Abstract
Bisphenol A (BPA), a component of some dialysis membranes, accumulates in CKD. Observational studies have linked BPA exposure to kidney and cardiovascular injury in humans, and animal studies have described a causative link. Normal kidneys rapidly excrete BPA, but insufficient excretion may sensitize patients with CKD to adverse the effects of BPA. Using a crossover design, we studied the effect of dialysis with BPA-containing polysulfone or BPA-free polynephron dialyzers on BPA levels in 69 prevalent patients on hemodialysis: 28 patients started on polysulfone dialyzers and were switched to polynephron dialyzers; 41 patients started on polynephron dialyzers and were switched to polysulfone dialyzers. Results were grouped for analysis. Mean BPA levels increased after one hemodialysis session with polysulfone dialyzers but not with polynephron dialyzers. Chronic (3-month) use of polysulfone dialyzers did not significantly increase predialysis serum BPA levels, although a trend toward increase was detected (from 48.8±6.8 to 69.1±10.1 ng/ml). Chronic use of polynephron dialyzers reduced predialysis serum BPA (from 70.6±8.4 to 47.1±7.5 ng/ml, P<0.05). Intracellular BPA in PBMCs increased after chronic hemodialysis with polysulfone dialyzers (from 0.039±0.002 to 0.043±0.001 ng/106 cells, P<0.01), but decreased with polynephron dialyzers (from 0.045±0.001 to 0.036±0.001 ng/106 cells, P<0.01). Furthermore, chronic hemodialysis with polysulfone dialyzers increased oxidative stress in PBMCs and inflammatory marker concentrations in circulation. In vitro, polysulfone membranes released significantly more BPA into the culture medium and induced more cytokine production in cultured PBMCs than did polynephron membranes. In conclusion, dialyzer BPA content may contribute to BPA burden in patients on hemodialysis.
Keywords: hemodialysis, oxidative stress, ESRD
2,2-Bis-(4-hydroxyphenyl) propane (bisphenol A [BPA]) was synthesized in the 1930s as a synthetic estrogen,1,2 but was displaced by diethylstilbestrol. Currently, BPA is a component of polycarbonate plastics and epoxy resins and is considered an environmental toxin. BPA contains aromatic rings with structural similarity to phenols, and BPA metabolism and disposal may have common characteristics with uremic toxins such as p-Cresol.3 BPA may be absorbed in the gastrointestinal tract after ingesting products packed in plastic containers, polycarbonate bottles, or cans of food and beverages. Like other exogenous phenols, BPA is conjugated by glucuronic acid in the bowel and liver and excreted in urine as BPA glucuronide.4 In this regard, BPA may also be considered a uremic toxin, because it accumulates in CKD and there is evidence that its accumulation is harmful.3
BPA is a chemical switch in endocrine processes, and may impact reproduction, weight, and development. Very low concentrations of BPA alter steroidogenesis5 and cell functions.6 In this regard, BPA exposure has been linked to the development of obesity, insulin resistance, metabolic syndrome, diabetes, and atherosclerosis.6–9
An epidemiologic association was observed between high urinary BPA levels and cardiovascular disease. Each increase in urinary BPA of 4.5 μg/L was associated with a 13% increase in the incidence of coronary heart disease within 10 years, although the significance was lost after adjustment for traditional cardiovascular risk factors.10 In healthy adult Americans, urinary BPA levels >4 μg/L were associated with a 50% increase in the prevalence of hypertension compared with levels <1.5 μg/L.11 Urinary BPA levels >1.4 μg/L were associated with a 23% higher risk of microalbuminuria than in healthy adults with levels <0.5 μg/L.12 Experimental studies have identified potential mechanisms of BPA nephrotoxicity through injury of glomerular podocytes, oxidative stress, inflammation, and induction of arterial hypertension.13–15
One argument by government agencies for considering safe the use of BPA in the general population is the almost complete and rapid urinary elimination of the conjugated molecule, which decreases the risks of exposure to BPA.16 Thus, BPA exposure is assessed as urinary BPA concentration in the general population because serum levels are very low when renal function is normal. However, serum BPA levels increase with decreasing renal function and are highest in individuals on hemodialysis,17 reflecting the accumulation of BPA in CKD. For this reason, studies on the causes and consequences of BPA accumulation in CKD patients are needed.
BPA is found in the housing (polycarbonate) and/or membranes of some commonly used dialyzers. The amount of BPA in polyester polymer alloy dialyzers is 12.2 μg/g, in polysulfone 8–34.5 μg/g, in polycarbonate 47.2 μg/g, in polymethylmethacrylate 0.008 μg/g, in cellulose triacetate 0.008 μg/g, and in cellulose 0.016 μg/g.17,18 BPA is released from dialyzers, although at lower levels than the limits stipulated by health authorities. We have now evaluated in a crossover study the impact of the choice of dialyzer (BPA-free versus BPA-containing) on serum and intracellular BPA levels and on inflammation and oxidative stress markers.
Results
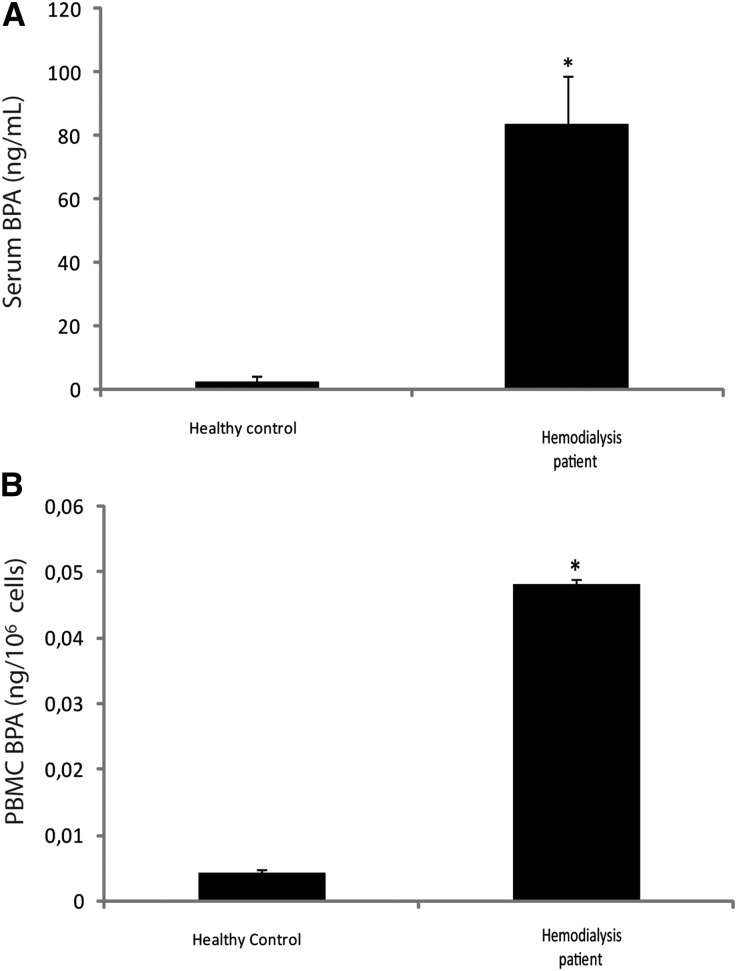
Serum and Intracellular BPA Levels are Higher in Patients on Hemodialysis than in Healthy Controls
Both serum and intracellular BPA were higher in patients on hemodialysis than in healthy controls (35-fold and 12-fold, respectively; Figure 1, A and B).
Figure 1.
Bisphenol serum and intracellular concentration. (A) Serum and (B) intracellular BPA in healthy controls and patients on hemodialysis. *P<0.05 versus control.
Polysulfone Membranes Increase Serum BPA Levels Following a Single Hemodialysis Session while Chronic Use of Polynephron Membranes for 3 Months Decreases Serum BPA
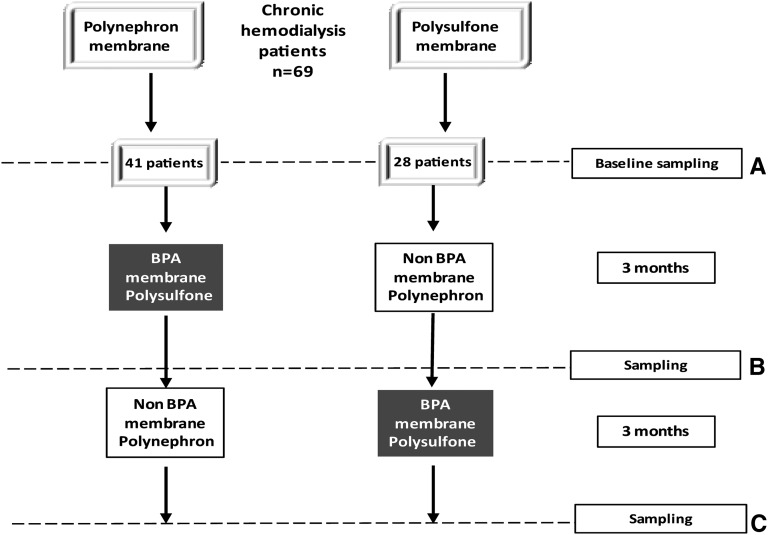
In a crossover design, prevalent patients chronically dialyzed with polysulfone (baseline polysulfone, n=28) were switched for 3 months to polynephron and then switched back for 3 months to polysulfone (Figure 2). Conversely, patients chronically dialyzed with polynephron (baseline polynephron, n=41) were switched for 3 months to polysulfone and then switched back for 3 months to polynephron (Figure 2).
Figure 2.
Study design. (A and B) In this crossover study, prevalent patients on hemodialysis were evaluated and sampled at baseline and next switched to a different dialysis membrane for 3 months. At that point, samples were taken and the membrane switched to the baseline one. (C) At 6 months, new sampling took place.
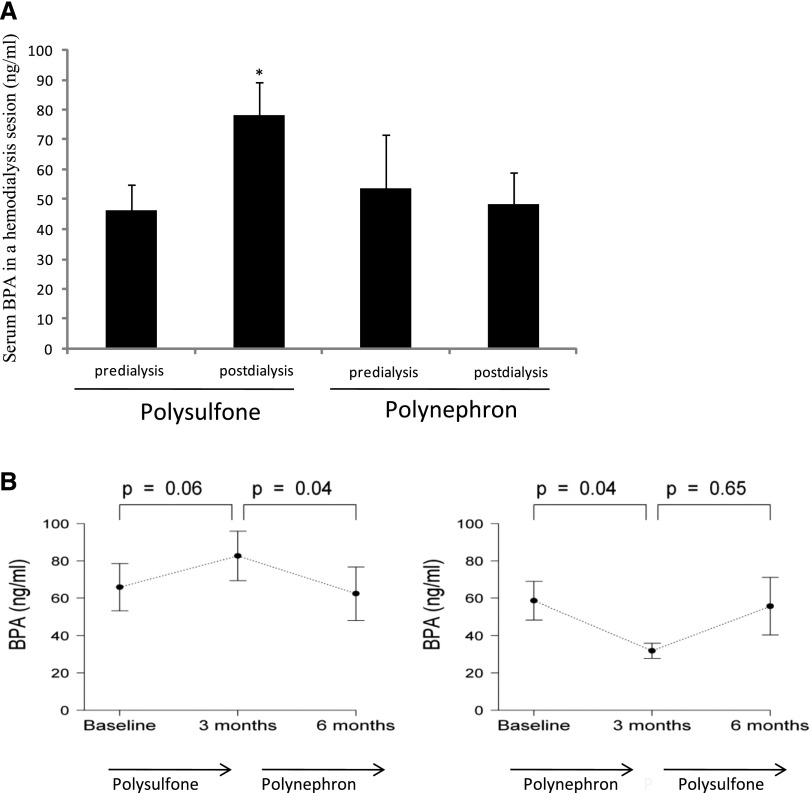
Mean BPA levels increased from 46.3±8.5 ng/ml to 78.4±11.0 ng/ml (68% increase over predialysis baseline values, P<0.05) following a single hemodialysis session with polysulfone dialyzers, while no changes were observed with polynephron membranes (Figure 3A). A trend toward an increase in outflow over inflow BPA levels was also observed with polysulfone, but not with polynephron membranes (Supplemental Figure 1).
Figure 3.
Serum BPA in patients on hemodialysis dialyzed with polysulfone or polynephron dialyzers. (A) Change in serum BPA following a single hemodialysis session with polysulfone or polynephron dialyzer. Mean+SEM of postdialysis versus predialysis values. *P<0.002 versus predialysis values. (B) Mean predialysis serum BPA over time for 6 months.
The continuous use of a polysulfone membrane for 3 months tended to increase predialysis serum mean BPA levels (from 48.8±6.8 to 69.1±10.1 ng/ml, a 44% increase for the combined 0–3 and 3–6 month groups, NS). By contrast, chronic use of polynephron membranes for 3 months reduced predialysis serum BPA (from 70.6±8.4 to 47.1±7.5 ng/ml, a 33% decrease for the combined 0–3 and 3–6 month groups, P<0.05; Figure 3B).
A smaller study of shorter duration (3 weeks for each dialyzer) in incident patients disclosed a similar trend toward increased serum BPA levels with polysulfone membranes (Supplemental Figure 2).
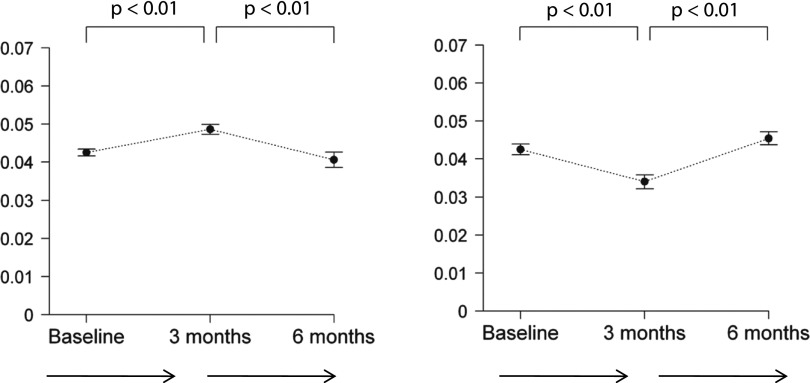
Chronic Use of Polysulfone Membranes Increases and of Polynephron Membranes Decreases Intracellular BPA
Chronic hemodialysis with polysulfone membranes for 3 months increased predialysis intracellular BPA in PBMCs (from 0.039±0.002 to 0.043±0.001 ng/106 cells, a 10% increase for the combined 0–3 and 3–6 month groups, P<0.01), whereas polynephron membranes decreased intracellular BPA (from 0.045±0.001 to 0.036±0.001 ng/106 cells, a 20% decrease for the combined 0–3 and 3–6 month groups, P<0.01; Figure 4).
Figure 4.
Intracellular BPA in PBMCs from patients on hemodialysis dialyzed with polysulfone or polynephron dialyzers. Predialysis intracellular BPA values over time for 6 months.
Oxidative Stress and Inflammation Markers
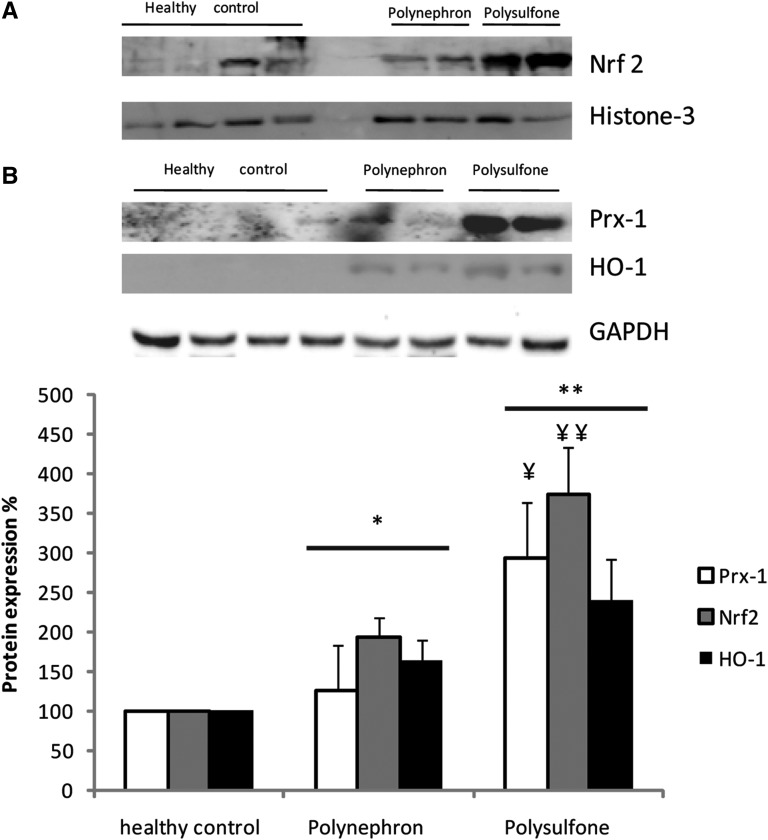
Oxidative stress markers were measured in 10 randomly selected patients. Oxidative stress activates the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription factor, which in turn promotes the expression of genes encoding proteins that protect the cell from oxidative stress, such as heme oxygenase 1 (HO-1) and peroxiredoxin 1 (Prx-1). The expression of oxidative stress markers was significantly higher after 3 months of hemodialysis with polysulfone than with polynephron membranes (Figure 5).
Figure 5.
Expression of oxidative stress proteins Nrf2, HO-1, and Prx-1 in PBMCs from patients dialyzed for 3 months with polysulfone or polynephron membranes. (A) Nrf2 Western blot using nuclear fraction and a histone-3 loading control. (B) HO-1 and Prx-1 Western blot using cytosolic fraction and a GAPDH loading control. (C) Quantification of Western blotting results. Values expressed as percentage increase over healthy controls. *P<0.05 versus healthy control; **P<0.001 versus healthy control; ¥P<0.05 versus polynephron; ¥¥P<0.01 versus polynephron.
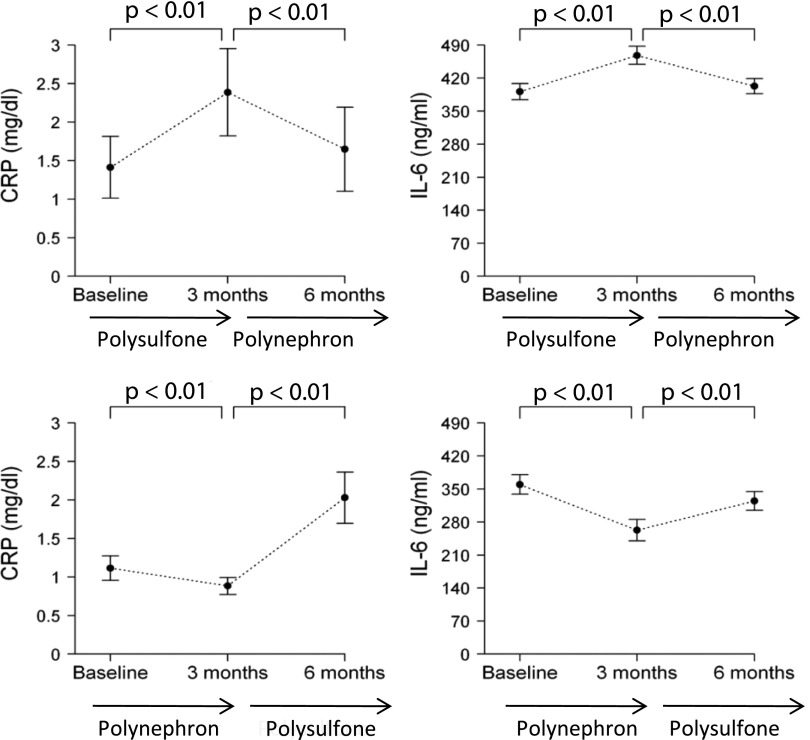
Inflammation markers were assessed in all patients. Three months of hemodialysis with polysulfone membranes increased circulating C-reactive protein (CRP) and IL-6 while polynephron membranes decreased CRP and IL-6 (P<0.001; Figure 6).
Figure 6.
Circulating inflammatory biomarkers in patients dialyzed with polysulfone or polynephron dialyzers for 3 months.
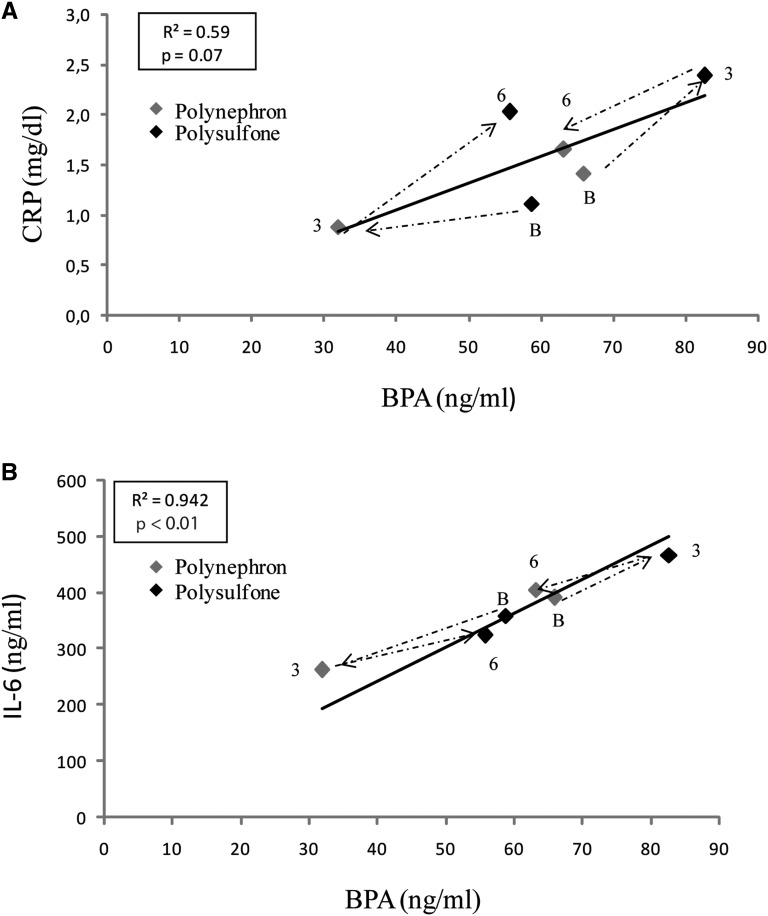
Changes in the concentration of circulating inflammatory biomarkers correlated with changes in BPA concentrations and both inflammatory markers and serum BPA increased when polysulfone membranes were employed (Figure 7).
Figure 7.
Correlation between serum BPA levels and inflammatory markers. Mean values for each group and time point are shown. BPA and inflammatory markers (A) CRP and (B) IL-6 were assessed in the same samples. Dashed lines represent timelines for the two independent groups. The first data point (B) along the dashed line represents baseline values, the second (3) the 3-month time point, and the third (6) the 6-month time point. Arrows represent the sequence of dialyzers. The dialyzer is color-coded. This was a crossover study of prevalent patients on hemodialysis and each independent study group was dialyzed at baseline with a different dialyzer. Thus, one group changed from polysulfone at baseline to polynephron and back to polysulfone, and the other group changed from polynephron at baseline to polysulfone and back to polynephron.
BPA and Polysulfone BPA-Containing Membranes Increase Inflammatory Markers in Cultured PBMCs
BPA dose-dependently increased cytokine mRNA expression in cultured PBMCs from healthy donors within a concentration range relevant for patients on hemodialysis (Supplemental Figure 3).
Coincubation of mashed polysulfone dialyzers dose-dependently increased cytokine mRNA expression and secretion in cultured healthy donor PBMCs (Supplemental Figure 4, A–D). In this regard, within 24 hours of culture, BPA was dose-dependently released from polysulfone dialyzer fibers into the culture media (Supplemental Figure 5). Thus, PBMCs may be exposed both to released BPA and to BPA within the membrane surface.
Discussion
This is the first prospective study to evaluate the impact of BPA-free dialyzers on serum BPA levels in patients on hemodialysis when compared with state-of-the-art BPA-containing dialyzers. The main finding is that the choice of dialyzer in terms of BPA content impacts on acute (after a single dialysis session) and chronic (after 3 months of continuous use of the same type of dialyzer) changes in serum BPA levels. This demonstrates that dialyzer BPA content may contribute to BPA burden in patients on hemodialysis. These patients are more sensitive to BPA accumulation and potential toxicity due to the loss of the physiologic BPA excretion mechanisms in urine.
BPA is used in the manufacturing of polycarbonate plastics and epoxy resins. The potential risk of BPA for human health has attracted much attention from regulatory agencies. To date, BPA is still considered safe enough in the general population, despite several red flags, as it is readily excreted in the urine. However, patients on hemodialysis are unable to excrete BPA in their urine,19 leading to BPA accumulation.20 Repeated loading of BPA during hemodialysis with BPA-containing membranes may aggravate the problem.17 We have found high serum BPA levels in patients on hemodialysis compared with patients with normal renal function, which is consistent with prior observations that serum BPA rises when the GFR falls below 60 ml/min.21 In this regard, our data support the concept that patients on hemodialysis represent a high-risk population for BPA overload, which may require special regulatory status with regards to BPA-containing medical devices.
BPA may migrate from dialyzers to the blood of patients on hemodialysis.16 However, no prospective, long-term studies have assessed the effect of chronic hemodialysis with state-of-the-art dialyzers with different BPA contents. The only similar study in patients on hemodialysis assessed the effect of hemodialysis for 4 weeks on serum BPA.21 In this prospective study both dialyzers had BPA-containing polycarbonate housing. Both the short duration of the study and the fact that both dialyzers contained BPA may explain the lack of differences between polysulfone and polynephron membranes.21 By contrast, we found differences in serum BPA after 12 weeks of use of BPA-free and BPA-containing dialyzers. Contrary to the 4-week study, the housing in the BPA-free polynephron dialyzers used in our study is also BPA-free. The combination of a longer exposure (3 months versus 1 month) and the use of completely BPA-free dialyzers in one arm of our study allowed the identification of a significant contribution of dialyzer BPA to the BPA burden of patients on hemodialysis. In this regard, in a shorter (3 weeks), smaller (n=7) pilot study in incident patients, the same trend toward increased serum BPA with polysulfone membranes was observed.
Prior studies have also suggested that dialyzer BPA may be released to the circulation, although these were in vitro studies or tested outdated membranes in only single-dialysis studies. Thus, a single-pass saline solution flushing released more BPA from polysulfone membrane dialyzers with polycarbonate (BPA-containing) housing than from polysulfone membrane dialyzers with BPA-free polystyrene housing, suggesting a potential role for housing on modulating serum BPA.18 However, the in vitro nature of the study precludes firm conclusions. In another study, a single hemodialysis session resulted in increased serum BPA levels in patients dialyzed with polysulfone but not in those dialyzed with cellulose membranes.20 However, cellulose membranes are now outdated and information is needed on state-of-the art membranes.
While the molecular mass is 228 D, due to high protein binding, the removal of BPA by hemodialysis is limited.21 The protein-bound fraction of plasma BPA in dialysis patients was estimated to be 74%±5%,21 similar to in vitro estimates of 95% binding at low concentrations.22 Moreover, a tissue:blood partition coefficient of 1.4 for nonadipose tissues and 3.3 for fat tissues further compromises the dialyzability of BPA.22 Thus, the observed decrease in BPA concentration following chronic but not acute dialysis with polynephron dialyzers may have an important component of decreased passage of BPA from the dialyzer into the blood, in addition to any removal of the compound.
Bloodlines are another potential source of BPA. All patients in this study used the same bloodlines made of polyvinyl chloride, (PVC), as they are the only ones currently available in our country. Flexible PVC contains BPA as an additive, although its contribution should be minimal (the manufacturer did not specify the BPA content); therefore, bloodlines are a potential additional source of BPA.
Intracellular BPA levels in PBMCs were higher in patients on hemodialysis than in healthy controls, suggesting that high serum BPA results in intracellular accumulation of the toxins in patients on dialysis. Furthermore, dialysis with polysulfone dialyzers further increased intracellular BPA, suggesting that the BPA burden from dialyzers may reach the intracellular space, where it may modulate gene expression.23
Our study argues against a significant role of hemodialysis in BPA clearance because no significant reduction of serum BPA was observed following a single dialysis session. This observation is in line with the higher serum BPA levels in patients on hemodialysis than in healthy controls. In addition, our results suggest that BPA-containing dialyzers may add to the BPA burden and chronic use of BPA-free dialyzers may decrease serum BPA levels, probably as consequence of decreased release of BPA from the dialyzer itself. Repetitive BPA exposure may lead to BPA accumulation in target organs, because the intravenous route bypasses the first-pass liver metabolism that helps detoxify BPA released from food containers and absorbed from the gastrointestinal tract.
Oxidative stress and inflammation contribute to cardiovascular disease in patients with CKD.24 Advanced CKD is characterized by increased oxidative stress and low-level systemic inflammation, best assessed by measuring CRP or IL-6.25–28 Both uremia itself and hemodialysis sessions may contribute to oxidative stress and inflammation. BPA exposure has been linked to inflammation and cardiovascular disease in humans and BPA induces oxidative stress and inflammation in rodents and cultured cells.29,30–33 Oxidative stress was associated with BPA-induced experimental hypertension.15 BPA induces mitochondrial dysfunction and this has been linked to BPA-associated inflammation.34–37 Urinary BPA concentration, a marker of BPA exposure in the general population, is associated with markers of oxidative stress (malondialdehyde and 8-hydroxydeoxyguanosine) and inflammation (white blood cell count and CRP levels) in certain subpopulations such as postmenopausal and pregnant women.30,38,39 In this regard, we found evidence of higher oxidative stress and inflammation in patients on hemodialysis dialyzed with BPA-containing polysulfone membranes than with BPA-free polynephron membranes. Inflammation correlated with changes in BPA concentrations in response to the different membranes. The proinflammatory effect of both BPA and BPA-containing membranes was also observed in cultured leukocytes. Furthermore, polysulfone dialyzers released BPA into the cell culture medium. The proinflammatory dose response of BPA encompasses the range of serum BPA values found in dialysis patients. Overall, the in vitro findings suggest that the BPA content of dialyzers may impact the inflammatory response of circulating leukocytes in vivo.
In conclusion, patients on hemodialysis have higher levels of serum and intracellular BPA than do healthy controls, and the choice of dialysis membrane impacts on serum and intracellular BPA levels. Dialyzers with BPA-containing membranes increase serum BPA levels over 3 months and even following every single hemodialysis session. The BPA increase after a single hemodialysis session suggests that hemodialysis does not compensate for the lack of urine BPA excretion. Rather, the use of BPA-containing dialysis membranes further adds to the BPA burden of patients on hemodialysis. In contrast, the chronic use of BPA-free dialyzers results in decreased BPA levels. Further studies should reveal the health consequences of BPA accumulation from dialysis membranes in patients on hemodialysis who cannot excrete excess BPA in urine. Meanwhile, our data suggest that patients on hemodialysis are a high-risk population for BPA overload, which may require special regulatory status with regards to BPA-containing medical devices.
Concise Methods
Clinical Design and Patient Recruitment
In a prospective 6-month, crossover study we compared BPA-free and BPA-containing dialyzers in 69 prevalent patients on hemodialysis. BPA-free high-flux polynephron (polynephron) membranes housed in polypropylene (Eliseo; Nipro Corp., Osaka, Japan) were compared with high-flux polysulfone (polysulfone) dialyzers that contain BPA (Fx80; Fresenius, Bad Homburg, Germany). The study was approved by the IIS-Fundación Jiménez Díaz Ethics Committee. Patients were enrolled after providing written informed consent.
Inclusion criteria were as follows: age >18 years; hemodialysis vintage >3 months; diuresis <0.5 L/24 h; informed consent; and absence of active inflammatory, infectious, or malignant diseases at the initiation or during the study. Baseline sampling and data were collected at consent. Two groups of patients were initially dialyzed for 3 months with a dialyzer different from the one they were previously using (prestudy dialyzer polynephron in 41 patients and polysulfone in 28 patients), and after 3 months blood was sampled predialysis and patients changed to the other dialyzer (crossover) for 3 months. A third predialysis blood sample was obtained at the end of the study (Figure 2). Bloodlines were made of PVC (Fresenius Medical Care) in all cases. Baseline patient clinical and biochemic basal characteristics are shown in Table 1. In 21 randomly selected patients, intracellular BPA was also measured. A healthy, age- and sex-matched control group with normal renal function was also studied.
Table 1.
Baseline clinical and biochemical characteristics
| N | Mean | SD | |
|---|---|---|---|
| Age | 69 | 65 | 13 |
| Dialysis vintage (months) | 69 | 63 | 92 |
| Kt/V urea | 68 | 1.54 | 0.38 |
| White blood cells (thousands/μl) | 69 | 5.6 | 1.6 |
| Hemoglobin (g/dl) | 69 | 11.5 | 1.3 |
| 25-OH-vitamin D (ng/ml) | 64 | 16 | 10 |
| Total proteins (g/dl) | 69 | 6.6 | 0.5 |
| Albumin (g/dl) | 69 | 3.76 | 0.59 |
| Calcium (mg/dl) | 69 | 9.1 | 0.5 |
| Phosphate (mg/dl) | 69 | 4.8 | 1.5 |
| Cholesterol (mg/dl) | 69 | 154 | 36 |
| Triglycerides (mg/dl) | 69 | 127 | 75 |
| CRP (mg/dl) | 69 | 8.6 | 14.2 |
| Parathormone (pg/ml)a | 69 | 235 | 123–414 |
| EPO dose (U/week) | 62 of 69 (90%) | 5199 | 7724 |
| Calcium-based P binders (mg/day) | 20 of 69 (29%) | 1329 | 859 |
| Sevelamer (mg/day) | 33 of 69 (48%) | 4388 | 2601 |
| Lanthanum (mg/day) | 23 of 69 (33%) | 2641 | 764 |
| Magnesium-based P binders (mg/day) | 8 of 69 (12%) | 822 | 397 |
EPO, erythropoietin; P, phosphate.
Median and interquartile range shown.
Dialysis was performed three times a week, with a blood flow >300 ml/min, and ultrapure dialysate flow 500 ml/min. Fasting blood samples was drawn just prior to a midweek dialysis treatment from the arteriovenous fistula and frozen at –80°C. Blood from ten patients was sampled before (predialysis) and after (postdialysis) a single dialysis session to determine BPA changes in one session using polynephron and in other session using polysulfone dialyzers. In addition, blood was sampled at the inflow and outflow of the dialyzer in five patients.
Basic demographic characteristics and clinical data (age, sex, hemodialysis vintage, and BP), Kt/V urea, and basic serum biochemistry parameters (calcium, phosphate, parathormone, vitamin D, cholesterol, triglycerides, CRP, IL-6, and PBMC oxidative stress markers Nrf2, PRX-1, and HO-1) were assessed.
A pilot (n=7 patients) study in incident patients on hemodialysis (Supplemental Table 1) evaluated serum BPA levels at baseline and following three weeks of dialysis with polysulfone and then polynephron membranes.
PBMCs
For coincubation experiments and intracellular BPA measurements, 10 ml of peripheral blood were obtained from healthy volunteers (blood bank, IIS-Fundacion Jimenez Diaz) after informed consent. Blood was drawn into EDTA tubes and cells were collected and used for isolation of PBMCs by Ficoll density gradient centrifugation.40
BPA Assessment
BPA was measured by a high-sensitivity ELISA (Abnova), following the manufacturer’s instructions. The intra- and interassay coefficients of variation were 6.5% and 10.5%, respectively. For intracellular BPA measurements, PBMCs were resuspended in 100 μl lysis buffer (Tris-HCl 50 mM, NaCl 150 mM, EDTA 2 mM, EGTA 2 mM, Triton X-100 0.2%, NaF 0.2%, NP-40, and PMSF 0.1 mM).
Cell Culture
PBMCs were washed, counted, and adjusted to 2–4×106 cells·ml−1 in culture medium RPMI 1640 supplemented with 1% FBS containing 100 U/ml−1 of penicillin 100 μg/ml−1. PBMCs were incubated with different amounts of both mashed dialyzers extracts (polynephron and polysulfone) or BPA (Sigma-Aldrich, St. Louis, MO) for 24 hours.
RNA and Protein Extraction
Total RNA was obtained using the Tripure isolation reagent (Roche Diagnostic GmbH, Mannheim, Germany) according to the manufacturer’s instructions. The quantity and purity of extracted RNA was assessed by measuring absorbance at 260 nm and the ratio A260:A280 in a UV spectrophotometer (NanoDrop Inc., Wilmington, DE). Only samples with an A260:A280 ratio up to 1.8 were considered valid for real-time PCR. Nrf2 translocation analysis were performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions.
RT-PCR and Real-Time PCR
Synthesis of cDNA by RT-PCR, and quantitative real-time PCR were performed as described previously.41 Multiplex real-time PCR was performed using Applied Biosystems expression assays for IL-6 (Hs00174131_m1) and TNF-α (Hs00174128_m1). Data were normalized with 18S eukaryotic ribosomal RNA expression (4310893E).
Protein Quantitation
ELISA or Western blotting was used to assess protein levels. ELISA kits were used to quantify IL-6 and TNF-α (Preprotech) according to the manufacturer’s instructions. Western blotting was performed on PBMC nuclear and cytoplasmic lysates using 30 μg protein per lane. Primary antibodies were rabbit anti-human Nrf2 (1:500, sc-722, Santa Cruz Biotechnology), anti–HO-1 (1:1000, SPA-896;, Enzo), anti–Prx-1 (1:200, sc-21948, Santa Cruz Biotechnology), anti-GAPDH (1:5000, MAB 374; EMD Millipore), and anti-histone 3 (EMD Millipore, 09–838, 1:1000), and secondary antibodies were horseradish peroxidase–labeled anti-mouse (DAKO Cytomation) or anti-rabbit (Santa Cruz Biotechnology). Quantitation was performed using ImagenQuant TL software (GE healthcare) after image acquisition on an Image Quant LAS 4000 system (GE Healthcare).
Statistical Analyses
Variables were described at baseline and at 3- and 6-month follow-up for each arm of the study as mean and SEM. Changes from baseline to 3 months and from 3 months to 6 months were calculated for each line of treatment. In addition, pooled data encompassing both periods using the same dialyzer (the 0–3 months on one dialyzer of patients on one arm of the study and the 3–6 months on the same dialyzer of patients on the other arm of the study) were also analyzed. Comparisons between values were performed using paired sample t test or Wilcoxon signed-rank test. All comparisons used the bilateral hypothesis test and a significance level of 0.05.
Disclosures
This study was funded by a grant from Nipro corporation. The funders had no role in the design of the study, interpretation of the results, or the writing of the manuscript.
Supplementary Material
Acknowledgments
A.O. received funding from Programa Intensificacion Actividad Investigadora (ISCIII/Agencia Lain-Entralgo/CM). This work was supported by a grant from Nipro corporation. Renal, vascular and diabetes laboratory get funding from the Spanish government: PI13/00047, MS12/03262, CP10/00479, PI13/00802, and PI14/00883; European Regional Development Fund (ERDF/FEDER) grants (ISCIII-RETIC RED in REN/RD06/0016, and RD12/0021), Comunidad de Madrid (S2010/BMD-2378), Spanish Nephology Society (SENEFRO), and Fundación Renal Íñigo Álvarez de Toledo (FRIAT).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030312/-/DCSupplemental.
References
- 1.Casajuana N, Lacorte S: New methodology for the determination of phthalate esters, bisphenol A, bisphenol A diglycidyl ether, and nonylphenol in commercial whole milk samples. J Agric Food Chem 52: 3702–3707, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Dodds EC, Goldberg L, Lawson W, Robinson R: OEstrogenic Activity of Certain Synthetic Compounds. Nature 141: 247–248, 1938 [Google Scholar]
- 3.González-Parra E, Herrero JA, Elewa U, Bosch RJ, Arduán AO, Egido J: Bisphenol a in chronic kidney disease. Int J Nephrol 2013: 437857, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekant W, Völkel W: Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol 228: 114–134, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Castro B, Sánchez P, Torres JM, Preda O, del Moral RG, Ortega E: Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS ONE 8: e55905, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G: Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118: 1055–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim ME, Park HR, Gong EJ, Choi SY, Kim HS, Lee J: Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem Toxicol 49: 3383–3389, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D: Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300: 1303–1310, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Yang Y-J, Lee S-Y, Kim K-Y, Hong Y-P: Acute testis toxicity of bisphenol A diglycidyl ether in Sprague-Dawley rats. J Prev Med Pub Health 43: 131–137, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Li M, Bi Y, Qi L, Wang T, Xu M, Huang Y, Xu Y, Chen Y, Lu J, Wang W, Ning G: Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int 81: 1131–1139, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Boeniger MF, Lowry LK, Rosenberg J: Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54: 615–627, 1993 [DOI] [PubMed] [Google Scholar]
- 12.You L, Zhu X, Shrubsole MJ, Fan H, Chen J, Dong J, Hao CM, Dai Q: Renal function, bisphenol A, and alkylphenols: results from the National Health and Nutrition Examination Survey (NHANES 2003-2006). Environ Health Perspect 119: 527–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowder SJT: Nephrotoxicity of bisphenol A (BPA)--an updated review. Curr Mol Pharmacol 6: 163–172, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Olea-Herrero N, Arenas MI, Muñóz-Moreno C, Moreno-Gómez-Toledano R, González-Santander M, Arribas I, Bosch RJ: Bisphenol-A induces podocytopathy with proteinuria in mice. J Cell Physiol 229: 2057–2066, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Saura M, Marquez S, Reventun P, Olea-Herrero N, Arenas MI, Moreno-Gómez-Toledano R, Gómez-Parrizas M, Muñóz-Moreno C, González-Santander M, Zaragoza C, Bosch RJ: Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J 28: 4719–4728, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Shintani H: Determination of the endocrine disrupter bisphenol-A in the blood of uremia patients treated by dialysis. Chromatographia 53: 331–333, 2001 [Google Scholar]
- 17.Haishima Y, Hayashi Y, Yagami T, Nakamura A: Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J Biomed Mater Res 58: 209–215, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki H, Nagake Y, Makino H: Determination of bisphenol a in effluents of hemodialyzers. Nephron 88: 376–378, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U: Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol 41: 263–291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami K, Ohashi A, Hori H, Hibiya M, Shoji Y, Kunisaki M, Akita M, Yagi A, Sugiyama K, Shimozato S, Ito K, Takahashi H, Takahashi K, Yamamoto K, Kasugai M, Kawamura N, Nakai S, Hasegawa M, Tomita M, Nabeshima K, Hiki Y, Sugiyama S: Accumulation of bisphenol A in hemodialysis patients. Blood Purif 25: 290–294, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Krieter DH, Canaud B, Lemke HD, Rodriguez A, Morgenroth A, von Appen K, Dragoun GP, Wanner C: Bisphenol A in chronic kidney disease. Artif Organs 37: 283–290, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Csanády GA, Oberste-Frielinghaus HR, Semder B, Baur C, Schneider KT, Filser JG: Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Arch Toxicol 76: 299–305, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho SM: Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS ONE 9: e90332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Cruz DN, de Cal M, Ronco C: Oxidative stress and anemia in chronic hemodialysis: the promise of bioreactive membranes. Contrib Nephrol 161: 89–98, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Elewa U, Sanchez-Niño MD, Martin-Cleary C, Fernandez-Fernandez B, Egido J, Ortiz A: Cardiovascular risk biomarkers in CKD: the inflammation link and the road less traveled. Int Urol Nephrol 44: 1731–1744, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Schindler R: Causes and therapy of microinflammation in renal failure. Nephrol Dial Transplant. 19[Suppl 5]: V34–V40, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ooe H, Taira T, Iguchi-Ariga SMM, Ariga H: Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol Sci 88: 114–126, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD: Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health 218: 212–219, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin F, Jiang L, Liu X, Geng C, Wang W, Zhong L, Yang G, Chen M: Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen 769: 29–33, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Aboul Ezz HS, Khadrawy YA, Mourad IM: The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology 67: 145–155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang C, Ning B, Waqar AB, Niimi M, Li S, Satoh K, Shiomi M, Ye T, Dong S, Fan J: Bisphenol A exposure enhances atherosclerosis in WHHL rabbits. PLoS ONE 9: e110977, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y, Liu J, Li Y, Chang H, Li G, Xu B, Chen X, Li W, Xia W, Xu S: Prenatal exposure to bisphenol A at the reference dose impairs mitochondria in the heart of neonatal rats. J Appl Toxicol 34: 1012–1022, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Kaur K, Chauhan V, Gu F, Chauhan A: Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic Biol Med 76: 25–33, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S: Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 4: e460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y, Mao Z, Chang H, Li G, Xu B, Chen X, Xu S: Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS ONE 9: e90443, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babu S, Uppu S, Claville MO, Uppu RM: Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: implications to BPA-related oxidative stress and toxicity. Toxicol Mech Methods 23: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Yang YJ, Hong YC, Oh SY, Park MS, Kim H, Leem JH, Ha EH: Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res 109: 797–801, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Simon TM, Lorber A, Holmes HT: A rapid method for preparing Ficoll-hypaque step gradients and gradient overlays. J Immunol Methods 12: 193–195, 1976 [DOI] [PubMed] [Google Scholar]
- 41.Mas S, Martínez-Pinna R, Martín-Ventura JL, Pérez R, Gomez-Garre D, Ortiz A, Fernandez-Cruz A, Vivanco F, Egido J: Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes 59: 1292–1301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.