Abstract
We identified five unrelated individuals with significant global developmental delay and intellectual disability (ID), dysmorphic facial features and frequent microcephaly, and de novo predicted loss-of-function variants in chromosome alignment maintaining phosphoprotein 1 (CHAMP1). Our findings are consistent with recently reported de novo mutations in CHAMP1 in five other individuals with similar features. CHAMP1 is a zinc finger protein involved in kinetochore–microtubule attachment and is required for regulating the proper alignment of chromosomes during metaphase in mitosis. Mutations in CHAMP1 may affect cell division and hence brain development and function, resulting in developmental delay and ID.
Keywords: congenital microcephaly; intellectual disability, severe; severe global developmental delay
INTRODUCTION
Whole-exome sequencing (WES) provides a comprehensive strategy to identify novel disease-associated genetic variants in patients with genetically heterogeneous conditions, including developmental abnormalities and intellectual disability (ID) (Veltman and Brunner 2012; Yang et al. 2013, 2014; Gilissen et al. 2014). Clinical WES is a powerful tool to identify de novo and inherited rare and novel variants (Yang et al. 2013, 2014) for individuals in whom initial diagnostic genetic evaluation is unrevealing and/or in individuals without distinguishing clinical features suggestive of a diagnosis. Mutations in CHAMP1 have recently been associated with global developmental delay, ID, hypotonia, and dysmorphic features (Hempel et al. 2015). Here we describe five unrelated individuals who have novel heterozygous variants in CHAMP1 that are predicted to be deleterious. CHAMP1 is located on Chromosome 13q34 and encodes a mammalian zinc finger protein involved in the proper alignment and segregation of chromosomes during mitosis (Itoh et al. 2011). We have identified five de novo novel predicted pathogenic variants in CHAMP1 that are all associated with neurodevelopmental disorders and dysmorphic features and frequently associated with microcephaly.
RESULTS
Clinical Presentation
Clinical WES was performed on 2144 individuals with developmental delay and/or intellectual disabilities, 148 of whom also had microcephaly. In three affected individuals from three unrelated families, we identified variants in CHAMP1 as potentially causative for the phenotype. The fourth individual was identified at Greenwood Genetic Center and through GeneMatcher (https://genematcher.org/) (Sobreira et al. 2015). We identified another additional proband, for a total of five affected individuals in five families. All of the families were sequenced with WES performed as either a proband–parent trio or family analysis using affected or unaffected siblings in the segregation analysis. The father of one individual was a sperm donor, and we were unable to determine whether this particular variant is de novo.
Exome sequencing from three affected individuals’ samples analyzed in the original clinical laboratory at GeneDx produced an average of ∼12 Gb of sequence per sample (Table 1). Mean coverage of the captured regions was ∼170× per sample, with >98% covered with at least 10× coverage, an average of >91% of base call quality of Q30 or greater, and an overall average mean quality score of >Q35. Filtering of common SNPs (>10% frequency present in 1000 Genomes [1000G] database) resulted in approximately 4500 variants per proband sample. We evaluated each of these variants by variant allele frequency in reference populations and filtered out variants with a minor allele frequency >1%, known disease association, pattern of inheritance, and similarity of clinical phenotype among probands. Candidate disease causing variants in the CHAMP1 gene were confirmed by Sanger sequencing. All five variants identified in the five families are rare and are highly conserved throughout across species. They include three nonsense and two frameshift deletion mutations. All five variants are predicted to be deleterious. No loss-of-function CHAMP1 variants were detected in ExAC, 1000G, NHLBI Exome Sequencing Project (ESP), or our own internal database of 15,716 exomes.
Table 1.
Sequencing results
| Patient | 10× cov (%) | Mean cov | Yield (Gb) | Q30 | MeanQ | Filtered var | CHAMP1 mean CDS cov | Var total fam cov | Samples | Mean per-sample var cov |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 98.80 | 167 | 12.3 | 91 | 35 | 4597 | 302 | 2053 | 3 | 684 |
| 2 | 99.14 | 184 | 14.1 | 93 | 36 | 4652 | 313 | 1157 | 3 | 386 |
| 3 | 96.33 | 157 | 11.3 | 90 | 35 | 4310 | 239 | 238 | 2 | 119 |
| Mean | 98.82 | 169 | 12.6 | 91 | 36 | 4520 | 285 | 1149 | 3 | 431 |
Results from individuals identified at GeneDx.
cov, coverage; CDS, coding sequence; var, variant; fam, family.
The five female individuals with CHAMP1 pathogenic variants are all significantly intellectually disabled, three have congenital microcephaly, and one showed acquired microcephaly first noted at 6 mo of age (<3%) (Table 2). They range in age from 4 to 23 yr old and are either nonverbal or minimally verbal, using signs and communication devices. Concerns were evident in the neonatal period with congenital microcephaly, hypotonia, and feeding difficulties. In addition, spasticity was a common feature, and one individual who was born prematurely and delivered at 34 wk had respiratory difficulties and apneic episodes. Some individuals, but not all, had congenital anomalies including choanal atresia, intestinal malrotation, bicuspid aortic valve, and a ventricular septal defect. All individuals exhibit short stature. However, weight ranges dramatically from underweight to overweight.
Table 2.
Clinical features of patients with mutations in CHAMP1
| Patient | Age | Sex | Mutation | Inheritance | Muscle tone | Spasticity | Head circumference | Current HT, WT | DD | Age at sitting | Age at walking | Verbal skills | Vision | Hearing | Brain MRI | Seizure | Abnormal Behavior |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 yo | F | c.1044delG p.Trp348* | De novo | Hypotonia | Spastic quadriplegia | Congenital microcephaly (<3 %ile) | HT = 152 cm (3rd %ile); WT = 72.4 kg (88th %ile) | Y | Unknown | 18 mo | Nonverbal | Normal | Moderate-severe BL SNHL | Hypoplastic corpus callosum | None | Aggressive, occasionally self-injurious |
| 2 | 7 yo | F | c.542_543delCT p.Ser181CysfsX5 | De novo | Normal | Spastic quadriplegia | Relative microcephaly (10th %ile) | HT = 115.3 cm (10th %ile); WT = 28.5 kg (90th %ile) | Y | 1 yo | 2 yo | Put two words together at 5 yo | Strabismus, corrected | Normal | N/A | Febrile seizures (resolved) | Skin-picking, rituals, food-foraging |
| 3 | 4 yo | F | c.1945C>T p.Gln649* | De novo | Central hypotonia | Spastic | Normal (75th %ile) | HT = 107 cm (<95th %ile); WT = 18.5 kg (<90th %ile) | Y | 6 mo | 2 yo | Nonverbal | Ocular albinism | Mild hearing loss in left ear | Normal | None | ADD/ADHD |
| 4 | 12 yo | F | c.1969C>T p.Gln657* | De novo | Hypotonia | None | Congenital microcephaly (<3rd %ile) | HT = 124.46 cm (<5th %ile); WT = 30 kg (<5th %ile) | Y | 18 mo | Nonambulatory | Has 2 words | Strabismus | Normal | Mildly decreased white matter, possible hypopituitarism | Seizures at 3 yo | Inappropriate laughter |
| 5 | 6 yo | F | c.2029G>T p.Glu677X | De novo | Severe truncal hypotonia in infancy, improved | None | Acquired microcephaly (<3rd %ile) | HT = 109.7 cm (<25th %ile); WT = 19.1 kg (<50th %ile) | Y | 10 mo | 2 yo | Nonverbal | Alternating exotropia, accommodative esotropia | Mild hearing loss in one ear | Mild cerebellar atrophy with mild inferior vermian hypogenesis | None | Hyperactivity |
| Hempel et al. #1 | 4 yo | M | c.1866_1867delCA p.Asp622Glufs*8 | De novo | Truncal and orofacial hypotonia | N/A | Congenital microcephaly (<3rd %ile) | HT = 111.5 cm (97th %ile); WT = 16.3 kg (50th %ile) | Y | 1 yo | 4 yo | Has 3 words | Strabismus, hyperopia | Normal | Mild brain atrophy and cerebellar cortical dysplasia | None | Frequent hand fluttering, jactitation, very friendly |
| Hempel et al. #2 | 3 yo | M | c.1768C > T p.Gln590* | De novo | Severe hypotonia, improved | N/A | Microcephaly (<3rd %ile) | HT = 86 cm (<3rd %ile); WT = 14.7 kg (<75th %ile) | Y | N/A | 3 yo | Nonverbal | Impaired | Normal | Slightly delayed myelination | Frontotemporal epilepsy | Turning, twisting movements of arms and hands, sighing, shaking, friendly |
| Hempel et al. #3 | 18 yo | M | c.1192C > T p.Arg398* | De novo | Truncal and orofacial hypotonia | N/A | Microcephaly (<3rd %ile) | HT = 160 cm (<3rd %ile); WT = 50 kg (<3rd %ile) | Y | 1 yo | 2 yo | Short sentences with slurred speech | Exotropia, hyperopia | Normal | Normal | None | Friendly |
| Hempel et al. #4 | 3 yo | F | c.635delC p.Pro212Leufs*7 | De novo | Mild truncal hypotonia | N/A | Congenital microcephaly (<3rd %ile) | HT = 93.5 cm (<50th %ile); WT = 14.7 kg (75th %ile) | Y | N/A | 1 yo | Impaired speech development | Hyperopia, astigmatism | Normal | Normal | None | Friendly, hand stereotypies, tactile hypersensitivity, sexual self-stimulation |
| Hempel et al. #5 | 9 yo | F | c.1192C > T p.Arg398* | De novo | Truncal and orofacial hypotonia | N/A | Normal (<75th %ile) | HT = 139 cm (>95th %ile); WT = 52 kg (>95th %ile) | Y | N/A | 1 yo | Three word sentences | Hyperopia, astigmatism | Normal | Normal | None | Friendly |
See Supplemental Table 1 for additional details of clinical presentations.
DD, developmental delay; yo, years old; BL, bilateral; SNHL, sensorineural hearing loss; ADD, attention deficit disorder; ADHD, attention deficit hyperactivity disorder; N/A, not available; mo, months.
Brain structure by MRI in two patients suggested no specific structural abnormalities, but there was decreased brain volume and white matter in one individual, hypoplastic corpus callosum in another, and cerebellar atrophy with mild inferior vermian hypogenesis in a third (Table 2). Seizures were observed in two individuals: febrile seizures that resolved in one and generalized seizures that started at age 3 and were adequately treated with levetiracetam in another. There were a range of abnormal behaviors including self-injurious behavior in the oldest individual, repetitive behaviors, and inappropriate laughter. Four individuals had difficulty with sleeping and remaining asleep. Two individuals had strabismus, one individual had foveal hypoplasia, blond fundus, and nystagmus, and a fourth had alternating exotropia and accommodative esotropia. One individual had bilateral sensorineural hearing loss and two others have decreased hearing unilaterally. Dysmorphic features were common to all the individuals and included hypertelorism, epicanthal folds, short philtrum, and upslanting or downslanting palpebral fissures (Fig. 1).
Figure 1.
Photographs of patients. (A,B) Patient 1. (C,D) Patient 3. (E,F) Patient 4. (G) Patient 5 at 1 yr of age. (H,I) Patient 5 at 6 yr. Note midface hypoplasia, upslanted palpebral fissures (A), and clinodactyly (B) in Patient 1, hypertelorism in Patients 1 and 3, short philtrum and pointed chin in Patient 5, and widely spaced teeth in Patients 1, 3, and 4, and broad nasal bridge in all four patients.
DISCUSSION
Five individuals with a shared phenotype of global developmental delay, significant ID, and dysmorphic features were all found to have predicted loss-of-function novel variants in CHAMP1 identified by WES, all of which have been confirmed as de novo. A recent report of five patients with similar clinical features of ID, hypotonia, severe speech impairment, and dysmorphisms identified de novo mutations in CHAMP1, including two frameshift and two nonsense mutations (Hempel et al. 2015). One of the nonsense mutations, c.1192C>T (p.Arg398*), was found in two affected individuals (Hempel et al. 2015). CHAMP1 encodes a zinc finger protein that plays a key role in chromosome alignment during metaphase in mitosis and has been identified as a candidate gene involved in ID (Gilissen et al. 2014). One study identified the identical c.1192C>T (p.Arg398*) de novo nonsense variant in CHAMP1 in two individuals with severe nonsyndromic ID by WES (Rauch et al. 2012). Another large-scale WES study implicated 12 novel genes enriched for damaging de novo variants with evidence for a role in developmental disorders. WES of 1133 children with severe, undiagnosed developmental disorders and their parents identified CHAMP1 loss-of-function variants in two individuals (Deciphering Developmental Disorders Study 2015). Two heterozygous loss-of-function frameshift deletion variants, c.1450delT (p.Phe485fs*100) and c.1596delC (p.Glu533fs*52) have also been reported from colon cancer samples in the Catalog of Somatic Mutations in Cancer (COSMIC; http://cancer.sanger.ac.uk/cosmic). Our additional five patients expand the number of individuals and the associated phenotypes of de novo CHAMP1 loss-of-function alleles.
CHAMP1 is an 812-amino acid protein first identified in human embryonic kidney 293 (HEK293) cells in a cellular screen for mitotic arrest deficient-like 2 (MAD2L2) protein interactors. The protein encoded by MAD2L2 is a component of the mitotic spindle assembly checkpoint complex, which regulates the onset of anaphase by monitoring the proper alignment of chromosomes at the metaphase plate during mitosis. MAD2L2 also regulates DNA repair activity in multiple settings by promoting nonhomologous end joining (NHEJ) at telomeres and double-strand breaks (Boersma et al. 2015). CHAMP1 binds to MAD2L2 and is required for localization of MAD2L2 to the mitotic spindle. Depletion of CHAMP1 results in misaligned chromosomes and abnormal chromosome segregation potentially resulting in mitotic arrest or mitotic errors such as aneuploidy (Itoh et al. 2011).
Previous reports of deletions in the 13q33-34 region including 11 common genes have been associated with ID, microcephaly, distinct dysmorphic facial features, and congenital heart disease (Walczak-Sztulpa et al. 2008; McMahon et al. 2015). CHAMP1 is located at 13q34 and is expressed during fetal brain development (Nagase et al. 2001) and throughout all fetal and adult tissues, including specific brain regions (Hawrylycz et al. 2012). CHAMP1 encodes a highly evolutionarily conserved zinc finger protein in mammals with no homolog in worms, flies, or yeast (Itoh et al. 2011). The protein consists of five C2H2 zinc-finger domains and several characteristic repeat motifs, including the SPE (consensus: PxxSPExxK), WK (SPxxWKxxP), and FPE (FPExxK) motifs (Fig. 2). Itoh et al. (2011) demonstrated the FPE region facilitates kinetochore–microtubule attachment and is necessary for proper chromosome alignment. Proteins involved in the proper alignment of the mitotic spindle are important in maintaining symmetric and asymmetric cell divisions and for controlling cell proliferation. Spindle orientation defects and aberrant functioning centromere proteins, such as Centromere Protein F (CENPF), have been associated with neurological and brain diseases including lissencephaly and primary microcephaly (MCPH) (Bakhoum and Compton 2012; Noatynska et al. 2012; Waters et al. 2015) and could account for the microcephaly observed in individuals with CHAMP1 variants. Twelve genes, MCPH1–MCPH12, are implicated in MCPH, of which nine are directly involved in regulating mitosis or cell cycle progression (Barbelanne and Tsang 2014). Accurate chromosome segregation is required for progression of mitosis and normal development. Missegregation is associated with aneuploidy and premature chromatid separation (PCS) (MIM #176430) caused by defects in the mitotic checkpoint protein BUB1 mitotic checkpoint serine/threonine kinase B (BUB1B). Multiple congenital abnormalities, dysmorphism, and mosaic variegated aneuploidy (MIM #257300), a rare cytogenetic disorder characterized by mosaicism for several different aneuploidies involving many different chromosomes, arise from dysregulation of mitotic checkpoint proteins, including BUB1B, and defects in kinetochore–microtubule complex proteins (Bakhoum and Compton 2012). Individuals with PCS and mosaic-variegated aneuploidies have severe ID, microcephaly, and growth retardation. We hypothesize that abnormal or decreased binding of CHAMP1 to its targets in coordinating kinetochore–microtubule attachment may be responsible for altered brain development and function and the dysmorphisms seen in individuals with mutations in CHAMP1. Additional studies of CHAMP1 are necessary to elucidate the molecular mechanism of CHAMP1-associated encephalopathy and growth retardation, but we hypothesize that the variants we describe result in haploinsufficiency of CHAMP1 and expand the genes involved in mitosis and cell cycle progression that can result in microcephaly and intellectual disabilities when mutated.
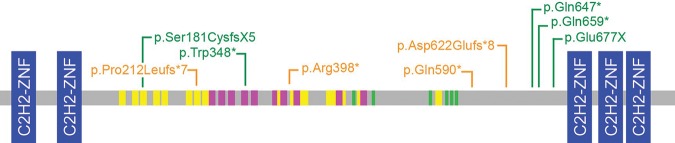
Figure 2.
Variants in CHAMP1. Diagram of CHAMP1 with C2H2-type zinc finger domains (blue), SPE motifs (yellow), WK motifs (pink), and FPE motifs (green). Gene disrupting nonsense and frameshift variants identified in our patients are in green and previously identified mutations are shown in orange (Rauch et al. 2012; Hempel et al. 2015).
METHODS
Whole-Exome Sequencing
Genomic DNA was extracted from whole blood from the affected children and their parents. For three of the individuals, exome sequencing was performed at GeneDx on exon targets isolated by capture using the Agilent SureSelect Human All Exon V4 (50 Mb) kit (Agilent Technologies). One microgram of DNA from blood specimen was sheared into 350–400-bp fragments, which were then repaired, ligated to adaptors, and purified for subsequent PCR amplification. Amplified products were then captured by biotinylated RNA library baits in solution following the manufacturer's instructions. Bound DNA was isolated with streptavidin-coated beads and reamplified. The final isolated products were sequenced using the Illumina HiSeq 2000 or 2500 sequencing system with 100-bp paired-end reads (Illumina). DNA sequence was mapped to the published human genome build UCSC hg19/GRCh37 reference sequence using BWA with the latest internally validated version at the time of sequencing, progressing from BWA v0.5.8 through BWA-MEM v0.7.8 (Li and Durbin 2009; Li 2012). Targeted coding exons and splice junctions of known protein-coding RefSeq genes were assessed for average depth of coverage with a minimum depth of 10× required for inclusion in downstream analysis. Local realignment around insertion–deletion sites was performed using the Genome Analysis Toolkit v1.6 (DePristo et al. 2011). Variant calls were generated simultaneously on all sequenced family members using SAMtools v0.1.18 (Li et al. 2009). All coding exons and surrounding intron/exon boundaries were analyzed. Automated filtering removed common sequence changes (defined as >10% frequency present in 1000 Genomes database). The targeted coding exons and splice junctions of the known protein-coding RefSeq genes were assessed for the average depth of coverage and data quality threshold values. WES data for all sequenced family members was analyzed using GeneDx's XomeAnalyzer (a variant annotation, filtering, and viewing interface for WES data), which includes nucleotide and amino acid annotations, population frequencies (NHLBI Exome Variant Server and 1000 Genomes databases), in silico prediction tools, amino acid conservation scores, and mutation references. Variants were filtered based on inheritance patterns, gene lists of interest, phenotype, and population frequencies, as appropriate. Resources including the Human Gene Mutation Database (HGMD), 1000 Genomes database, NHLBI Exome Variant Server, OMIM, PubMed, and ClinVar were used to evaluate genes and detected sequence changes of interest. The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (http://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/). Additional searches were performed using specific gene lists related to the patients’ clinical features. Identified sequence changes of interest were confirmed in all members of the trio by conventional di-deoxy DNA sequence analysis using an ABI3730 (Life Technologies) and standard protocols with a new DNA preparation.
ADDITIONAL INFORMATION
Ethics Statement
The study was approved by the Institutional Review Board of Columbia University and written consent was obtained from the patients.
Database Deposition and Access
Whole-exome sequencing data are not publicly available because patient consent could not be obtained. The CHAMP1 variants found in this study have been deposited in ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/) under accession numbers SCV000256075, SCV000256076, SCV000256077, SCV000256073, and SCV000256072.
Acknowledgments
We thank the patients and families for their generous contributions.
Author Contributions
A.J.T. and M.T.C. analyzed the data and drafted and critically reviewed the manuscript. K.R. generated and analyzed the data and critically reviewed the manuscript. J.R.J. and Y.-H.J. provided and analyzed the clinical data and critically reviewed the manuscript. C.N., J.D., A.M.-R., G.B.S., and J.K. provided the clinical data and critically reviewed the manuscript. A.T., B.F., G.D., and K.G.M. analyzed the data and critically reviewed the manuscript. W.K.C. conceived of the study, analyzed the data, drafted and critically reviewed the manuscript.
Funding
This work was supported in part by a grant from the Simons Foundation.
Competing Interest Statement
M.T.C., K.R., A.T., B.F., G.D., and K.G.M. are employees of GeneDx. W.K.C. is a consultant to BioReference Laboratories.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Bakhoum SF, Compton DA. 2012. Kinetochores and disease: keeping microtubule dynamics in check! Curr Opin Cell Biol 24: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbelanne M, Tsang WY. 2014. Molecular and cellular basis of autosomal recessive primary microcephaly. Biomed Res Int 2014: 547986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma V, Moatti N, Segura-Bayona S, Peuscher MH, van der Torre J, Wevers BA, Orthwein A, Durocher D, Jacobs JJ. 2015. MAD2L2 controls DNA repair at telomeres and DNA breaks by inhibiting 5′ end resection. Nature 521: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study. 2015. Large-scale discovery of novel genetic causes of developmental disorders. Nature 519: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, et al. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C, Hehir-Kwa JY, Thung DT, van de Vorst M, van Bon BW, Willemsen MH, Kwint M, Janssen IM, Hoischen A, Schenck A, et al. 2014. Genome sequencing identifies major causes of severe intellectual disability. Nature 511: 344–347. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, et al. 2012. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel M, Cremer K, Ockeloen CW, Lichtenbelt KD, Herkert JC, Denecke J, Haack TB, Zink AM, Becker J, Wohlleber E, et al. 2015. De novo mutations in CHAMP1 cause intellectual disability with severe speech impairment. Am J Hum Genet 97: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh G, Kanno S, Uchida KS, Chiba S, Sugino S, Watanabe K, Mizuno K, Yasui A, Hirota T, Tanaka K. 2011. CAMP (C13orf8, ZNF828) is a novel regulator of kinetochore–microtubule attachment. EMBO J 30: 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2012. Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics 28: 1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CJ, Breathnach C, Betts DR, Sharkey FH, Greally MT. 2015. De novo interstitial deletion 13q33.3q34 in a male patient with double outlet right ventricle, microcephaly, dysmorphic craniofacial findings, and motor and developmental delay. Am J Med Genet A 167A: 1134–1141. [DOI] [PubMed] [Google Scholar]
- Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. 2001. Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 8: 85–95. [DOI] [PubMed] [Google Scholar]
- Noatynska A, Gotta M, Meraldi P. 2012. Mitotic spindle (DIS)orientation and DISease: cause or consequence? J Cell Biol 199: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Albrecht B, Bartholdi D, Beygo J, Di Donato N, et al. 2012. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380: 1674–1682. [DOI] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A. 2015. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36: 928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman JA, Brunner HG. 2012. De novo mutations in human genetic disease. Nat Rev Genet 13: 565–575. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Wisniewska M, Latos-Bielenska A, Linné M, Kelbova C, Belitz B, Pfeiffer L, Kalscheuer V, Erdogan F, Kuss AW, et al. 2008. Chromosome deletions in 13q33-34: report of four patients and review of the literature. Am J Med Genet A 146A: 337–342. [DOI] [PubMed] [Google Scholar]
- Waters AM, Asfahani R, Carroll P, Bicknell L, Lescai F, Bright A, Chanudet E, Brooks A, Christou-Savina S, Osman G, et al. 2015. The kinetochore protein, CENPF, is mutated in human ciliopathy and microcephaly phenotypes. J Med Genet 52: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, Braxton A, Beuten J, Xia F, Niu Z, et al. 2013. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med 369: 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, et al. 2014. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312: 1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.