Abstract
Mutations in the vacuolar–type H+-ATPase B1 subunit gene ATP6V1B1 cause autosomal–recessive distal renal tubular acidosis (dRTA). We previously identified a single-nucleotide polymorphism (SNP) in the human B1 subunit (c.481G>A; p.E161K) that causes greatly diminished pump function in vitro. To investigate the effect of this SNP on urinary acidification, we conducted a genotype-phenotype analysis of recurrent stone formers in the Dallas and Bern kidney stone registries. Of 555 patients examined, 32 (5.8%) were heterozygous for the p.E161K SNP, and the remaining 523 (94.2%) carried two wild–type alleles. After adjustment for sex, age, body mass index, and dietary acid and alkali intake, p.E161K SNP carriers had a nonsignificant tendency to higher urinary pH on a random diet (6.31 versus 6.09; P=0.09). Under an instructed low–Ca and low–Na diet, urinary pH was higher in p.E161K SNP carriers (6.56 versus 6.01; P<0.01). Kidney stones of p.E161K carriers were more likely to contain calcium phosphate than stones of wild-type patients. In acute NH4Cl loading, p.E161K carriers displayed a higher trough urinary pH (5.34 versus 4.89; P=0.01) than wild-type patients. Overall, 14.6% of wild-type patients and 52.4% of p.E161K carriers were unable to acidify their urine below pH 5.3 and thus, can be considered to have incomplete dRTA. In summary, our data indicate that recurrent stone formers with the vacuolar H+-ATPase B1 subunit p.E161K SNP exhibit a urinary acidification deficit with an increased prevalence of calcium phosphate–containing kidney stones. The burden of E161K heterozygosity may be a forme fruste of dRTA.
Keywords: renal tubular acidosis, human genetics, kidney stones
Urinary acidification is achieved by H+ secretion from α-intercalated cells in the collecting duct.1 In these cells, H+ furnished by the cytosolic carbonic anhydrase is translocated against a concentration gradient by the ATP-driven vacuolar H+-ATPase (V-ATPase) into the urine. Contemporaneous with apical H+ secretion, carbonic anhydrase liberates HCO3− ions, which exit the α-intercalated cell basolaterally through the AE1 chloride-base exchanger to complete transepithelial acid-base transport. Dysfunction of α-intercalated cells causes distal renal tubular acidosis (dRTA), which is characterized by metabolic acidosis in the presence of unduly alkaline urine, growth retardation, rickets, nephrolithiasis, nephrocalcinosis, hypokalemia, and progressive renal failure.2 dRTA can be acquired or inherited. Mutations in the a4 and B1 subunits of the V-ATPase, the AE1 chloride-base exchanger, and the carbonic anhydrase type II enzyme were thus far identified as causes of inherited dRTA in humans.3–6 Mutations in a4 and B1 subunits cause early–onset, autosomal–recessive disease, whereas AE1 mutations cause autosomal–dominant or autosomal-recessive, later–onset disease. Partial impairment of distal tubular acidification is known as incomplete dRTA and has been associated with osteoporosis and nephrolithiasis.7–9 In contrast to dRTA, which is characterized by overt metabolic acidosis, blood pH and HCO3− are normal in incomplete dRTA, but maximal urinary acidification is impaired when challenged.7,10 The etiology of incomplete dRTA is unknown but may be, in part, caused by allelic variants of genes involved in H+ secretion in α-intercalated cells.
The V-ATPase consists of two multisubunit complexes, the V1 (head) and V0 (membrane–anchored) subunits.11 The 640-kD V1 subunit is composed of subunits A–H. Mammals have two B subunits, the ubiquitous B2 isoform and the B1 isoform, which is restricted to specialized epithelia of the inner ear, epididymis, and intercalated cells.12 Numerous homozygous and compound heterozygous missense, nonsense, frameshift, and splice site mutations along the B1 subunit gene ATP6V1B1 have been reported in individuals with congenital dRTA.3,13
We previously investigated disease–causing missense B1 subunit mutations in vitro.14 In B subunit–defective yeast, wild–type human B1 but not mutant B1 subunits rescued the growth phenotype by functional complementation. With one exception (p.G316E mutation), all mutant B1 subunits studied caused disrupted V-ATPase assembly in cell–based and biochemical assays (i.e., the failure to assemble in mammalian cells and inability to complement in yeast were concordant features). The extension of our analysis to common ATP6V1B1 single–nucleotide polymorphisms (SNPs) unexpectedly revealed that the SNP (c.481G>A; p.E161K) exhibits greatly diminished pump function in vitro with the yeast growth assay, despite intact assembly in mammalian cells.14 This lack of concordance between pump assembly and function is unique and raises the question of the whether this is truly a functional variant or some phenomenon specific to yeast hosts. If this variant allele affects urinary acidification in vivo is currently unknown. To address this question, we conducted a genotype-phenotype analysis in recurrent stone formers (SFs).
Results
Screening and Identification of p.E161K Carriers
To identify p.E161K carriers, we conducted a search in the kidney stone registries at the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research, the University of Texas Southwestern Medical Center, and the Division of Nephrology, Hypertension and Clinical Pharmacology, University Hospital of Bern. The kidney stone registry in Bern was initiated in 2004 with the Dallas registry as a template. Thus, both stone registries contain patients who underwent the same detailed metabolic workup protocols, including a 1-week controlled Ca (15–20 mmol/d) and Na (100 mmol/d) diet intervention protocol (details in Concise Methods). Patients in the Bern registry also underwent dual–energy x-ray absorptiometry (DEXA) analysis of bone mineral density (BMD); 555 patients in both registries met the inclusion criteria (age ≥18 years old, written informed consent, and at least one episode of kidney stone disease) and had no exclusion criterion (known medications and disease states interfering with urinary acidification; details in Concise Methods). All 555 patients were genotyped for the c.481G>A; p.E161K SNP using bidirectional Sanger sequencing of exon 6 of ATP6V1B1. To exclude additional mutations that could confound the analysis, we sequenced the coding regions and intron-exon boundaries of the two genes associated with autosomal-recessive dRTA in humans (ATP6V1B1 and ATPV0A4) in all p.E161K heterozygous SFs included in the study. To distinguish benign from likely disease–causing variants, we evaluated each variant individually on the basis of strict criteria as described in Concise Methods. The sequencing of the ATP6V1B1 and ATPV0A4 genes revealed no additional mutations and no additional likely disease–causing variants.
Baseline Characteristics of the Study Population
Characteristics of the patients included in the analysis are depicted in Table 1; 32 of 555 recurrent SFs included in the analysis were heterozygous (5.8%) for the p.E161K SNP. None of 555 recurrent SFs analyzed were homozygous for the SNP. The frequency of p.E161K heterozygosity was similar in both registries (5.5% in the Dallas registry and 5.9% in the Bern registry). As expected for recurrent SFs, there were two times as many men as women overall in the two cohorts. There were no significant sex differences between wild-type and heterozygous patients (74.2% men versus 68.8% men, respectively; P=0.50). However, heterozygous SFs were younger at first presentation at the stone clinic (median, 38.3 versus 42.7 years; P=0.04), had a lower body mass index (BMI; median, 25.0 versus 26.5 kg/m2; P=0.02), and were more likely to have a positive family history of kidney stone disease (60.7% versus 41.3%; P=0.04). Furthermore, calculi of p.E161K carriers were significantly more likely to contain calcium phosphate (CaP) (70.0% versus 38.6%; P<0.01).
Table 1.
Baseline patient characteristics according to E161K genotype (n=555)
| Characteristic | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|
| Total stone patients | 523 | 94.2% (of total) | 32 | 5.8% (of total) | |
| Center Bern | 351 | 67.11% | 22 | 68.75% | 0.85 |
| Center Dallas | 172 | 32.89% | 10 | 31.25% | |
| Age at first presentation (yr) | 514 | 42.65 (33.9–53.0) | 31 | 38.30 (32.2–46.3) | 0.04 |
| Men | 382 | 74.2% | 22 | 68.8% | 0.50 |
| BMI (kg/m2) | 479 | 26.5 (23.4–29.6) | 29 | 25.0 (22.4–27.1) | 0.02 |
| Positive family history of stones | 179 | 41.3% | 17 | 60.7% | 0.04 |
| Patients with stones available for analysis | 369 | 71.3% | 20 | 62.5% | 0.29 |
| Stones containing calcium oxalate | 346 | 94.0% | 18 | 90.0% | 0.47 |
| Stones containing calcium phosphate | 142 | 38.6% | 14 | 70.0% | <0.01 |
| Stones containing uric acid | 19 | 5.2% | 0 | 0% | 0.30 |
| T score lumbar spine | 303 | −0.51±1.05 | 20 | −0.48±1.61 | 0.94 |
| T score femur neck | 300 | −0.54±1.05 | 20 | −0.57±0.97 | 0.92 |
| T score tibia diaphysis | 301 | 0.40±1.08 | 19 | 0.38±0.91 | 0.93 |
| T score tibia epiphysis | 300 | −0.58±0.96 | 19 | −0.65±0.87 | 0.74 |
| Osteoporosis presenta | 21 | 6.9% | 1 | 4.8% | 0.71 |
| Osteopenia presenta | 162 | 53.1% | 13 | 61.9% | 0.44 |
The number of SFs is indicated for each characteristic stratified by the genotype. Categorical variables are further described by percentage, and continuous variables are further described by their means±SDs or their medians (25th to 75th percentiles). Between-group differences are determined by Welch t, Mann–Whitney U, or chi-squared test as appropriate, and the corresponding P values are indicated.
Osteoporosis was defined as T score <−2.5, and osteopenia was defined as T score <−1.0 and >−2.5.
DEXA analysis revealed no relevant differences in BMD between the two groups of patients. The prevalence of osteoporosis and osteopenia was equal in both groups.
Blood and Urinary Biochemistries on Random Outpatient Diet
Blood and urinary biochemistries of patients on a random outpatient diet are depicted in Table 2. There were no differences in plasma electrolytes or renal function between the two groups. Random blood glucose was higher in wild-type patients (median, 5.0 versus 4.8 mmol/L; P=0.02), and plasma bicarbonate was lower in heterozygous patients (mean, 25.2 versus 26.1 mmol/L; P=0.02). Heterozygous SFs displayed nonsignificant trends to higher urinary pH (mean, 6.31 versus 6.09; P=0.09) and a lower 24-hour citrate excretion (median, 2.01 versus 2.79 mmol/24 h; P=0.08). Normalization to K, K − P, and net gastrointestinal absorption (NGIA), all surrogates of alkali intake, also showed numerically lower citrate values in heterozygotes that did not reach statistical significance; 24-hour sulfate excretion as a measure of animal protein intake, 24-hour urinary ammonium (NH4), net acid excretion, and NGIA were comparable between the two groups of patients.
Table 2.
Blood and urine parameters according to E161K genotype on random outpatient diet
| Characteristic | Normal Range | Unit | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|---|---|
| Plasma Na | 132–142 | mmol/L | 480 | 140.5±2.2 | 29 | 140.6±2.1 | 0.87 |
| Plasma K | 3.5–4.7 | mmol/L | 480 | 3.9 (3.7–4.1) | 29 | 4 (3.8–4.1) | 0.28 |
| Plasma Cl | 97–108 | mmol/L | 475 | 104 (102–106) | 29 | 105 (102–107) | 0.25 |
| Plasma Ca total | 2.10–2.55 | mmol/L | 481 | 2.4 (2.3–2.4) | 29 | 2.4 (2.3–2.5) | 0.13 |
| Plasma Ca ionized | 1.13–1.30 | mmol/L | 343 | 1.2 (1.2–1.2) | 22 | 1.2 (1.2–1.2) | 0.14 |
| Plasma P | 0.84–1.45 | mmol/L | 481 | 1±0.2 | 29 | 1.1±0.2 | 0.11 |
| Plasma Mg | 0.75–1.00 | mmol/L | 401 | 0.8 (0.8–0.9) | 24 | 0.8 (0.8–0.9) | 0.23 |
| Plasma creatinine | 59–104 | µmol/L | 481 | 80 (70–89) | 29 | 80 (69–88) | 0.52 |
| eGFR CKD-EPI | >90 | ml/min per 1.73 m2 | 481 | 98.1 (83.8–109.9) | 28 | 99 (87.5–114.1) | 0.39 |
| Plasma urea | 3.2–8.1 | mmol/L | 348 | 5.3 (4.5–6.3) | 20 | 4.9 (4.3–5.6) | 0.10 |
| Plasma uric acid | 202–416 | µmol/L | 475 | 325±79.3 | 29 | 308.6±81.4 | 0.30 |
| Plasma random glucose | 3.33–5.55 | mmol/L | 474 | 5.0 (4.6–5.6) | 28 | 4.8 (4.4–5) | 0.02 |
| Blood pH | 7.35–7.45 | — | 344 | 7.392±0.029 | 22 | 7.398±0.032 | 0.38 |
| Plasma bicarbonate | 18.0–29.0 | mmol/L | 469 | 26.1±2.4 | 30 | 25.2±2 | 0.02 |
| Plasma anion gap | 8–16 | mmol/L | 317 | 10.3±2.5 | 21 | 9.8±2.2 | 0.31 |
| Serum PTH | 15–65 | pg/ml | 427 | 38 (30–46) | 26 | 34 (23.8–60.8) | 0.74 |
| Serum 25-OH-vitamin D | 49–134 | nmol/L | 222 | 40 (24.3–56) | 10 | 35.5 (26.3–40.5) | 0.29 |
| Serum 1,25-OH-vitamin D | 48–160 | pmol/L | 343 | 97 (72.5–125.5) | 21 | 91 (70–132) | 0.64 |
| Plasma alkaline phosphatase | 35–105 | units/L | 350 | 65 (55–76) | 21 | 63 (57–74) | 0.80 |
| Urine Dpd/creatinine | 2.5–9.0 | nmol/mmol | 311 | 4 (3.4–4.9) | 19 | 4.8 (3.8–5.5) | 0.14 |
| Urine pyridinium crosslinks | — | nmol/L | 316 | 37.3 (23.4–56) | 20 | 33 (19.8–49.1) | 0.54 |
| Serum TSH | 0.27–4.20 | milliunits/L | 347 | 1.3 (0.9–1.8) | 22 | 1.3 (0.8–1.5) | 0.55 |
| Urinary volume | — | ml | 502 | 1998 (1493–2618) | 30 | 1887 (1609–2111) | 0.67 |
| Urine pH | — | — | 476 | 6.09±0.64 | 30 | 6.31±0.68 | 0.09 |
| Urine anion gap | — | mmol/L | 329 | 41.3±14.3 | 22 | 41.3±12.8 | 0.98 |
| Urine Na | 40–220 | mmol/24 h | 501 | 184 (141–236) | 30 | 171.8 (130.1–230.6) | 0.35 |
| Urine K | 25–125 | mmol/24 h | 501 | 60.5 (46–77) | 30 | 58.3 (43.5–66.4) | 0.08 |
| Urine Cl | 110–250 | mmol/24 h | 472 | 172.3 (128.4–225.5) | 27 | 141 (117.5–209.7) | 0.14 |
| Urine Ca | 2.50–7.50 | mmol/24 h | 500 | 6.26 (4.46–8.82) | 30 | 6.82 (5.55–8.88) | 0.52 |
| Urine P | 13.0–42.0 | mmol/24 h | 500 | 30.8 (24.1–38.9) | 30 | 30.1 (26.1–36.1) | 0.52 |
| Urine Mg | 2.50–8.50 | mmol/24 h | 497 | 4.34 (3.29–5.42) | 30 | 4.35 (3.8–4.93) | 0.59 |
| Urine uric acid | <5900 | µmol/24 h | 500 | 3500 (2935–4270) | 30 | 3216 (2633–3594) | 0.004 |
| Urine urea | 96–556 | mmol/24 h | 349 | 410.6 (329.3–509.3) | 22 | 364.4 (288.6–513.2) | 0.21 |
| Urine creatinine | 8600–19,400 | µmol/24 h | 502 | 14,334±4046 | 30 | 13,519±3300 | 0.20 |
| Urine citrate | 1.65–6.6 | mmol/24 h | 487 | 2.79 (1.81–4.02) | 28 | 2.01 (1.36–3.09) | 0.08 |
| Urine citrate/K | — | (mmol/24 h)/(mmol/24 h) | 487 | 0.046 (0.032–0.064) | 28 | 0.041 (0.023–0.05) | 0.12 |
| Urine K-P | — | mmol/24 h | 500 | 28.4 (17.3–41.9) | 30 | 22.6 (14.3–37.1) | 0.08 |
| Urine citrate/K-P | — | (mmol/24 h)/(mmol/24 h) | 485 | 0.098 (0.063–0.173) | 28 | 0.094 (0.043–0.162) | 0.41 |
| NGIA | — | mmol/24 h | 468 | 30.76±29.67 | 27 | 26.51±28.62 | 0.46 |
| Urine SO4 | — | mmol/24 h | 487 | 22.2 (16.3–28.5) | 28 | 20.4 (15.5–28) | 0.74 |
| Urine oxalate | <500 | µmol/24 h | 492 | 382.4 (286.1–559.4) | 28 | 358 (268.8–482.8) | 0.08 |
| Urine glycolate | 150–600 | µmol/24 h | 331 | 371.5 (148.5–574.9) | 20 | 357.5 (182.4–499.3) | 0.56 |
| Urine NH4 | 10–107 | mmol/24 h | 96 | 36.8±15.9 | 4 | 31.9±4.7 | 0.32 |
| NAE | — | mmol/24 h | 96 | 90.1 (63.3–103.8) | 4 | 74.2 (67.32–80.9) | 0.20 |
| Urine NH4/SO4 | — | (mmol/24 h)/(mmol/24 h) | 96 | 1.82 (1.41–2.18) | 4 | 1.9 (1.77–2.9) | 0.46 |
| Urine NH4/NAE | — | (mmol/24 h)/(mmol/24 h) | 96 | 0.405±0.092 | 4 | 0.447±0.125 | 0.56 |
The number of SFs is indicated for each characteristic stratified by the genotype. Continuous variables are further described by their means±SDs or their medians (25th to 75th percentiles) as appropriate. Between-group differences are determined by Welch t or Mann–Whitney U test as appropriate, and the corresponding P value is indicated. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; PTH, parathormone; Dpd deoxypyridinoline; TSH, thyroid stimulating hormone; SO4, sulfate; NAE, net acid excretion.
Blood and Urinary Biochemistry on an Outpatient Diet Low in Na and Ca
Blood and urinary biochemistries on an instructed outpatient diet low in Na and Ca are depicted in Table 3. This diet is not meant as therapy but rather, to control extrinsic dietary factors, allowing one to observe the endogenous characteristics of the subjects.15 Compared with the random outpatient diet, there were clear reductions in both groups of patients in 24-hour urinary Na and Ca excretion on the low–Ca and low–Na diet, indicating adherence to the dietary instructions. With the exception of plasma P, which was higher in the heterozygous patients (mean, 1.01 versus 0.92 mmol/L; P=0.04), blood chemistries revealed no relevant differences between the two groups. Heterozygous patients exhibited a significantly higher 24-hour urine pH (median, 6.555 versus 6.005; P<0.01); however, there was no difference in 24-hour citrate excretion when diet was controlled (median, 2.372 versus 2.653 mmol/24 h; P=0.32). As was the case under random diet, 24-hour sulfate excretion, 24-hour urinary NH4, net acid excretion, and NGIA were comparable between the two groups under the low–Na and low–Ca diet.
Table 3.
Blood and urine parameters according to E161K genotype on a low–Ca and low–Na diet
| Characteristic | Normal Range | Unit | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|---|---|
| Plasma Na | 132–142 | mmol/L | 317 | 139.2±1.8 | 21 | 139.4±1 | 0.39 |
| Plasma K | 3.5–4.7 | mmol/L | 317 | 3.813±0.262 | 21 | 3.848±0.186 | 0.43 |
| Plasma Cl | 97–108 | mmol/L | 214 | 101.7±2.3 | 10 | 101.1±1.2 | 0.16 |
| Plasma Ca total | 2.10–2.55 | mmol/L | 311 | 2.31 (2.25–2.39) | 21 | 2.34 (2.3–2.44) | 0.12 |
| Plasma Ca ionized | 1.13–1.30 | mmol/L | 303 | 1.194±0.048 | 19 | 1.206±0.041 | 0.23 |
| Plasma P | 0.84–1.45 | mmol/L | 316 | 0.92±0.174 | 21 | 1.012±0.191 | 0.04 |
| Plasma Mg | 0.75–1.00 | mmol/L | 212 | 0.845 (0.8–0.89) | 10 | 0.825 (0.775–0.848) | 0.16 |
| Plasma creatinine | 59–104 | µmol/L | 312 | 81 (71–90) | 21 | 77 (73–89) | 0.93 |
| Plasma uric acid | 202–416 | µmol/L | 287 | 363±89 | 19 | 337±63 | 0.10 |
| Blood pH | 7.35–7.45 | — | 292 | 7.387±0.029 | 19 | 7.391±0.042 | 0.68 |
| Plasma bicarbonate | 18.0–29.0 | mmol/L | 275 | 26±2.1 | 18 | 26.3±2.1 | 0.51 |
| Plasma anion gap | 8–16 | mmol/L | 198 | 11.5±1.8 | 10 | 11.8±1.7 | 0.63 |
| Urinary volume | — | ml | 453 | 2200 (1620–2840) | 30 | 1903 (1405–2558) | 0.17 |
| Urine pH | — | — | 392 | 6.005 (5.63–6.4) | 24 | 6.555 (5.958–6.875) | <0.01 |
| Urine anion gap | — | mmol/L | 295 | 30 (22.2–39.2) | 21 | 30.1 (24.4–47.6) | 0.31 |
| Urine Na | 40–220 | mmol/24 h | 452 | 92.5 (62–129) | 30 | 90 (47.5–118.5) | 0.16 |
| Urine K | 25–125 | mmol/24 h | 450 | 54 (39–70.8) | 30 | 43 (27.8–60.8) | 0.15 |
| Urine Cl | 110–250 | mmol/24 h | 419 | 80 (55–119) | 26 | 67 (41.8–101.3) | 0.10 |
| Urine Ca | 2.50–7.50 | mmol/24 h | 452 | 4.04 (2.485–6.113) | 30 | 3.56 (2.603–5.643) | 0.79 |
| Urine P | 13.0–42.0 | mmol/24 h | 451 | 26.1 (19.9–32.5) | 30 | 21 (18.2–28.6) | 0.09 |
| Urine Mg | 2.50–8.50 | mmol/24 h | 445 | 3.87 (2.96–4.75) | 30 | 3.32 (2.828–4.09) | 0.14 |
| Urine uric acid | <5900 | µmol/24 h | 451 | 3403 (2641–4260) | 29 | 3011 (2396–3886) | 0.04 |
| Urine urea | 96–556 | mmol/24 h | 314 | 355.2 (266.8–445.8) | 21 | 345.4 (192.1–418) | 0.44 |
| Urine creatinine | 8600–19,400 | µmol/24 h | 451 | 14,248 (10,841–17,092) | 30 | 13,678 (10,208–16,163) | 0.29 |
| Urine citrate | 1.65–6.6 | mmol/24 h | 442 | 2.653 (1.668–3.784) | 27 | 2.372 (1.623–3.299) | 0.32 |
| Urine citrate/K | — | (mmol/24 h)/(mmol/24 h) | 441 | 0.048 (0.034–0.068) | 27 | 0.056 (0.033–0.071) | 0.56 |
| Urine K-P | — | mmol/24 h | 450 | 26.7 (13.1–44.2) | 30 | 19.3 (5.3–38.9) | 0.86 |
| Urine citrate/K-P | — | (mmol/24 h)/(mmol/24 h) | 440 | 0.092 (0.057–0.153) | 27 | 0.099 (0.043–0.135) | 0.66 |
| NGIA | — | mmol/24 h | 413 | 25.6 (10.1–40.9) | 26 | 24.7 (6.4–45.5) | 0.51 |
| Urine SO4 | — | mmol/24 h | 443 | 18.3 (13–23.3) | 25 | 19.5 (11.5–21.3) | 0.96 |
| Urine oxalate | <500 | µmol/24 h | 448 | 418 (311–605) | 26 | 395 (290–638) | >0.99 |
| Urine glycolate | 150–600 | µmol/24 h | 310 | 320 (168–467) | 18 | 329 (154–458) | 0.83 |
| Urine NH4 | 10–107 | mmol/24 h | 80 | 37.4 (28.8–47.5) | 3 | 35.7 (27.5–48.7) | 0.91 |
| NAE | — | mmol/24 h | 80 | 85.9 (67.8–108.1) | 3 | 70.4 (65.9–83.8) | 0.37 |
| Urine NH4/SO4 | — | (mmol/24 h)/(mmol/24 h) | 80 | 1.895 (1.631–2.515) | 3 | 2.38 (1.904–5.04) | 0.50 |
| Urine NH4/NAE | — | (mmol/24 h)/(mmol/24 h) | 80 | 0.438 (0.378–0.487) | 3 | 0.507 (0.41–0.57) | 0.71 |
The number of SFs is indicated for each characteristic stratified by the genotype. Continuous variables are further described by their means±SDs or their medians (25th to 75th percentiles) as appropriate. Between-group differences are determined by Welch t or Mann–Whitney U test as appropriate, and the corresponding P value is indicated. SO4, sulfate; NAE, net acid excretion.
Multivariate Analyses
In the unadjusted analysis, heterozygous carriers of the p.E161K SNP exhibit signs of impaired urinary acidification. These include increased CaP content of calculi, lower plasma bicarbonate on a random outpatient diet, and lower urinary pH on a more controlled diet. We next performed a multivariate analysis to adjust for known predictors of acid-base homeostasis, including sex, age, BMI, and dietary acid and alkali intake, taking between-center variability into account.16–18 As shown in Table 4, after this adjustment, urinary pH on low–Ca and low–Na diet remained significantly different between the two groups of patients. However, plasma bicarbonate under random outpatient diet was no longer different between the two groups. Because of the relatively low number of heterozygotes, logistic regression for stone analysis could not be performed with inclusion of all covariates. Table 5 shows associations between p.E161 carriers and kidney stone type as odds ratios on the basis of mixed effects logistic regression models, alternatively adjusting for sex, age, BMI, or combinations of two of these variables, taking center variability into account. E161K heterozygosity remains significantly associated with an increased CaP content of calculi.
Table 4.
Associations between the E161K polymorphism and plasma and urinary acid-base parameters estimated by linear regression models and mixed effects linear regression models
| Response Variable | E161K Unadjusted | E161K Adjusted | ||||
|---|---|---|---|---|---|---|
| β | 95% Confidence Interval | P Value | β | 95% Confidence Interval | P Value | |
| Under random diet | ||||||
| Plasma bicarbonate (mmol/L; n=439) | −0.66 | −1.63 to 0.30 | 0.18 | −0.70 | −1.61 to 0.21 | 0.13 |
| Urinary pH (n=442) | 0.31 | 0.03 to 0.58 | <0.05 | 0.22 | −0.03 to 0.48 | 0.09 |
| Urinary 24-h citrate (mmol/24 h; n=465)a | −0.22 | −0.44 to −0.01 | <0.05 | −0.14 | −0.33 to 0.06 | 0.17 |
| Urinary 24-h ammonium (mmol/24 h; n=83)b | −5.82 | −23.88 to 12.23 | 0.52 | 2.08 | −9.25 to 13.41 | 0.71 |
| Under low–calcium and low–sodium diet | ||||||
| Plasma bicarbonate (mmol/L; n=281)c | 0.44 | −0.72 to 1.61 | 0.46 | 0.59 | −0.51 to 1.68 | 0.29 |
| Urinary pH (n=346) | 0.51 | 0.21 to 0.80 | <0.001 | 0.38 | 0.12 to 0.64 | <0.01 |
| Urinary 24-h citrate (mmol/24 h; n=433)a | −0.10 | −0.32 to 0.12 | 0.39 | −0.07 | −0.28 to 0.15 | 0.54 |
| Urinary 24-h ammonium (mmol/24 h; n=64)b | −3.29 | −25.89 to 19.31 | 0.77 | 8.24 | −6.99 to 23.47 | 0.28 |
The estimate of E161K has the wild type as the reference group. For plasma bicarbonate, urinary pH, and urinary 24-hour citrate, the between-center variability was taken into account as a random effect for all models. Adjusted models were created by backward selection from a full additive model containing sex, age, BMI, 24-hour urinary sulfate excretion (as a marker for dietary acid intake), and net gastrointestinal alkali absorption (as a marker for dietary alkali intake), including nonlinear terms and interactions where appropriate as described in the statistical part.
Square root transformation was applied.
Variables available in the Dallas center only.
Variables available in the Bern center only.
Table 5.
Associations between the E161K polymorphism and kidney stones estimated by mixed effects logistic regression models
| Kidney Stones | Unadjusted | Sex | Age | BMI | Sex and Age | Sex and BMI | Age and BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Calcium oxalate (present) | 0.57 (0.15 to 3.74) | 0.47 | 0.62 (0.16 to 4.10) | 0.54 | 1.06 (0.22 to 21.69) | 0.88 | 1.32 (0.25 to 2.45) | 0.79 | 1.21 (0.23 to 22.46) | 0.86 | 1.34 (0.25 to 2.49) | 0.78 | 1.3 (0.24 to 2.45) | 0.80 |
| Calcium phosphate (present) | 3.71 (1.45 to 10.69) | <0.01 | 3.60 (1.37 to 10.57) | <0.05 | 2.89 (1.10 to 8.45) | <0.05 | 3.28 (1.23 to 9.69) | <0.05 | 2.90 (1.08 to 8.66) | <0.05 | 3.32 (1.21 to 10.04) | <0.05 | 2.78 (1.03 to 8.28) | <0.05 |
| Uric acid (present) | <0.001 | 0.86 | <0.001 | 0.87 | <0.001 | 0.99 | <0.001 | 0.96 | <0.001 | 0.95 | <0.001 | 0.31 | <0.001 | 0.97 |
The estimate of E161K has the wild type as the reference group. The between-center variability was taken into account as a random effect in all models. Complete data of 365–388 kidney SFs were available for inclusion in the regression models. OR, odds ratio; 95% CI, 95% confidence interval.
Response to Acute Acid Loading
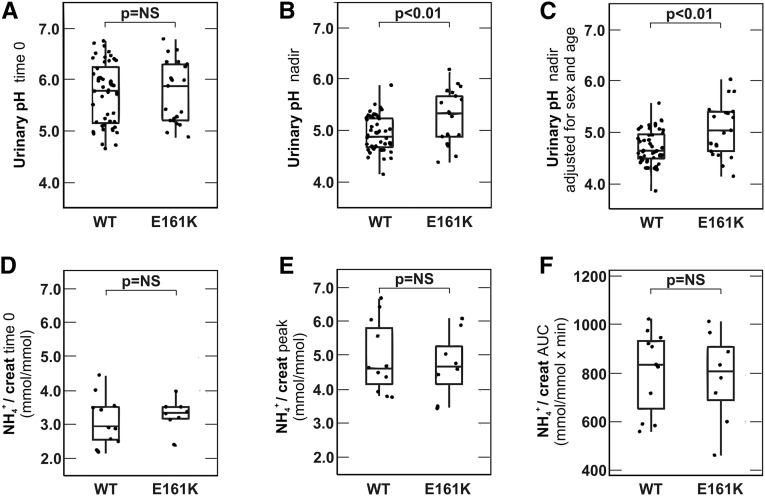
We next challenged the renal acid excretory capacity in wild-type (n=48) and heterozygous (n=21) SFs by performing the standard NH4Cl loading test.7 There were no significant age and sex differences between the two groups (Table 6). Urinary pH was measured at baseline and hourly for 6 consecutive hours after ingestion of NH4Cl gelatin capsules (0.1 g/kg body wt). As shown in Figure 1A, there was no difference in the median fasting urinary pH at the beginning of the test (5.78 in wild-type versus 5.87 in heterozygous patients; P=0.71). However, we observed a clear difference in the trough urinary pH reached between the two groups of patients (median, 4.89 in wild-type versus 5.34 in heterozygous patients; P=0.01; Mann–Whitney U test) (Figure 1B). This significant difference in trough urinary pH persisted even after adjustment for sex and age (Figure 1C); 7 of 48 wild-type (14.58%) and 11 of 21 heterozygous patients (52.38%) were unable to acidify their urine below 5.3 and thus, had incomplete dRTA (P=0.001; chi-squared test). Baseline and peak urinary NH4 concentrations and urinary NH4 excretion profiles (area under the curve) were not different between the two groups of patients (Figure 1, D–F).
Table 6.
Characteristics of patients who underwent ammonium chloride loading according to E161K genotype (n=69)
| Characteristic | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|
| Center Bern | 37 | 77.1% | 13 | 61.9% | 0.19 |
| Center Dallas | 11 | 22.9% | 8 | 38.1% | |
| Age (yr) | 48 | 41.84 (34.5–50.6) | 21 | 39.5 (25.4–44.8) | 0.17 |
| Men | 33 | 68.8% | 15 | 71.4% | 0.82 |
The number of SFs is indicated for each characteristic stratified by the genotype. Categorical variables are further described by percentage, and continuous variables are further described by their medians (25th to 75th percentiles). Between-group differences are determined by Mann–Whitney U or chi-squared test as appropriate, and the corresponding P value is indicated.
Figure 1.
p.E161K heterozygous SFs have higher UpH after acid loading. 48 WT and 21 p.E161K heterozygous (E161K) SFs underwent NH4Cl loading, and 68.8% of WT and 71.4% of E161K heterozygous SFs tested were men (P=0.82). Median ages of WT and E161K patients were 41.8 (interquartile range, 34.5–50.6) and 39.5 years old (interquartile range, 25.4–44.8), respectively (P=0.17). (A) Urinary pH at the beginning of the test before ingestion of NH4Cl capsules (time 0). (B) Trough urinary pH (nadir pH) reached during the test. (C) Trough urinary pH reached during the test adjusted for sex and median age. For this conditional plot, a linear regression model adjusted for sex and age was used. Age was set to the median of 41.8 years old, and sex was set to the most common category: men. (D) Urinary NH4 at the beginning of the test before ingestion of NH4Cl capsules (time 0). (E) Peak urinary NH4 reached during the test. (F) Urinary NH4 excretion profile (area under the curve). Urinary NH4 levels were not available in all test participants. Between-group differences are determined by Mann–Whitney U test or chi-squared test where appropriate, and the corresponding P value is indicated. AUC, area under the curve; creat, creatinine.
Blood and Urinary Biochemistry in Non-SFs with the p.E161K SNP
To assess the effect of the p.E161K SNP on urinary pH in non-SFs, we performed an analysis of blood and urinary parameters of wild-type and p.E161K heterozygous non-SFs. Biochemical data and DNA of these individuals are deposited in the Dallas stone registry as non-SF controls. Inclusion and exclusion criteria for non-SFs were identical to the ones used for SFs, with the exception that history of renal stone disease was an exclusion criterion. We identified a total of 148 wild-type and 14 p.E161K heterozygous non-SFs in the database who met the inclusion criteria and had no exclusion criterion. There was no difference between the two groups with regards to median age (39.9 versus 35.7 years; P=0.37) or sex (40.5% men versus 28.6% men; P=0.64). Blood and 24-hour urinary biochemistries of non-SFs on a random outpatient diet are depicted in Table 7. There were no differences in plasma electrolytes or renal function between the two groups. Median plasma bicarbonate tended to be lower in heterozygous than in wild–type non-SFs (24 versus 27 mmol/L), but the difference did not reach statistical significance (P=0.15). Median urinary pH in heterozygous non-SFs was not different from that of wild–type non-SFs on a random outpatient diet (6.2 versus 6.1; P=0.44). However, similar to the observation made in SFs, heterozygous non-SFs displayed a significantly higher 24-hour urine pH than wild–type non-SFs (median, 6.7 versus 6.2; P<0.05) on an instructed diet (Table 8). This difference in 24-hour urine pH persisted even after adjustment for sex and age (P=0.05).
Table 7.
Blood and urine parameters of non-SFs according to E161K genotype on random outpatient diet
| Characteristic | Normal Range | Unit | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|---|---|
| Plasma Na | 132–142 | mmol/L | 98 | 138 (137–139) | 9 | 138 (138–139) | 0.57 |
| Plasma K | 3.5–4.7 | mmol/L | 98 | 4.3 (4.1–4.5) | 9 | 4.2 (4.1–4.4) | 0.69 |
| Plasma Cl | 97–108 | mmol/L | 98 | 106 (105–108) | 9 | 106 (104–106) | 0.50 |
| Plasma Ca total | 2.10–2.55 | mmol/L | 98 | 2.4 (2.3–2.4) | 9 | 2.3 (2.3–2.4) | 0.89 |
| Plasma P | 0.84–1.45 | mmol/L | 96 | 1.1 (0.9–1.2) | 9 | 1.1 (1–1.3) | 0.33 |
| Plasma Mg | 0.75–1.00 | mmol/L | 87 | 0.9 (0.8–0.9) | 7 | 0.9 (0.7–0.9) | 0.24 |
| Plasma creatinine | 45–84 | µmol/L | 88 | 80 (62–88) | 8 | 80 (77–88) | 0.66 |
| eGFR CKD-EPI | >90 | ml/min per 1.73 m2 | 86 | 96 (80–104) | 8 | 88 (84–92) | 0.43 |
| Plasma uric acid | 202–416 | µmol/L | 79 | 298 (238–354) | 8 | 283 (171–341) | 0.40 |
| Plasma random glucose | 3.33–5.55 | mmol/L | 83 | 5 (4.6–5.3) | 8 | 4.8 (4.6–4.9) | 0.15 |
| Plasma bicarbonate | 18.0–29.0 | mmol/L | 95 | 27 (25–28) | 9 | 24 (24–28) | 0.15 |
| Serum PTH | 15–65 | pg/ml | 61 | 30 (24–39) | 2 | 17 (15–20) | — |
| Urinary volume | — | ml | 75 | 1715 (1173–2558) | 8 | 1700 (1579–2356) | 0.49 |
| Urine pH | — | — | 75 | 6.1 (5.8–6.4) | 8 | 6.2 (6–6.3) | 0.44 |
| Urine Na | 40–220 | mmol/24 h | 75 | 131 (90–188) | 8 | 149 (125–184) | 0.50 |
| Urine K | 25–125 | mmol/24 h | 74 | 49 (35–63) | 8 | 48 (47–54) | 0.64 |
| Urine Cl | 110–250 | mmol/24 h | 71 | 130 (80–176) | 7 | 132 (127–173) | 0.38 |
| Urine Ca | 2.50–7.50 | mmol/24 h | 75 | 3.4 (1.9–4.9) | 8 | 3.7 (2.6–4.4) | 0.58 |
| Urine P | 13.0–42.0 | mmol/24 h | 74 | 26 (17.8–33.1) | 8 | 24.4 (21.8–27.3) | 0.90 |
| Urine Mg | 2.50–8.50 | mmol/24 h | 75 | 3.7 (2.8–4.4) | 8 | 3.6 (3.2–4) | 0.78 |
| Urine uric acid | <5900 | µmol/24 h | 68 | 3015 (2353–3972) | 4 | 2927 (2183–3823) | 0.92 |
| Urine creatinine | 6300–19,400 | µmol/24 h | 75 | 11,378 (9101–15,265) | 8 | 12,264 (8890–13,199) | 0.99 |
| Urine citrate | 1.65–6.6 | mmol/24 h | 72 | 3.2 (2.29–4.28) | 7 | 2.54 (2.2–2.67) | 0.20 |
| Urine citrate/K | — | (mmol/24 h)/(mmol/24 h) | 72 | 0.065 (0.045–0.085) | 7 | 0.047 (0.036–0.064) | 0.21 |
| Urine K-P | — | mmol/24 h | 74 | 23 (12–35) | 8 | 22 (18–31) | 0.68 |
| Urine citrate/K-P | — | (mmol/24 h)/(mmol/24 h) | 72 | 0.118 (0.06–0.198) | 7 | 0.102 (0.06–0.161) | 0.69 |
| NGIA | — | mmol/24 h | 71 | 18.9 (7.4–36.4) | 7 | 30.6 (22.9–32.4) | 0.22 |
| Urine SO4 | — | mmol/24 h | 68 | 17 (13–24) | 4 | 18 (16–21) | 0.71 |
| Urine oxalate | <500 | µmol/24 h | 68 | 284 (193–359) | 4 | 299 (282–306) | 0.79 |
The number of participants is indicated for each characteristic stratified by the genotype. Continuous variables are further described by their medians (25th to 75th percentiles). Between-group differences are determined by Mann–Whitney U test, and the corresponding P value is indicated. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; PTH, parathormone; SO4, sulfate.
Table 8.
Blood and urine parameters of non-SFs according to E161K heterozygosity on low–Ca and low–Na diet
| Characteristic | Normal Range | Unit | N | Wild Type | N | Heterozygote | P Value |
|---|---|---|---|---|---|---|---|
| Urinary volume | — | ml | 40 | 1985 (1428–2590) | 5 | 1900 (1500–1925) | 0.75 |
| Urine pH | — | — | 39 | 6.2 (5.7–6.4) | 5 | 6.7 (6.3–6.7) | 0.05 |
| Urine Na | 40–220 | mmol/24 h | 39 | 120 (94–174) | 5 | 144 (119–266) | 0.15 |
| Urine K | 25–125 | mmol/24 h | 39 | 47 (37–66) | 5 | 71 (42–104) | 0.29 |
| Urine Cl | 110–250 | mmol/24 h | 31 | 111 (95–165) | 5 | 132 (117–239) | 0.30 |
| Urine Ca | 2.50–7.50 | mmol/24 h | 39 | 3.1 (2.1–4.2) | 5 | 4.2 (3.5–4.9) | 0.24 |
| Urine P | 13.0–42.0 | mmol/24 h | 39 | 24 (18–28) | 5 | 26 (26–33) | 0.29 |
| Urine Mg | 2.50–8.50 | mmol/24 h | 39 | 3.6 (2.6–4.6) | 5 | 3.7 (3.3–4) | 0.85 |
| Urine uric acid | <5900 | µmol/24 h | 33 | 3147 (2594–3683) | 5 | 3176 (3052–3295) | 0.61 |
| Urine creatinine | 6300–13,400 | µmol/24 h | 39 | 12,314 (9233–14,666) | 5 | 9980 (8847–17,079) | 0.97 |
| Urine citrate | 1.65–6.6 | mmol/24 h | 35 | 3 (2.1–4.9) | 5 | 3 (2.7–3.4) | 0.84 |
| Urine citrate/K | — | (mmol/24 h)/(mmol/24 h) | 34 | 0.067 (0.05–0.091) | 5 | 0.048 (0.021–0.071) | 0.24 |
| Urine K-P | — | mmol/24 h | 39 | 26 (18–37) | 5 | 38 (16–86) | 0.52 |
| Urine citrate/K-P | — | (mmol/24 h)/(mmol/24 h) | 34 | 0.123 (0.081–0.2) | 5 | 0.025 (0.012–0.09) | 0.04 |
| NGIA | — | mmol/24 h | 30 | 14 (−4–31) | 5 | 45 (8–91) | 0.15 |
| Urine SO4 | — | mmol/24 h | 33 | 19 (15–24) | 5 | 21 (18–23) | 0.83 |
| Urine oxalate | <500 | µmol/24 h | 33 | 289 (228–411) | 5 | 304 (200–322) | 0.60 |
The number of SFs is indicated for each characteristic stratified by the genotype. Continuous variables are further described by their medians (25th to 75th percentiles). Between-group differences are determined by Mann–Whitney U test, and the corresponding P value is indicated. SO4, sulfate.
Discussion
Classically, dRTA due to B1 mutation has been considered an autosomal-recessive disease. A recent study showed that heterozygous carriers in a large family with a B1 truncation mutation (p.Phe468fsX487) were not normal but exhibited features of urinary acidification deficit and elevated stone risk.19 The phenotype in heterozygotes of this peculiar mutation was attributed to haploinsufficiency, because in vitro studies were not compatible with negative dominance of this mutation. Zhang et al.19 also failed to find evidence of negative dominance in vitro of other known B1 missense mutations and two common SNPs, including p.E161K. If the other known B1 subunit missense mutations also cause a detectable deficit in urinary acidification in a heterozygous state is currently unknown. The functional role of the B1 subunit p.E161K SNP has not been studied in vivo.
The goal of this study was to investigate the phenotype of recurrent SFs with the B1 subunit p.E161K SNP. To our knowledge, this is the first study with a comprehensive analysis of the effect of a V-ATPase B1 SNP on urinary acidification. By combining patient data of the Dallas and Bern stone registries, we identified 32 heterozygous p.E161K carriers in a total of 555 recurrent SFs, corresponding to an allele frequency of 5.8%. This is slightly lower compared with a previous study that found the c.481G>A; p.E161K SNP to be in Hardy–Weinberg equilibrium with an allele frequency of 10%.13 We identified no homozygous patients in our cohort of 555 recurrent SFs. Assuming Hardy–Weinberg equilibrium, the observed frequency of heterozygous patients in our cohort would correspond to a homozygosity frequency of 0.000883%. Thus, approximately 1100 recurrent SFs would be needed for the identification of one homozygous p.E161K carrier.
Hildebrandt and colleagues20 recently studied a cohort of recurrent SFs by high–throughput mutation analysis of 30 genes believed to cause kidney stones. Hildebrandt and colleagues20 detected in 14.9% of patients likely causative mutations in 14 of 30 genes analyzed, indicating that monogenetic causes of recurrent nephrolithiasis are more prevalent than currently appreciated. Interestingly, one patient in that analysis with recurrent nephrolithiasis, hypercalciuria, and diminished plasma bicarbonate was found to be homozygous for the p.E161K SNP.
Our analysis indicates that heterozygous p.E161K SFs also exhibit subtle defects in urinary acidification. p.E161K heterozygous SFs had a higher 24-hour urinary pH under a diet low in Ca and Na and a tendency to higher urinary 24-hour urinary pH under random outpatient diet than wild-type SFs. In support of these findings, p.E161K heterozygous non–SFs also exhibited a higher urinary pH under a diet low in Ca and Na than wild–type non-SFs.
Challenging renal acid excretory capacity by acute acid loading unmasked the suspected acidification deficit in p.E161K heterozygous SFs and unveiled a high prevalence of incomplete dRTA in this group of patients. Compatible with the findings of an increased urinary pH, the CaP content of calcareous stones was significantly higher in p.E161K SNP carriers. An alkaline urine favors CaP stone formation but decreases the risk of uric acid precipitation. Using computer-based methods (EQUIL221 and JESS22) as well as empirical physicochemical methods, Pak et al.23 showed that raising urinary pH from 6.0 to 6.5, such as we have observed in our two groups, will increase the brushite relative supersaturation ratio, saturation index, and concentration product ratio from 2.1 to 4.5, from 1.8 to 2.3, and from 1.4 to 2.5, respectively, thereby escalating risk of calcium phosphate stones. Thus, the p.E161K SNP may augment the individual risk for development of renal calculi. However, because of the opposing effect on CaP and uric acid stone risk, the prevalence of the p.E161K SNP in the general population may not greatly differ from the one observed in recurrent SFs. Clearly, additional studies will be needed to answer this question.
In addition to defects in urinary acidification, B1 subunit mutations are associated with sensorineural hearing loss.3,13 In this study, we did not detect obvious hearing abnormalities in recurrent SFs with the p.E161K carriers, but audiometric investigations were not performed, and thus, the effect of the p.E161K SNP on inner ear function remains unknown at the moment.
Patients with overt dRTA exhibit low BMD, mainly because of low bone formation.24 The case may be different in patients with a mild impairment in urinary acidification and normal systemic acid-base balance. A recent study detected no difference in BMD in recurrent SFs with incomplete dRTA compared with SFs without dRTA.25 We also did not observe differences in BMD between p.E161K heterozygous and wild-type SFs. This may be related to the fact that these subjects do not have sustained systemic acidosis to alter bone biology.
In summary, our data indicate that recurrent SFs with the V-ATPase B1 subunit p.E161K SNP exhibit a urinary acidification deficit with an increased prevalence of CaP–containing kidney stones. The burden of E161K heterozygosity may be a forme fruste of dRTA.
Concise Methods
Patients and Study Protocol
The study was conducted with patients recruited at the Division of Nephrology, Hypertension and Clinical Pharmacology at the University Hospital of Bern and the General Clinical Research Center at the University of Texas Southwestern Medical Center with approval by the Institutional Review Board (Dallas) and the Ethical Committee of the Kanton Bern (Bern). All participants provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. Patients in the registries were seen at the clinic between March of 2004 and March of 2014, suffered from at least one stone episode, and underwent a three–visit mineral metabolism workup. Mineral metabolism workup included a 24-hour urine on a random outpatient diet and one 24-hour urine after 1 week under an instructed low–Ca (15–20 mmol/d) and low–Na (100 mmol/d) diet according to a protocol first established by Pak et al.26,27 Urine and blood analyses were performed at the Central Laboratory of the University Hospital of Bern or the Center of Mineral Metabolism and Clinical Research University of Texas Southwestern Medical Center using standard laboratory methods. Blood gas analysis was done on venous blood samples. eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration.28 Osteodensitometry was performed at the Department of Osteoporosis at the University Hospital of Bern by DEXA (Hologic QDR 4500A; Hologic, Bedford, MA) at the lumbar spine, the nondominant femoral neck, the proximal femur, the distal tibial diaphysis, and the epiphysis. Charts of all patients in the registry were reviewed manually for this study. Inclusion criteria were informed consent and at least one stone episode. Exclusion criteria for analysis were absence of informed consent, genetic diseases causing dRTA, cystinuria, primary hyperoxaluria, primary or secondary hyperparathyroidism, autoimmune diseases, renal diseases, malignancy, hypo- or hyperthyroidism, liver diseases, short bowel syndrome or bariatric surgery, chronic urinary tract infection, pregnancy, mineralocorticoid deficiency, anorexia nervosa, chronic diarrheal syndromes, or medications interfering with urinary acidification during the investigation.
Biochemical data and DNA of non-SFs used in the study are deposited in the Dallas stone registry as non-SF controls. Inclusion and exclusion criteria for non-SFs included in this study were identical to the ones used for SFs, with the exception that history of renal stone disease was an exclusion criterion.
DNA Extraction and Genotyping
Genomic DNA was extracted from peripheral blood leukocytes using a Nucleospin Blood L (Macherey-Nagel) DNA Extraction Kit. In all subjects included in the study (SFs and non-SFs), exon 6 with adjacent exon-intron boundaries of the ATP6V1B1 gene was individually amplified by PCR (AmpliTaq Gold System; Applied Biosystems) using primers described previously.3 The DNA sequence of both strands was determined by Sanger sequencing at Microsynth AG. In addition, in all of 32 p.E161K heterozygous SFs, coding sequences and intron-exon boundaries of ATP6V0A4 and ATP6V1B1 were sequenced using TruSeq Custom Amplicon v1.5 (Illumina) on a MiSeq (Illumina). Data were analyzed using integrated MiSeq Reporter v2.5.1. Mutations were considered to be known if they were deposited in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php). Nonsynonymous variants were considered as likely disease causing according to the following inclusion and exclusion criteria.20 Inclusion criteria were (1) truncating mutation (stop gained, abrogation of start or stop codon, abrogation of obligatory splice site, or frameshift) or (2) missense mutation if one of the following is applied: (1) in silico prediction by Polyphen2-HumVar with a score >0.90, suggesting a probably damaging effect on the protein level,29 or (2) the given disease–causing allele is supported by functional data. Exclusion criterion was that the allele is present in healthy controls of the Exome Variant Server database with a minor allele frequency of >5.0%.
Acute Acid Loading
All p.E161K carriers were invited to participate in the acute acid loading test. Recruitment of wild-type SFs for NH4Cl loading was as follows: all patients referred to our stone clinic for metabolic workup of stone disease who met the inclusion criteria but did not meet the exclusion criterion (see Concise Methods, Patients and Study Protocol) were consecutively enrolled irrespective of baseline urinary pH, sex, or age. Genotyping was performed after acid loading. Acid loading was performed using the short NH4Cl loading test as described.30 NH4Cl gelatin capsules (0.1 g/kg body wt) were given to fasting subjects at 0800 hours with water in the presence of the nursing staff. During the test, fluid intake was ad libitum. Venous blood samples were obtained for chemistry, pH, and blood gases at 0800, 1000, and 1200 hours. Urine was collected hourly from 0800 to 1400 hours. Urine pH was measured immediately after collection with an electrode pH meter that was calibrated every day. Urinary ammonium was assessed enzymatically by the glutamate dehydrogenase method.
Statistical Analyses
Continuous variables with normal distribution are expressed as the means±SDs and in case of skewed distribution, as the medians (25th to 75th percentiles). Categorical variables are expressed as numbers and frequencies in percentages. The shape of the distribution of each continuous variable was visually inspected, and square root, log, or inverse transformations were applied to ensure normality for statistical analyses. For a few variables, no appropriate transformation was found. Welch t, Mann–Whitney U, or chi-squared tests were performed where appropriate to compare baseline characteristics for continuous and categorical variables. The statistical tests were two sided, and a P value <0.05 was considered to indicate a statistically significant difference.
To estimate the associations between the p.E161K SNP and acid-base parameters or kidney stone type, we first applied univariable linear or logistic regression analyses taking the between-center variability into account where available. We next analyzed the independent associations of the p.E161K SNP with the response variables and assumed that sex, age, BMI, dietary acid, and alkali intake are most likely to influence these parameters and selected them as explanatory covariables. We, therefore, performed a multivariate analysis of the influence of the p.E161K SNP adjusted for the main effects sex, age, BMI, urinary sulfate excretion, and net gastrointestinal alkali absorption using linear regression and mixed effects linear and logistic regression models. We considered the presence of nonlinear effects and interactions and therefore, included quadratic terms and interactions involving up to three different factors into the full model. Age and urinary sulfate excretion were square root transformed, and BMI was transformed as its negative inverse. All continuous explanatory variables were centered to zero to address the potential problem of collinearity that may be induced by adding squared terms and interaction terms to the models. No important collinearities between the prediction variables were detected on inspection of scatterplot and correlation matrices and variance inflation factors. Backward selection was carried out to eliminate interaction terms and quadratic terms with a P value =0.10 or higher. All parent terms of significant higher–order terms were kept in the model. All main effects were also kept in the model irrespective of their significance. Because of the small number of heterozygotes, the mixed effects logistic regression models did not converge when applying the full model. Therefore, for binary outcomes of kidney stone parameters, mixed effects logistic regression models were run with the p.E161K SNP as the only fixed covariate, while adjusting for, at most, two alternating additional covariates at a time and taking the between-center variability into account. Estimates for logistic regression with more than three fixed effects and one random effect were not available because of a noninvertible singular Hessian (the matrix of second derivatives). Models were validated graphically if appropriate for (1) homogeneity by plotting residuals versus fitted values, (2) normality by a histogram of the residuals and a quantile-quantile plot, and (3) homoscedasticity by plotting residuals against each explanatory variable used in the final models. These visual inspections did not reveal any obvious deviations from homoscedasticity or normality. The statistical analysis was performed by using the R Statistics software, version 3.0.2.
Disclosures
D.G.F. has served on an advisory board for Otsuka Pharmaceuticals and received unrestricted research grants from Novartis, Abbvie and Otsuka.
Acknowledgments
We thank Christian Schindler, senior statistician at the Swiss Tropical and Public Health Institute (Basel, Switzerland) for expert statistical advice.
O.W.M. was supported by National Institutes of Health Grants R01 DK091392, R01 DK081423, and R01 DK092461; O’Brien Kidney Research Center Grant P30-DK-07938; the Simmons Family Foundation; and the Charles and Jane Pak Foundation. D.G.F. was supported by the Swiss National Centres of Competence in Research Kidney.CH and TransCure, Swiss National Science Foundation Grants 31003A_135503 and 31003A_152829, and a Medical Research Position Award of the Foundation Prof. Dr. Max Cloëtta.
Parts of the results were presented at the 46th Annual Meeting of the Swiss Society of Nephrology 2014 held December 5, 2014 in Interlaken, Switzerland.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Wagner CA, Devuyst O, Bourgeois S, Mohebbi N: Regulated acid-base transport in the collecting duct. Pflugers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Laing CM, Unwin RJ: Renal tubular acidosis. J Nephrol 19[Suppl 9]: S46–S52, 2006 [PubMed] [Google Scholar]
- 3.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP: Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ: Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE: Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 80: 2752–2756, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE: Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Wrong O, Davies HE: The excretion of acid in renal disease. Q J Med 28: 259–313, 1959 [PubMed] [Google Scholar]
- 8.Backman U, Danielson BG, Sohtell M: Urine acidification capacity in renal stone formers. Scand J Urol Nephrol 35[Suppl]: 49–61, 1976 [PubMed] [Google Scholar]
- 9.Backman U, Danielson BG, Johansson G, Ljunghall S, Wikström B: Incidence and clinical importance of renal tubular defects in recurrent renal stone formers. Nephron 25: 96–101, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Dedmon RE, Wrong O: The excretion of organic anion in renal tubular acidosis with particular reference to citrate. Clin Sci 22: 19–32, 1962 [PubMed] [Google Scholar]
- 11.Smith AN, Lovering RC, Futai M, Takeda J, Brown D, Karet FE: Revised nomenclature for mammalian vacuolar-type H+ -ATPase subunit genes. Mol Cell 12: 801–803, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD: V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch AB, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE: Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet 39: 796–803, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuster DG, Zhang J, Xie XS, Moe OW: The vacuolar-ATPase B1 subunit in distal tubular acidosis: Novel mutations and mechanisms for dysfunction. Kidney Int 73: 1151–1158, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Pak CY, Odvina CV, Pearle MS, Sakhaee K, Peterson RD, Poindexter JR, Brinkley LJ: Effect of dietary modification on urinary stone risk factors. Kidney Int 68: 2264–2273, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY: Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65: 1422–1425, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Maalouf NM, Cameron MA, Moe OW, Sakhaee K: Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 5: 1277–1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meschi T, Maggiore U, Fiaccadori E, Schianchi T, Bosi S, Adorni G, Ridolo E, Guerra A, Allegri F, Novarini A, Borghi L: The effect of fruits and vegetables on urinary stone risk factors. Kidney Int 66: 2402–2410, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Fuster DG, Cameron MA, Quiñones H, Griffith C, Xie XS, Moe OW: Incomplete distal renal tubular acidosis from a heterozygous mutation of the V-ATPase B1 subunit. Am J Physiol Renal Physiol 307: F1063–F1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halbritter J, Baum M, Hynes AM, Rice SJ, Thwaites DT, Gucev ZS, Fisher B, Spaneas L, Porath JD, Braun DA, Wassner AJ, Nelson CP, Tasic V, Sayer JA, Hildebrandt F: Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol 26: 543–551, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Rodgers A, Allie-Hamdulay S, Jackson G: Therapeutic action of citrate in urolithiasis explained by chemical speciation: Increase in pH is the determinant factor. Nephrol Dial Transplant 21: 361–369, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Pak CY, Moe OW, Maalouf NM, Zerwekh JE, Poindexter JR, Adams-Huet B: Comparison of semi-empirical and computer derived methods for estimating urinary saturation of brushite. J Urol 181: 1423–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, Sirikulchayanonta V, Ongphiphadhanakul B, Radinahamed P, Karnsombut P, Kunkitti N, Ruang-raksa C, Rajatanavin R: Bone mineral density and histology in distal renal tubular acidosis. Kidney Int 59: 1086–1093, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Arampatzis S, Röpke-Rieben B, Lippuner K, Hess B: Prevalence and densitometric characteristics of incomplete distal renal tubular acidosis in men with recurrent calcium nephrolithiasis. Urol Res 40: 53–59, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Pak CY, Kaplan R, Bone H, Townsend J, Waters O: A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N Engl J Med 292: 497–500, 1975 [DOI] [PubMed] [Google Scholar]
- 27.Pak CY, Britton F, Peterson R, Ward D, Northcutt C, Breslau NA, McGuire J, Sakhaee K, Bush S, Nicar M, Norman DA, Peters P: Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 69: 19–30, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh SB, Shirley DG, Wrong OM, Unwin RJ: Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int 71: 1310–1316, 2007 [DOI] [PubMed] [Google Scholar]