Abstract
There is increasing interest in the colonic microbiota as a relevant source of uremic retention solutes accumulating in CKD. Renal disease can also profoundly affect the colonic microenvironment and has been associated with a distinct colonic microbial composition. However, the influence of CKD on the colonic microbial metabolism is largely unknown. Therefore, we studied fecal metabolite profiles of hemodialysis patients and healthy controls using a gas chromatography-mass spectrometry method. We observed a clear discrimination between both groups, with 81 fecal volatile organic compounds detected at significantly different levels in hemodialysis patients and healthy controls. To further explore the differential impact of renal function loss per se versus the effect of dietary and other CKD-related factors, we also compared fecal metabolite profiles between patients on hemodialysis and household contacts on the same diet, which revealed a close resemblance. In contrast, significant differences were noted between the fecal samples of rats 6 weeks after 5/6th nephrectomy and those of sham-operated rats, still suggesting an independent influence of renal function loss. Thus, CKD associates with a distinct colonic microbial metabolism, although the effect of renal function loss per se in humans may be inferior to the effects of dietary and other CKD-related factors. The potential beneficial effect of therapeutics targeting colonic microbiota in patients with CKD remains to be examined.
Keywords: chronic kidney disease, intestine, nutrition
The human intestinal tract is colonized by hundreds of trillions of microbes, collectively possessing hundreds of times as many genes as coded for by the human genome. The combined genetic potential of the endogenous microbiota, referred to as the “microbiome,” supplements the host with trophic, metabolic, and protective signals.1 Accordingly, the mammalian plasma metabolome is a composite of endogenous metabolites and metabolites originating from the colonic microbiota.2
The colonic microbiota is also a significant contributor to the metabolome in patients with CKD.3 Widely studied uremic retention solutes as p-cresyl sulfate and indoxyl sulfate are actually microbiome-human cometabolites. p-Cresyl sulfate results from the combined actions of bacterial fermentation of tyrosine to p-cresol and endogenous sulfate conjugation. Likewise, indoxyl sulfate is the end product of bacterial fermentation of tryptophan to indole followed by endogenous oxidation and sulfate conjugation.4,5
Loss of renal function leads to retention of these solutes.6,7 Notably, renal function is not the sole determinant of serum concentrations of these colon-derived uremic retention solutes. In a recent study, both eGFR and intestinal generation independently determined serum concentrations of p-cresyl sulfate and indoxyl sulfate, with intestinal generation showing substantial interindividual variability.8 This interplay between intestinal generation and renal excretion has been coined the gut-kidney axis.9
In a pivotal study, Vaziri et al. have already demonstrated that the colonic microbial composition itself is altered in CKD. Notable increases in the number of bacterial operational taxonomic units belonging to the Brachybacterium, Catenibacterium, Enterobacteriaceae, Halomonadaceae, Moraxellaceae, Nesterenkonia, Polyangiaceae, Pseudomonadaceae, and Thiothrix families were found.10 To isolate the effect of renal function loss from CKD-related dietary restrictions, drug therapy and comorbid conditions (e.g., diabetes mellitus), they additionally used a 5/6th nephrectomy rat model, again observing significant differences in the colonic microbial composition.
As different microbial species can share similar functional gene profiles, so-called functional redundancy, knowledge of the microbial composition alone does not necessarily lead to an understanding of its metabolic activity.11 Moreover, the colonic microbial composition is not the sole determinant of the microbial metabolism. Apart from the microbial composition, physicochemical characteristics of the fermentable substrate, amount of substrate available, intraluminal pH, colonic transit time and other factors all affect the way substrates are utilized and fermentation products are formed.12–14 It must be noted that CKD profoundly affects the gastrointestinal environment, thereby potentially influencing colonic microbial metabolism. We previously demonstrated that small intestinal protein assimilation is disturbed in patients with CKD.15 In addition, intraluminal pH is higher due to a higher ammonia concentration. Also, colonic transit time is prolonged.16 Furthermore, patients with renal dysfunction receive intestinal targeted drug therapy (e.g., phosphate binders), as well as dietary restrictions, mostly fruit and vegetables, which prevent potassium overload, but as a trade-off result in a significantly decreased fiber intake.17
Therefore, we questioned whether the colonic microbial metabolism is CKD-specific. As CKD extends beyond renal function loss, with associated differences in diet, age, drug therapy, and comorbidity, the second aim was to disentangle the effect of renal function loss per se versus the overall impact of CKD (“renal phenotype”).
RESULTS
Baseline Characteristics of Patients on Hemodialysis and Control Groups
We included 20 patients on maintenance hemodialysis with a median age of 74 years (interquartile range [IQR], 64–81) and a dialysis vintage of 22 months (IQR, 9–30). All patients were on a stable hemodialysis regime (4 hours, 3 times weekly) with a mean single-pool Kt/V of 1.77 (SD 0.25) reflecting adequate dialysis treatment. Most of the patients on hemodialysis (70%) were treated with phosphate binders and all patients received nutritional counseling to restrict dietary intake of fluid, sodium, phosphate, and potassium. Baseline characteristics of the hemodialysis group and three control groups (i.e., unrelated healthy subjects, unrelated age-matched healthy subjects, and household contacts on the same diet) are summarized in Table 1.
Table 1.
Baseline characteristics of patients on hemodialysis and control groups
| Variable | Patients on Hemodialysis (n=20) | Healthy Controls (n=20) | Age-Matched Healthy Controls (n=20) | Household Contacts (n=20) |
|---|---|---|---|---|
| Age (years) | 74 (64–81) | 25 (23–32)a | 72 (66–78)b | 69 (64–77)b |
| Gender: female/male (%) | 10/10 (50/50) | 17/3 (85/15)a | 10/10 (50/50)b | 10/10 (50/50)b |
| Body mass index (kg/m2) | 26.34 (5.08) | 21.32 (1.55)a | 27.14 (3.75)b | 27.61 (6.46)b |
| Diabetes mellitus: yes/no (%) | 6/14 (30/70) | 0/20 (0/100)a | 0/20 (25/75)a | 5/15 (25/75)b |
Data are expressed as mean (SD) or median (IQR), as appropriate. Differences between groups were tested using Student’s t test, Wilcoxon rank sum test, or chi-squared test, as appropriate.
P value patients on hemodialysis versus control group <0.05.
P value patients on hemodialysis versus control group nonsignificant.
CKD versus Healthy Controls
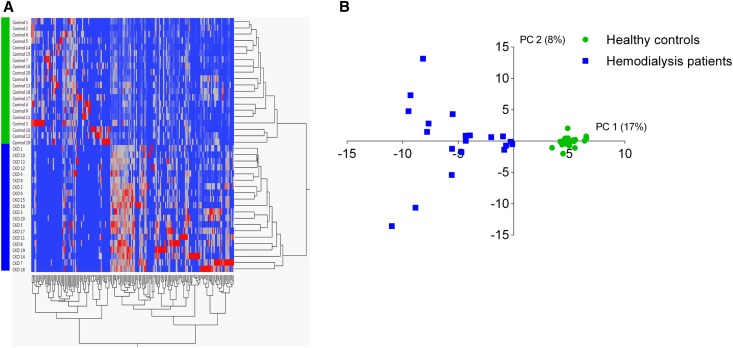
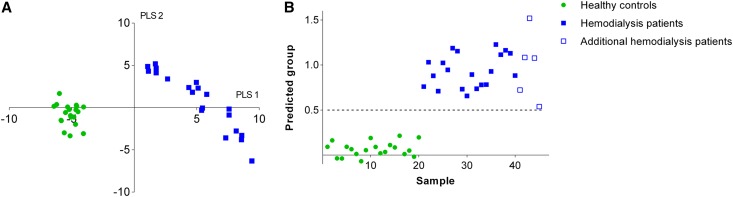
We measured fecal metabolite profiles of patients on hemodialysis and healthy controls using a previously reported gas chromatography-mass spectrometry (GC-MS) method.18 This technique allows for untargeted metabolomics of fecal volatile organic compounds (VOCs). In total, we identified 243 different VOCs. Of these, 48 VOCs were subject-specific and 25 VOCs were found in all subjects. In addition, there was a significantly higher number of VOCs per sample in healthy controls compared with patients on hemodialysis (mean of 98 [SD 8] versus 79 [SD 5] VOCs, respectively, P<0.001). At first, data were analyzed by principal component analysis (PCA) and hierarchical cluster analysis, both unsupervised methods, already demonstrating substantial differences between fecal metabolite profiles of patients on hemodialysis and healthy controls (Figure 1). Next, we built a partial least-square discriminant analysis (PLS-DA) model to optimize differences between these two groups, resulting in a clear discrimination between patients on hemodialysis and controls (Figure 2A). This PLS-DA model was further validated using leave-one-out cross-validation. As can be observed in Figure 2B, all samples could precisely be allocated to one of both groups. As a final validation step, we included five unrelated fecal samples of patients on hemodialysis, allowing us to perform a prediction analysis for these five samples (Figure 2B). Again, each sample could accurately be classified as belonging to the group of patients on hemodialysis, demonstrating stability and predictive performance of the obtained model.
Figure 1.
Fecal metabolite profiles of patients on hemodialysis and healthy controls. (A) Hierarchical cluster analysis. Rows represent fecal metabolite profiles of patients on hemodialysis (CKD, solid blue) and healthy controls (control, green). Red indicates increased abundance of individual VOCs relative to internal standard and blue indicates decreased abundance. (B) PCA score plot of fecal metabolite profiles of patients on hemodialysis (solid blue square) and healthy controls (green circle).
Figure 2.
PLS-DA of fecal metabolite profiles of hemodialysis patients and healthy controls. (A) PLS-DA score plot of fecal metabolite profiles of patients on hemodialysis (solid blue square) and healthy controls (green circle). (B) Leave-one-out cross-validation and prediction analysis from the PLS-DA model for patients on hemodialysis (solid blue square), healthy controls (green circle) and five additional unrelated patients on hemodialysis (blue box); the predicted group of patients on hemodialysis has a target value of y=1, the group of healthy controls has a target value of y=0, and the discriminant threshold (y=0.5) is the dashed line.
We then explored individual VOCs responsible for discrimination of fecal metabolite profiles. After adjustment for the false discovery rate (FDR), we identified a total of 81 individual VOCs being significantly different between patients on hemodialysis and healthy controls. Of these, 53 VOCs were upregulated and 28 VOCs were downregulated in patients on hemodialysis (Tables 2 and 3). As can be derived from Table 2, both p-cresol and indole were also upregulated in the hemodialysis group. When grouping individual metabolites according to chemical classes with inspection of the correlation loading plot, we observed an upregulation of aldehydes, benzenes, branched-chain fatty acids, furans, indoles, medium-chain fatty acids, and short-chain fatty acids, while ketones were downregulated in patients on hemodialysis.
Table 2.
Relative levels of VOCs upregulated in fecal samples of patients on hemodialysis versus healthy controls (median [IQR])
| Metabolite | Patients on Hemodialysis | na | Healthy Controls | na | P valueb |
|---|---|---|---|---|---|
| Diphenyl sulfide | 0.0187 (0.0140–0.0207) | 20 | ND | 0 | <0.001 |
| o-Cymene | 0.0920 (0.0301–0.3262) | 20 | ND | 0 | <0.001 |
| Benzoic acid, 4-ethoxy-, ethyl ester | 0.0096 (0.0058–0.0125) | 20 | 0.0002 (0–0.0003) | 14 | <0.001 |
| Hexadecanal | 0.0700 (0.0511–0.0925) | 20 | 0.0011 (0.0001–0.0016) | 15 | <0.001 |
| Propanal | 0.2318 (0.1850–0.4000) | 20 | 0.0506 (0.0369–0.0595) | 20 | <0.001 |
| Carbon disulfide | 0.0486 (0.0377–0.0614) | 20 | 0.0040 (0.0032–0.0052) | 20 | <0.001 |
| Tetradecanal | 0.1353 (0.0818–0.1911) | 20 | 0.0065 (0.0043–0.0095) | 0 | <0.001 |
| Furan | 0.0440 (0.0243–0.0512) | 20 | 0.0078 (0.0064–0.0138) | 20 | <0.001 |
| Oxirane, hexadecyl- | 0.1346 (0.0525–0.2575) | 20 | 0.0051 (0.0034–0.0137) | 20 | <0.001 |
| Dodecanal | 0.0528 (0.0248–0.0835) | 20 | 0.0033 (0.0012–0.0062) | 19 | <0.001 |
| Tridecanal | 0.0044 (0.0023–0.0105) | 17 | 0 (0–0) | 2 | <0.001 |
| ϒ-Dodecalactone | 0.0036 (0.0006–0.0108) | 17 | 0 (0–0) | 2 | <0.001 |
| 4-Hydroxy-2-methylacetophenone | 0.0033 (0.0023–0.0057) | 16 | 0 (0–0) | 1 | <0.001 |
| 2-Furancarboxaldehyde, 5-methyl- | 0.0104 (0.0056–0.0143) | 19 | 0.0006 (0.0003–0.0012) | 20 | <0.001 |
| Benzene, 1,3,5-trimethyl- | 0.0024 (0.0016–0.0042) | 19 | 0.0003 (0.0002–0.0005) | 20 | <0.001 |
| Cumene | 0.0089 (0.0043–0.0162) | 20 | 0.0021 (0.0010–0.0025) | 20 | <0.001 |
| Furan, 2-methyl- | 0.0535 (0.0327–0.0752) | 20 | 0.0082 (0.0055–0.0119) | 20 | <0.001 |
| Propanal, 2-methyl- | 0.2629 (0.1480–0.3990) | 20 | 0.0603 (0.0312–0.1109) | 20 | <0.001 |
| Butanoic acid, 2-methyl- | 1.7851 (1.1153–2.1031) | 20 | 0.4009 (0.2690–0.6865) | 20 | <0.001 |
| Butanoic acid, 3-methyl- | 1.9240 (1.2099–2.4170) | 20 | 0.3947 (0.3106–0.8021) | 20 | <0.001 |
| Unknown ether | 0.0333 (0.0079–0.0441) | 18 | 0.0007 (0–0.0021) | 14 | <0.001 |
| Indole | 0.0045 (0.0017–0.0093) | 19 | 0.0008 (0.0004–0.0011) | 20 | <0.001 |
| Cyclododecane | 0.0039 (0.0019–0.0111) | 16 | 0 (0–0) | 3 | <0.001 |
| Propanoic acid, 2-methyl- | 0.5095 (0.3770–0.6937) | 20 | 0.1705 (0.1351–0.3057) | 20 | <0.001 |
| Bromochloronitromethane | 0.0017 (0.0008–0.0033) | 20 | 0.0004 (0.0003–0.0007) | 20 | <0.001 |
| p-Cresol | 0.7123 (0.5485–0.9055) | 20 | 0.1214 (0.0797–0.1694) | 20 | <0.001 |
| Methanethiol | 0.0475 (0.0236–0.0699) | 18 | 0.0078 (0.0044–0.0124) | 17 | <0.001 |
| Methane, tribromo- | 0.0030 (0.0018–0.0058) | 18 | 0.0006 (0.0004–0.0007) | 20 | <0.001 |
| 9-Hexadecenal | 0.0007 (0–0.0023) | 12 | ND | 0 | <0.001 |
| Furan, tetrahydro- | 0.5948 (0.3757–1.0087) | 20 | 0.2250 (0.1486–0.4011) | 20 | <0.001 |
| Acetaldehyde | 1.0375 (0.8149–1.3758) | 20 | 0.5494 (0.2662–0.6825) | 20 | <0.001 |
| Benzaldehyde | 0.4652 (0.3334–0.6707) | 20 | 0.1450 (0.0821–0.2110) | 20 | <0.001 |
| Benzene, 1,2,4-trimethyl- | 0.0032 (0–0.0060) | 13 | 0 (0–0) | 2 | <0.001 |
| Styrene, 3,4-dimethyl- | 0.0026 (0–0.0173) | 13 | 0 (0–0) | 2 | <0.001 |
| Unknown aldehyde | 0.0788 (0.0144–0.1369) | 18 | 0.0028 (0.0011–0.0079) | 17 | <0.001 |
| Pentanoic acid | 0.6949 (0.4082–1.0076) | 20 | 0.2249 (0.1837–0.3160) | 20 | <0.001 |
| 3,4-Dimethyl-2-(3-methyl-butyryl)-benzoic acid, methyl ester | 0.0001 (0–0.0006) | 11 | ND | 0 | <0.001 |
| Phenol, 3,5-dimethyl- | 0.0030 (0.0018–0.0055) | 18 | 0.0010 (0.0006–0.0019) | 20 | 0.002 |
| Acetic acid | 0.3123 (0.1873–0.4586) | 20 | 0.1472 (0.1139–0.1887) | 20 | 0.003 |
| Propanoic acid | 0.2419 (0.1210–0.4632) | 20 | 0.0980 (0.0721–0.1359) | 20 | 0.01 |
| Benzyl alcohol | 0 (0–0.0009) | 8 | ND | 0 | 0.01 |
| Nonanal | 0 (0–0.0006) | 8 | ND | 0 | 0.01 |
| p-Xylene | 0.0022 (0–0.0041) | 14 | 0.0002 (0–0.0007) | 10 | 0.01 |
| Benzeneacetaldehyde | 0.0074 (0.0018–0.0143) | 16 | 0.0018 (0.0011–0.0025) | 18 | 0.01 |
| Benzene, 1,2,3,5-tetramethyl- | 0 (0–0.0022) | 7 | ND | 0 | 0.01 |
| 1-Butanol | 0.0549 (0.0159–0.2146) | 20 | 0.0067 (0.0023–0.0205) | 20 | 0.02 |
| 1H-Indole, 3-methyl- | 0.1199 (0.0307–0.2992) | 19 | 0.0185 (0.0025–0.0657) | 20 | 0.02 |
| α-Terpineol | 0.0090 (0.0035–0.0439) | 19 | 0.0025 (0.0013–0.0069) | 20 | 0.02 |
| p-Menth-1-en-4-ol | 0.0241 (0.0039–0.0465) | 16 | 0.0029 (0.0002–0.0064) | 15 | 0.02 |
| Toluene | 0.0081 (0.0068–0.0184) | 17 | 0.0034 (0.0019–0.0043) | 20 | 0.04 |
| Butanoic acid | 1.3062 (0.6324–1.7350) | 20 | 0.5292 (0.3271–0.8490) | 20 | 0.05 |
| Butanoic acid, 2,2-dimethyl- | 0 (0–0.0157) | 5 | ND | 0 | 0.05 |
| 7-Hexadecene | 0 (0–0.0002) | 5 | ND | 0 | 0.05 |
ND, nondetectable.
Number of subjects in which VOC was detected (/20).
FDR-adjusted P value.
Table 3.
Relative levels of volatile organic compounds downregulated in fecal samples of patients on hemodialysis versus healthy controls (median [IQR])
| Metabolite | Patients on Hemodialysis | na | Healthy Controls | na | P Valueb |
|---|---|---|---|---|---|
| β-Cymene | ND | 0 | 0.0092 (0.0048–0.0212) | 20 | <0.001 |
| 2-Pentanone | ND | 0 | 0.0138 (0.0023–0.0263) | 20 | <0.001 |
| Ethyl ether | ND | 0 | 0.0038 (0.0013–0.0056) | 17 | <0.001 |
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl ester | ND | 0 | 0.0005 (0.0003–0.0054) | 16 | <0.001 |
| Propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl ester | 0 (0–0) | 2 | 0.0006 (0.0002–0.0081) | 19 | <0.001 |
| 5-Hepten-2-one, 6-methyl- | ND | 0 | 0.0003 (0.0000–0.0010) | 15 | <0.001 |
| Thiophene, 2-methyl- | 0 (0–0) | 2 | 0.0003 (0.0002–0.0005) | 17 | <0.001 |
| 2-Pentanol | ND | 0 | 0.0007 (0–0.0013) | 14 | <0.001 |
| 2-Carene | ND | 0 | 0.0005 (0–0.0027) | 14 | <0.001 |
| Hexane | ND | 0 | 0.0038 (0–0.0689) | 14 | <0.001 |
| Disulfide, methyl propyl- | 0 (0–0) | 1 | 0.0010 (0–0.0043) | 14 | <0.001 |
| Trichloromethane | 0 (0–0.0036) | 5 | 0.0122 (0.0034–0.0412) | 20 | <0.001 |
| Ethylbenzene | 0 (0–0) | 2 | 0.0009 (0–0.0014) | 14 | <0.001 |
| Unknown component | 0 (0–0) | 3 | 0.0008 (0.0005–0.0011) | 18 | <0.001 |
| 1-Butene, 3,3-dimethyl- | ND | 0 | 0.0002 (0–0.0006) | 10 | 0.002 |
| Phenol, 2-ethyl- | 0 (0–0) | 1 | 0.0002 (0–0.0009) | 12 | 0.003 |
| Disulfide, dimethyl- | 0.1176 (0.0747–0.1713) | 20 | 0.2447 (0.1582–0.3505) | 20 | 0.004 |
| Methane, bromodichloro- | 0 (0–0) | 2 | 0.0001 (0–0.0001) | 14 | 0.004 |
| Hexanal | 0 (0–0.0029) | 8 | 0.0048 (0.0029–0.0116) | 17 | 0.004 |
| Pyrrole | 0 (0–0) | 2 | 0.0002 (0–0.0003) | 13 | 0.01 |
| Isopropyl alcohol | ND | 0 | 0 (0–0.0011) | 7 | 0.01 |
| Pentanal | 0 (0–0.0014) | 5 | 0.0059 (0–0.0131) | 14 | 0.02 |
| 3-Phenyl-4-penten-2-ol | ND | 0 | 0 (0–0.0001) | 6 | 0.03 |
| Furan, 2-pentyl- | 0 (0–0) | 4 | 0.0003 (0.0001–0.0004) | 16 | 0.03 |
| β-Pinene | 0 (0–0) | 1 | 0 (0–0.0020) | 8 | 0.03 |
| 1-Butanone, 1-(2-furanyl)- | 0 (0–0) | 3 | 0.0001 (0–0.0003) | 12 | 0.05 |
| Thiocyanic acid, methyl ester | ND | 0 | 0 (0–0.0000) | 5 | 0.05 |
| 3-Buten-2-one, 3-methyl- | ND | 0 | 0 (0–0.0003) | 5 | 0.05 |
ND, nondetectable.
Number of subjects in which VOC was detected (/20).
FDR-adjusted P value.
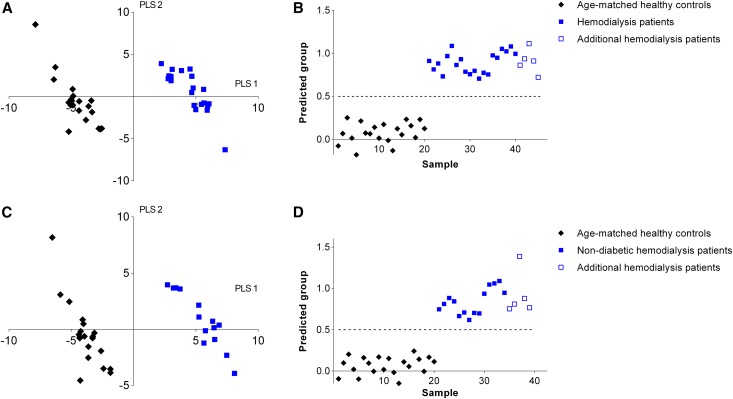
As there were significant differences in baseline characteristics between patients on hemodialysis and healthy controls, potentially contributing to the observed discrimination of fecal metabolite profiles, we included a second healthy control group with a similar age, gender, and body mass index distribution. As demonstrated in Figure 3A, a pronounced discrimination between fecal metabolite profiles of patients on hemodialysis and the second healthy control group was noted in the corresponding PLS-DA model. In addition, each left-out sample and each sample of the five unrelated patients on hemodialysis could accurately be classified in leave-one-out cross-validation and prediction analysis, respectively (Figure 3B). The same was also observed when performing a secondary analysis in nondiabetic patients on hemodialysis (Figure 3, C and D). When investigating discriminating metabolites, we identified a total of 55 significant VOCs (FDR-adjusted) of which 17 VOCs were also different between patients on hemodialysis and the other healthy control group (Supplemental Tables 1 and 2).
Figure 3.
PLS-DA of fecal metabolite profiles of patients on hemodialysis and age-matched healthy controls. (A) PLS-DA score plot of fecal metabolite profiles of patients on hemodialysis (solid blue square) and age-matched healthy controls (black diamond). (B) Leave-one-out cross-validation and prediction analysis from the PLS-DA model for patients on hemodialysis (solid blue square), age-matched healthy controls (black diamond), and five additional unrelated patients on hemodialysis (blue box). (C) PLS-DA analysis score plot of fecal metabolite profiles of nondiabetic patients on hemodialysis (solid blue square) and age-matched healthy controls (black diamond). (D) Leave-one-out cross-validation and prediction analysis from the PLS-DA model for nondiabetic patients on hemodialysis (solid blue square), age-matched healthy controls (black diamond), and five additional unrelated patients on hemodialysis (blue box); the predicted group of hemodialysis patients has a target value of y=1, the group of healthy controls has a target value of y=0, and the discriminant threshold (y=0.5) is the dashed line.
CKD versus Household Contacts on the Same Diet
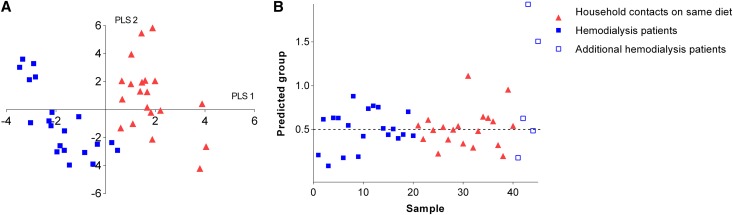
As patients with CKD are subjected to substantial dietary restrictions and other therapeutic interventions, it is difficult to elucidate the impact of renal function loss per se on the colonic microbial metabolism. Therefore, we included a second control group of household contacts sharing the dietary habits of the patients on hemodialysis. In addition, there were neither significant between-group differences in age, gender, body mass index nor in the prevalence of diabetes. In this population, we identified a total of 203 different VOCs with 53 subject-specific VOCs and 28 VOCs that were found in all subjects. There was no significant difference in number of VOCs per sample between patients on hemodialysis and household contacts on the same diet (mean of 79 [SD 5] versus 77 [SD 9] VOCs, respectively, P=0.38). When performing PCA on fecal metabolite profiles of both groups, there was no discrimination between samples of patients on hemodialysis and household contacts (data not shown). By assigning group information with the PLS-DA model (Figure 4A), a discrimination could be observed between the two groups, which was, however, not as pronounced as in the previous analysis. Classification of left-out samples in the leave-one-out cross-validation was also inaccurate (sensitivity 45%, specificity 50%) and prediction analysis of unrelated patients on hemodialysis failed to correctly predict three out of five samples (Figure 4B).
Figure 4.
Fecal metabolite profiles of patients on hemodialysis and household contacts on the same diet. (A) PLS-DA score plot of fecal metabolite profiles of patients on hemodialysis (solid blue square) and household contacts on the same diet (red triangle). (B) Leave-one-out cross-validation and prediction analysis from the PLS-DA model for patients on hemodialysis (solid blue square), household contacts on the same diet (red triangle), and five additional unrelated patients on hemodialysis (blue box); the predicted group of patients on hemodialysis has a target value of y=0, the group of household contacts on the same diet has a target value of y=1, and the discriminant threshold (y=0.5) is the dashed line.
Again, we explored individual VOCs being different between patients on hemodialysis and household contacts on the same diet. Now, we only identified two VOCs (benzofuran and dimethyl sulfide), albeit not formally significant after FDR adjustment. When taking into account chemical classes and examining the correlation loading plot for possible shifts, we found an upregulation of aldehydes and furans in the group of patients on hemodialysis.
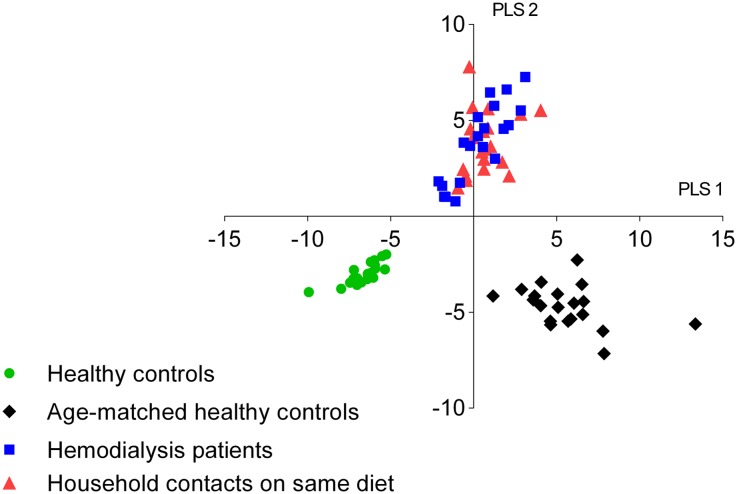
The differential impact of the renal phenotype (including differences in diet, age, and other CKD-related factors) versus renal function loss itself was also clearly visualized in the score plot of the combined PLS-DA model, simultaneously incorporating all four groups (patients on hemodialysis, healthy subjects, age-matched healthy subjects, household contacts on the same diet; Figure 5). While there was a clear discrimination between fecal metabolite profiles of patients on hemodialysis and both healthy control groups, this difference faded when considering household contacts on the same diet.
Figure 5.
Fecal metabolite profiles of patients on hemodialysis, healthy controls, age-matched healthy controls, and household contacts on the same diet. PLS-DA score plot of fecal metabolite profiles of patients on hemodialysis (solid blue square), healthy controls (green circle), age-matched healthy controls (black diamond), and household contacts on the same diet (red triangle).
Animal Data
To further explore the sole impact of renal function loss we compared rats with CKD 6 weeks after induction of 5/6th nephrectomy with control rats 6 weeks after sham operation. As expected, serum creatinine and 24-h proteinuria were significantly higher in the CKD group than in the control group. Although both groups gained weight during the study, the final body weight of rats with CKD was lower, probably due to a lower cumulative food intake (Table 4). When analyzing their fecal metabolite profiles, we observed a total of 227 individual VOCs. Of these, 20 were animal-specific and 60 were found in all fecal samples. There were no significant differences in number of VOCs per sample between fecal samples of rats with CKD and those of control rats (mean 136 [SD 7] versus 133 [SD 6] VOCs, respectively, P=0.36). PCA demonstrated discrimination between fecal samples of both groups (data not shown), which was even more pronounced when also including group information within the PLS-DA model (Figure 6A). In leave-one-out cross-validation, apart from one sample in each group, all samples could be correctly classified (sensitivity of 90% and specificity of 90%; Figure 6B).
Table 4.
Characteristics of rats with CKD and control rats 6 weeks after 5/6th nephrectomy or sham operation
| Variable | Rats with CKD (n=10) | Control Rats (n=10) | P Value |
|---|---|---|---|
| Serum creatinine (mg/dl) | 1.58 (±0.27) | 0.84 (±0.24) | <0.001 |
| Creatinine clearance (ml/min) | 0.80 (±0.29) | 1.67 (±0.36) | <0.001 |
| Proteinuria (mg/24 h) | 79 (40–160) | 12 (8–14) | <0.001 |
| Body weight (g) | 370 (±36) | 406 (±25) | 0.02 |
| Cumulative food intake (g per 6 weeks) | 996 (±50) | 1090 (±38) | <0.001 |
Figure 6.
Fecal metabolite profiles of rats 6 weeks after induction of 5/6th nephrectomy and after sham operation. (A) PLS-DA score plot of fecal metabolite profiles of rats 6 weeks after induction of 5/6th nephrectomy (CKD rats, solid blue square) and after sham operation (control rats, green circle). (B) Leave-one-out cross-validation from the PLS-DA model for rats with CKD (solid blue square) and control rats (green circle); the predicted group of rats with CKD has a target value of y=1, the group of control rats has a target value of y=0, and the discriminant threshold (y=0.5) is the dashed line.
We also explored individual metabolites responsible for the discrimination. In unadjusted analysis, 58 VOCs differed between rats with CKD and control rats. After FDR adjustment, four VOCs remained significantly different (Table 5). Of these, levels of ethylbenzene were downregulated in rats with CKD, in agreement with the patients on hemodialysis. For p-cresol and indole, there were no significant differences between rats with CKD and control rats (both unadjusted P value of 0.10). When stratified according to chemical classes in the correlation loading plot, induction of renal insufficiency by 5/6th nephrectomy resulted in a predominant upregulation of alkanes, branched-chain fatty acids, phenols, and sulfides, while short-chain fatty acids and esters were downregulated.
Table 5.
Relative levels of VOCS differentially expressed in fecal samples of rats with CKD and controls rats (median [IQR])
| Metabolite | Rats with CKD | na | Control Rats | na | P Valueb | |
|---|---|---|---|---|---|---|
| Up | Benzoic acid | 0.0004 (0.0002–0.0007) | 8 | 0 (0–0) | 0 | 0.04 |
| Down | 1-Hexadecanol | 0 (0–0) | 0 | 0.0024 (0.0019–0.0094) | 9 | 0.04 |
| Propanoic acid | 0.1388 (0.1038–0.1582) | 10 | 0.2244 (0.1919–0.2613) | 10 | 0.04 | |
| Ethylbenzene | 0 (0–0) | 2 | 0.0030 (0.0015–0.0053) | 10 | 0.04 |
Number of rat samples in which volatile organic compound was detected (/10).
FDR-adjusted P value.
DISCUSSION
In this study we explored the influence of CKD on the colonic microbial metabolism. The key findings are that CKD is associated with a distinct colonic microbial metabolism. The CKD-related differences in the human colonic microbial metabolism can be attributed to a large extent to dietary restrictions and to a lesser extent to loss of renal function.
Lately, there is increasing interest in the colonic microbial metabolism as a contributor to the uremic retention solutes accumulating in CKD.4,5 Both p-cresyl sulfate and indoxyl sulfate are representatives of this group of solutes and have been associated with overall mortality, cardiovascular disease, and progression of CKD.6,7,19–22 Recently, it has been demonstrated that CKD profoundly alters the colonic microbial composition.10 However, the impact of CKD on the colonic microbial metabolism is largely unexplored.
In a first analysis, we demonstrated substantial differences in the fecal metabolite profiles of patients on hemodialysis and healthy controls, indicative of a distinct colonic microbial metabolism in CKD. When looking into more detail, we identified a total of 81 fecal metabolites being significantly different between patients on hemodialysis and healthy controls. Interestingly, generation of both p-cresol and indole as precursors of p-cresyl sulfate and indoxyl sulfate, respectively, was also upregulated in patients on hemodialysis, which confirms and extends a previous observation of higher levels of p-cresol and indole producing microbiota in patients with CKD.23 In addition, there was an overall upregulation of aldehydes, benzenes, branched-chain fatty acids, furans, indoles, medium-chain fatty acids, and short-chain fatty acids, while the generation of ketones was blunted in patients on hemodialysis. The clinical relevance of these fecal metabolite shifts is unknown. It may be hypothesized that alterations in fecal metabolite profiles may contribute to CKD-associated intestinal barrier dysfunction, possibly leading to bacterial translocation and endotoxemia as a mechanism behind systemic inflammation and cardiovascular disease in patients with CKD.24–26 In addition, this may cause a paradigm shift in the traditional view of CKD as a state of accumulating potentially toxic solutes due to diminished renal excretion. Indeed, these findings may suggest that CKD also affects the intestinal exposure to different metabolites. Linking these fecal metabolite shifts to concomitant changes in serum levels in patients with CKD may therefore be relevant to further elucidate the importance of the gut-kidney axis.
As CKD not only implies a loss of renal function, but goes along with differences in age and comorbidity (e.g., diabetes mellitus), as well as substantial restrictions in diet, we performed additional analyses in an attempt to elucidate the differential impact of renal function loss itself versus the renal phenotype. First, we included an age-matched healthy control group, again demonstrating a pronounced discrimination with patients on hemodialysis. Next, we compared patients on hemodialysis with household contacts on the same diet who were also similar with respect to age, gender, body mass index, and presence of diabetes mellitus. Fecal metabolite profiles of household contacts closely resembled those of patients on hemodialysis. As the household contacts were no direct relatives of the patients on hemodialysis, a common genetic background cannot explain the resemblance in fecal metabolite profiles, thus pointing to similarity in external influences. In this regard, it has been noted that cohabitation is associated with a more comparable colonic microbial composition.27,28 Although this has mainly been explained by shared dietary habits as also present in the household contacts of our study, there may also be possible involvement of the mutual physical environment and social interaction.29,30 Additionally, as it remains challenging to explore the sole effect of human renal function loss, we studied fecal metabolite profiles in rats 6 weeks after 5/6th nephrectomy as well as after sham operation with both groups receiving regular dietary supply. In agreement with a previous experimental study by Meinardi et al.,31 there were substantial differences in fecal metabolite profiles between CKD and control rats, pointing to an important and independent influence of renal function loss per se. Although interspecies differences cannot be excluded, these findings suggest that dietary and other CKD-related factors have a substantial impact on colonic microbial metabolism in patients with CKD and may even outweigh the impact of renal function loss itself.
As nutrient intake is one of the most important factors driving colonic microbial behavior in the general population, it may not be surprising that dietary differences have a substantial impact on colonic microbial metabolism in patients with CKD.13,32 However, changing nutrient intake in patients with CKD may not be simple, as these patients are at risk for hyperkalemia, hyperphosphatemia, and malnutrition. Therefore, targeting the colonic microbial metabolism may be more feasible than interfering with current standard-of-care dietary restrictions. We previously explored the potential beneficial effect of prebiotics consisting of a mixture of inulin and oligofructose in patients on hemodialysis, resulting in reduced serum levels and generation rates of p-cresyl sulfate.33 Small studies with probiotics also demonstrated their potential to reduce the generation of certain microbial-derived uremic retention solutes (e.g., indoxyl sulfate).34,35 However, it must be noted that although these results are encouraging, there are no studies investigating the benefit of these therapeutics on hard clinical end points.
There are limitations to our study. First, to investigate colonic microbial metabolism we studied metabolite profiles in fecal samples, probably more reflecting distal colonic microbial metabolism than that of the entire colon. In addition, we cannot exclude a concomitant effect of altered colonic metabolite transport due to CKD. Third, although household contacts were selected based on a statement of similar dietary habits, we did not formally include dietary assessments. Therefore, minor dietary differences between patients on hemodialysis and household contacts cannot be excluded, but would probably not substantially alter the abovementioned conclusions. Availability of dietary information may also have enabled us to link changes in fecal metabolite profiles to specific CKD-related dietary differences. Fourth, our study population mainly consisted of patients of Caucasian origin. Care must be taken to extrapolate our data to other patient populations. Finally, a direct comparison between animal and human data may be challenging when considering interspecies differences in colonic microbial metabolism. Thus, further studies are still required to establish the sole effect of human renal function loss on fecal metabolite profiles.
In conclusion, the colonic microbial metabolism is altered in CKD. While this can be attributed in part to loss of kidney function per se, dietary and other CKD-related factors also affect the colonic microbial metabolism and even outweigh the importance of a reduced GFR. These observations challenge the conventional paradigm of accumulation of potentially toxic solutes solely due to diminished renal excretion. Our findings suggest that CKD also affects exposure to metabolites through altered colonic microbial metabolism.
CONCISE METHODS
Patients and Controls
Patients with CKD, treated with maintenance hemodialysis therapy for at least 3 months at the dialysis unit of the University Hospitals Leuven, ≥18 years and able to provide consent, were eligible for inclusion (clinicaltrials.gov NCT01874210). We included three control groups composed of unrelated healthy subjects, unrelated age-matched healthy subjects, as well as household contacts on the same diet. Each group consisted of 20 subjects. Patients with known gastrointestinal disease (e.g., inflammatory bowel disease) and previous colorectal surgery were excluded. Use of antibiotics, prebiotics or probiotics in the past 4 weeks was not allowed. Both patients on hemodialysis and control subjects were asked to bring a fecal sample to the laboratory within 12 h of defecation. After collection of the fecal sample, an aliquot was immediately frozen and stored at –20°C until further analysis. Preliminary testing demonstrated no differences between fecal metabolite profiles of fresh and frozen (–20°C) samples. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the University Hospitals Leuven. Informed consent was obtained from all patients.
Animals
Experimental procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals 85–23 (1996) and approved by the ethics committee of the University of Antwerp (Permit number: 2012–13). Fourteen male Wistar rats (Charles River, Lille, France) were subjected to 5/6th nephrectomy, consisting of the ligation of 2/3 of the extrarenal branches of the renal artery of the left kidney, followed by nephrectomy of the right kidney one week later. Before surgery, rats were anesthetized by intraperitoneal injection of 60 mg/kg Nembutal (Ceva Santé Animale, Libourne, France). During study, rats were fed a standard rodent diet (SSNIFF Spezialdiäten, Soest, Germany) and had free access to food and water. Animals were housed two per cage and bedding was changed twice weekly. At 6 weeks post 5/6th nephrectomy, 10 animals were still alive and included for further analysis. At that moment, blood was drawn by puncture of the tail vein. Furthermore, urine and fecal matter were collected for 24 h using metabolic cages. As a control group, we used 10 male Wistar rats undergoing sham operation and fed the same diet. Again, 6 weeks after the procedure, collection of blood, urine, and fecal matter was performed.
Analytical Methods
Fecal metabolite profiles were studied using a dedicated GC-MS method, allowing for untargeted metabolomics of fecal VOCs with identification of a highly relevant subgroup, including p-cresol and indole as precursors of p-cresyl sulfate and indoxyl sulfate, respectively.18 Immediately before analysis, fecal aliquots were thawed and 0.25 g fecal sample was suspended in 4870 µl of water. 2-Ethylbutyrate (40 µl; 250 mg/100 ml) was added as internal standard. A magnetic stirrer, a pinch of sodium sulfate, and 130 µl of sulfuric acid were added to the sample to salt out and acidify the solution, respectively. To prevent crossover from one sample to another, water samples were extracted after each sample. The VOCs were analyzed on a GC-MS (Trace GC, Thermoquest, Rodano, Italy and DSQ II, Thermo Electron, San José, CA), which was coupled online to a purge-and-trap system (Velocity, Teledyne Tekmar, Mason, OH). Metabolites were purged out the sample for 20 min with a helium flow rate of 40 ml/min, carried over a dry flow column (Trap Tenax tbv Velocity, Interscience, Louvain-la-Neuve, Belgium) for 3 min to control moisture transfer, and concentrated on a second polar trap column (Trap Vocarb tbv Velocity, Interscience). Consequently, the VOCs were desorbed from the trap by raising the trap temperature to 250°C for 5 min. After desorption, the trap temperature was further raised to 270°C for 10 min to remove any contamination of tailing compounds. The desorbed compounds were conducted via the transfer line (175°C) to the injector of the gas chromatograph, where they were separated on an analytical column, AT Aquawax DA (30 m×0.25 mm internal diameter, 0.25 µm film thickness, Grace, Deerfield, IL). Helium was used as the carrier gas with a constant flow of 10 ml/min. The oven temperature was maintained at 35°C (isothermal for 2 min) and increased with 5°C/min to 100°C and with 10°C/min to 240°C. This final temperature was held for 5 min. Mass spectroscopy was performed in full scan mode from m/z 30 to m/z 500 at two scans per second. Xcalibur software (Thermo Scientific, Breda, The Netherlands) was used for the automation of the GC-MS and for data acquisition. The resulting chromatograms were processed using Automatic Mass Spectral Deconvolution and Identification Software (AMDIS, version 2.71) provided by the National Institute of Standards and Technology (NIST, Gaithersburg, MD). Identification of the metabolites in the samples was achieved by comparing the mass spectra of unknown peaks with the NIST library. Compounds showing mass spectra with match factors ≥90% were positively identified. Differential peak identities were further confirmed for retention time and mass spectra from our in-house standards library. All compounds were relatively quantified compared with 2-ethylbutyrate.
Statistical Analysis
Data are expressed as mean (±SD) for normally distributed variables or median (IQR) for non-normally distributed variables. Between-group differences of baseline characteristics were tested using Student’s t test, Wilcoxon rank sum test, or chi-squared test, as appropriate. P values less than 0.05 were considered significant.
For the statistical processing of fecal metabolite profiles, sample-specific VOCs, that is, compounds that were detected in only one subject, were discarded from statistical analysis because they do not exert any discriminatory power due to their low occurrence rate and introduce noise if implemented into the classification model.36 Multivariate statistical analysis consisted of PCA, hierarchical cluster analysis, and PLS-DA. Data were weighed by their SD to give them equal variances. PCA and hierarchical cluster analysis, both unsupervised methods, were carried out for first data exploration and clustering of metabolite profiles without knowledge of group membership. PLS-DA, a supervised learning technique, was then applied to cluster samples with similar metabolite profiles after assignment of group information. The obtained models were validated using leave-one-out cross-validation and the additional inclusion of five unrelated samples of patients with hemodialysis also allowed testing of the predictive performance of these models. Between-group differences of VOCs were explored with the correlation loading plot and Wilcoxon rank sum test. The Benjamini–Hochberg FDR was applied to correct for multiple testing and FDR-adjusted P values of less than 0.05 were considered significant.37 All statistical analyses were performed using SAS (version 9.3, the SAS institute, Cary, NC) and Unscrambler (Version 10.3, CAMO A/S, Trondheim, Norway).
DISCLOSURE
None.
Supplementary Material
Acknowledgments
Technical assistance by G. Vandermeulen and E. Houben is highly appreciated.
RP is the recipient of a fellowship of the Research Foundation – Flanders (FWO) (grant 11E9813N). Part of the research has been funded by the Research Foundation – Flanders (FWO) (grant G077514N).
Part of this work was presented at the American Society of Nephrology Kidney Week, November 5–10, 2013, Atlanta, GA and at the ERA-EDTA Congress, May 31–June 3, 2014, Amsterdam, the Netherlands.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030279/-/DCSupplemental.
References
- 1.Shanahan F: The gut microbiota in 2011: Translating the microbiota to medicine. Nat Rev Gastroenterol Hepatol 9: 72–74, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G: Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106: 3698–3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114: S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P: p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5: 1182–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B: Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8: 1508–1514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijers BK, Evenepoel P: The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 26: 759–761, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R: Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macfarlane GT, Macfarlane S: Models for intestinal fermentation: association between food components, delivery systems, bioavailability and functional interactions in the gut. Curr Opin Biotechnol 18: 156–162, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Smith EA, Macfarlane GT: Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 81: 288–302, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ: The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32: 2094–2101, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int 64: 2196–2203, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ: Colonic transit time in long-term dialysis patients. Am J Kidney Dis 44: 322–327, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G: Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 12: 17–31, 2002 [DOI] [PubMed] [Google Scholar]
- 18.De Preter V, Van Staeyen G, Esser D, Rutgeerts P, Verbeke K: Development of a screening method to determine the pattern of fermentation metabolites in faecal samples using on-line purge-and-trap gas chromatographic-mass spectrometric analysis. J Chromatogr A 1216: 1476–1483, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS: p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 26: 938–947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND: Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T: Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32: 754–759, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS: Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant 27: 2686–2693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK: Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, Gordon JI, Fierer N, Knight R: Cohabiting family members share microbiota with one another and with their dogs. eLife 2: e00458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI: Human gut microbiome viewed across age and geography. Nature 486: 222–227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, Metcalf JL, Ursell LK, Vázquez-Baeza Y, Van Treuren W, Hasan NA, Gibson MK, Colwell R, Dantas G, Knight R, Gilbert JA: Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345: 1048–1052, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung J, Barreiro LB, Burns MB, Grenier JC, Lynch J, Grieneisen LE, Altmann J, Alberts SC, Blekhman R, Archie EA: Social networks predict gut microbiome composition in wild baboons. eLife 4: 05224, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meinardi S, Jin KB, Barletta B, Blake DR, Vaziri ND: Exhaled breath and fecal volatile organic biomarkers of chronic kidney disease. Biochim Biophys Acta 1830: 2531–2537, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Tremaroli V, Bäckhed F: Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Takayama F, Taki K, Niwa T: Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 41[Suppl 1]: S142–S145, 2003 [DOI] [PubMed] [Google Scholar]
- 36.De Preter V, Ghebretinsae AH, Abrahantes JC, Windey K, Rutgeerts P, Verbeke K: Impact of the synbiotic combination of Lactobacillus casei shirota and oligofructose-enriched inulin on the fecal volatile metabolite profile in healthy subjects. Mol Nutr Food Res 55: 714–722, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y: Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc, Series B 57: 289–300, 1995 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.