Abstract
High body mass index (BMI) is paradoxically associated with better outcome in hemodialysis (HD) patients. Persistent inflammation commonly features in clinical conditions where the obesity paradox is described. We examined the relationship between BMI and mortality in HD patients, accounting for inflammation, in a historic cohort study of 5904 incident HD patients enrolled in 2007–2009 (312 facilities; 15 European countries) with ≥3 months of follow-up. Patients were classified by presence (n=3231) or absence (n=2673) of inflammation (C-reactive protein ≥10 mg/l and/or albumin ≤35 g/l). Patients were divided into quintiles by BMI (Q1–Q5: <21.5, 21.5–24.0, >24.0–26.4, >26.4–29.8, and >29.8 kg/m2, respectively). Noninflamed patients in BMI Q5 formed the reference group. During a median follow-up period of 36.7 months, 1929 deaths occurred (822 cardiovascular), with 655 patients censored for renal transplantation and 1183 for loss to follow-up. Greater mortality was observed in inflamed patients (P<0.001). In fully adjusted time-dependent analyses, the all-cause mortality risk in noninflamed patients was higher only in the lowest BMI quintile (hazard ratio [HR, 1.80; 95% confidence interval [95% CI], 1.26 to 2.56). No protective effect was associated with higher BMI quintiles in noninflamed patients. Conversely, higher BMI associated with lower all-cause mortality risk in inflamed patients (HR [95% CI] for Q1: 5.63 [4.25 to 7.46]; Q2: 3.88 [2.91 to 5.17]; Q3: 2.89 [2.16 to 3.89]; Q4: 2.14 [1.59 to 2.90]; and Q5: 1.77 [1.30 to 2.40]). Thus, whereas a protective effect of high BMI was observed in inflamed patients, this effect was mitigated in noninflamed patients.
Keywords: chronic dialysis, chronic inflammation, obesity
The impact of obesity and high body mass index (BMI) in disease processes is demonstrably more complicated than previously appreciated. Although obesity increases disease prevalence and has well documented devastating consequences, emerging data show that a high BMI is associated with a paradoxic survival advantage in the very same chronic debilitating diseases that it is a risk factor for. The ‘obesity paradox’ has been observed in ESRD1,2 as in earlier CKD stages,3 coronary heart disease,4 stroke,5 rheumatoid arthritis,6 type II diabetes,7 cancer,8 dementia,9 and congestive heart failure.10 Epidemiologic studies supporting the obesity paradox have been criticized11 as most are cross-sectional and do not account for obesity quantity or type, muscle mass, disease severity, or comorbidities. It was recently suggested that the obesity paradox results from methodologic pitfalls, such as population selection bias, lack of control for the severity of associated diseases,12 confounding by an underweight and sicker reference population, and by smoking.13
Nevertheless, based on findings on the relation between BMI and outcome, two important and relevant questions arise. First, by which mechanisms does high BMI mediate a survival advantage? Second, should weight gain and obesity be promoted in the patient groups where the obesity paradox exists? As persistent low-grade inflammation is a common feature in all the chronic debilitating diseases where the obesity paradox has been reported, its impact on the association between BMI and outcome needs further consideration. In this study we evaluated whether higher C-reactive protein (CRP) and/or lower serum albumin (denoted as inflammation) would affect the documented association between high BMI and better outcome in dialysis patients.
Results
Patient Characteristics
Baseline characteristics are presented in Supplemental Table 1. As expected, the presence of inflammation (n=3231: CRP≥10 mg/l n=2559; serum albumin ≤35 g/l n=1622; both n=950) was associated with older age, more catheter use, more prevalent cardiovascular disease (CVD) and cancer history, more hospitalization, lower hemoglobin, lower creatinine, and higher ferritin levels. Included patients were broadly similar to excluded patients (Supplemental Table 1). Patients were divided into quintiles by BMI (Q1–Q5: <21.5, 21.5–24.0, >24.0–26.4, >26.4–29.8, and >29.8 kg/m2, respectively). Noninflamed patients in BMI Q5 formed the reference group. Dialysis vintage (median days [Q1, Q3]) was similar among included (5 [1, 16]) and excluded (5 [1, 19]) patients, and by inflamed (6 [1, 17]) and noninflamed (5 [1, 15]) status among included patients. As a large proportion of patients were excluded for missing BMI, CRP, or serum albumin values it is unsurprising that excluded patients were more likely to have missing data for other observed parameters. Of note, no BMI differences were observed across groups. Mortality rates in all included and excluded patients, and by inflammatory status in included patients, are provided in Supplemental Table 2.
Baseline-Stratified Statistical Analysis
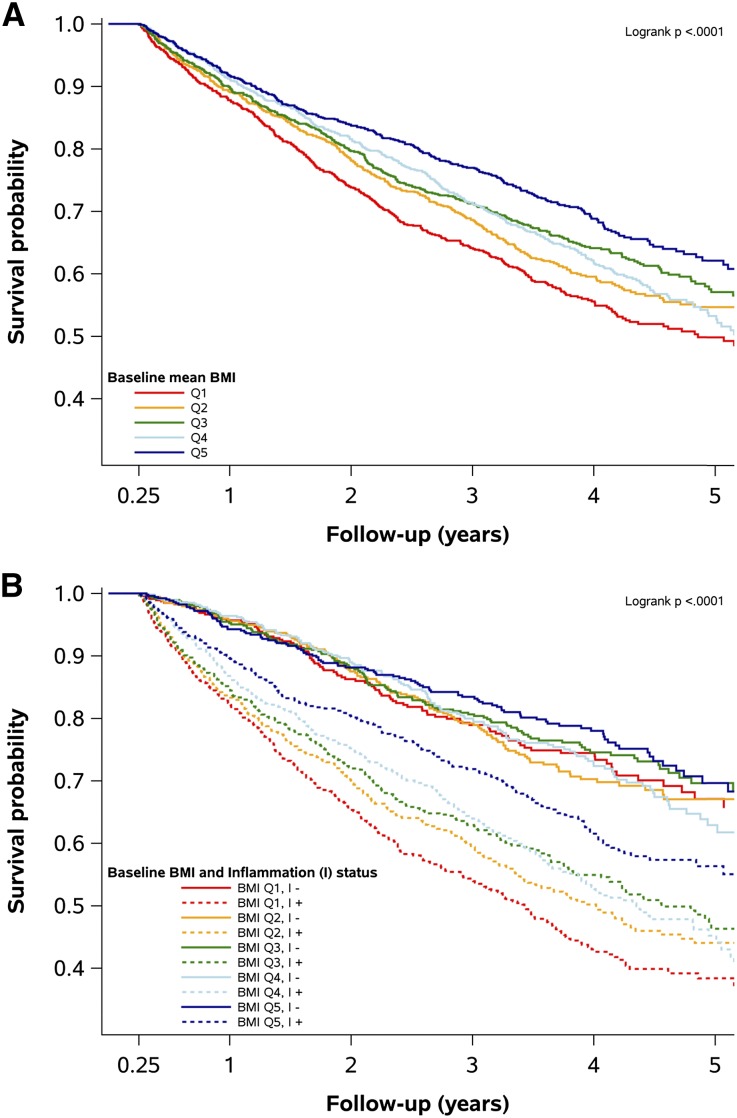
The relationship between baseline BMI levels and survival irrespective of inflammation status is depicted in Figure 1A. Separation of strata was apparent within the first year and continued throughout follow-up. Changes were more apparent in the extremes, with poorer survival in the middle quintiles (Q2–Q4) relative to the highest BMI quintile (Q5) from approximately 18 months, and poorer survival in the lowest quintile compared with all other patients from around 12 months.
Figure 1.
Baseline survival analysis. (A) Kaplan Meier curves showing all-cause mortality by basal BMI in Q1–5. (B) Same analysis stratified by inflammation status showing how it affects the relationships between BMI and outcome.
The relative survival by inflammation status is shown in Supplemental Figure 1. Poorer survival in inflamed patients was apparent almost immediately during follow-up. The relationship between BMI and mortality was markedly different depending on inflammation status (Figure 1B): mortality in those without inflammation was no different across BMI strata, and this was confirmed by Cox proportional hazard modeling (Supplemental Table 3, nonadjusted model 0), while a near-linear relationship between decreasing BMI and mortality was observed for patients with baseline inflammation. Compared with noninflamed patients in Q5, the hazard for inflamed patients in the same quintile was 79% higher, whereas for inflamed patients in Q1 it was over 300% higher. Mortality rates were attenuated with increasing adjustment but the overall hazard ratios (HRs) were largely maintained (Supplemental Table 3, adjusted models 1 and 2). A residual effect of BMI on mortality was observed in noninflamed patients in the partially and fully adjusted baseline analysis, with a significantly higher HR in patients in Q1 when compared with Q5.
Time-Dependent Statistical Analysis
While the proportional hazards assumption held for BMI (Supplemental Figure 2A), the effect of inflammation varied with time (Supplemental Figure 2B), necessitating a time-dependent approach. The above relationships were enhanced (Table 1). Compared with noninflamed patients, inflamed patients in the highest BMI quintile (Q5) experienced a 77% increased hazard over the study period; for those in Q1 the relative HR was over 500%. The residual effect of BMI on noninflamed patients described above was also apparent. The complete separation of mortality rates in the unadjusted model was maintained when the model was adjusted for demographic factors (model I) and rates were significantly higher for the three lowest BMI strata (Q1–Q3) in the fully adjusted model II (Figure 2A). A sensitivity analysis (unadjusted time-dependent all-cause mortality) where either CRP (>10 mg/L) or serum albumin (≤35 g/L) alone, defined inflammation showed no major differences in HRs compared with the combined group (Supplemental Table 4).
Table 1.
All-cause mortality by BMI and inflammation; time-dependent Cox regression analysis
| Model | BMI Quintile (kg/m2) | Hazard Ratio (95% Confidence Interval) | |
|---|---|---|---|
| Inflammation Present | Inflammation Absent | ||
| 0 | 1 (<21.5 kg/m2) | 10.83 (8.22 to 14.26)a | 1.51 (1.06 to 2.14) |
| 2 (21.5–24.0 kg/m2) | 7.21 (5.43 to 9.57)a | 0.95 (0.66 to 1.39) | |
| 3 (24.1–26.4 kg/m2) | 5.03 (3.76 to 6.72)a | 1.04 (0.73 to 1.49) | |
| 4 (26.5–29.8 kg/m2) | 3.67 (2.72 to 4.95) | 0.97 (0.68 to 1.40) | |
| 5 (>29.8 kg/m2) | 2.96 (2.19 to 4.01) | 1 | |
| I | 1 (<21.5 kg/m2) | 10.02 (7.58 to 13.24)a | 1.75 (1.23 to 2.49) |
| 2 (21.5–24.0 kg/m2) | 6.39 (4.80 to 8.51)a | 1.01 (0.70 to 1.47) | |
| 3 (24.1–26.4 kg/m2) | 4.55 (3.40 to 6.10)a | 1.07 (0.75 to 1.53) | |
| 4 (26.5–29.8 kg/m2) | 3.21 (2.38 to 4.33) | 0.94 (0.65 to 1.35) | |
| 5 (>29.8 kg/m2) | 2.78 (2.05 to 3.76) | 1 | |
| II | 1 (<21.5 kg/m2) | 5.63 (4.25 to 7.46)a | 1.80 (1.26 to 2.56) |
| 2 (21.5–24.0 kg/m2) | 3.88 (2.91 to 5.17)a | 1.03 (0.71 to 1.50) | |
| 3 (24.1–26.4 kg/m2) | 2.89 (2.16 to 3.89)a | 1.16 (0.81 to 1.66) | |
| 4 (26.5–29.8 kg/m2) | 2.14 (1.59 to 2.90) | 1.07 (0.74 to 1.54) | |
| 5 (>29.8 kg/m2) | 1.77 (1.30 to 2.40) | 1 | |
Model 0: nonadjusted. Model I: adjusted for demographics and medical history: age, gender, region (western or eastern Europe), dialysis vintage, smoking status, CKD etiology, diabetes, CVD, and cancer history. Model II: Model I plus adjustment for modifiable factors: hospitalization, catheter use, Kt/V, actual blood flow, and systolic blood pressure.
Wald chi-squared test versus Q5 BMI with inflammation present; P<0.001,
Figure 2.
Time-updated all-cause (top), cardiovascular (middle), and noncardiovascular (bottom) death by BMI (Q1–Q5) and inflammation (present=red, absent=blue) in an unadjusted model (model 0), following adjustment for demographics and medical history (model I), and following additional adjustment for modifiable factors (model II). PY, patients years.
Cause-Specific Analyses
Complementary analyses of cardiovascular and noncardiovascular mortality revealed similar findings (Figure 2, B and C). Notwithstanding the lower statistical power in these subsets, the effect of BMI on the outcomes, and the modification of this by inflammation status, was less apparent for cardiovascular mortality and greater for noncardiovascular mortality. No cause-specific differences in noncardiovascular international classification of diseases (ICD)-deaths in inflamed patients were apparent (Supplemental Table 5). Deaths were notified to the dialysis clinic either directly from hospital if the patient had an acute fatal episode, or via the patient’s family if death occurred at home; the latter made up the majority. Cause of death was determined from the hospital discharge sheet, or from the death certificate, taking into account the clinic physician’s knowledge of the patient’s clinical history. The clinic physician entered the ICD-10 code into the European Fresenius Medical Care (EU-FMC) database. Data on cause of death were missing or invalid in approximately 30% of patients (Supplemental Table 5). A time-dependent analysis of mortality rates by diabetes status revealed a limited impact on the findings (Supplemental Figure 3). As smoking status was missing for 33.4% of patients (Supplemental Table 1) an all-cause mortality sensitivity analysis excluding patients with missing smoking data was performed (Supplemental Figure 4). No major differences were observed when compared with the complimentary whole-population analysis.
Discussion
In 1999, Fleischmann et al.1 first reported that obesity was protective in hemodialysis (HD) patients. Subsequently most, but not all,14 studies based on large American,2,15 Asian,16 and European17 dialysis cohorts have shown that high BMI confers a survival advantage. A recent study on 123,383 North American HD patients showed that higher BMI was associated with lower mortality across all age and dialysis vintage groups.18 Our data from a large European incident HD cohort confirm the existence of the obesity paradox in HD patients with lower BMI levels than observed in North America. Although persistent inflammation is a common feature in the uremic milieu,19 the impact of inflammation on the association between high BMI and outcome has, to our knowledge, never been evaluated. Addressing this question, we found that the inverse relationship between BMI and mortality in HD patients is largely absent in the absence of inflammation.
Inflammation in dialysis patients is caused by multiple factors, including those related to the uremic milieu, the dialysis procedure, and comorbidity.19 We confirm the high prevalence of inflammation in HD patients, with approximately 54% of the cohort having either an elevated CRP (≥10 mg/l) or a decreased albumin concentration (≤35 g/l), and its association with subsequent mortality. Importantly we found that inflammation impacts greatly on the relationship between BMI and survival. When inflammation was classified according to elevated CRP or lower serum albumin only, the relationship between BMI and survival did not differ substantially. Whereas a high BMI is protective in inflamed HD patients, it does not confer a survival advantage in noninflamed HD patients (Figure 2A). We also report that the protective effects of high BMI in inflamed patients are stronger for noncardiovascular than cardiovascular mortality (Figure 2, B and C). All the observed associations persisted after adjustment for demographics, medical history, and modifiable factors during dialysis treatment; although we concede that unmeasured confounding could influence these findings. As avoiding obesity remains a common clinical approach for diabetic patients, the lack of effect modification by diabetic status (Supplemental Figure 3) is noteworthy.
We also report that, among noninflamed patients, those in the lowest BMI quintile still had an 80% higher mortality than patients with higher BMIs. Further studies should identify the clinical characteristics of the noninflamed dialysis group with low BMI and the reasons for their higher mortality. As depression is linked to malnutrition in dialysis patients20 we speculate that psychosocial factors, such as depression and/or drug abuse, may be more common in noninflamed dialysis patients with low BMI and may contribute to the increased mortality risk.
Several mechanisms may explain why high BMI is protective in inflamed dialysis patients. Firstly, although a high BMI can be associated with muscle wasting and catabolism,21 it likely reflects preserved energy stores and patients with a preserved appetite. When renal function deteriorates and a uremic milieu develops, well preserved energy stores become increasingly important.22 Diminished appetite is a poor prognostic sign in dialysis patients.23,24 Second, as uremic toxin production is relatively higher in patients with low body weight,25 this factor may relate to better outcome in large-sized, inflamed patients. Finally, as endothelial progenitor cell density is related to obesity26 when not complicated by diabetes,27 it is possible that this endogenous repair mechanism is better preserved.28 Indeed, inflammation attenuates endothelial progenitor survival and function.29 Additional mechanistic hypotheses include better cardiopulmonary exercise testing,30 associations to genetic traits with survival advantage,31 less dependence of cardiovascular properties on catecholamines and the renin-angiotensin system,32 and more efficient binding of endotoxins due to higher lipoprotein levels.33
Because the majority of CKD patients die before reaching ESRD,34 dialysis patients are likely to constitute a select group of survivors. From an evolutionary perspective, predisposition to insulin resistance and fat mass accumulation constitute a survival advantage during periods of external stress, such as infectious complications and shortage of food.35 However, because an increase in BMI may also reflect more lean body mass, the association between increased BMI and better outcome does not necessarily imply that fat mass is protective. The resilient protection of a high BMI is confined to long-term dialysis patients with indices of normal or high muscle mass.36–38 Both increased fat mass and lean body mass were associated with better outcome in Japanese dialysis patients.39 Obese sarcopenia is common in the aging population and constitutes a risk factor for poor health outcome, functional decline, and frailty.40 Persistent inflammation is a major risk factor for muscle mass loss,41 and obese sarcopenia is common and associated with both inflammation and poor outcome in incident dialysis patients.21 The present data should also be considered in the context of the ongoing debate about the value and indication of kidney transplantation in obese dialysis patients. Because BMI is a poor reflection of body composition, we believe this debate cannot be resolved if no precise assessment of body composition (fat mass versus muscle mass) is performed.22
The present study has several strengths. We present data on a large group of incident HD patients with at least three months of follow-up after dialysis initiation. The use of noninflamed patients with the highest BMI (Q5) as the reference group for statistical comparisons is logical, as previous studies confirm that dialysis patients with the highest BMI exhibit the best survival rate.1,2,15 These consistent findings persist after multiple corrections. Clearly, time-dependent models are more dynamic than baseline Cox models, since CRP, serum albumin levels, and BMI are considered over each and every time period (90-day segments).
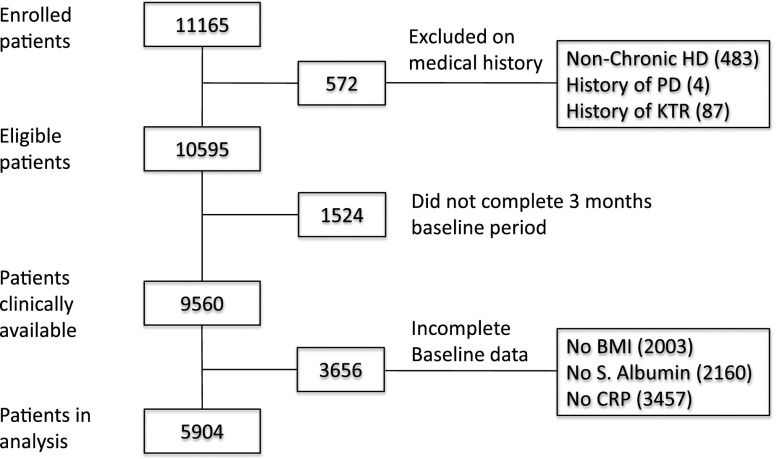
Some limitations also need to be considered. First, 4696 dialysis patients in the original second Analyzing Data, Recognizing Excellence and Optimizing Outcomes cohort (AROii) could not be evaluated, mainly due to incomplete data (Figure 3). However, a comparison of included and excluded patients (Supplemental Table 1) showed that the two groups were almost identical with regard to age, gender, and BMI and that the differences in CRP, serum albumin, and smoking status were of small magnitude and with little clinical relevance. A higher missingness rate in patients excluded mostly on incomplete BMI, CRP, and serum albumin data suggests a degree of interrelationship with regard to missing data. We find it unlikely that the observed difference in geographic region between included and excluded patients could have affected the results. The greater observed prevalence of CVD and diabetes among included patients may reflect more frequent clinical investigation in these populations, lower chance of missingness, and hence greater likelihood for inclusion. A second limitation is the high prevalence (33.4%) of missing smoking data. As smoking is associated with decreased body weight and increased mortality risk, smoking is a well established concern in analyses of BMI in relation to mortality.42 However, exclusion of patients with missing smoking data did not affect the findings (Supplemental Figure 4); although the prognostic value of missingness with regards to this variable is unknown.
Figure 3.
Study population derivation. Out of 11,165 HD patients 572 were excluded based on medical history, 1524 patients did not complete the 3 months baseline period and 3656 patients did not have complete baseline data. KTR, Kidney transplant.
A third limitation may be uncertainty of the exact cause of death using ICD-10 codes. In the complex setting of ESRD in which many patients have concurrent clinical signs of wasting, and infectious and cardiovascular complications,43 the exact cause of death may be difficult to assess. Diabetes, a common comorbidity in dialysis patients that is related to both BMI and inflammation, could have confounded the results. The presence of obesity in diabetic HD patients may eliminate the beneficial effect of obesity.44 However, diabetic status was included in the multivariate regression model, and analyses stratified by diabetic medical history had limited impact. While the crude definition of inflammation is a potential limitation, we used a similar definition to Liu et al.45 on the modifying effect of inflammation on the association between cholesterol and outcome. Although the present study and the study by Liu et al.45 differ with regard to number of included dialysis patients (5904 versus 823), ethnicity (Caucasians versus mixed population), death rate (10.9 versus 16.4% per year), and prevalence of inflammation (54.7 versus 77.0%), both studies demonstrate a dramatic catalytic effect46 of inflammation on the association between two traditional risk factors (BMI and cholesterol) and outcome in the uremic milieu. It should also be acknowledged that whereas IL-6 was included in the definition of inflammation in the study by Liu et al.,45 this elaborate inflammatory marker was missing in the present study. It is well established that the inflammatory biomarker CRP is a ‘moving target’ in the uremic milieu47 and affected by numerous processes.19 However, as time-dependent statistical modeling was used, the inflammation status was updated over observation periods. Although serum albumin is still considered a biomarker of nutritional status in dialysis patients, it correlates poorly with nutritional status markers in this patient group.48,49 Several groups have reported that the strong association between serum albumin and mortality in dialysis patients is explained by persistent inflammation rather than by malnutrition.49,50
Finally, BMI is a crude marker of body composition and does not accurately differentiate between lean and fat tissue. As the more metabolically active visceral fat depots promote insulin resistance, atherosclerosis, fatty liver, and type II diabetes, a more precise marker of fat mass distribution, such as waist/hip ratio, bioelectric impedance analysis, abdominal height, or conicity index22 would have been better, but these were unavailable. Since inflammation and wasting are often considered together in dialysis patients41,43 it is likely that a considerable portion of the inflamed patients in this study have an ongoing muscle wasting process. Unfortunately, no precise estimation of muscle mass was available.
In conclusion, the obesity paradox is modified by the presence of inflammation and does not exist in noninflamed dialysis patients. High BMI constitutes a survival advantage only in inflamed patients. While the underlying biologic mechanisms remain speculative, these findings point to a role of elevated BMI nullifying the negative consequences of chronic inflammation. From a clinical perspective, underweight inflamed dialysis patients represent a high-risk population. Our findings indicate that inflammation is a major effect modifier that should be addressed in the clinical situation when weight management of obese dialysis patients is discussed.
Concise Methods
Study Population
The ARO research initiative is described elsewhere.51 The present study focuses on the AROii cohort, which enrolled European (15 participating countries) incident (maximum <183 days since initiation) adult HD patients from 312 EU-FMC facilities and followed up on prospectively.52 Anonymized patient-level medical history, longitudinal laboratory, dialysis, and medication data, plus ICD-10-coded hospitalization and death data, are captured in a validated clinical database.53 All ethical and regulatory obligations concerning patient data were met locally and informed consent was obtained from all patients.51
Between 2007 and 2009 11,165 incident patients were recruited into AROii. Patients who, either alone or combined, never commenced HD (4.3%), or had a history of renal transplant (0.8%) or peritoneal dialysis (0.04%) on admission were excluded, leaving 10,595 patients. A further 13.6% did not complete baseline. Of the remaining 9560, 3656 had either incomplete baseline BMI data (n=2003), and/or lacked CRP (n=3457) and/or serum albumin values (n=2160). These patients were excluded, leaving 5904 for analysis (Figure 3). The remaining patients were stratified into quintile BMI groups (Q1–Q5: <21.5, 21.5–24.0, >24.0–26.4, >26.4–29.8, and >29.8 kg/m2).
Measurements
Fasting blood samples were drawn routinely and processed locally at time of dialysis session start to determine CRP, serum albumin, hemoglobin, ferritin, blood lipids, creatinine, calcium, phosphate, and parathyroid hormone levels. Postdialysis body weight and height were used to calculate BMI. The use of objective, cohort-specific, data-driven BMI cutoff points is preferential over traditional methods, e.g., World Health Organization-defined grouping as these were designed for the general population and are less applicable to ESRD patients.
Follow-Up
Follow-up commenced on patients’ first EU-FMC dialysis session and patients accrued time-at-risk from baseline end until they experienced the event of interest or were censored (the earliest date of either kidney transplantation, loss-to-follow-up [>45 days without continuous EU-FMC dialysis treatment], or study end [March 31, 2013]).
Statistical Analyses
Statistical analyses were performed using SAS (Windows version 9.2; SAS Institute Inc., Cary, NC) and were reproduced independently. Means and SDs for continuous variables and counts and frequencies for categorical data described baseline study variables. Ordinal or skewed continuous variables were described using a median and interquartile range, or were categorized. First, the association between BMI and inflammation (defined by CRP [≥10 mg/l] and/or serum albumin [≤35 g/l]) were described separately. Kaplan-Meier methods were used to estimate time-to-event for patients according to baseline BMI categories and/or inflammation status. The linear trend between categories was assessed using the log-rank test for trends.
For subsequent analyses, the effect of inflammation was nested within BMI quintiles, creating a ten-point ‘BMI-inflammation’ categorical variable. The effect of this exposure on outcomes of interest was modeled in two ways. Firstly, having assessed over-dispersion, Poisson regression was undertaken to derive all-cause and cause-specific mortality rate estimates, with 95% confidence intervals (95% CIs), for each stratum. Secondly, Cox proportional hazards regression modeling was performed to estimate HRs and 95% CI for baseline BMI-inflammation when compared with noninflamed patients in the highest BMI quintile. The proportional hazards assumption was checked graphically by plotting Schoenfeld residuals and Loess smoothing.54 Where time-dependent Cox or Poisson regression analyses were warranted, person-time was split into consecutive 90-day periods, explanatory variables recalculated, and analyses repeated. Three levels of adjustment were performed. Model 0 was unadjusted. Model I was adjusted for demography (age, gender, and region) and medical history (dialysis vintage, smoking status, CKD etiology, diabetes [captured on EU-FMC admission], CVD, and cancer history). Model II augmented model I by including modifiable factors (hospitalization, catheter-based vascular access, dialysis adequacy assessed by Kt/V, extracorporeal actual blood flow, and predialysis session systolic blood pressure). Missing data were recorded as such in baseline analyses; for time-dependent analyses, missing BMI data were imputed using the last-observation-carried-forward approach, and for missing inflammation status, a more complex algorithm was employed (Supplemental Figure 5). There was no further imputation for missing data when using the composite BMI-inflammation variable.
Disclosures
Drs. Stenvinkel, Kronenberg, Drueke, Schernthaner, Eckardt, Floege, and Anker received consultancy fees from Amgen. Ms. Addison and Dr. Gillespie are full-time Amgen employees. Dr. Froissart was a full-time Amgen employee and Mr. Tunks was a contractor to Amgen at time the study was completed. Dr. Marcelli is a full-time EU-FMC employee. Study Collaborators and Sponsors are listed in the Supplemental Material.
Supplementary Material
Acknowledgments
The sponsors were responsible for data collection (EU-FMC, Amgen) and data management (EU-FMC), providing resources for statistical and epidemiologic analysis, and participating in data interpretation and manuscript preparation (FMC).
The ARO CKD Research Initiative is a joint observational research commitment from Amgen and FMC, fully funded by Amgen (Europe; GmbH, Zug, Switzerland).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Obesity Paradox and the Role of Inflammation,” on pages 1270–1272.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030252/-/DCSupplemental.
References
- 1.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK: Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 55: 1560–1567, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP: Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 25: 2088–2096, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr: The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 39: 578–584, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK: Body mass index and poststroke mortality. Neuroepidemiology 30: 93–100, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Michaud K: Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 1471–1479, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR: Association of weight status with mortality in adults with incident diabetes. JAMA 308: 581–590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM: Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 132: 625–634, 2013 [DOI] [PubMed] [Google Scholar]
- 9.García-Ptacek S, Kåreholt I, Farahmand B, Cuadrado ML, Religa D, Eriksdotter M: Body-mass index and mortality in incident dementia: a cohort study on 11,398 patients from SveDem, the Swedish Dementia Registry. J Am Med Dir Assoc 15: 447.e1–447.e7, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA: Body mass index and mortality in heart failure: a meta-analysis. Am Heart J 156: 13–22, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Dixon JB, Egger GJ, Finkelstein EA, Kral JG, Lambert GW: ‘Obesity paradox’ misunderstands the biology of optimal weight throughout the life cycle. Int J Obes 39: 82–84, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Dehlendorff C, Andersen KK, Olsen TS: Body mass index and death by stroke: no obesity paradox. JAMA Neurol 71: 978–984, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Shah RV, Abbasi SA, Yamal JM, Davis BR, Barzilay J, Einhorn PT, Goldfine AB; ALLHAT Collaborative Research Group : Impaired fasting glucose and body mass index as determinants of mortality in ALLHAT: is the obesity paradox real? J Clin Hypertens (Greenwich) 16: 451–458, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mutsert R, Snijder MB, van der Sman-de Beer F, Seidell JC, Boeschoten EW, Krediet RT, Dekker JM, Vandenbroucke JP, Dekker FW: Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol 18: 967–974, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Johansen KL, Kutner NG, Young B, Chertow GM: Association of body size with health status in patients beginning dialysis. Am J Clin Nutr 83: 543–549, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, Levin NW, Nissenson AR, Lee JS, Kalantar-Zadeh K: Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc 88: 479–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D: Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 24: 2871–2876, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Vashistha T, Mehrotra R, Park J, Streja E, Dukkipati R, Nissenson AR, Ma JZ, Kovesdy CP, Kalantar-Zadeh K: Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am J Kidney Dis 63: 612–622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrero JJ, Stenvinkel P: Inflammation in end-stage renal disease--what have we learned in 10 years? Semin Dial 23: 498–509, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Koo JR, Yoon JW, Kim SG, Lee YK, Oh KH, Kim GH, Kim HJ, Chae DW, Noh JW, Lee SK, Son BK: Association of depression with malnutrition in chronic hemodialysis patients. Am J Kidney Dis 41: 1037–1042, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, Stenvinkel P, Lindholm B: Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr 86: 633–638, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Stenvinkel P, Zoccali C, Ikizler TA: Obesity in CKD--what should nephrologists know? J Am Soc Nephrol 24: 1727–1736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrero JJ, Qureshi AR, Axelsson J, Avesani CM, Suliman ME, Kato S, Bárány P, Snaedal-Jonsdottir S, Alvestrand A, Heimbürger O, Lindholm B, Stenvinkel P: Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr 85: 695–701, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD: Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 80: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Sarkar SR, Kuhlmann MK, Kotanko P, Zhu F, Heymsfield SB, Wang J, Meisels IS, Gotch FA, Kaysen GA, Levin NW: Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int 70: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG: Influence of BMI on level of circulating progenitor cells. Obesity (Silver Spring) 19: 1722–1726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graziani F, Leone AM, Basile E, Cialdella P, Tritarelli A, Bona RD, Liuzzo G, Nanni G, Iaconelli A, Iaconelli A, Mingrone G, Biasucci LM, Crea F: Endothelial progenitor cells in morbid obesity. Circ J 78: 977–985, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Ramirez R, Carracedo J, Merino A, Nogueras S, Alvarez-Lara MA, Rodríguez M, Martin-Malo A, Tetta C, Aljama P: Microinflammation induces endothelial damage in hemodialysis patients: the role of convective transport. Kidney Int 72: 108–113, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ: C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109: 2058–2067, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Horwich TB, Leifer ES, Brawner CA, Fitz-Gerald MB, Fonarow GC; HF-ACTION Investigators : The relationship between body mass index and cardiopulmonary exercise testing in chronic systolic heart failure. Am Heart J 158[Suppl]: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavebratt C, Wahlqvist S, Nordfors L, Hoffstedt J, Arner P: AHSG gene variant is associated with leanness among Swedish men. Hum Genet 117: 54–60, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Weber MA, Neutel JM, Smith DH: Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol 37: 169–174, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD: Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 353: 1838–1842, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Johnson RJ, Stenvinkel P, Martin SL, Jani A, Sánchez-Lozada LG, Hill JO, Lanaspa MA: Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology. Obesity (Silver Spring) 21: 659–664, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beddhu S, Pappas LM, Ramkumar N, Samore M: Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14: 2366–2372, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD: The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc 85: 991–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K: Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 5: 2258–2268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kakiya R, Shoji T, Tsujimoto Y, Tatsumi N, Hatsuda S, Shinohara K, Kimoto E, Tahara H, Koyama H, Emoto M, Ishimura E, Miki T, Tabata T, Nishizawa Y: Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int 70: 549–556, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ: Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 68: 1001–1007, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Manson JE, Stampfer MJ, Hennekens CH, Willett WC: Body weight and longevity. A reassessment. JAMA 257: 353–358, 1987 [PubMed] [Google Scholar]
- 43.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Deger SM, Ellis CD, Bian A, Shintani A, Ikizler TA, Hung AM: Obesity, diabetes and survival in maintenance hemodialysis patients. Ren Fail 36: 546–551, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Carrero JJ, Stenvinkel P: Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol 4[Suppl 1]: S49–S55, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Snaedal S, Heimbürger O, Qureshi AR, Danielsson A, Wikström B, Fellström B, Fehrman-Ekholm I, Carrero JJ, Alvestrand A, Stenvinkel P, Bárány P: Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis 53: 1024–1033, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Friedman AN, Fadem SZ: Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 21: 223–230, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Gama-Axelsson T, Heimbürger O, Stenvinkel P, Bárány P, Lindholm B, Qureshi AR: Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol 7: 1446–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW; Netherlands Cooperative Study on the Adequacy of Dialysis-II Study Group : Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 19: 127–135, 2009 [DOI] [PubMed] [Google Scholar]
- 51.de Francisco AL, Kim J, Anker SD, Belozeroff V, Canaud B, Chazot C, Drüeke TB, Eckardt KU, Floege J, Kronenberg F, Macdougall IC, Marcelli D, Molemans B, Passlick-Deetjen J, Schernthaner G, Stenvinkel P, Wheeler DC, Fouqueray B, Aljama P: An epidemiological study of hemodialysis patients based on the European Fresenius Medical Care hemodialysis network: results of the ARO study. Nephron Clin Pract 118: c143–c154, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU: Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 87: 996–1008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steil H, Amato C, Carioni C, Kirchgessner J, Marcelli D, Mitteregger A, Moscardo V, Orlandini G, Gatti E: EuCliD--a medical registry. Methods Inf Med 43: 83–88, 2004 [PubMed] [Google Scholar]
- 54.Schoenfeld D: Partial residuals for the proportional hazards regression model. Biometrika (1982) 69: 239–241, 1982 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.