Abstract
Assessment of deceased-donor organ quality is integral to transplant allocation practices, but tools to more precisely measure donor kidney injury and better predict outcomes are needed. In this study, we assessed associations between injury biomarkers in deceased-donor urine and the following outcomes: donor AKI (stage 2 or greater), recipient delayed graft function (defined as dialysis in first week post-transplant), and recipient 6-month eGFR. We measured urinary concentrations of microalbumin, neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver-type fatty acid binding protein (L-FABP) from 1304 deceased donors at organ procurement, among whom 112 (9%) had AKI. Each biomarker strongly associated with AKI in adjusted analyses. Among 2441 kidney transplant recipients, 31% experienced delayed graft function, and mean±SD 6-month eGFR was 55.7±23.5 ml/min per 1.73 m2. In analyses adjusted for donor and recipient characteristics, higher donor urinary NGAL concentrations associated with recipient delayed graft function (highest versus lowest NGAL tertile relative risk, 1.21; 95% confidence interval, 1.02 to 1.43). Linear regression analyses of 6-month recipient renal function demonstrated that higher urinary NGAL and L-FABP concentrations associated with slightly lower 6-month eGFR only among recipients without delayed graft function. In summary, donor urine injury biomarkers strongly associate with donor AKI but provide limited value in predicting delayed graft function or early allograft function after transplant.
Keywords: acute renal failure, cadaver organ transplantation, delayed graft function
For patients with ESRD, kidney transplantation provides longer survival and better quality of life at lower cost than chronic dialysis.1–3 The number of kidney transplants from deceased donors increased from 7639 in 1994 to 11,570 in 2014.4 This growth was achieved, in part, by using expanded criteria donor kidneys, which have shorter allograft survival because of donor age and comorbidities,5 and by using kidneys donated after circulatory determination of death, which commonly have prolonged warm ischemia and delayed graft function (DGF).6 Despite this growth, >100,000 patients are waiting for kidney transplants, and >4000 die each year on the wait-list.7 Additionally, the greater procurement of lower quality kidneys has increased kidney discard rates and recipient complications among kidneys transplanted.8 These facts reveal the need to expand the pool of donated kidneys and for new tools to identify viable kidneys for transplantation.
Allograft quality may be jeopardized in the donor because of undiagnosed CKD, the traumatic events leading to donor death, the inflammatory milieu of brain death, or hemodynamic instability and nephrotoxic insults during hospitalization. Injury of the kidney during shipping, machine perfusion, or implantation may also harm allografts.9 Currently, transplant centers make use of the kidney donor profile index (KDPI), which includes donor age, terminal serum creatinine, and other characteristics to predict allograft survival.10 In some cases, organ procurement organizations (OPOs) and transplant centers use kidney biopsy histopathology and machine perfusion parameters to assess kidney quality. However, these characteristics do not fully capture the severity of kidney injury and have limited accuracy in predicting post-transplant function.10–13
In the context of substantial limitations to allograft quality assessment, donor urinary biomarkers offer potential advantages as precise measures of kidney injury that can be rapidly and reproducibly measured. Urinary biomarkers, such as neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver-type fatty acid binding protein (L-FABP), have been shown to be accurate markers of ischemic kidney injury in diverse experimental and clinical settings,14,15 whereas urinary albumin is used clinically to assess for presence and severity of CKD.16 Our group previously showed that higher urinary NGAL and IL-18 levels in deceased-donor kidney recipients on the first post-transplant day were associated with higher DGF rates and poor allograft function by 12 months.17,18 However, assessing kidney injury in deceased donors, rather than in the early post-transplant period, would have advantages in that this information could inform allograft selection and early perioperative recipient management.
Therefore, the aims of this prospective, multicenter study were to evaluate whether deceased-donor urinary biomarkers were associated with (1) donor AKI, (2) DGF among kidney transplant recipients, and (3) allograft renal function at 6 months after transplantation.
Results
The cohort was comprised of 1304 deceased donors and 2441 kidney transplant recipients. Figure 1 shows the cohort generation. As shown in Table 1, mean±SD donor age was 41±15 years. The cohort demonstrated wide variation in organ quality as manifested by the KDPI, with a mean±SD of 48.3±27.3.
Figure 1.
Enrollment of deceased kidney donors and recipients into the study cohort.
Table 1.
Deceased donor characteristics, stratified by AKI status
| Characteristic | All (n=1304) | No AKI (n=1192) | AKI (n=112) | P Valuea | |
|---|---|---|---|---|---|
| Age, yr | 41±15 | 42±15 | 39±13 | 0.08 | |
| Male | 787 (60) | 719 (60) | 68 (61) | 0.94 | |
| Black race | 208 (16) | 186 (16) | 22 (20) | 0.27 | |
| Height, cm | 171.17±10.8 | 171.1±10.9 | 171.81±9.68 | 0.70 | |
| Weight, kg | 83.31±22.08 | 82.98±21.42 | 86.79±28.12 | 0.37 | |
| Hypertension | 400 (31) | 364 (31) | 36 (32) | 0.73 | |
| Diabetes | 130 (10) | 120 (10) | 10 (9) | 0.70 | |
| Cause of death | Stroke | 429 (34) | 396 (34) | 33 (30) | 0.11 |
| Anoxia | 428 (34) | 380 (33) | 48 (44) | ||
| Head trauma | 397 (31) | 368 (32) | 29 (26) | ||
| Other | 18 (1) | 18 (2) | 0 (0) | ||
| Hepatitis C serostatus positive | 48 (4) | 43 (4) | 5 (4) | 0.60 | |
| Admission serum creatinine, mg/dl | 1.1±0.61 | 1.1±0.6 | 1.02±0.64 | 0.01 | |
| Terminal serum creatinine, mg/dl | 1.17±0.85 | 1.01±0.46 | 2.92±1.67 | <0.001 | |
| Admission creatinine>terminal creatinine | 625 (48) | 625 (52) | 0 (0) | <0.001 | |
| Extended criteria donor | 246 (19) | 226 (19) | 20 (18) | 0.78 | |
| Donation after circulatory determination of death | 206 (16) | 193 (16) | 13 (12) | 0.20 | |
| KDRI | 1.29±0.41 | 1.28±0.42 | 1.36±0.36 | 0.01 | |
| KDPI, % | 48.3±27.3 | 47.7±27.7 | 54.5±22.0 | 0.01 | |
| Kidneys transplanted | 1 | 167 (13) | 139 (12) | 28 (25) | <0.001 |
| 2 | 1137 (87) | 1053 (88) | 84 (75) | ||
| Total organs transplanted | 1 | 60 (5) | 48 (4) | 12 (11) | 0.05 |
| 2 | 330 (25) | 300 (25) | 30 (27) | ||
| 3 | 410 (31) | 374 (31) | 36 (32) | ||
| 4 | 282 (22) | 262 (22%) | 20 (18) | ||
| 5 | 145 (11) | 136 (11%) | 9 (8) | ||
| 6 | 72 (6) | 67 (6%) | 5 (4) | ||
| 7 | 5 (0) | 5 (0%) | 0 (0) | ||
Values reported are mean±SD, n (%), or as otherwise indicated. AKI was defined as at least a 2-fold increase in donor serum creatinine from the admission to terminal value.
Wilcoxon rank-sum test for continuous variables and chi-squared or Fisher exact test for categorical variables.
Donor AKI
AKI occurred in 9% of donors. As shown in Supplemental Figure 1, urinary microalbumin, NGAL, KIM-1, IL-18, and L-FABP were all highly associated with donor AKI in univariate and adjusted analyses. The adjusted relative risks (RRs) for the highest versus lowest urinary biomarker tertile were 9.38 (95% confidence interval [95% CI], 4.53 to 19.45) for microalbumin, 8.33 (95% CI, 4.34 to 15.99) for NGAL, 1.71 (95% CI, 1.06 to 2.74) for KIM-1, 5.49 (95% CI, 3.05 to 9.89) for IL-18, and 7.28 (95% CI, 3.78 to 14.02) for L-FABP. To examine whether associations of biomarkers with donor AKI were affected by urine dilution, we performed secondary analyses in which biomarker concentrations were divided by urine creatinine concentration. Supplemental Table 1 shows that these associations remained consistent when the biomarkers were indexed to urinary creatinine.
Recipient Outcomes
Table 2 shows kidney transplant recipient characteristics. Mean age±SD was 53±15 years, 61% were men, and 39% were black race. Thirty-one percent experienced DGF. By the 6-month follow-up, 89 (4%) recipients had experienced allograft failure, and 73 (3%) died. Mean 6-month eGFR was 55.7±23.5 ml/min per 1.73m2.
Table 2.
Kidney transplant recipient characteristics, stratified by DGF
| Characteristic | All (n=2441) | Non DGF (n=1685) | DGF (n=756) | P Valuea | |
|---|---|---|---|---|---|
| Age, yr | 53±15 | 52±16 | 55±13 | <0.001 | |
| Male | 1497 (61) | 1001 (59) | 496 (66) | 0.004 | |
| Black race | 963 (39) | 592 (35) | 371 (49) | <0.001 | |
| Hispanic ethnicity | 280 (11) | 202 (12) | 78 (10) | 0.24 | |
| Cause of ESRD | Diabetes | 711 (29) | 484 (29) | 227 (30) | 0.05 |
| Hypertension | 666 (27) | 436 (26) | 230 (30) | ||
| Other or unknown | 510 (21) | 370 (22) | 140 (19) | ||
| GN | 395 (16) | 286 (17) | 109 (14) | ||
| Graft failure | 159 (7) | 109 (6) | 50 (7) | ||
| HLA mismatch level | 0 | 155 (6) | 128 (8) | 27 (4) | <0.001 |
| 1 | 19 (1) | 8 (0) | 11 (1) | ||
| 2 | 85 (3) | 65 (4) | 20 (3) | ||
| 3 | 294 (12) | 203 (12) | 91 (12) | ||
| 4 | 644 (26) | 452 (27) | 192 (25) | ||
| 5 | 824 (34) | 554 (33) | 270 (36) | ||
| 6 | 414 (17) | 270 (16) | 144 (19) | ||
| Panel reactive antibody | 0% | 1553 (64) | 1052 (62) | 501 (66) | 0.23 |
| 1%–10% | 126 (5) | 85 (5) | 41 (5) | ||
| 11%–80% | 379 (16) | 272 (16) | 107 (14) | ||
| >80% | 383 (16) | 276 (16) | 107 (14) | ||
| Pretransplant blood transfusion | 426 (17) | 265 (16) | 161 (21) | <0.001 | |
| Cold ischemia time, h | 15.3±7.09 | 14.44±6.89 | 17.21±7.15 | <0.001 | |
| Recipient not receiving chronic dialysis | 275 (11) | 247 (15) | 28 (4) | <0.001 | |
| Serum creatinine at transplant, mg/dl | 7.77±3.35 | 7.46±3.36 | 8.44±3.22 | <0.001 | |
Values reported are mean±SD, n (%), or as otherwise indicated. HLA, human leukocyte antigen.
Wilcoxon rank-sum test for continuous variables and chi-squared or Fisher exact test for categorical variables.
As shown in Supplemental Table 2, the concentrations of all five donor biomarkers were higher among recipients with DGF versus recipients without DGF. Table 3 shows that in fully adjusted analyses, higher donor urinary NGAL concentration remained significantly associated with recipient DGF with a RR of 1.21 (95% CI, 1.02 to 1.43) for highest versus lowest tertile of urinary NGAL concentration. For KIM-1, in fully adjusted analyses, the association with recipient DGF was only significant for the middle biomarker tertile. Supplemental Table 3 shows that changes in the area under the curve, the net reclassification improvement, and the integrated discrimination improvement statistics were small and nonsignificant after the addition of urinary biomarkers to models predicting DGF. Supplemental Table 4 shows associations of creatinine-indexed urinary biomarkers with DGF.
Table 3.
RR of DGF after kidney transplantation associated with donor urinary biomarker concentrations
| Biomarker | Quantile | Event Rate (%) | Range | RR (95% CI) for DGF | |||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Donor Variables Onlyb | Adjusted for Donor Variables and SCrc | Adjusted for Donor, Transport, and Recipient Variablesd | ||||
| Microalbumin | n1=755 | 192 (25) | 0.50–0.89 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| n2=751 | 247 (33) | 0.90–3.84 | 1.29 (1.08 to 1.53)a | 1.14 (0.97 to 1.34) | 1.08 (0.92 to 1.27) | 1.05 (0.89 to 1.23) | |
| n3=754 | 251 (33) | 3.86–44.86 | 1.30 (1.10 to 1.55)a | 1.12 (0.95 to 1.33) | 0.95 (0.80 to 1.13) | 0.94 (0.80 to 1.11) | |
| NGAL | n1=813 | 193 (24) | 0.00–18.10 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| n2=811 | 246 (30) | 18.20–83.40 | 1.32 (1.10 to 1.58)a | 1.19 (1.00 to 1.42)a | 1.15 (0.97 to 1.36) | 1.13 (0.96 to 1.34) | |
| n3=813 | 317 (39) | 83.60–8792.38 | 1.67 (1.41 to 1.98)a | 1.51 (1.28 to 1.77)a | 1.24 (1.04 to 1.47)a | 1.21 (1.02 to 1.43)a | |
| KIM-1 | n1=813 | 213 (26) | 58.96–843.30 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| n2=814 | 266 (33) | 844.28–2430.76 | 1.23 (1.04 to 1.46)a | 1.20 (1.02 to 1.41)a | 1.17 (1.00 to 1.37)a | 1.17 (1.00 to 1.36)a | |
| n3=812 | 277 (34) | 2432.46–37,759.01 | 1.25 (1.05 to 1.49)a | 1.17 (0.99 to 1.38) | 1.12 (0.95 to 1.32) | 1.06 (0.90 to 1.24) | |
| IL-18 | n1=812 | 207 (25) | 2.58–26.59 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| n2=813 | 255 (31) | 26.63–76.69 | 1.23 (1.03 to 1.47)a | 1.16 (0.98 to 1.37) | 1.14 (0.97 to 1.35) | 1.11 (0.94 to 1.30) | |
| n3=812 | 294 (36) | 77.41–1448.69 | 1.43 (1.21 to 1.70)a | 1.27 (1.08 to 1.50)a | 1.13 (0.96 to 1.32) | 1.07 (0.91 to 1.25) | |
| L-FABP | n1=815 | 214 (26) | 1.00–5.60 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| n2=807 | 237 (29) | 6.00–30.00 | 1.08 (0.91 to 1.29) | 1.05 (0.89 to 1.24) | 1.01 (0.86 to 1.19) | 1.00 (0.85 to 1.17) | |
| n3=812 | 304 (37) | 30.40–250.00 | 1.40 (1.19 to 1.66)a | 1.28 (1.09 to 1.50)a | 1.09 (0.93 to 1.28) | 1.04 (0.89 to 1.22) | |
SCr, serum creatinine.
P<0.05.
Donor variables used for adjustment: age (years), height (cm), weight (kg), black race, history of hypertension, history of diabetes, stroke as cause of death, hepatitis C serostatus, and donation after circulatory determination of death status.
Includes donor variables plus terminal Scr.
Includes all listed donor variables, Scr, cold ischemia time, and the following recipient variables: age (years), black race, sex, previous kidney transplant, diabetes as the cause of ESRD, need for pretransplant blood transfusion, number of human leukocyte antigen mismatches, panel reactive antibody (%), body mass index, and pretransplant dialysis.
DGF was associated with lower 6-month eGFR. As shown in Supplemental Table 5, compared with recipients without DGF, recipients with DGF had an eGFR that was 11.53 ml/min per 1.73 m2 lower at 6 months in unadjusted analyses and 7.53 ml/min per 1.73 m2 lower in analyses adjusted for donor, transport, and recipient variables.
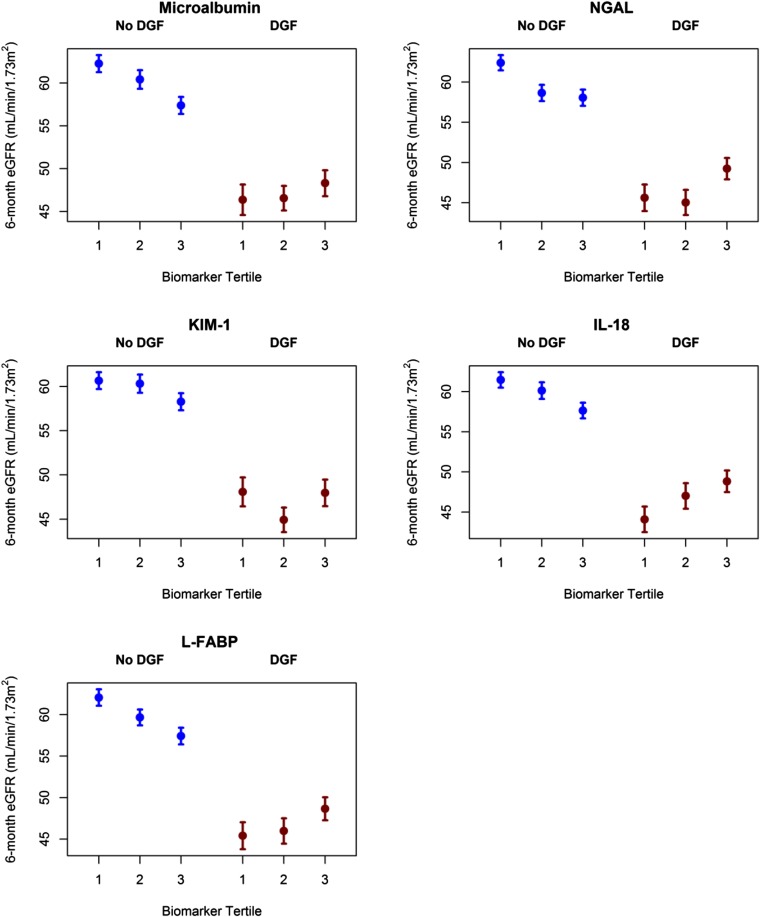
In unadjusted models, DGF modified associations between four donor urinary biomarkers and recipient 6-month eGFR; the interaction was not statistically significant for KIM-1 (P values for biomarker×DGF interaction: microalbumin, P=0.02; NGAL, P<0.001; KIM-1, P=0.29; IL-18, P=0.01; L-FABP, P=0.01).
Figure 2 shows differences in observed 6-month eGFR across donor biomarker tertiles for recipients stratified by DGF status. For recipients without DGF, there was a general pattern of lower 6-month eGFR as biomarker concentration tertiles increased. For recipients with DGF, the opposite pattern manifested for all biomarkers except KIM-1. As urinary biomarker tertiles increased, 6-month eGFR was higher.
Figure 2.
Donor urinary biomarkers and the outcome of 6-month recipient eGFR, stratified by recipient DGF status. See Supplemental Tables 6 and 7 for number of patients and biomarker ranges in each tertile. Patients without DGF are represented in blue, and patients with DGF are represented in red. Bars represent SEM.
Supplemental Table 6 shows fully adjusted linear regression models for the outcome of 6-month eGFR for recipients without DGF. After adjustment for donor characteristics other than terminal serum creatinine, patients without DGF who received kidneys with the highest tertile of NGAL concentration had –3.15 ml/min per 1.73 m2 (95% CI, –5.81 to –0.50) eGFR compared with kidneys with the lowest concentration of NGAL. Non-DGF recipients of kidneys with the highest tertile of L-FABP concentration had –3.56 ml/min per 1.73 m2 (95% CI, –6.30 to –0.83) eGFR compared with kidneys with the lowest concentration of L-FABP. However, these associations were not significant after further adjustment for donor terminal serum creatinine and recipient characteristics.
Supplemental Table 7 shows that among recipients with DGF, higher donor urinary concentrations (analyzed as tertiles) were not associated with recipient eGFR. Finally, Supplemental Figure 2 displays spline plots showing the association of recipient eGFR and donor urinary biomarkers across their entire distributions.
Discussion
Assessment of organ quality is of paramount importance to efficient organ allocation and informed consent for recipients; however, tools to predict outcomes after kidney transplantation have considerable limitations. In this prospective multicenter study, urinary biomarkers measured in deceased donors at organ procurement were highly associated with donor AKI. Higher donor urinary NGAL was associated with a modestly increased RR of recipient DGF. At 6 months after transplantation, however, donor urinary biomarkers added minimal value in predicting recipient allograft function. These findings suggest that new efforts to profile deceased-donor kidney quality should not focus exclusively on ischemic kidney injury biomarkers at organ procurement or should consider injury at later time points, such as during organ transport or at reperfusion. It is still possible that other markers than the ones tested here are able to predict long-term renal function.
This research was motivated by a strong conceptual model that donor AKI could affect post-transplant kidney allograft outcomes. In the nontransplant population, AKI is strongly associated with long-term outcomes, including CKD, ESRD, and death.19 Recent research in cardiac surgery has revealed that subclinical kidney injury (defined as patients who do not meet conventional AKI criteria according to serum creatinine, but have high urinary biomarker concentrations) is associated with lower survival.20 In transplantation, severe forms of ischemic kidney injury resulting in DGF are also associated with adverse long-term outcomes, including >40% increased risk of graft loss at 1 year compared with the absence of DGF.21 Early kidney allograft injury leads to immune cell infiltration and proinflammatory cytokine release, which likely primes the alloimmune response (increasing the risk of acute and chronic rejection) and drives interstitial fibrosis, tubular atrophy, and glomerulosclerosis, ultimately resulting in functional kidney impairment and premature allograft failure.22
A range of urine biomarkers have been identified to detect specific regions of nephron damage. As an example, NGAL is expressed at very low levels in the distal tubule and is induced in stimulated epithelia. NGAL predicted AKI during cardiac surgery and in an emergency department and was also associated with severity of polycystic kidney disease, IgA nephropathy, and cardiovascular disease.23–26 In a previous study from our group, elevated urine NGAL concentrations among kidney transplant recipients on the first post-transplant morning predicted DGF and poor allograft function at 1 year.17,18
This study showed a very robust association between all five donor urinary biomarkers and donor AKI. Notably, across the AKI and non-AKI groups of donors, the urinary NGAL and IL-18 concentrations were higher than those reported in a recent study that measured these biomarkers in a population of stable hospitalized patients awaiting cardiac surgery.27 These high concentrations of injury biomarkers are plausible given the multiple potential pathways for kidney injury that often characterize the events preceding deceased kidney donation, including the traumatic episode surrounding brain injury, which may include hypotension and exposure to nephrotoxins (e.g., contrast, antibiotics) during the donation hospitalization.
Our finding of a modest association between donor urinary NGAL and recipient DGF (RR of 1.19 for highest versus lowest tertiles of NGAL) should be considered in the context of prior research. In a cohort of 99 deceased donors and 176 kidney transplant recipients, Hollmen et al. found NGAL measured in deceased-donor urine samples did not predict DGF as defined by any dialysis in the first week of transplant, but higher donor urine NGAL concentrations were associated with a longer duration of post-transplant dialysis and lower 1-year allograft survival.28 A potential explanation for the contrasting findings is that our cohort had substantially greater power to detect the association with DGF. We also did not have detailed data on duration of post-transplant dialysis in the entire cohort, precluding our ability to examine DGF severity.
In this cohort, donor urinary biomarkers provided little value in predicting recipient eGFR at 6 months. In stratified analyses, donor urinary L-FABP and NGAL were associated with slightly lower eGFR at 6 months, but these associations were only present among recipients without DGF and were no longer evident after adjustment for transport and recipient variables. These findings do not support our original conceptual model, which proposed that donor AKI would lead to recipient DGF and worse allograft function particularly in kidneys with DGF.
To explain the lack of association between urinary biomarkers and 6-month eGFR in the setting of DGF, we hypothesize that donor AKI could provide limited protective effects through ischemic preconditioning and upregulation of anti-ischemic regulatory proteins in clinical settings that result in DGF.29,30 This explanation would support the pattern of higher observed eGFRs associated with higher donor urinary biomarkers among recipients with DGF (shown in Figure 2), which is the opposite pattern observed among recipients without DGF who might benefit less from ischemic preconditioning. Alternatively, associations between donor injury biomarkers and recipient eGFR may be confounded by greater discard rates for kidneys at risk for DGF and subsequent adverse outcomes. Finally, a robust body of evidence has shown that DGF is a manifestation of ischemia-reperfusion injury that activates diverse biologic pathways after transplantation, including cell death programs, stimulation of innate and adaptive immune mechanisms, and clinical decisions, including changes in immunosuppression and dialysis treatment that may lead to further injury (e.g., through hypotension).9 These post-transplant events are difficult to measure and could mask the association between donor biomarkers and eGFR. Therefore, it may be that by 6 months, the limited adverse effects of donor AKI (as manifested by biomarkers) are only evident in recipients who do not experience DGF.
We acknowledge study limitations. We only measured urinary biomarkers at the time of organ procurement; however, donors could have experienced kidney injury at earlier points during the hospitalization, and injury biomarker levels might have declined before procurement surgery. Donors may receive aggressive fluid resuscitation or otherwise have dilute urine caused by diabetes insipidus, leading to dilution of biomarkers. To address this possibility, we examined urinary biomarker concentrations indexed to urinary creatinine. Similar to unindexed analyses, creatinine-indexed concentrations of microalbumin and NGAL revealed strong associations with donor AKI. Because we measured the urinary biomarkers just before organ procurement, the biomarkers would also not capture injury during the agonal phase of donation after cardiac death. We did not assess biomarkers in recipients post-transplantation, limiting our ability to assess reperfusion injury or patterns of injury across the entire donor-to-recipient spectrum of care.31 As with other observational studies, unmeasured confounding of associations between donor biomarkers and the outcomes are possible. For example, recipient immune responses to the allograft, such as the generation of de novo donor-specific antibody, will plausibly affect the outcome of 6-month recipient allograft function,32 but we do not have data about immune activation.
These results may illuminate productive new research directions. The high prevalence of donor kidney injury suggests the potential for improved donor management strategies to protect donors from kidney injury during hospitalization. However, consistent with work by our group and others, we find only minor adverse effects of donor AKI on post-transplant recipient outcomes.33,34 This study provides additional evidence that transplantation using deceased-donor kidneys with AKI often leads to good allograft function and that transplant centers should consider acceptance of these kidneys. On the other hand, the development of better tools to profile donor kidney quality and extend the survival of transplanted kidneys remains a high priority for the field of transplantation. New research may instead focus on variation in mechanisms involved in recovery from injury, such as repair pathways, fibrogenesis, or alternatively, events at the time of reperfusion, which may generate substantial inflammation or immune activation.
In conclusion, this large prospective study showed that deceased-donor urinary biomarkers were strongly associated with donor AKI. However, these donor injury biomarkers had limited utility to predict outcomes among kidney transplant recipients.
Concise Methods
The investigator team worked with five OPOs (Gift of Life Donor Program, Philadelphia, PA; New Jersey Sharing Network, New Providence, NJ; Gift of Life Michigan, Ann Arbor, MI; New York Organ Donor Network, New York, NY; and New England Organ Bank, Waltham, MA) that collected urine samples from deceased donors at the time of organ procurement between May 2010 and December 2013. The cohort was comprised of donors at least 16 years of age (if at least one kidney underwent transplantation) whose surrogates provided consent for research and the recipients of these kidneys.
OPO personnel followed institutional protocols for managing donors. The study was approved by OPO scientific review committees and institutional review boards for the investigators.
Data Sources
To ascertain donor and recipient characteristics and outcomes, we linked study databases to the United Network for Organ Sharing (UNOS) database. UNOS data were supplied by the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN, and has been described elsewhere.35 The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Through review of OPO charts, we augmented donor data with additional important elements, including admission serum creatinine.
Donor AKI was defined on the basis of admission to terminal serum creatinine (irrespective of time between measurements or urine output) as ≥2-fold increase.36 This increase corresponds to at least stage 2 AKI according to the AKI Network.36
Recipient outcomes were ascertained using data submitted by transplant centers to UNOS through routine post-transplant forms. DGF was defined as dialysis in the first week after transplantation. The outcome of recipient 6-month eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation and using the serum creatinine reported in the UNOS 6-month follow-up form.37 Few recipients experienced graft failure (defined as return to chronic dialysis or retransplantation) or death before 6 months. For recipients with graft failure, we imputed 6-month eGFR as 10 ml/min per 1.73 m2. For recipients who died with a functioning allograft, we carried forward the last available serum creatinine to calculate 6-month eGFR.
Biomarker Measurement
There were 10 ml of fresh donor urine collected from the indwelling urinary catheter tube in the operating room just before organ procurement and transported on ice to the individual OPOs and stored at −80°C until monthly batch shipments to the Yale University Biorepository. The median time from collection of urine base samples to freezing was 6.3 hours (interquartile range, 4.9–8.0). On the basis of prior work from our group, urinary biomarkers measured for this study should remain stable for >24 hours.38 Samples were then processed following a single controlled thaw, separated into 1-ml barcoded aliquots, and stored at −80°C.
Urine NGAL measurement was performed with the Architect platform (Abbott Diagnostics). Urinary KIM-1 and IL-18 were measured using the Meso Scale Discovery platform (Meso Scale diagnostics, Gaithersburg, MD), which uses electrochemiluminescence detection combined with patterned arrays. Urine L-FABP was measured by a new approach used in clinical chemistry analyzers, on the basis of latex-enhanced immunoturbidimetry with anti-human L-FABP mouse monoclonal antibodies (Sekisui Medial). Urine albumin was measured using the RxDaytona immunoturbidimetry assay (Randox Laboratories, Boston, MA).
Supplemental Figure 2 provides further information about assays for biomarker measurement.
Statistical Analyses
Descriptive statistics were reported as mean±SD or median (interquartile range) for continuous variables and as frequency (%) for categorical variables. For comparisons of categorical attributes between groups, we used chi-squared or Fisher exact tests as appropriate. Continuous variables were compared with a Wilcoxon rank-sum test. For each biomarker, we empirically divided the donors into tertiles of approximately equal size. We calculated the kidney donor risk index (KDRI)10 and converted KDRI, as per convention,39 to obtain the KDPI. We fit multivariable modified Poisson regression models to estimate the RR between donor urinary biomarkers and donor AKI.40 These models were adjusted for donor variables the KDRI includes, including age (years), height (cm), weight (kg), race (black/nonblack), history of hypertension, history of diabetes, hepatitis C serostatus, stroke as cause of death, and determination of death status. These models were not adjusted for donor terminal serum creatinine because change in creatinine defines the AKI outcome.
We also fit multivariable modified Poisson regression models to estimate the RR of DGF associated with donor urinary biomarkers. Subsequently, we fit multivariable linear regression models to test for associations between recipient DGF and 6-month recipient eGFR and between donor biomarkers and 6-month recipient eGFR.
All regression analyses involving recipient outcomes were clustered at the level of the kidney donor. Models were first adjusted for the KDRI variables previously listed. The second model also adjusted for terminal serum creatinine. The third model additionally adjusted for recipient variables of age, race, sex, prior kidney transplant, diabetes as cause of ESRD, pretransplant blood transfusion, panel reactive antibody, human leukocyte antigen mismatch, body mass index (kg/m2), pretransplant dialysis status (i.e., preemptive transplantation versus dialysis before transplantation), and organ transport variable of cold ischemia time (hours). Therefore, the third models adjusted for donor, recipient, and transport attributes, all of which are important to discern an independent relationship between donor urinary biomarkers and recipient outcomes.
For analyses of the outcome of DGF, we calculated the change in area under the curve, the net reclassification improvement, and the integrated discrimination improvement after the addition of urinary biomarker levels to the clinical model composed solely of donor characteristics.41 In the net reclassification improvement analysis, risk categories were defined as low (<5%), medium (5%–10%), or high (>10%) risk.
For analyses of eGFR, we fit prespecified stratified models according to recipient DGF status and performed tests for effect modification between donor biomarkers and DGF on 6-month eGFR.
Analyses were completed using SAS 9.3 software for Windows (SAS Institute, Cary, NC) and R 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided with a significance level of <0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to acknowledge important advice and support from the study Advisory Board members: Peter Abt, Amit Garg, Peter Heeger, and Fadi Lakkis. The authors also thank Drs. Richard Formica and Sanjay Kulkarni for valuable input. The authors thank Isabel Butrymowicz and Rowena Kemp for their assistance with data and sample coordination for this multicenter study. We appreciate the assistance of Dr. Eoin Cotter and Dr. Peter Doran in the UCD CRC Biomarker Laboratory for performance of NGAL assays. We are tremendously grateful for the study participation of partners at five organ procurement organizations, including Gift of Life Philadelphia (Sharon West, Vicky Reilly), New York Organ Donor Network (Harvey Lerner, Anthony Guidice, Allison Hoffman), Michigan Organ and Tissue Donation Program (Burton Mattice, Susan Shay), New Jersey Sharing Network (William Reitsma, Cindy Godfrey, Alene Steward, Joel Padilla Benitez), and New England Organ Bank (Christopher Curran, Brandon McKown).
This work was supported by the National Institutes of Health (grant nos. RO1DK-93770 and K24DK090203), a Roche Organ Transplantation Research Foundation Award to Dr. Parikh, an award from the American Heart Association to Dr. Hall, a Yale Kidney O’Brien Center Award (P30DK79337), and the Health Resources and Services Administration (contract no. 234-2005-37011C).
Dr. Parikh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Parikh also affirms that he has listed everyone who made substantial contributions to this work. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. NGAL assays were donated by Abbott Diagnostics and measured at University of Ireland. L-FABP assays were donated by Sekisui Medical. The companies did not participate in design, analysis, or interpretation of study results. The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040345/-/DCSupplemental.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Dew MA, Switzer GE, Goycoolea JM, Allen AS, DiMartini A, Kormos RL, Griffith BP: Does transplantation produce quality of life benefits? A quantitative analysis of the literature. Transplantation 64: 1261–1273, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Schnitzler MA, Lentine KL, Burroughs TE: The cost effectiveness of deceased organ donation. Transplantation 80: 1636–1637, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Health Resources and Services Administration, U.S. Department of Health & Human Services: Organ Procurement and Transplantation Network, Data Reports Builder. Transplants by Donor Type: Kidney. Available at: http://optn.transplant.hrsa.gov/converge/LatestData/step2.asp. Accessed December 19, 2014
- 5.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA: Outcomes of kidneys from donors after cardiac death: Implications for allocation and preservation. Am J Transplant 7: 1797–1807, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, Badger GJ, Lynch ME: Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 103: 1009–1018, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B: Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int 59: 1539–1543, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Louvar DW, Li N, Snyder J, Peng Y, Kasiske BL, Israni AK: “Nature versus nurture” study of deceased-donor pairs in kidney transplantation. J Am Soc Nephrol 20: 1351–1358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuno N, Sakurai E, Tamaki I, Uchiyama M, Kozaki K, Kozaki M: The effect of machine perfusion preservation versus cold storage on the function of kidneys from non-heart-beating donors. Transplantation 57: 293–294, 1994 [PubMed] [Google Scholar]
- 13.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, Drachenberg CB, Thom KA, Perencevich EN, Haririan A, Rasetto F, Cooper M, Campos L, Barth RN, Bartlett ST, Philosophe B: The Maryland aggregate pathology index: A deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant 8: 2316–2324, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; NGAL Meta-analysis Investigator Group : Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Siew ED, Ware LB, Ikizler TA: Biological markers of acute kidney injury. J Am Soc Nephrol 22: 810–820, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 17.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR: Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 7: 1224–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, Shlipak MG, Parikh CR; TRIBE-AKI Consortium : Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25: 1063–1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Land WG: The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 79: 505–514, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol 123: 227–234, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S: Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant 22: 295–296, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollmen ME, Kyllönen LE, Inkinen KA, Lalla ML, Merenmies J, Salmela KT: Deceased donor neutrophil gelatinase-associated lipocalin and delayed graft function after kidney transplantation: A prospective study. Crit Care 15: R121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gassanov N, Nia AM, Caglayan E, Er F: Remote ischemic preconditioning and renoprotection: From myth to a novel therapeutic option? J Am Soc Nephrol 25: 216–224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Lang XB, Zhang P, Lv R, Wang YF, Chen JH: Remote ischemic preconditioning for prevention of acute kidney injury: A meta-analysis of randomized controlled trials. Am J Kidney Dis 64: 574–583, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Hall IE, Parikh CR: Human models to evaluate urinary biomarkers of kidney injury. Clin J Am Soc Nephrol 5: 2141–2143, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD: Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16: 2804–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Hall IE, Schrӧppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, Thiessen-Philbrook H, Reese PP, Parikh CR: Associations of Deceased Donor Kidney Injury With Kidney Discard and Function After Transplantation. Am J Transplant 15: 1623–1631, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farney AC, Rogers J, Orlando G, al-Geizawi S, Buckley M, Farooq U, al-Shraideh Y, Stratta RJ: Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg 216: 645–655; discussion 645–655, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Dickinson DM, Bryant PC, Williams MC, Levine GN, Li S, Welch JC, Keck BM, Webb RL: Transplant data: Sources, collection, and caveats. Am J Transplant 6[Suppl 9]: 13–26, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, Reeves WB, Devarajan P, Kimmel PL, Siew ED, Liu KD; ASSESS-AKI Study Investigators : Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis 63: 567–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.OPTN: Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting KDPI. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf. Accessed May 14, 5014
- 40.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, Thiessen-Philbrook H: Key concepts and limitations of statistical methods for evaluating biomarkers of kidney disease. J Am Soc Nephrol 25: 1621–1629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.