraction for real-time PCR (QPCR), protein extraction for Western blotting, or mitochondria isolation.
Diet-Induced Obesity Mice
Eight-week-old C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). They were maintained on a 12-h light/12-h dark cycle. They were fed for 12 weeks a low (10 kcal %) fat diet from Research Diets (D12450), or a high (60 kcal %) fat diet from Research Diets (D12492) supplemented with no addition or the TGR5 agonist 6α-ethyl-23(S)-methylcholic acid (6-EMCA, INT-777), 30 mg/kg body weight/day. At the end of the treatment period, mice were studied as above.
TGR5 KO Mice
TGR5 generalized KO mice and their littermates were a generous gift from Dr. Johan Auwerx and Dr. Kristina Schoonjans, Lausanne, Switzerland.21
FXR KO Mice
FXR generalized KO mice, on the C57BL/6J genetic background, originally generated by Frank Gonzalez,56 were obtained from The Jackson Laboratory (Bar Harbor, ME).
Animal studies and relative protocols adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of Colorado Denver.
Glomerular and Tubulo-Interstitial Cell Isolation from Mice
Mouse glomeruli were isolated from kidney cortex using the iron oxide injection method as described elsewhere.57 Those not collected for glomeruli in the purification process were saved as the tubular fraction. The purity of glomerular and tubular preparation was verified under microscopy as well as podocin (glomerular) and tubular (NaPi-2a) markers.
Human Kidney Samples
Formalin-fixed paraffin-embedded (FFPE) tissue specimen renal biopsy material was obtained from the archives of the Columbia Renal Pathology Laboratory and from pathologic archives of the Department of Pathology at Rabin Medical Center. Kidney samples were obtained from leftover portions of diagnostic kidney biopsies of patients with DN (n=34) or ORG (n=33) and normal kidneys (n=17).
Control biopsies were renal biopsies that appeared normal by histologic, immunofluorescence, and electron microscopic examination. These controls were either obtained from renal biopsies performed for minimal isolated proteinuria or hematuria or tissue from uninvolved portions of a kidney at the time of nephrectomy for tumor or from candidate renal donors.58 DN kidney biopsies were from Rabin Medical Center.
ORG was defined morphologically as focal segmental glomerulosclerosis and glomerulomegaly occurring in obese patients with a body mass index higher than 30 kg/m.59,60 Nine biopsy-proven ORG were from Rabin Medical Center archive and 24 were from Columbia University.
Total RNA was isolated using RNeasy FFPE columns (Qiagen, Valencia, CA). The manufacturer’s protocol was followed with the exception of increased (overnight) proteinase K digestion time. RNA quantity and quality were determined by measuring OD at 260 and 280 nm on a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA was converted to cDNA using RevertAid First Strand cDNA Synthesis Kit (Fermentas); cDNA was then amplified using TaqMan PreAmp Master Mix (Applied Biosystems) for 14 cycles of pre-amplification according to the manufacturer’s protocol using target gene assays (Applied Biosystems).
Candidate gene expression was analyzed by real-time RT-PCR, performed as described previously,61 using the TaqMan and SYBR system based on real-time detection of accumulated fluorescence (ABI Prism Step One; PerkinElmer, Foster City, CA). Fluorescence for each cycle was quantitatively analyzed by an ABI Step One sequence detection system (PerkinElmer). In order to control for variation in the amount of DNA that was available for PCR in the different samples, gene expression of the target sequence was normalized in relation to the expression of an endogenous control, 18S rRNA or RPLPO (large ribosomal protein).
Definition of Slow Versus Rapid Progression of eGFR Decline in Relationship of Initial TGR5 mRNA Level
This is based on initial and follow-up eGFR. In the whole patient cohort, the median rate of GFR decline was 0.6 ml/min/month (Supplemental Table 1). To study the association of TGR5 mRNA with longitudinal eGFR decline, we analyzed TGR5 expression after classifying the patients into two groups: (A) slow progressors: eGFR decline below 0.6 ml/min/month versus (B) fast progressors: eGFR decline above 0.6 ml/min/month. Median time for starting dialysis was 18 months in the fast progressors group versus 60 months for the slow progressors group. On biopsy day the group’s demographic data were different only in HbA1c levels and age on the biopsy day. The faster eGFR decline group showed significantly lower expression of TGR5 (Figure 8H).
Laser Capture Microdissection (LCM)
LCM was performed using the PALM Micro Beam instrument (PALM, Carl Zeiss, Germany). FFPE blocks used were cut into sections (5 μm thick) onto PALM membrane slides (PALM, Carl Zeiss), baked and deparaffinized with xylene, lightly stained with hematoxylin and eosin, and air-dried. All the glomeruli or the tissue surrounding the captured glomeruli were microdissected and captured on PALM adhesive cap tubes followed by total RNA extraction using the RNeasy FFPE Kit (Qiagen).
The study was approved by the Rabin Medical Center Institutional Ethics Committee and by the Columbia University Institutional Review Board.
Urine Chemistry
Urine albumin and creatinine concentrations were determined using kits from Exocell (Philadelphia, PA). Urinary H2O2 and TBARS level was measured using the kits from Thermo Fisher Scientific (Rockford, IL) and Bioassay Systems (Hayward, CA), respectively.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed as previously described.47–49,62–67 Primer sequences are listed in Supplemental Table 2.
Western Blotting
Western blotting was performed as previously described.47–49,62–67 The antibodies against PGC-1α (EMD Millipore, Billerica, MA), p-AMPK, p-ACC, AMPK, SIRT1, SIRT3 (Cell Signaling Technology, Danvers, MA), ERRα (Epitomics, Burlingame, CA), SOD2 (Enzo Life Sciences, Farmingdale, NY), acetylated SOD2,68 and acetylated IDH2 (Genetel Laboratories, Madison, WI) were used for Western blotting.
Mitochondria Isolation
Freshly excised kidneys were homogenized with a small Dounce homogenizer in isolation buffer (50 ml 0.1 M Tris-MOPS, 5 ml 0.1 M EGTA-Tris, 100 ml 1 M sucrose in 500 ml nanopure water, pH 7.4). The homogenate was centrifuged first at 800 g, 4°C for 10 min to remove tissue debris. The supernatant was centrifuged at 8000 g, 4°C for 10 min twice to obtain a mitochondrial pellet. The preparation was resuspended in a small amount of isolation buffer. The mitochondrial protein concentration was measured with a BCA protein assay kit (Pierce, Rockford, IL). The isolated mitochondria were used immediately for ROS measurements or were stored at −80°C until further analysis.
Mitochondrial ROS Measurement
H2O2 generation was used to measure the ROS in isolated mitochondria using Amplex Red (Life Technologies) as specified.69
SOD Activity Measurement
The total SOD and mitochondrial SOD activities were measured by using SOD Assay kit from Cayman Chemical (Ann Arbor, MI).
Histology Staining and Immunofluorescence Microscopy
Sections (2 μm thick) cut from 10% formalin-fixed, paraffin-embedded kidney samples were used for periodic acid-Schiff (PAS) staining and immunohistochemistry for p5770 (performed by Kelly Hudkins and Charles Alpers, University of Washington, Seattle). Frozen sections were used for oil red staining of neutral lipid (cholesterol esters and triglycerides) deposits or for immunostaining for fibronectin and type IV collagen (Sigma-Aldrich), nephrin (a gift from Dr. Lawrence Holzman, University of Pennsylvania, Philadelphia, PA), synaptopodin (Sigma-Aldrich), and CD68 (AbD Serotec, Raleigh, NC), and imaged with a laser scanning confocal microscope (LSM 510; Carl Zeiss, Jena, Germany). TGR5 immunofluorescence was performed on human renal biopsy sections (University of Colorado Denver) with antibody from R&D Systems (Minneapolis, MN) (catalog number MAB4286).
Immunohistochemical Staining
De-identified renal biopsy sections (Columbia University, John Hopkins University, and Rabin Medical Center (Petah Tikva, Israel)) were used under IRB guidelines. Briefly, staining was performed on 5 μm formalin-fixed paraffin-embedded sections. Heat-induced antigen retrieval (pH 9) and peroxide block was performed in preparation for incubation with anti-TGR5 rabbit polyclonal antibody (1:200 for 2 h; Sigma-Aldrich, product number HPA062890) and detection with DAB.
Quantification of Morphology
All quantifications were performed in a masked manner. Using coronal sections of the kidney, 30 consecutive glomeruli per mouse, six mice per group were examined for evaluation of glomerular mesangial expansion. The index of the mesangial expansion was defined as ratio of mesangial area/glomerular tuft area. The mesangial area was determined by assessment of the PAS-positive and nucleus-free area in the mesangium using ScanScope image analyzer (Aperio Technologies, Vista, CA). ImageJ software was used to quantify the synaptopodin or nephrin immunofluorescence by measuring the percentage of the positive staining area in glomerular tuft area. p57 staining quantification was as described and the glomerular tuft area was used as a denominator to assess podocyte number.71
Podocyte Culture
Human podocytes were maintained in RPMI 1640, 1% Insulin-Transferrin-Selenium, 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin at 33°C as previously described.72–77 Podocyte differentiation was induced by thermo-shifting the cells from 33°C to 37°C for 10 days. Podocytes were then treated for 48 h with control (10 mM glucose + 20 mM mannitol as osmotic control), high glucose (30 mM), or high glucose plus INT-777 (10 µM). Mouse podocytes (line AI) were cultured similarly, with the addition of 30 units/ml of mouse interferon gamma.78
Proximal Tubule Cell Culture
Human proximal tubules were maintained in RPMI 1640, 1% Insulin-Transferrin-Selenium, 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin at 33°C. Cell differentiation was induced by thermo-shifting the cells from 33°C to 37°C for 3–5 days. Proximal tubules were then treated for 48 h with 10 mM normal glucose control, high glucose (30 mM), or high glucose plus INT-777 (10 µM).
Mitochondrial Oxygen Consumption Rate
Measurements of mitochondrial oxygen consumption rate were performed on Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA). For these measurements 1.75 × 104 podocytes per well were seeded in 24-well microplates for 7 days. Baseline OCR was measured in control (vehicle) or INT-777 (10 μM or as indicated) treated cells. To determine fatty acid β-oxidation cells were treated with BAS-complexed palmitate (0.5 mM) followed by treatment with control or INT-777 (10 μM).
Podocyte Staining for Mitochondrial ROS and Mitochondrial Number
Human podocytes were cultured in 35 mm glass bottom culture dishes (MatTek Ashland, MA) for 10 days at 37°C. After culturing in the control or high glucose with or without INT-777 treatment, cells were incubated with (1) 200 nM Mitotracker deep green (Mito Green) (Life Technologies, Grand Island, NY) for 20 min to determine mitochondrial number or (2) 5 μM MitoSox Red (Life Technologies) for 10 min to determine mitochondrial ROS at 37°C before taking images with a laser scanning confocal microscope LSM 510 (Carl Zeiss, Jena, Germany). Alternatively, human podocytes seeded in black-wall clear-bottom 96-well plates for 10 days were used for direct fluorescence measurement of Mito Green or MitoSox Red staining with Synergy 2 Microplate Readers (BioTek, Winooski, VT).
CARS Microscopy
We performed CARS microscopy for label-free imaging of lipid deposits in podocytes as previously described.79–81 Briefly, the CARS images were acquired using our custom CARS-TPAF (two-photon autofluorescence) multimodal platform Olympus FV-1000 microscope (Olympus America, Center Valley, PA) coupled to a picoEmerald™ system (APE; Angewandte Physik und Elektronik, Berlin, Germany) which is internally pumped by a picosecond laser (High-Q Laser, Rankweil, Austria; part of Newport Corporation, Irvine, CA) generating two beams, approximately 5 W at 532 nm and 1 W at 1064 nm. The repetition rate is 80 MHz with a pulse width of approximately 6 ps. The 532 nm output feeds an optical parametric oscillator (OPO) that generates an 816 nm laser beam with a maximum of approximately 1 W of power. The generated 816 nm laser beam (pump and probe) is combined with the 1064 nm laser beam (Stokes) and sent into the microscope to excite the sample for imaging. The pump/probe wavelength was chosen to excite the CH2 Raman vibrational stretch at 2845 cm−1 present in lipids. The resulting CARS signal is at approximately 662 nm. A 60× 1.2 NA objective (U Plan S Apo 60× IR W, Olympus America, Center Valley, PA) is used to focus the laser beams onto the sample. The laser powers measured at the objective are typically <70 mW for both the 816 nm and 1064 nm laser beams depending on the power necessary to image the sample. The pump/probe power is set at twice the Stokes power because this laser must serve both the pump and probe functions in generating a CARS signal. An external non-descanned detector in the epi direction is used to measure the TPAF signal from the sample through a 420–520 nm emission filter (hq470/100m-2p; Chroma Technology, Bellows Falls, VT). The CARS signal is measured in the forward direction with another non-descanned external detector through a 640–680 nm emission filter (hq660/40m-2p; Chroma Technology). The pixel dwell time is 10 µs and the image pixel resolution is 1600 by 1600. A Kalman averaging filter set to three scans/line time is used during image acquisition to improve the signal-to-noise ratio of the acquired images.
Statistical Analysis
Results are presented as the means±SEM for at least three independent experiments. Data were analyzed by ANOVA and Student–Newman–Keuls tests for multiple comparisons or by t test for unpaired data between two groups. Statistical significance was accepted at the P<0.05 level.
Disclosures
Moshe Levi is a recipient of grant support from Intercept through the University of Colorado. Luciano Adorini is an employee of Intercept.
Supplementary Material
Acknowledgments
We thank Dr. Stephen Hewitt and Kris Ylaya (Experimental Pathology Laboratory, NCI at National Institutes of Health [NIH]) for IHC support.
These studies were supported by grants from NIH (1R01-DK098336, to M. Levi, X. Wang, Y. Luo, and E. Dobrinskikh), VA (1I01BX001954, to M. Levi, X. Wang, Y. Luo, and E. Dobrinskikh), NIH (U24-DK076169, to M. Herman Edelstein, V. D. D’Agati, and M. Levi), and Intercept (to M. Levi).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014121271/-/DCSupplemental.
References
- 1.Dietz WH: Implications of the energy gap for the prevention and treatment of childhood obesity. Am J Prev Med 42: 560–561, 2012 [DOI] [PubMed] [Google Scholar]
- 2.May AL, Kuklina EV, Yoon PW: Prevalence of cardiovascular disease risk factors among US adolescents, 1999-2008. Pediatrics 129: 1035–1041, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF: Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odermatt A: The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol 301: F919–F931, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 6: 2364–2373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobulescu IA: Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19: 393–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gurley SB, Coffman TM: The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27: 144–152, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Hunley TE, Ma LJ, Kon V: Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens 19: 227–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauer SM: Structural-functional correlations of diabetic nephropathy. Kidney Int 45: 612–622, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC 3rd: From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57: 1439–1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan XZ, Varghese Z, Moorhead JF: An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol 5: 713–721, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Weinberg JM: Lipotoxicity. Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Usui HK, Sharma K: Regulation of transforming growth factor beta in diabetic nephropathy: implications for treatment. Semin Nephrol 27: 153–160, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS: Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K: Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7: 678–693, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Tiwari A, Maiti P: TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today 14: 523–530, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M: A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K: Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714–719, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K: TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsuma S, Hirasawa A, Tsujimoto G: Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L: Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol 78: 617–630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J: Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R: The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45: 695–704, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K: TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 14: 747–757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianco AC, Kim BW: Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116: 2571–2579, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki-Anzai S, Masuda M, Levi M, Keenan AL, Miyazaki M: Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One 9: e108270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellicciari R, Sato H, Gioiello A, Costantino G, Macchiarulo A, Sadeghpour BM, Giorgi G, Schoonjans K, Auwerx J: Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J Med Chem 50: 4265–4268, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Roda A, Pellicciari R, Gioiello A, Neri F, Camborata C, Passeri D, De Franco F, Spinozzi S, Colliva C, Adorini L, Montagnani M, Aldini R: Semisynthetic bile acid FXR and TGR5 agonists: physicochemical properties, pharmacokinetics, and metabolism in the rat. J Pharmacol Exp Ther 350: 56–68, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Hardie DG, Ross FA, Hawley SA: AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K: AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finck BN, Kelly DP: PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handschin C, Spiegelman BM: Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Marcos PJ, Auwerx J: Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarpulla RC: Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813: 1269–1278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soriano FX, Liesa M, Bach D, Chan DC, Palacín M, Zorzano A: Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55: 1783–1791, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Villena JA, Kralli A: ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab 19: 269–276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranhotra HS: Estrogen-related receptor alpha and mitochondria: tale of the titans [published online ahead of print September 15, 2014]. J Recept Signal Transduct Res doi:10.3109/10799893.2014.959592 [DOI] [PubMed] [Google Scholar]
- 40.Huss JM, Torra IP, Staels B, Giguère V, Kelly DP: Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24: 9079–9091, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D: Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets 14: 622–636, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Houtkooper RH, Pirinen E, Auwerx J: Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenmoehl J, Hoeflich A: Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13: 755–761, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH: Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92: 1479–1514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bause AS, Haigis MC: SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 48: 634–639, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Kwak SH, Park KS, Lee KU, Lee HK: Mitochondrial metabolism and diabetes. J Diabetes Investig 1: 161–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M: Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56: 2485–2493, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M: The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol 297: F1587–F1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, Urbanek C, Solis N, Scherzer P, Lewis L, Gonzalez FJ, Adorini L, Pruzanski M, Kopp JB, Verlander JW, Levi M: Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59: 2916–2927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzameli I: The evolving role of mitochondria in metabolism. Trends Endocrinol Metab 23: 417–419, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK: Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24: 1901–1912, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng Z, Almeida FA: Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link. Cell Cycle 13: 890–897, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towler DA: Mitochondrial ROS deficiency and diabetic complications: AMP[K]-lifying the adaptation to hyperglycemia. J Clin Invest 123: 4573–4576, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D: Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ: Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Feng X, Lu TC, Chuang PY, Fang W, Ratnam K, Xiong H, Ouyang X, Shen Y, Levy DE, Hyink D, Klotman M, D’Agati V, Iyengar R, Klotman PE, He JC: Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, Eichinger F, Vining C, Berthier CC, Kretzler M, D’Agati VD: A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177: 1674–1686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD: Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Tobar A, Ori Y, Benchetrit S, Milo G, Herman-Edelstein M, Zingerman B, Lev N, Gafter U, Chagnac A: Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One 8: e75547, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U: Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55: 561–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang T, Liebman SE, Lucia MS, Li J, Levi M: Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int 68: 2608–2620, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Jiang T, Liebman SE, Lucia MS, Phillips CL, Levi M: Calorie restriction modulates renal expression of sterol regulatory element binding proteins, lipid accumulation, and age-related renal disease. J Am Soc Nephrol 16: 2385–2394, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M: Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Wang XX, Jiang T, Shen Y, Santamaria H, Solis N, Arbeeny C, Levi M: Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am J Physiol Renal Physiol 300: F801–F810, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M: Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54: 2328–2335, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Sun L, Halaihel N, Zhang W, Rogers T, Levi M: Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem 277: 18919–18927, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D: Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Starkov AA: Measurement of mitochondrial ROS production. Methods Mol Biol 648: 245–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin SV, Krofft RD, Pippin JW, Shankland SJ: Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int 67: 977–986, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Yanez D, Floege A, Lichtnekert J, Krofft RD, Liu ZH, Pippin JW, Shankland SJ: ACE-inhibition increases podocyte number in experimental glomerular disease independent of proliferation. J Renin Angiotensin Aldosterone Syst 16: 234–248, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P: Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: a model for diabetic podocytopathy. Diabetes 60: 1779–1788, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ: Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB: Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299: C464–C476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsumoto T, Hess S, Kajiyama H, Sakairi T, Saleem MA, Mathieson PW, Nojima Y, Kopp JB: Proteomic analysis identifies insulin-like growth factor-binding protein-related protein-1 as a podocyte product. Am J Physiol Renal Physiol 299: F776–F784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakairi T, Abe Y, Kopp JB: TGF-beta1 reduces Wilms’ tumor suppressor gene expression in podocytes. Nephrol Dial Transplant 26: 2746–2752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kajiyama H, Titus S, Austin CP, Chiotos K, Matsumoto T, Sakairi T, Kopp JB: Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol 29: 153–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim RS, Kratzer A, Barry NP, Miyazaki-Anzai S, Miyazaki M, Mantulin WW, Levi M, Potma EO, Tromberg BJ: Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J Lipid Res 51: 1729–1737, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim RS, Suhalim JL, Miyazaki-Anzai S, Miyazaki M, Levi M, Potma EO, Tromberg BJ: Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral CARS imaging. J Lipid Res 52: 2177–2186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suhalim JL, Chung CY, Lilledahl MB, Lim RS, Levi M, Tromberg BJ, Potma EO: Characterization of cholesterol crystals in atherosclerotic plaques using stimulated Raman scattering and second-harmonic generation microscopy. Biophys J 102: 1988–1995, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.