Abstract
Generating kidney organoids using human stem cells could offer promising prospects for research and therapeutic purposes. However, no cell-based strategy has generated nephrons displaying an intact three-dimensional epithelial filtering barrier. Here, we generated organoids using murine embryonic kidney cells, and documented that these tissues recapitulated the complex three-dimensional filtering structure of glomerular slits in vivo and accomplished selective glomerular filtration and tubular reabsorption. Exploiting this technology, we mixed human amniotic fluid stem cells with mouse embryonic kidney cells to establish three-dimensional chimeric organoids that engrafted in vivo and grew to form vascularized glomeruli and tubular structures. Human cells contributed to the formation of glomerular structures, differentiated into podocytes with slit diaphragms, and internalized exogenously infused BSA, thus attaining in vivo degrees of specialization and function unprecedented for donor stem cells. In conclusion, human amniotic fluid stem cell chimeric organoids may offer new paths for studying renal development and human podocyte disease, and for facilitating drug discovery and translational research.
Keywords: kidney regeneration, podocyte, human amniotic fluid stem cells, kidney organoids, glomerulogenesis, filtration slits
Producing kidney organoids from human stem cells would be an enormous breakthrough for researchers working on kidney disease, and would bring with it significant changes to how disease is studied and treated. Although several types of kidney cells have so far been derived from a variety of sources through in vitro differentiation protocols, a number of technical difficulties have prevented the creation of functional kidney tissue that may incorporate cells derived from humans. One of the major problems is that conventional culture systems cannot accurately replicate organogenesis in vitro, due to the kidney's intricate morphology and the multiple interactions that occur between different cell lineages during kidney development. A modified culture system that resembles in vivo organogenesis more closely is needed.
Unlike traditional in vitro monolayer cultures or embryoid body based methods, the mouse embryonic kidney has a special capacity to reconstruct itself after single-cell dissociation and reaggregation.1 This method consequently provides the possibility to establish chimeric organ cultures in which the three-dimensional (3D) nephrogenic potential of human stem cells or progenitors can be tested. Indeed, various versions of this reaggregation system have been employed to create chimeric 3D organoids in vitro using human cells from different sources, such as amniotic fluid stem cells (AFSCs),2 adult kidney cell-derived nephron-progenitor cells,3 and pluripotent stem cell (PSC)-derived kidney cells.4,5 These organoids possessed most of the features of fetal kidney anatomy, including nephrons, collecting ducts and renal stroma. However, the brief survival of organ cultures in vitro,6 and most importantly the insufficiency of these systems to support the development of vascularized glomeruli, do not allow for further maturation into a status that resembles adult kidneys. These facts limit the usefulness of chimeric organoid cultures to studies on embryonic developmental stages.
The complete reproduction of the glomerulus is probably the most difficult and indeed critical step for any cell-based attempt to establish functional kidney tissue. The most complex cellular component of the glomerular filter is the podocyte, a highly specialized cell type characterized by interdigitating foot processes that enmesh the glomerular capillary loop, and form a unique cell-cell junction termed the slit diaphragm. Slit diaphragms are traversed by pores that are heterogeneous both in size and shape within the junction, which selectively permit the passage of macromolecules.7 The correct assembly of this epithelial layer is critical in the generation and maintenance of the glomerular barrier to protein ultrafiltration.8–11
By using an optimized reaggregation system12 we recently showed that organoids constructed in vitro using suspensions of fully dissociated mouse kidney cells were integrated into a living recipient, and grew to form vascularized glomeruli that exhibited well formed capillary structures and filtration slits. These organoids were also competent at exerting kidney specific functions in terms of blood filtration, tubular reabsorption of macromolecules and erythropoietin production. Based on this evidence, and in combination with the ability of mouse organoids to host human stem cells in vitro, we may expect this technology to be applied to growing vascularized nephrons and efficient kidney tissue, starting with human stem cells.
Here we set out to answer whether the newly formed glomeruli, made using embryonic cell suspensions, developed the complete 3D structural framework of the slit diaphragm that could support filtering function and the restriction of macromolecular trafficking toward the tubular lumen. Additionally, we sought to establish whether the generation of self-forming organoids could be an efficient method for directing the differentiation of human AFSCs to such an extent that they could form 3D chimeric structures, potentially displaying mature morphology and exerting nephron-specific functions.
Results
Suspensions of Mouse Embryonic Kidney Cells Give Rise to 3D Organoids with Highly Specialized Renal Structures In Vivo
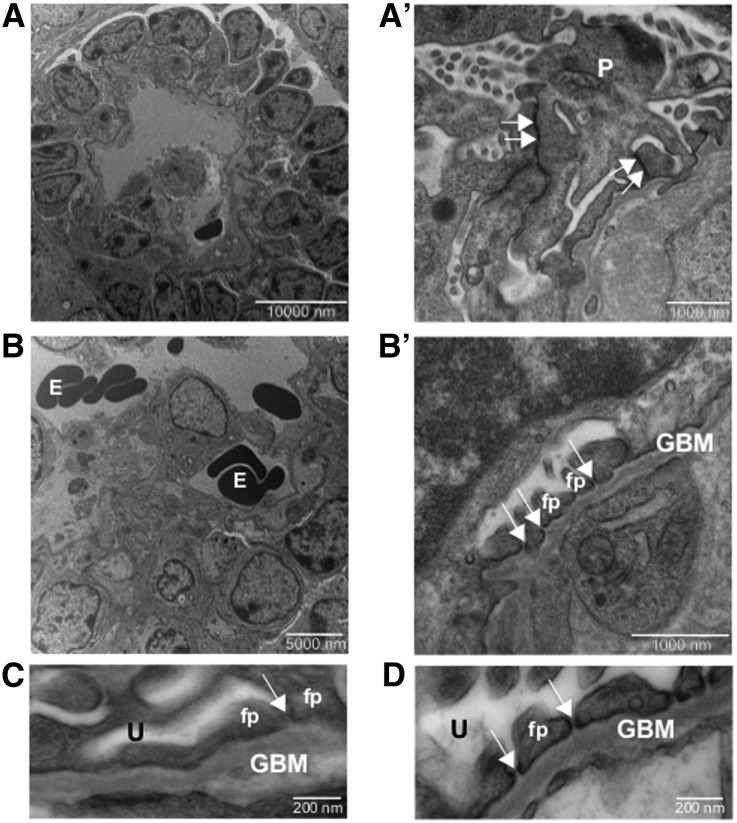
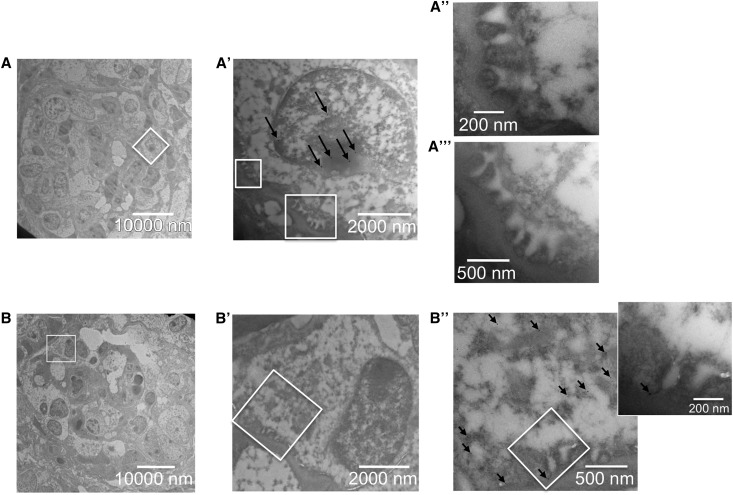
Organoids made from suspensions of embryonic kidney cells were cultured for 5 days and implanted beneath the renal capsules of unilaterally nephrectomized athymic rats. At 2 weeks post implantation, using survey histologic examination, the intragraft organoids developed both tubular and glomerular structures (Supplemental Figure 1A, insets and arrowheads, and Supplemental Figure 1B), and were efficiently connected with the recipient animal’s vascular system, as indicated by red blood cells found in the vascular and glomerular structures (Supplemental Figure 1A, arrows). Transmission electron microscopy (TEM) analysis showed high degrees of maturation, leading to the formation of vascularized glomeruli with capillary walls, mesangial-like regions, and layers of visceral and parietal cells (Figure 1). Early signs of cellular process interdigitation were seen in glomerular-like structures at less advanced stages. Most of the intercellular junctions of the maturing visceral epithelium were located apically, or where lateral membranes of tightly associated processes were in contact (Figure 1, A and A′, double arrows). With further maturation, podocytes showed regularly spaced processes with filtration slits, as is typical of foot processes, and the slit diaphragm became detectable (Figure 1, B, B′, and C, arrows). The mature filtration slit was well represented (Figure 1C, arrow), albeit less frequent as compared with those seen diffusely in the adult mouse glomerulus (Figure 1D, arrows), and the percentage of slit diaphragms showing a well recognizable central dense area was extremely high (80% in the most mature organoid-derived structure).
Figure 1.
Development of slit diaphragm in implanted renal organoids from single cell suspensions. Transmission electron micrographs of organoid-derived glomerular structure (A, A′) show immature podocytes bearing apically located and immature intercellular junctions along the contact sites between forming processes. In the glomerular epithelium in an advanced stage (B, B′), foot processes and filtration slits are seen. With maturation of the junction, the morphology of filtration slits and diaphragms (B′, C) is similar to that of the control adult mouse (D). Double arrows (in A′): maturing junctions; arrows (in B′, C, and D): slit diaphragms. E, erythrocyte; GBM, glomerular basement membrane; P, maturing podocyte; fp, foot process; U, ultrafiltrate-containing space.
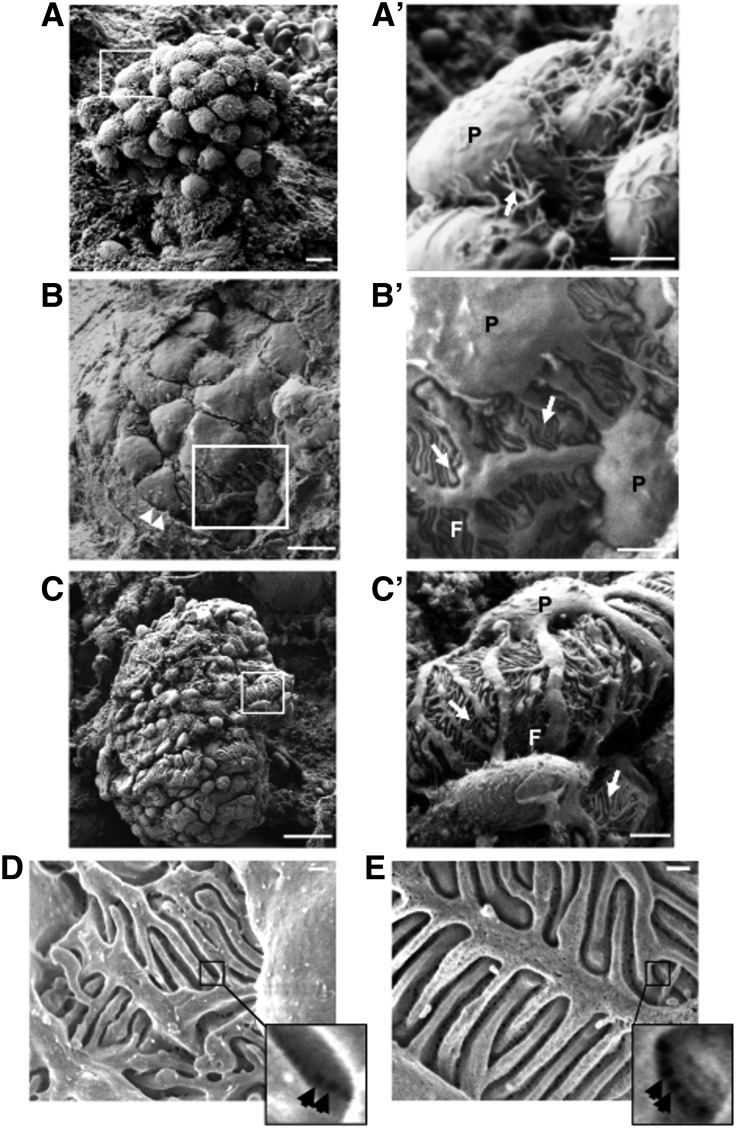
To better define the ultrastructural organization of the slit diaphragm in implanted organoids we used scanning electron microscopy (SEM). Results showed developing glomerular structures in early stages, displaying rounded and closely packed visceral epithelial cells, looking like a bunch of grapes (Figure 2A). Some of these cells were characterized by extensions resembling primary or elongating processes (Figure 2A′, arrow). In more mature nephrons, Bowman capsules (Figure 2B, arrowheads) and Bowman spaces surrounded more differentiated visceral epithelial cells (Figure 2B). Compared with cells at early developmental stages, these podocytes had a larger surface, were less clustered and showed both primary processes and interdigitating foot processes (Figure 2B, inset and Figure 2B′, arrows). The foot processes were tightly opposed to each other and the filtration slits between them were open (Figure 2B′). The appearance of these slits was normal (Figure 2, C and C′, arrows). This heterogeneous maturation pattern in developing glomeruli was also observed both in metanephroi at advanced developmental stages (E18.5, Supplemental Figure 2, A and A′) and in postnatal kidneys (2 days, Supplemental Figure 2, B and B′).
Figure 2.
Glomerular ultrastructural maturation by SEM in renal organoids. Scanning electron micrographs of organoid-derived (A, B) and adult control mouse (C) glomeruli, with the corresponding high magnification insets (A′–C′). (A, A′) Early developmental-stage glomerulus presenting packed visceral epithelial cells with elongating primary processes (arrow). (B, B′) An advanced developmental-stage glomerulus displays podocytes with primary processes and interdigitating foot processes (arrows), resembling those seen in the adult control mouse glomerulus (C, C′, arrows). (D) Representative image of epithelial filtration pores observed in podocyte junctions of organoid-derived glomerulus by using in-lens detector with SEM (D, inset, arrows). Size, shape, and distribution of these pores resembled those of adult control murine kidney (E, inset, arrows). Arrowheads (in B), Bowman's capsule. Scale bars: 5 μm (A, B), 2 μm (A′–C′), 20 μm (C), 200 nm (D, E). F, foot processes; P, podocyte.
By using SEM with a high-sensitivity (in-lens) detector we performed a more thorough analysis of the slit’s ultrastructure, which revealed the deepest region of the filtration slit and podocyte-podocyte junction, corresponding to the slit diaphragm, as could be observed from its urinary side. We obtained images of irregular pores, which were heterogeneous both in size and shape within the junction (Figure 2D, inset, arrows). The epithelial filtration pores were mainly located in the central region of the filtration slits and the dimensions of the pores resembled those of healthy adult murine kidneys (Figure 2E, inset, arrows).
Organoids Mature to Create Selective Glomerular Filtering Barrier and Tubular Reabsorption Capacity
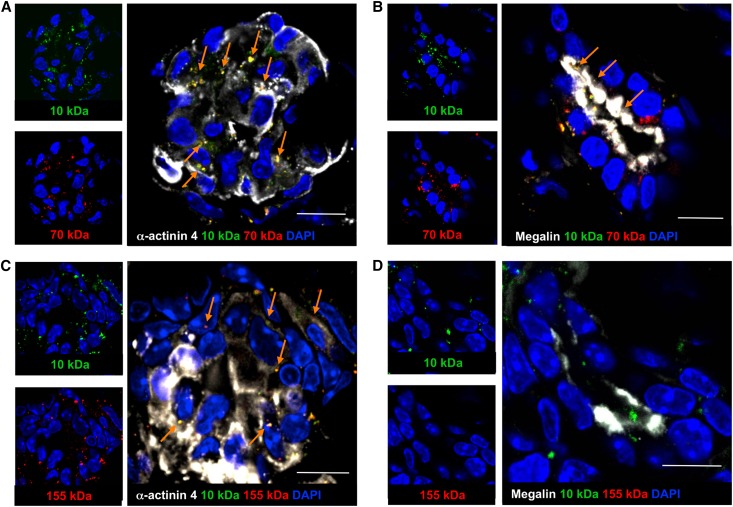
We previously documented that implanted organoids developed nephron structures able to absorb fluorescence isothiocyanate-conjugated (FITC)-dextran (10 kDa) from the tubular lumen upon glomerular ultrafiltration. However, it was not clear whether the degree of maturation of newly created podocytes was sufficiently advanced to selectively restrict the transglomerular passage of high molecular mass macromolecules to the tubular lumen. To test whether nephrons of the engineered tissue could exert permselective and reabsorptive function, we injected FITC and rhodamine isothiocyanate (RITC), or FITC- and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated dextrans (10 and 70 or 10 and 155 kDa, respectively) 15 seconds before euthanasia into the host blood system and performed localization studies using fluorescence microscopy (Figure 3). All dextrans were found in glomeruli that exhibited the expression of the podocyte marker α-actinin 4 (Figure 3, A and C, arrows), indicating that the injected probes were effectively delivered to glomerular structures, which were endowed with specialized epithelial barriers. Proximal tubule cells were exposed to ultrafiltered 10 and 70 kDa dextrans, as reflected by the detection of the FITC and RITC-conjugated tracers in the lumen and at the apical pole of the cell (Figure 3B). In contrast, high molecular mass dextran did not gain access to filtering tubules, as demonstrated by the absence of TRITC-conjugated tracer and the concomitant presence of coinjected FITC-conjugated tracer at the tubular level (Figure 3D). Both 10 kDa and 70 kDa tracers were colocalized with apical staining for megalin13 (Figure 3B, arrows), consistently indicating full competence of the cells to reabsorb these probes from the tubular lumen, whereas 155 kDa tracer had no access (Figure 3D). These findings were consistent even when dextrans remained in the host blood system for 30 minutes (Supplemental Figure 3, A–D), further confirming the exclusion of 155 kDa tracer from the glomerular filtration barrier. The pattern of all dextrans at the glomerular and tubular levels in the host tissue is shown in Supplemental Figure 3, E–H.
Figure 3.
Functional maturation of implanted organoids. (A, B) FITC and RITC-conjugated dextrans (10 and 70 kDa, respectively), injected into the femoral vein of the recipient animal 15 seconds before euthanasia, were found in α-actinin 4-positive glomeruli (A, white, arrows) and in tubular structures, where they colocalized with megalin (B, white, arrows). (C) FITC and TRITC-conjugated dextrans (10 and 155 kDa, respectively) were found in α-actinin 4-positive glomeruli (white, arrows). (D) No TRITC-dextran was found in FITC-tracer-positive tubular lumina of the graft. 4′,6-diamidino-2-phenylindole (DAPI; blue-stained nuclei). Scale bars: 10 μm.
Mixed Suspensions of Mouse Embryonic Cells and Human AFSCs Generate Chimeric Kidney Organoids In Vitro
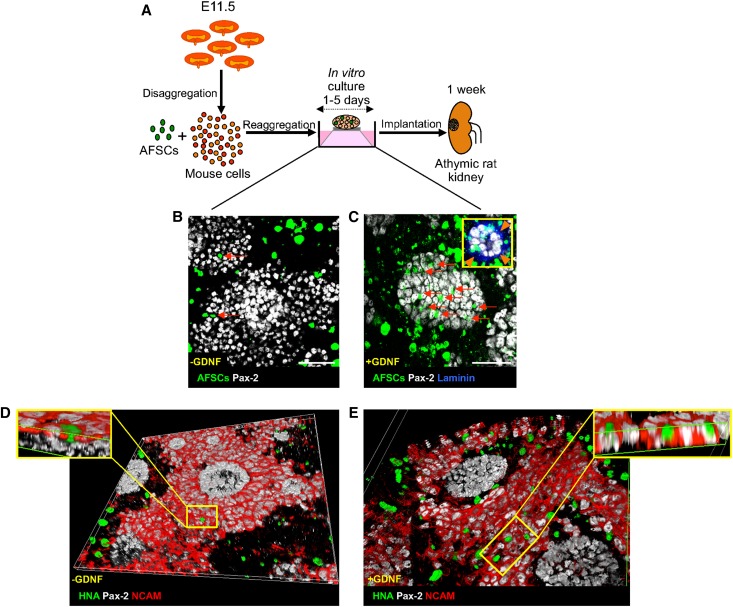
To construct chimeric organoids we mixed AFSCs labeled with a green fluorescent chloromethyl derivative of fluorescein diacetate probe and E11.5 mouse kidney cells. Chimeric organoids were cultured in vitro for up to 5 days without AFSCs having a negative effect on tissue development (Figure 4). At 2 days, AFSCs were homogenously distributed in the chimeric organoid and mainly localized in interstitial spaces between renal structures positive for the paired box 2 (Pax-2) transcription factor, which is a marker of both developing nephrons and ureteric buds (Figure 4B). To increase the integration of AFSCs into renal structures, we genetically modified cells to temporarily express glial cell line-derived neurotrophic factor (GDNF), a key factor expressed by the metanephric mesenchyme during the early stage of kidney organogenesis14 and previously shown to substantially enhance the integration of human mesenchymal stem cells in developing mouse metanephroi.15 At 2 days, GDNF-expressing AFSCs were abundantly incorporated into developing Pax-2-positive structures (Figure 4C). Although fewer AFSCs were detected in the chimeric organoid after 5 days, some of these were organized into developing Pax-2-positive structures, surrounded by laminin-positive basement membranes (Figure 4C, inset).
Figure 4.
In vitro construction of chimeric renal organoids. (A) Experimental design. (B, C) Integration of AFSCs into renal structures in vitro. (B) AFSCs (green, arrows) are mainly seen in interstitial areas and rarely in Pax-2-positive developing renal elements (white) at 2 days. (C) At the same time GDNF-expressing AFSCs (green, arrows) are abundantly incorporated into Pax-2-positive structures (white). After 5 days, human–mouse chimeric structures surrounded by the basement membrane marker laminin (blue, inset, arrowheads) are visible. (D, E) At 2 days, Z sectioning and 3D reconstruction indicate untransfected (D) or GDNF-expressing AFSCs (E) (HNA-positive, green) integrated into NCAM (red) and Pax-2-positive (white) caps of condensed metanephric mesenchyme surrounding NCAM-negative and Pax-2-positive ureteric bud tips. Scale bars: 50 μm (B, C).
To provide deeper insight into the distribution of AFSCs in the 3D renal structures, chimeric organoids made with unlabeled AFSCs were immunostained with the human nuclear antigen (HNA) marker, combined with Pax-2 and neural cell adhesion molecule (NCAM), a marker of condensed metanephric mesenchyme,16 and 3D reconstructions of confocal Z-stack images were performed. These experiments revealed that AFSCs were incorporated alongside the mouse cells within the NCAM and Pax-2-positive caps of the condensed metanephric mesenchyme and, consistent with results obtained using the fluorescent probe (Figure 4, B and C), presented only minimal ability to integrate with NCAM and Pax-2-positive structures (Figure 4D), unless they were transfected with GDNF (Figure 4E).
Human AFSCs Efficiently Integrate in Developing Neo-Nephrons and Generate Functional Podocytes In Vivo
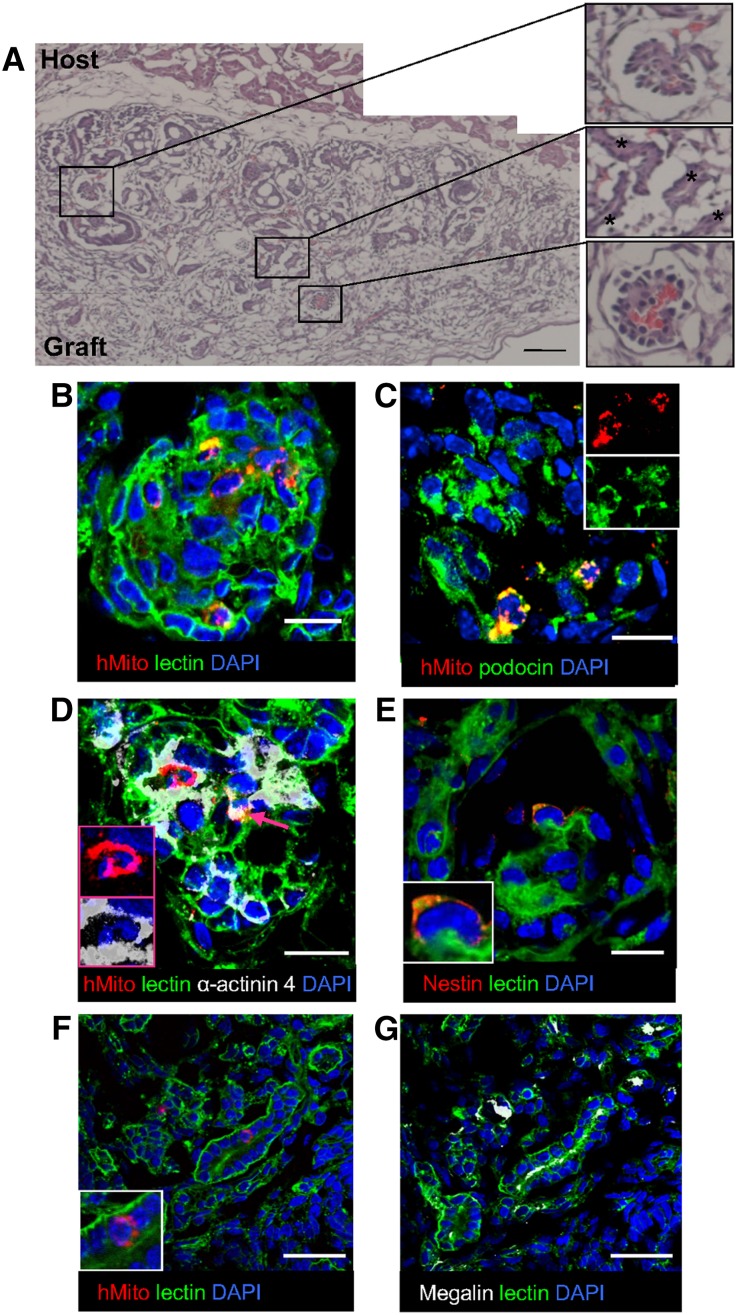
To study in vivo growth and maturation potential, chimeric organoids made of GDNF-expressing AFSCs were cultured for 1 or 5 days and implanted in athymic rats (Figure 4A). The histologic evaluation 1 week post implantation revealed that both grafts survived and increased in size (Figure 5A, Supplemental Figure 4), but only 1-day grafts developed well defined tubules and glomerular structures (Figure 5A). As expected, glomeruli and vessels of these organoids contained red blood cells, indicating vascular connection between graft and host (Figure 5A, insets). Immunofluorescence analysis of the graft tissue with a human-specific mitochondrial marker17 (Supplemental Figure 5D) showed that AFSCs were present in glomerular structures (Figure 5B), where they differentiated towards the podocyte epithelial lineage expressing podocin (Figure 5C, insets) and α-actinin-4 (Figure 5D, insets and arrow). Human cells were also positive for the podocyte marker nestin as revealed by an antibody that specifically recognizes the human nestin (Figure 5E, inset, Supplemental Figure 5, A–C). Very few AFSCs were found in tubules that were positive for megalin (Figure 5, F, inset, and G). Quantifying the efficiency of AFSC incorporation into glomerular structures using two different lines revealed that 62.2% of the counted glomeruli incorporated human cells. No significant differences were found between the tested lines, indicating that the model can be transferred across AFSC lines obtained from different donors.
Figure 5.
In vivo maturation of chimeric renal organoids. (A) Histology of organoids at 1 week shows glomerular structures containing red blood cells (upper and lower insets) and tubular structures (middle inset, asterisks). (B) AFSCs stained by specific human mitochondrial marker (red) are localized in the developing glomerulus. (C, D) AFSCs (red) expressing the podocyte proteins podocin (C, green, insets) and α-actinin 4 (D, white, insets and arrow). (E) A cell positive for the human-specific podocyte marker nestin (red). (F) An AFSC (red, inset) found in the proximal tubule, positive for megalin (white) (G). (F, G serial sections). 4′, 6-diamidino-2-phenylindole (DAPI; blue-stained nuclei). Wheat-germ agglutinin lectin (green) was used to help display renal structures. Scale bars: 100 μm (A), 10 μm (B–E), 25 μm (F, G). hMito, human mitochondria.
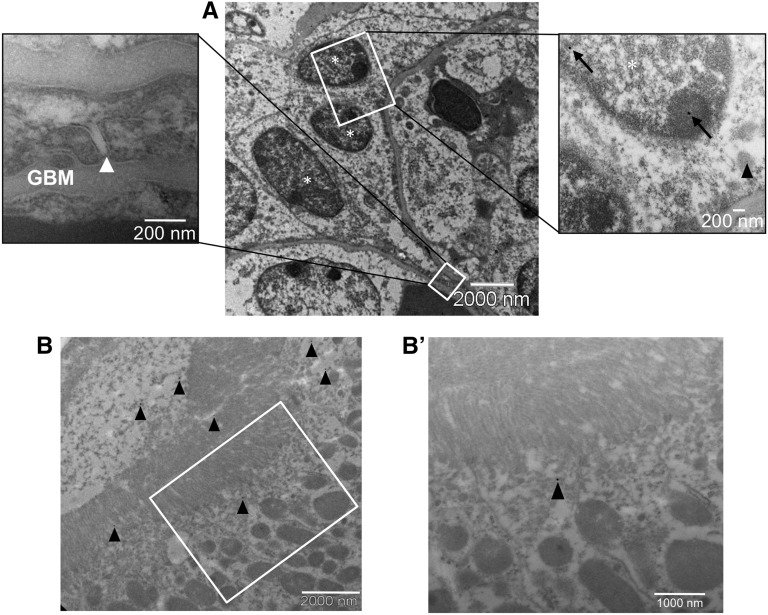
To investigate the fate of AFSCs in the glomerular epithelium, we first carried out an immuno-electron microscopy analysis of chimeric organoids to detect HNA marker, which is exclusively expressed in human cells (Supplemental Figure 6, A–C). Capillary tufts of chimeric glomeruli were covered by epithelium with prominent interdigitating process features (Figure 6). Immunogold labeling for HNA was revealed in the nuclei of many podocytes (Figure 6A′ and Figure 7A, gold particles per μm2 of nucleus in AFSC-derived podocytes 0.17±0.03 versus 0.25±0.04 of control human podocytes), and organized foot processes with intact interposed slit diaphragms were seen distinctly in the same cells (Figure 6, A′, A′′, and A′′′ and Figure 7A left inset, arrowheads). That foot processes originate from human AFSCs was further confirmed by immunogold staining with a human-specific nestin antibody (Supplemental Figure 6, D–F) that was found in the cytoplasm and foot processes of human AFSC-derived podocytes (Figure 6, B′, B′′, and inset). To test whether glomerular capillaries of vascularized chimeric structures might exert physiologic function, the host rat was intravenously injected with FITC-conjugated BSA, and double immunogold staining for BSA and HNA was performed. BSA was localized in cytoplasmic vesicles (Figure 7A, right inset, arrowhead), indicating established perfusion and early filtration in the chimeric glomerular structure. Both BSA and nuclear HNA (Figure 7A, right inset, arrowhead and arrows, respectively) were found within the same cell, which is clear evidence of active endocytosis by viable and functional human podocytes, as happens in normal podocytes in vivo.18 BSA was also detected in the tubular lumen and in subapical vesicles of proximal tubular cells (Figure 7, B and B′, arrowheads), indicating tubular reabsorption in the chimeric tissue. Unlike differentiated podocytes, in vitro cultured AFSCs were not able to take up FITC-BSA (Supplemental Figure 7), demonstrating that this property was acquired upon differentiation within the glomerular structures.
Figure 6.
Human AFSC-derived podocytes mature and display organized foot processes with interposed slit diaphragms. (A, B) Transmission electron micrographs of chimeric glomeruli. A human podocyte (A, box) shows both positive immunogold nuclear staining for HNA (A′, arrows) and organized foot processes overlying the glomerular basement membrane (A′). Between the foot processes the well formed slit diaphragms are clearly visible (A′′ and A′′′). In another chimeric glomerulus (B, box) an AFSC-derived podocyte is positive for human-specific nestin immunogold staining. Human nestin is seen in the human podocyte’s cytoplasm and foot processes, which are spaced by the intact slit diaphragm (B′, B′′, and inset). HNA and human-specific nestin were detected through the indirect immunogold technique using secondary antibodies conjugated with 25 nm and 12 nm gold particles, respectively.
Figure 7.
Ultrafiltration and reabsorption competence of chimeric tissue. (A) Transmission electron micrographs of a chimeric glomerulus displaying fully integrated podocytes (asterisks) of human origin (HNA-positive, right inset, arrows). Systemically injected BSA is localized here in an intracellular vesicle (right inset, arrowhead), indicating uptake by the AFSC-derived podocyte. A well formed slit diaphragm (left inset, white arrow) is clearly seen between foot processes in the human podocyte layer. (B, B′) In the proximal tubule, BSA is seen in the lumen and on the surface of microvilli (B, arrowheads), and in the subapical region of the cell (B′, arrowhead). HNA and BSA were detected through the indirect immunogold technique using secondary antibodies conjugated with 25 nm and 10 nm gold particles, respectively. GBM, glomerular basement membrane.
Discussion
Οrganoid model systems have been crucial for clarifying important questions about the developmental mechanisms of many organs, and have also been exploited to create new tissues in vitro, for future replacement therapies, or to model human diseases.19 To date, no functional kidney organoids of human origin have been described. Despite this, the results of studies on kidney differentiation approaches using human PSCs (either embryonic stem cells or induced PSCs),4,5,20 as well as the recently reported in vivo generation of a vascularized glomerulus using mouse embryonic stem cells, are mostly promising.20
Unlike PSCs, metanephric cells are already committed to a genetic program of renal development, obviating the need to preprogram cell fate. Previous studies have shown that renal primordial tissues continue to develop in vivo, become vascularized, and exhibit functional properties in neonatal21 or adult22,23 recipients. Similar approaches in recipients with different diseases have shown that embryonic kidneys can initiate regenerative processes in damaged kidney tissue,24 regulate arterial blood pressure,25 exhibit metabolic properties26 and prolong life in anephric rats.27,28 Although these methods showed promising therapeutic potential, they cannot be applied in experiments that seek to grow chimeric organs in vivo for testing the ability of stem cells to contribute to different structures in the organ and modeling human development or disease. In particular, the compact nature of the tissue makes the introduction of exogenous cells into the system difficult and their widespread dispersal to make fine-grained chimeras impossible. This problem could potentially be overcome by using a newly developed method that enables in vivo maturation of organoids that were previously dissociated into single cells.12
Here we have advanced this method by disclosing: (1) how far podocytes grown from single-cell suspensions, using reaggregation-based technology, can fully develop the normal ultrastructural and 3D anatomy of the glomerulus; (2) the downstream reuptake of selectively ultrafiltered probe by proximal tubular cells; and (3) how successfully this reaggregation system can be applied to generate chimeric organoids in vivo using human AFSCs, which can differentiate into podocytes and exert nephron-specific filtering functions.
Podocytes play fundamental roles in the complex cyto-architecture of the glomerular filter. Classic studies using TEM revealed the three layers of the glomerular capillary wall, consisting of the fenestrated endothelium, which is in contact with the blood, the glomerular basement membrane, and the epithelial foot processes with filtration slits and slit diaphragms. The slit diaphragm is the most specialized subcellular structure of the glomerular filter, probably the widest intercellular contact known to date and a unique junctional class with signaling properties.8 Here, the TEM finding that filtration slits of intragraft organoids, made with embryonic kidney cells, were highly enriched with slit diaphragms (as occurs in the normal kidney) indicated podocyte competence to efficiently build the filtration slit and its membrane. This was further supported by evidence, from conventional SEM analysis, that intact foot processes and slits became recognizable. In line with this high degree of phenotypic maturation, slit diaphragms showed circular and ellipsoidal pores, substantially supporting the completion of glomerulogenesis.7 The diaphragm’s 3D, porous ultrastructure is probably one of the best available morphologic correlates to the barrier’s permeability property and sieving function, which enables the filtration of high water flow and small solutes and, at the same time, differential retention of macromolecules within blood.29–32 Altogether, the high degree of maturation of the epithelial barrier in the implanted tissue offers unprecedented evidence of the efficiency of organoid-based approaches in terms of functional capacity.
In accordance with the morphologic data, analysis of injected tracer distribution revealed the differential passage of circulating molecules of varying molecular mass into the tubular lumen within the implanted organoid. The combined ultrastructural and tracer-based evidence suggests that the newly generated selective filtering function translated into restricted passage of high molecular mass macromolecules across the capillary wall into the urinary space. Thus, the apical co-localization of both megalin and lower molecular mass dextrans in the cells, along with the high molecular mass probe not gaining access to the graft’s tubular lumen, can be interpreted as indicating efficient tubular reabsorption of differentially ultrafiltered macromolecules.
Importantly, organoids from embryonic kidney cells exhibit the intrinsic ability to faithfully recapitulate glomerulogenesis and tubulogenesis developmental patterns.1,12 This property was exploited here to generate 3D chimeric organ cultures in which AFSCs were incorporated with mouse cells and acted together to form immature 3D kidney structures. Previous reports on cultivating AFSCs in combination with reaggregated2 or intact kidneys33 in vitro, have shown that a fraction of AFSCs harbor an inherent ability to contribute to renal structures. To bolster this ability, we induced AFSCs to temporarily produce GDNF, a manipulation previously shown to be indispensable for the integration of human mesenchymal stem cells into the developing rodent kidney.15 Indeed, our experiments showed that this step enhanced the incorporation of AFSCs into the developing renal structures. Subsequently, when chimeric organoids were implanted in vivo, they became vascularized and grew to form glomeruli containing red blood cells and tubular structures. Interestingly, human cells were primarily incorporated into the glomerular structures, where they differentiated into podocytes. This tendency, which was reproducible in different cell lines, suggests that these cells may have an intrinsic propensity for differentiation towards podocytes. Indeed previous studies have shown that though in vitro cultured AFSCs do not express classic renal markers such as aquaporins 1, 2, and 3, claudin 1, nephrin, Tamm-Horsfall protein, GDNF, and others,33 they do express WT1,2 a transcription factor that is highly expressed in both developing and postnatal podocytes and considered one of the key regulators of podocyte development.34 Therefore it can be argued that in our setting, the transient expression of GDNF (a molecule that is expressed by metanephric mesenchyme) first improves the capacity of AFSCs to interact with the condensing mesenchyme and integrate in nascent nephrons, while their inherent affinity with podocytes drives them to incorporate into the developing glomeruli and finally differentiate into specialized podocytes. Indeed, the combined ultrastructural evidence that AFSC-derived podocytes became fully integrated to form the slit diaphragm and were able to internalize BSA in vivo indicates that a very high degree of structural and functional specialization was reached. Remarkably, as documented in mouse-derived organoids, chimeric organoids obtained from human cells displayed the ability to efficiently reabsorb ultrafiltered probe, further documenting that physiologic filtering function was established.
The hitherto unknown evidence that a suspension of mouse kidney cells can be used to create functioning chimeric nephrons from human AFSCs, as well as the demonstration of both podocyte fate and ultrafiltering property in vivo, fuels expectations for future attempts at engineering 3D kidney tissues for various applications. For example, since amniocenteses are performed worldwide for prenatal genetic diagnosis of monogenetic diseases, it could be feasible to establish isogenic AFSC lines derived from pregnancies with specific genetic aberrations and to test the effect of these mutations on the development and function of the glomerular filtration barrier. For instance, mutations in the gene for α-actinin-4,35 are causative in an autosomal-dominant form of familial focal and segmental glomerulosclerosis. This is true for a wide spectrum of mutations targeting the glomerulus, such as NPHS1 and NPHS2, encoding the slit membrane proteins nephrin and podocin, respectively, which by disrupting glomerular filtration cause proteinuria and kidney disease.8,36,37 Therefore, this approach, with no major modifications, can immediately be used as a platform for recapitulating normal and pathologic differentiation processes without the need for prior processing to induce stem cell properties. For research purposes it will be highly useful to combine this method with gene-editing approaches, to recreate genetic mutations and alterations observed in patients for in vivo isogenic modeling of human kidney disease. In the future, it will also be particularly interesting to combine our system with PSC-differentiation technologies that have allowed for the generation of a fetal-like chimeric organoid containing nephron and ureteric bud-like structures, but have not induced further maturation because of the brief survival of renal structures in vitro.6
The technology described here, unlike that used in previous methods using whole embryonic kidneys or fragments, can be used as a platform for growing and transplanting human chimeras with filtering function, a capacity that may facilitate focused developmental studies and greatly augment the potential for modeling kidney diseases and cell-based therapeutic strategies. This unconventional system may lay the conceptual groundwork for the generation of other sophisticated chimeric tissues using human stem cells, which is currently unfeasible, particularly for highly vascularized multifunctional organs with complex 3D structures.
Concise Methods
Isolation, Culture, and GDNF-Transfection of Human AFSCs
AFSCs, also called AFS cells,38 were produced in the Stem Cells and Regenerative Medicine Laboratory, Azienda Ospedaliera Padova (protocol number 451P/32887) using samples from human amniocenteses of consenting volunteer donors, according to guidelines, by immunoselection of CD117, the type III tyrosine kinase receptor of the stem cell factor.38 CD117-positive AFSCs were grown in modified α-MEM (Gibco BRL, Gaithersburg, MD) containing 15% embryonic stem cells FBS (ES-FBS, Gibco, Invitrogen Corporation, Grand Island,NY), 1% L-glutamine (Invitrogen Corporation, Carlsbad, CA), and 1% penicillin/streptomycin (Invitrogen), supplemented with 18% Chang B and 2% Chang C (Irvine Scientific, Santa Ana, CA), as previously described.39,40 AFSCs were genetically modified to express GDNF by infection with the adenovirus AxCAh-GDNF, which was generated as previously described.15 The cells were infected with 250 moi of AxCAh-GDNF in medium without antibiotics and serum for 3 hours. Then, medium was replaced with fresh culture medium and AFSCs were used 48 hours later. For these experiments we used five AFSC lines obtained from five different donors and cultured them for up to 10 passages.
For the use of human biologic materials the institute complies with Legislative Decree 30/6/2003 n.196 (Italian Personal Data Protection Code); Guidelines for Data Processing within the Framework of Clinical Drug Trials (Italian Garante of Privacy) of July 24, 2008; Legislative Decree no. 211 of June 24, 2003 (Transposition of Directive 2001/20/EC relating to the implementation of Good Clinical Practice in the conduct of clinical trials on medicinal products for clinical use); and all national regulations about clinical trials with medicines currently in force.
Construction and Implantation of Mouse and Chimeric Organoids
E11.5 embryonic kidney rudiments were dissected and dissociated as described before.12 Briefly, E11.5 CD1 mouse (Charles River Italia SpA, Calco, Italy) embryonic kidneys were dissected in MEM (M5650; Sigma-Aldrich, St. Louis, MO), placed in 1× trypsin/EDTA (Biochrom AG, Berlin, Germany) for 3 minutes at 37°C and then dissociated by trituration into single-cell suspensions that were filtered through a 40-µm cell strainer (BD Falcon, Oxford, UK). A total of 4×105 freshly dissociated renal cells were centrifuged at 900g for 4 minutes and the pellet was placed on top of a 5-μm filter (Merck Millipore Ltd., Ireland) supported by a metal grid, at the air-medium interface in a humidified atmosphere with 5% carbon dioxide and at 37°C. Mouse organoids were cultured for 5 days and implanted under the kidney capsule of uninephrectomized athymic rats as previously described.12 Culture conditions and implantation experiments are described in detail in the Supplemental Material.
To generate chimeric organoids, mouse embryonic kidney cells were centrifuged in the presence of untransfected or GDNF-transfected AFSCs with a 10:1 ratio (mouse/human), and aggregates were cultured as above. For the in vitro studies, 1.2×104 AFSCs were used, and in some cases cells were labeled with the green fluorescent chloromethyl derivative of fluorescein diacetate probe cell tracker (Molecular Probes Inc., Eugene, OR), following the manufacturer’s instructions, before being mixed with mouse cells and centrifuged. Viability was evaluated through Trypan blue (Sigma-Aldrich) exclusion. Chimeric organoids were fixed with 4% paraformaldehyde for 10 minutes after 2 or 5 days in culture.
For in vivo studies, chimeric organoids consisting of 4×105 mouse cells and 4×104 AFSCs were cultured in vitro for 1 or 5 days and implanted as described above. Recipients of chimeric organoids were sacrificed after 1 week. Animal work was approved by the Ethics Committee of IRCCS, Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan, Italy and conducted in conformity with guidelines that are in compliance with national and international law and policies.
Immunofluorescence Analysis and Renal Histology
For the in vitro studies, fixed renal organoids were permeabilized with 100% cold methanol for 10 minutes and incubated with chicken anti-laminin (1:100; Sigma-Aldrich), rabbit anti–Pax-2 (1:80; Zymed Laboratories, San Francisco, CA), anti–FITC-conjugated HNA (1:100; Merck Millipore Ltd.) and 5B8 anti-NCAM (1:2; developed by Jessel T.M., Dodd J. and Brenner-Morton S. from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) overnight at 4°C followed by the specific secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) overnight at 4°C. Nuclei were stained with 4',6-diamidino-2-phenylindole (Sigma-Aldrich).
For functional studies, rats were injected into the femoral or tail vein with FITC, RITC or TRITC-conjugated dextran (33 mg in 0.5 ml saline; Sigma-Aldrich)12 of different molecular masses (approximate average 10, 70 and 155 kDa, respectively), 15 seconds21,41 or 30 minutes, respectively, before euthanasia. For immunofluorescence staining, 3-μm periodate-lysine paraformaldehyde–fixed cryosections were incubated with mouse anti-human mitochondria (1:50; Merck Millipore, Darmstadt, Germany), rabbit anti-Podocin (1:50, Sigma-Aldrich), rabbit anti–α-actinin-4 (1:50; OriGene Technologies, Inc., Rockville, MD), rabbit anti-human nestin (1:300, Merck Millipore), or goat anti-megalin (1:25; Santa Cruz Biotechnology, Santa Cruz, CA) followed by species-specific secondary antibodies (1:50; Jackson ImmunoResearch Laboratories). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (Sigma-Aldrich) and renal structures were labeled with fluorescein wheat-germ agglutinin or rhodamine lens culinaris agglutinin lectin (Vector Laboratories, Burlingame, CA). Negative controls were obtained by omitting the primary antibody on adjacent sections. Finally, slides were mounted using Dako Fluorescence Mounting Medium (DAKO, Denmark) and examined using an inverted confocal laser scanning microscope (LS 510 Meta; Carl Zeiss, Jena, Germany). Appropriate human and rodent controls are shown in Supplemental Figure 5.
Renal histology was performed as previously described.12 Additional details for this section are available in the Supplemental Material.
TEM Analysis
Glutaraldehyde-fixed fragments of graft and adjacent host tissue were washed repeatedly in cacodylate buffer, postfixed in 1% osmium tetroxide, dehydrated through ascending grades of alcohol, and embedded in Epon resin. Semi thin sections were stained with toluidine blue in borax and examined using light microscopy. Thin sections (100–120 nm) were stained with uranyl acetate for morphologic analysis using a Philips Morgagni transmission electron microscope (Morgagni 268D; Philips, Brno, Czech Republic). Epithelial filtration slit frequency was evaluated on digitized TEM images as previously described.42 To identify human AFSCs and FITC-BSA through TEM, immunogold labeling was performed. Fragments of 3.5% paraformaldehyde-0.01% glutaraldehyde-fixed organoids were dehydrated through ascending grades of alcohol and embedded in LR white resin (Electron Microscopy Sciences, Hatfield, PA), sectioned and transferred to nickel grids coated with Formvar (Electron Microscopy Sciences). For human nestin immunogold staining, nonspecific sites were blocked with 1% BSA for 30 minutes and sections incubated overnight with rabbit anti-human nestin antibody (1:10, Merck Millipore) followed by 12 nm gold-conjugated goat anti-rabbit secondary antibody (1:50, Electron Microscopy Sciences). For HNA and BSA staining, nonspecific sites were blocked with 3% BSA for 30 minutes and sections were incubated for 1 hour with mouse anti-HNA antibody (1:4000, Merck Millipore) followed by 25 nm gold-conjugated goat anti-mouse IgG secondary antibody (1:50, Electron Microscopy Sciences). Then, sections were incubated for 1 hour with mouse anti-FITC 10 nm gold-conjugated antibody (1:150, Electron Microscopy Sciences). All sections were finally stained with 2% aqueous uranyl acetate before being examined using TEM. To determine the extent of HNA labeling, gold particles per µm2 of nuclear area were quantified (mean±SEM) using interactive image editing software (ImageJ; National Institutes of Health, http://rsbweb.nih.gov/ij/). Gold particle counts were obtained from three normal human glomeruli and three chimeric glomeruli. Appropriate positive and negative controls for these experiments are shown in Supplemental Figure 6.
SEM Analysis
For SEM analysis, fragments of the grafts, mouse metanephroi, postnatal kidneys (2 days after birth), and normal adult mouse kidney were fixed overnight in 2.5% glutaraldehyde (buffered with 0.1 M sodium cacodylate buffer, pH 7.4), washed in cacodylate buffer, and postfixed in 1% osmium tetroxide for 1 hour. Fixed specimens were dehydrated with increasing concentrations of alcohol. Immediately after dehydration, samples were rinsed with liquid carbon dioxide with a Bal-Tec 030 critical point dryer (BAL-TEC AG, Balzers, Liechtenstein) and observed at SEM (Supra 55; Carl Zeiss, Oberkochen, Germany). The in-lens detector was used to generate images of secondary electrons.
Disclosures
C.X. is a cofounder and managing director of Biorenovo Ltd; however, he received no compensation for this role. C.X. received research funding from Bellco Srl.
Supplementary Material
Acknowledgments
The authors wish to thank Kerstin Mierke for proofreading and editing the manuscript, Elena Gagliardini for helpful comments, Valerio Brizi and Raquel Rodrigues-Diez for helping with the cell cultures, and Manuela Passera for technical assistance.
The authors would also like to thank Prof. Erich Cosmi and Dr. Silvia Visentin (Department of Women’s and Children’s Health SDB, Obstetric Clinic, Padova) for providing human amniotic fluid samples.
The authors are also grateful to Dr. Peter Mathieson and Moin Saleem (University of Bristol, UK) for providing immortalized human podocytes.
V.B. and P.R. are recipients of fellowships from Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR), Bergamo, Italy.
This study was partially supported by the Associazione per la Ricerca sul Diabete Italia, the ERC-2010-AdG-268632 RESET Grant and European Commission grant no. HEALTH-F4-2012-305436 (STELLAR project).
The authors gratefully thank Bellco Srl for continued financial support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Amniotic Fluid Stem Cells within Chimeric Kidney Rudiments Differentiate to Functional Podocytes after Transplantation into Mature Rat Kidneys,” on pages 1266–1268.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030316/-/DCSupplemental.
References
- 1.Unbekandt M, Davies JA: Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77: 407–416, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, Davies JA, Lubec G, Hengstschläger M: Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet 19: 3320–3331, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, Little MH: Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol 24: 1424–1434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH: Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16: 118–126, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC: Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15: 1507–1515, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Sancho-Martinez I, Nivet E, Rodriguez Esteban C, Campistol JM, Izpisua Belmonte JC: The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc 9: 2693–2704, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Gagliardini E, Conti S, Benigni A, Remuzzi G, Remuzzi A: Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J Am Soc Nephrol 21: 2081–2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm – from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Schnabel E, Dekan G, Miettinen A, Farquhar MG: Biogenesis of podocalyxin – the major glomerular sialoglycoprotein – in the newborn rat kidney. Eur J Cell Biol 48: 313–326, 1989 [PubMed] [Google Scholar]
- 10.Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanwar YS, Danesh FR, Chugh SS: Contribution of proteoglycans towards the integrated functions of renal glomerular capillaries: a historical perspective. Am J Pathol 171: 9–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G: In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23: 1857–1868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermüller N, Kränzlin B, Blum WF, Gretz N, Witzgall R: An endocytosis defect as a possible cause of proteinuria in polycystic kidney disease. Am J Physiol Renal Physiol 280: F244–F253, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumäe U, Meng X, Lindahl M, Pachnis V, Sariola H: Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124: 4077–4087, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T: Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A 102: 3296–3300, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bard JB, Gordon A, Sharp L, Sellers WI: Early nephron formation in the developing mouse kidney. J Anat 199: 385–392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K: Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4: 1367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyre J, Ioannou K, Grubb BD, Saleem MA, Mathieson PW, Brunskill NJ, Christensen EI, Topham PS: Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol 292: F674–F681, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lancaster MA, Knoblich JA: Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345: 1247125, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R: Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Woolf AS, Palmer SJ, Snow ML, Fine LG: Creation of a functioning chimeric mammalian kidney. Kidney Int 38: 991–997, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Dilworth MR, Clancy MJ, Marshall D, Bravery CA, Brenchley PE, Ashton N: Development and functional capacity of transplanted rat metanephroi. Nephrol Dial Transplant 23: 871–879, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Yokoo T, Yokote S, Utsunomiya Y, Ohashi T, Hosoya T: Functional development of a transplanted embryonic kidney: effect of transplantation site. J Nephrol 25: 50–55, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Imberti B, Corna D, Rizzo P, Xinaris C, Abbate M, Longaretti L, Cassis P, Benedetti V, Benigni A, Zoja C, Remuzzi G, Morigi M: Renal primordia activate kidney regenerative events in a rat model of progressive renal disease. PLoS One 10: e0120235, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokote S, Yokoo T, Matsumoto K, Utsunomiya Y, Kawamura T, Hosoya T: The effect of metanephros transplantation on blood pressure in anephric rats with induced acute hypotension. Nephrol Dial Transplant 27: 3449–3455, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Yokote S, Yokoo T, Matsumoto K, Ohkido I, Utsunomiya Y, Kawamura T, Hosoya T: Metanephros transplantation inhibits the progression of vascular calcification in rats with adenine-induced renal failure. Nephron, Exp Nephrol 120: e32–e40, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Marshall D, Dilworth MR, Clancy M, Bravery CA, Ashton N: Increasing renal mass improves survival in anephric rats following metanephros transplantation. Exp Physiol 92: 263–271, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Rogers SA, Hammerman MR: Prolongation of life in anephric rats following de novo renal organogenesis. Organogenesis 1: 22–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deen WM, Lazzara MJ, Myers BD: Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–F596, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Edwards A, Daniels BS, Deen WM: Ultrastructural model for size selectivity in glomerular filtration. Am J Physiol 276: F892–F902, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Deen WM, Bridges CR, Brenner BM, Myers BD: Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Physiol 249: F374–F389, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Remuzzi A, Puntorieri S, Battaglia C, Bertani T, Remuzzi G: Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J Clin Invest 85: 541–549, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo RE: Renal differentiation of amniotic fluid stem cells. Cell Prolif 40: 936–948, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein – nephrin – is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 96: 7962–7967, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A: Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100–106, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio AS, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M: Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev 21: 1911–1923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzobon M, Piccoli M, Schiavo AA, Atala A, De Coppi P: Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol Biol 1035: 191–198, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Steinhausen M, Wayland H, Fox JR: Renal test dyes. V. Quantitative analysis of tubular passage of FITC-dextrans in kidneys of rats. Pflugers Arch 369: 273–279, 1977 [DOI] [PubMed] [Google Scholar]
- 42.Ruggenenti P, Cravedi P, Sghirlanzoni MC, Gagliardini E, Conti S, Gaspari F, Marchetti G, Abbate M, Remuzzi G: Effects of rituximab on morphofunctional abnormalities of membranous glomerulopathy. Clin J Am Soc Nephrol 3: 1652–1659, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.