Abstract
The idea that serotonergic synaptic transmission plays an essential role in the control of mood and the pharmacotherapy of anxiety and depression is one of the cornerstones of modern biological psychiatry. As a result, there is intense interest in understanding the mechanisms controlling the activity of serotonin-synthesizing (serotonergic) neurons. One of the oldest and most durable ideas emerging from this work is that serotonergic neurons are capable of autonomously regulating their own basal firing rate. Serotonergic neurons express on their surface 5-HT1A receptors (autoreceptors) that, when activated, induce the opening of potassium channels that hyperpolarize and thereby inhibit cell firing. Activity-dependent release of serotonin within serotonergic nuclei is thought to activate these autoreceptors, thus completing an autoinhibitory feedback loop. This concept, which was originally proposed in the 1970s, has proven to be enormously fruitful and has guided the interpretation of a broad range of clinical and preclinical work. Yet, remarkably, electrophysiological studies seeking to directly demonstrate this phenomenon, especially in in vitro brain slices, have produced mixed results. Here, we critically review this work with a focus on electrophysiological studies, which directly assess neuronal activity. We also highlight recent work suggesting that 5-HT1A receptor-mediated autoinhibition may play other roles in the control of firing besides acting as a feedback regulator for the pacemaker-like firing rate of serotonergic neurons.
Keywords: Serotonin, 5-HT1A autoreceptors, Raphe nuclei, autoinhibition, firing rate
Graphical abstract
Serotonergic neurons constitute a very small fraction of the neurons in the mammalian brain and are specifically localized to a series of midline brainstem structures collectively known as the Raphe nuclei.1 These neurons appear to be divided into two main groups, a large rostral group and a smaller caudal cell group,2 which, in turn, can be subdivided further based on their location3 or embryological origin.4 The serotonergic innervation of the forebrain, including the cerebral cortex, originates predominantly from the rostral cell group and especially from the serotonergic neurons located in the dorsal raphe nucleus (DRN). These cells are derived mostly from rhombomere 14 and represent a large group of serotonergic neurons, features that have greatly facilitated their study. Consequently, our understanding of the cellular physiology of serotonergic neurons is based overwhelmingly on the neurons of this cell group.
CELL FIRING AND THE MACHINERY OF SEROTONERGIC AUTOINHIBITION IN THE DRN
In anesthetized rodents, serotonergic neurons of the DRN fire at relatively low frequencies, generally on the order of a 1–3 action potentials per second, in what is often described as a pacemaker-like firing pattern5,6 (reviewed in ref 1). For the purposes of study, this firing has often been integrated and averaged over multisecond epochs5–7 and referred to as the serotonergic cell firing rate. A similar pattern of activity is recorded from DRN serotonergic neurons in freely moving animals, although under these conditions their firing rate can be seen to depend on the sleep–wake–arousal cycle of the animal.8–10 Recent studies have additionally shown that in freely moving animals the spontaneous firing of serotonergic neurons exhibits phasic changes in response to behaviorally relevant contingencies over a time frame much briefer than that used to determine the overall cell firing rate.11–14
Serotonergic neurons, in spite of their spontaneous activity seen in vivo, are not intrinsic pacemakers but rather depend on extrinsic inputs to drive their firing. In anesthetized animals, serotonergic cell firing requires noradrenergic inputs acting on α1-adrenergic receptors and depends only marginally on fast excitatory glutamate-mediated synaptic inputs.15–17 Activation of α1-adrenergic receptors on serotonergic neurons of the DRN elicits a robust depolarization/inward current.18 Thus, it seems likely that in anaesthetized animals, where serotonin neurons receive a reduced excitatory synaptic drive,19 serotonin cell firing is sustained by the membrane depolarization elicited by the activation of α1-adrenergic receptors. In contrast, in freely moving animals, serotonin cell firing appears to be mostly independent of α1-adrenergic receptor activation.20 In this case, multiple factors, probably including phasic synaptic inputs,8,14,19 likely interact to determine the timing and overall frequency (firing rate) of serotonergic cell firing.
Serotonergic neurons of the DRN express both 5-HT1A and 5-HT1B receptors, which are often referred to as autoreceptors. 5-HT1A autoreceptor expression is restricted to the somatodendritic compartment of serotonergic neurons, whereas 5-HT1B autoreceptors are strongly targeted to axonal terminals.21,22 Administration of serotonin to serotonergic neurons of the DRN activates 5-HT1A autoreceptors to elicit an outward current carried through potassium channels of the Kir3 (GIRK) family (reviewed in ref 23). Activation of this potassium current results in a membrane hyperpolarization and the inhibition of serotonergic neuron firing. Importantly, in vivo and in vitro fast-scan cyclic voltammetry studies have demonstrated stimulus-evoked serotonin release in the DRN,24–27 whereas electrophysiological studies have shown that the stimulus-evoked release of endogenous serotonin can activate 5-HT1A autoreceptors to elicit a robust membrane hyperpolarization.28,29 All together, these results leave little doubt that serotonergic neurons can engage in 5-HT1A receptor-mediated autoinhibition. However, exactly how this 5-HT1A receptor-mediated autoinhibition regulates the neuronal activity of serotonergic neurons remains poorly understood.
5-HT1A RECEPTOR-MEDIATED AUTOINHIBITION AND THE REGULATION OF SEROTONIN CELL FIRING IN THE DRN
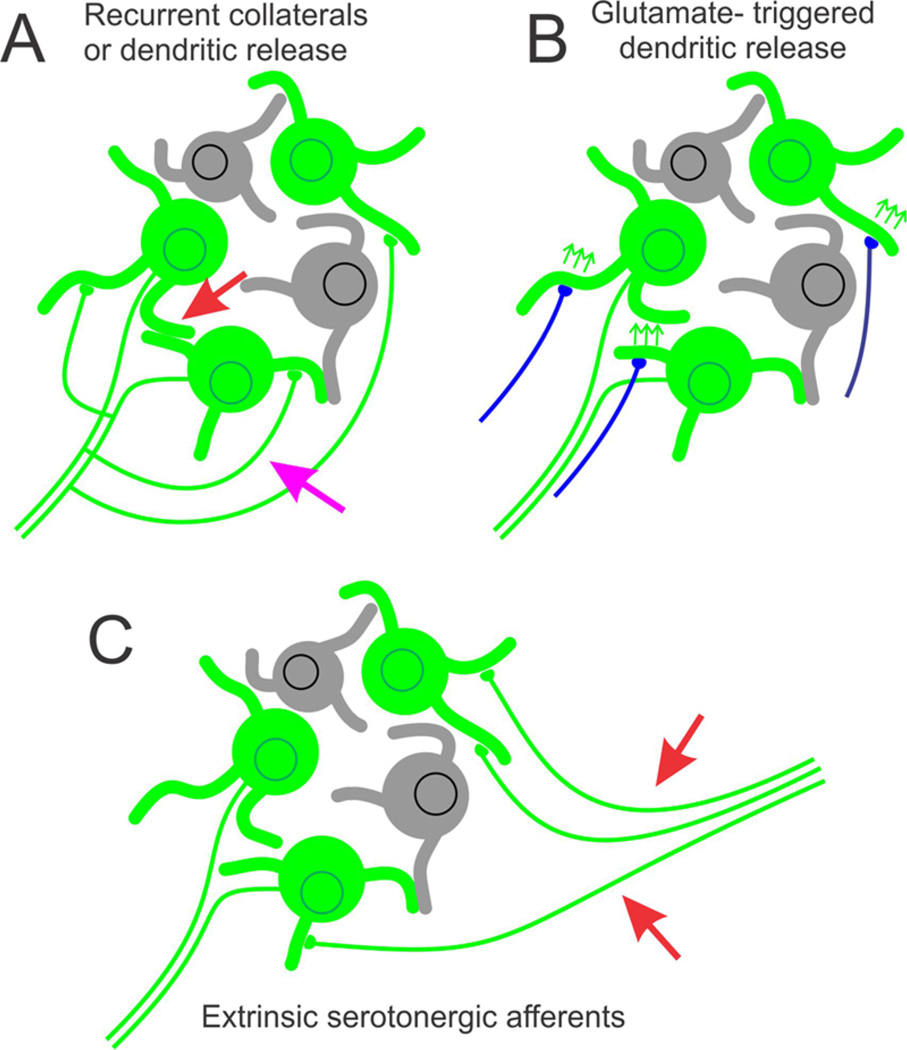
The initial and most durable idea on the functional role of 5-HT1A receptor-mediated autoinhibition in the DRN originates in the seminal work of Aghajanian and colleagues30 during the 1960s and 1970s. This work, which was conducted entirely in vivo, showed that serotonergic neuron cell firing appeared to be homeostatically regulated in vivo such that manipulations that increase the synaptic availability of serotonin inhibit serotonergic neuronal firing rate, whereas manipulations that decrease the synaptic availability of serotonin accelerate firing rates. In a parallel set of experiments, the same group showed that serotonin cells were themselves inhibited by serotonin and that antidromic activation of the serotonergic neurons of the DRN resulted in a period of postactivation inhibition that appeared to be serotonin-dependent.31,32 These results were interpreted to indicate that serotonergic neurons engaged in serotonin-mediated autoinhibition. At a mechanistic level, this autoinhibition was originally envisioned to be mediated through axonal recurrent collaterals (Figure 1A). Subsequent work using electron microscopy, however, revealed the existence of serotonin and of synaptic vesicles in the serotonergic cell dendrites.33–35 This led to the suggestion that autoinhibition could also be mediated via dendritic release of serotonin (Figure 1A).
Figure 1.
5-HT1A receptor-mediated autoinhibition in the DRN: some possible models. The DRN contains both serotonergic neurons (green) and nonserotonergic neurons (gray). (A) The traditional model proposes that serotonin autoinhibition is activity-dependent and is mediated through serotonin release from axonal collaterals (pink arrow) or dendrites (red arrow). (B) An alternative model proposes that serotonin is released secondary to local dendritic depolarizations elicited by glutamatergic synapses51,52 (blue). (C) Finally, it is possible that serotonergic autoinhibition may reflect serotonergic afferents to the DRN from caudal serotonergic cell groups54 (red arrow).
Thus, by the early 1980s, an extensive body of work supported the notion that serotonin neuron firing rate was homeostatically regulated. Similarly, a strong consensus had emerged that serotonin neurons could engage in serotonin-mediated self-regulation. At the time, these two phenomena were assumed to be linked, and the idea took hold that serotonergic autoinhibition was a key feedback control mechanism regulating the pacemaker-like firing rates of serotonergic neurons. In retrospect, while acknowledging the enormous insights that emerged from this early work, we can see limitations in this functional conclusion. Most notably, while serotonin autoinhibition could, in principle, act as a feedback control to homeostatically set the pacemaker-like firing of serotonergic neurons, this does not mean that it does so. Unfortunately, the experimental approaches available at the time, coupled with the lack of selective 5-HT1A receptor antagonists, made it difficult to test these ideas.
DOES 5-HT1A RECEPTOR-MEDIATED AUTOINHIBITION REGULATE FIRING RATE: IN VIVO STUDIES
One of these limitations was removed in the 1990s, with the development of effective 5-HT1A receptor antagonists.36,37 The question of 5-HT1A receptor control of firing rate was explicitly reexamined by Fornal et al. in the mid 1990s by taking advantage of the availability of the potent and selective 5-HT1A receptor antagonist WAY-100635.38 Using extracellular recordings in freely moving cats, they showed that blockade of 5-HT1A receptors, which was ascertained by antagonism of exogenous 8-OHDPAT, resulted in a modest increase in firing rate in animals in a quiet waking state but surprisingly not in slow wave sleep. This remarkable state-dependent effect of WAY-100635 suggested a complex role for 5-HT1A receptors in the regulation of firing rate, perhaps related to different mechanisms driving spontaneous firing during these different behavioral states (see above).
A drawback of the use of 5-HT1A receptor antagonists is that they block 5-HT1A autoreceptors as well as 5-HT1A receptors located on postsynaptic neurons. This limitation, however, can be bypassed by using modern genetic approaches that allow for the selective targeting of 5-HT1A autoreceptors in serotonergic neurons. In an elegant study, Richarson-Jones et al. used such approaches to generate two mouse strains differing in 5-HT1A autoreceptor expression by approximately 30% (1A-High and 1A-Low).39 As part of a large battery of tests, this group compared the firing rate of DRN neurons in these two strains using extracellular recordings in anesthetized animals.39 If 5-HT1A receptor-mediated autoinhibition homeostatically regulates firing rate, then the expectation would be that serotonin neurons in 1A-High mice should exhibit slower firing rates than cells in the 1A-low mice. Consistent with this expectation, neurons in the 1A-Low mouse exhibited higher firing rates than those seen in 1A-High mice. This would appear to provide strong support for the idea that 5-HT1A autoreceptors regulate firing rate. However, it is important to note that the increase in firing rate detected in 1A-Low mice resulted not from an overall increase in firing but from the appearance of a subpopulation of fast firing neurons, whereas the remaining cells fired in the same range as those in the 1A-High mouse. This could reflect mosaicism, but it is striking that the firing rate of the fast firing cells in the 1A-Low mouse is anomalously high and falls well outside firing rates normally associated with serotonergic neurons, even after 5-HT1A receptor blockade or 5-HT1A receptor deletion.38,40,41 The identification of serotonergic neurons in in vivo recordings is not trivial and becomes especially difficult if two of the essential diagnostic criteria, slow regular firing and sensitivity to serotonin, can no longer be relied upon for identification, as was the case in the Richardson-Jones et al. study.39 This raises the possibility that the 1A-Low cell sample could have included faster-firing nonserotonergic neurons. Consistent with this possibility, extracellular serotonin levels measured using dialysis in the hippocampus and frontal cortex, a proxy for serotonin cell firing rate, did not differ in these two mice strains.39 Clearly, these electrophysiological studies need to be replicated and expanded using a physiological tag to unambiguously identify serotonergic neurons.42 Nevertheless, in our view, in aggregate these studies contain sufficient anomalies to question the idea that they provide unambiguous support for the traditional view that 5-HT1A receptor-mediated autoinhibition functions simply as a negative feedback for the homeostatic regulation of the pacemaker-like firing rate of serotonergic neurons in the DRN.
DOES 5-HT1A RECEPTOR-MEDIATED AUTOINHIBITION REGULATE FIRING RATE: IN VITRO STUDIES
An alternative approach for the study of the relationship between 5-HT1A receptor-mediated autoinhibition and pacemaker-like firing rate emerged in the 1990s with the maturation of in vitro brain slice methodologies. The power of this approach is that, by isolating the serotonergic neurons of the DRN from their targets, it allows for the direct examination of the control of cell firing through local mechanisms, including 5-HT1A receptor-mediated autoinhibition. A complication with this electrophysiological approach, however, is that serotonergic neurons in in vitro brain slices are electrically quiescent, the result of their deafferentation and loss of noradrenergic tone onto α1-adrenergic receptors (see above). Therefore, initial studies relied on the addition of the α1-adrenergic agonist phenylephrine (PE) to re-establish spontaneous pacemaker-like firing. The consensus at the time was that, upon activation, serotonergic neurons would engage in autoinhibition that should curtail their firing rate. This autoinhibition should then be detectable as an acceleration of the cell firing rates upon administration of 5-HT1A receptor antagonists. This conjecture became testable with the development of 5-HT1A receptor antagonists such as WAY-100635. Surprisingly, blockade of 5-HT1A autoreceptors in DRN brain slices was found by multiple groups to have essentially no effect on the firing of the serotonergic neurons37,43,44 (reviewed in ref 45). This result was clearly inconsistent with the idea that 5-HT1A receptor-mediated autoinhibition is part of the intrinsic machinery controlling the pacemaker-like firing rate of serotonergic neurons.
One explanation for these unexpected results could be that they represent an artifact of in vitro brain slices. Three groups argued for this possibility and, more specifically, that the failure to detect 5-HT1A autoreceptor-mediated feedback regulation of serotonergic cell firing reflected a loss of serotonin synthesis (and hence serotonin) in this preparation.45–47 Support for this hypothesis came from a demonstrable time-dependent loss of serotonin in brain slices.45–47 Importantly, this loss was palliated by adding tryptophan to the bath, a manipulation that itself results in an inhibition of firing, as expected if tryptophan rescued autoinhibition by enabling serotonin synthesis. Furthermore, tryptophan-induced inhibition was reversed by the 5-HT1A receptor antagonist WAY-100635, consistent with the idea that tryptophan supplementation enabled 5-HT1A receptor-mediated autoinhibition which, in turn, acts as a negative feedback on firing. While these results are highly suggestive, the need for conversion of tryptophan to serotonin appears to have never been directly tested experimentally, and electrochemical studies in in vitro brain slices have consistently shown serotonin release without tryptophan supplementation.24–29 However, the main limitation with this idea, as discussed above, is the absence of the predicted robust increase in firing rate in response to WAY-100635. In our view, these findings cast doubt on the idea that loss of serotonin in slice preparations can account for the failure to observe robust 5-HT1A autoreceptor-mediated regulation of firing rates.
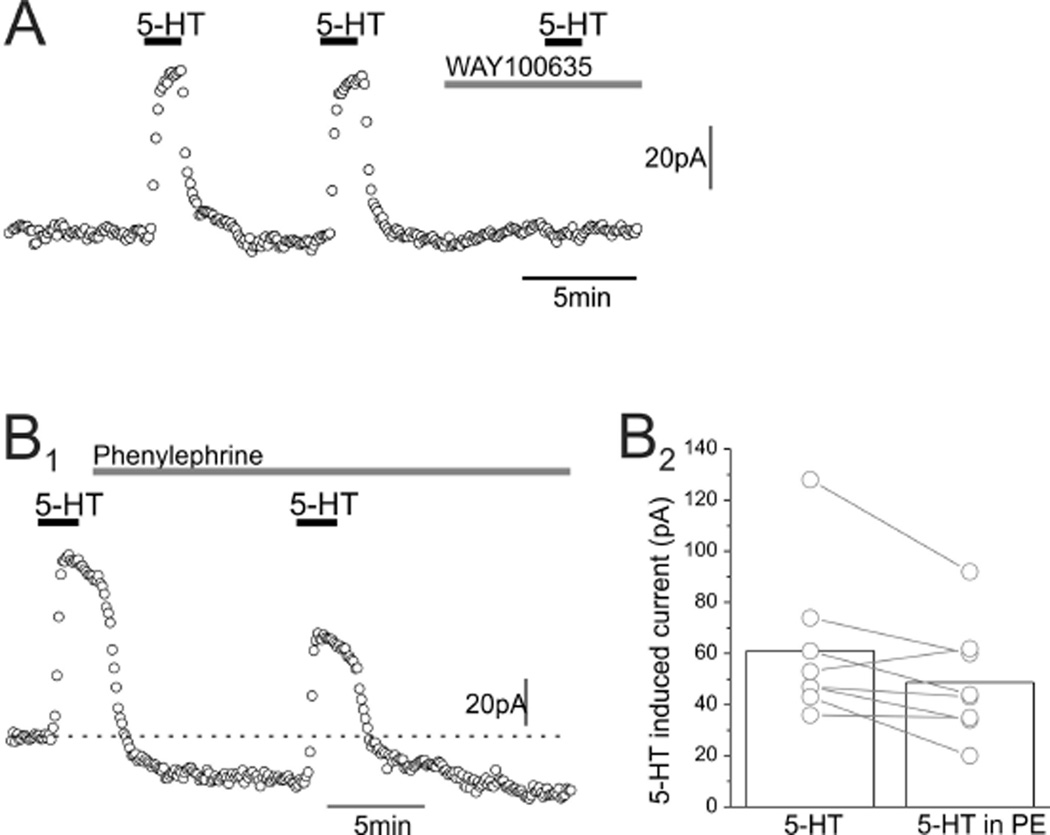
The failure to detect the predicted robust effect of 5-HT1A autoreceptors on firing rate in slices could alternatively be due to the reliance in these experiments on α-1 adrenergic receptors to elicit repetitive firing in serotonergic neurons. These receptors signal through Gαq-11 to activate phospholipase Cβ, leading to the breakdown of PtdIns(4,5)P2. This phospholipid, however, is essential for the function of Kir3 (GIRK) channels downstream from the 5-HT1A autoreceptors48,49 (and references therein), and previous studies have shown that Kir3 (GIRK) channel activity is suppressed by activation of Gαq-11-coupled receptors.50 Therefore, it is possible that the reliance on PE to activate serotonergic cells resulted in an impairment in the ability of 5-HT1A autoreceptors to activate Kir3 (GIRK) channels and hence enact autoinhibition. We have recently tested this possibility by determining the effect of PE on serotonin-induced (i.e., 5-HT1A autoreceptor-mediated) outward currents in serotonergic neurons of the DRN (Figure 2A,B). Administration of PE elicited only a very small, albeit statistically significant, effect on the amplitude of serotonin-induced currents. This suggests to us that PtdIns(4,5)P2 depletion is also unlikely to account for the difficulty in detecting a robust effect of 5-HT1A receptor-mediated autoinhibition on firing rate.
Figure 2.
Effect of 5-HT and PE on serotonergic neurons of the DRN. Whole-cell recordings were obtained from serotonergic neurons of the DRN in mouse brain slices. (A) Bath application of 5-HT (10 µM) elicits an outward current that is completely blocked by WAY-100635 (1 µM), indicating that it is mediated by 5-HT1A autoreceptors. (B1, B2) Administration of PE (10 µM) induces an inward current and also a small but statistically significant (p = 0.04) reduction in the amplitude of the outward current elicited by 5-HT (30 µM).
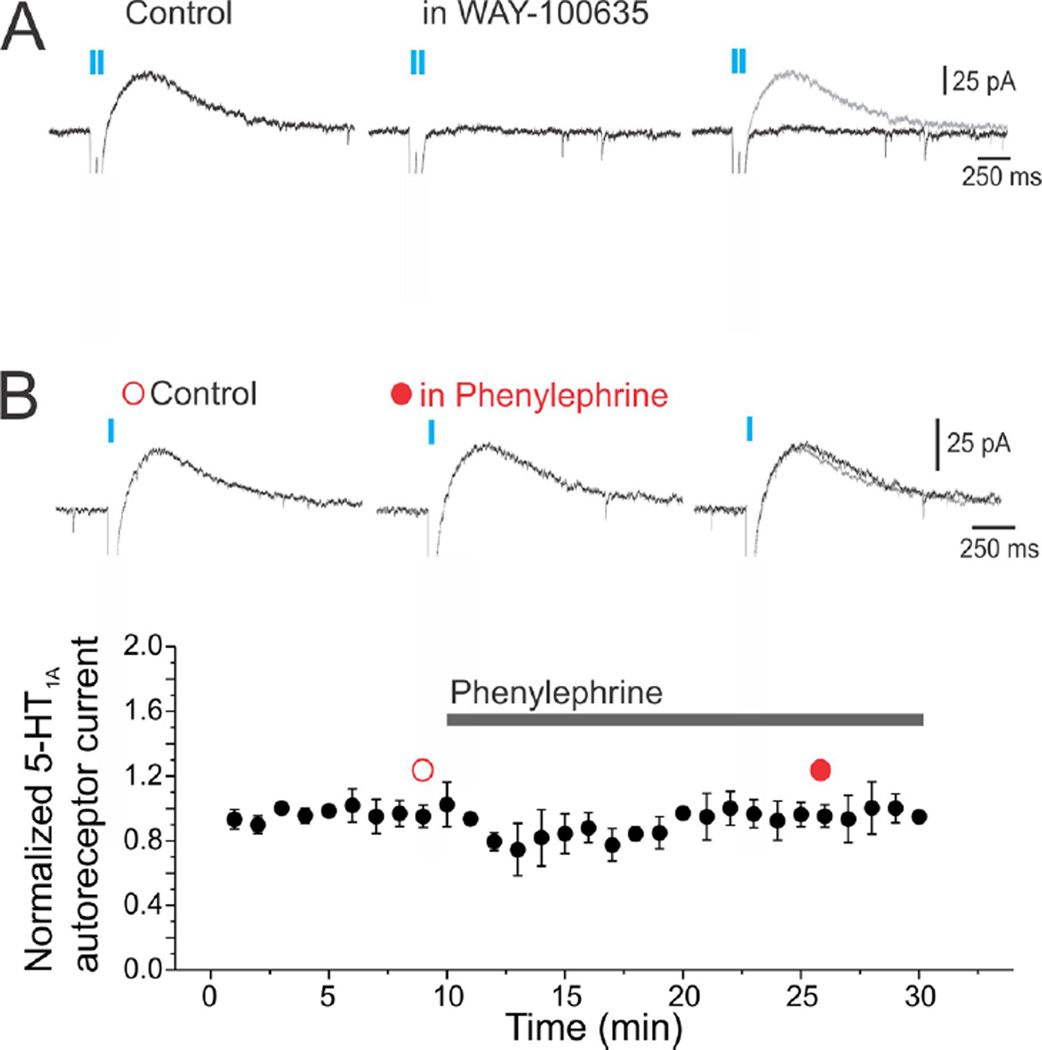
A third possibility is that the prolonged application of PE typically used in experiments examining the regulation of firing rate may have inadvertently resulted in the depletion of releasable serotonin in the slice. In experiments examining firing rate, neurons can be expected to fire many hundreds of action potentials, which could, in principle, lead to the depletion of the pool of serotonin-containing vesicles available for release. Presumably, this would not happen in experiments directly assessing serotonin release since those experiments use vastly lower stimulation frequencies. We have recently examined the stability of 5-HT1A receptor-mediated autoinhibition after PE administration by taking advantage of optogenetic approaches that allow for the selective excitation of serotonergic cellular elements in brain slices using light. As illustrated in Figure 3A, repeated light stimulation of DRN slices derived from mice expressing channelrhodopsin-2-(H143R)(ChR) in serotonergic neurons resulted in transient inward currents (ChR currents) followed by slower, outward currents mediated by the activation of 5-HT1A autoreceptors. We used these light-evoked 5-HT1A autoreceptor-mediated responses, which reflect synaptic release of endogenous serotonin, to assess the possible depletion of serotonin when slices are stimulated with PE. As illustrated in Figure 3B, administration of PE, which should have resulted in activation of the serotonergic neurons of the DRN, failed to elicit a significant reduction in the amplitude of the 5-HT1A autoreceptor currents. These experiments suggest that neither serotonin depletion nor loss of autoinhibition due to PtdIns(4,5)P2 depletion likely account for the failure to observe 5-HT1A autoreceptor control of firing rates in brain slices stimulated with PE.
Figure 3.
Effect of PE on 5-HT1A autoreceptor-mediated synaptic currents in serotonergic neurons of the DRN. Whole-cell recordings were obtained from brain slices derived from a mouse expressing ChR in serotonergic neurons (SERT-Cre driver [MMRRC] × Ai32 ChR reporter [Jackson Laboratories]). (A) Light stimulation (blue bars) elicits transient (ChR) inward currents that are followed by a slower transient outward current. This outward current is blocked by WAY-100635 (3 µM), indicating it is mediated by the activation of 5-HT1A autoreceptors. (B) Administration of PE (10 µM) has no detectable effect on these 5-HT1A autoreceptor-mediated synaptic currents. In separate experiments, we confirmed that PE elicits a robust inward current and serotonin cell spiking under our recording conditions (not shown).
POSSIBLE ALTERNATIVE ROLES FOR 5-HT1A RECEPTOR-MEDIATED AUTOINHIBITION IN THE DRN
The in vivo and in vitro results reviewed above indicate that serotonergic neurons are capable of robust 5-HT1A receptor-mediated autoinhibition. At the same time, in our opinion, they do not support the idea that 5-HT1A receptor-mediated autoinhibition functions as a homeostatic feedback mechanism regulating the pacemaker-like firing rate of serotonergic neurons. Could 5-HT1A receptor-mediated autoinhibition have other roles in serotonergic neurons?
One possibility is that 5-HT1A receptor-mediated autoinhibition may regulate serotonin cell activity by regulating synaptic inputs to the DRN or perhaps even synchronizing neuronal activity within DRN networks. Of course, such actions could manifest themselves as changes in firing rate under some conditions, which could have contributed to a misinterpretation of their role, but they would not be part of a homeostatic feedback loop controlling the spontaneous pacemaker firing of these cells.
One specific implementation of these ideas emerges from the work of de Kock and colleagues,51 who suggested that serotonin is released from dendrites following calcium influx through NMDA receptors opened in response to glutamate-mediated synaptic events (Figure 1B). A variant of this mechanism has been proposed by Colgan, Levitan, and associates,52,53 who have argued that serotonin release from dendrites is secondary to calcium influx through L-type calcium channels that open in response to the local dendritic depolarization elicited by synaptically released glutamate. Both of these mechanisms postulate that serotonin release, and hence 5-HT1A receptor-mediated autoinhibition, is engaged by excitatory glutamatergic inputs to the DRN. As such, they posit that 5-HT1A receptor-mediated autoinhibition is engaged by glutamate synaptic inputs to the DRN, via locally triggered calcium influx, rather than by neuronal firing. This offers a parsimonious hypothesis that uncouples 5-HT1A receptor-mediated autoinhibition from the regulation of pacemaker firing and, in doing so, reconciles most previous experimental observations to date. However, one problem with this hypothesis is that electrically evoked serotonin release persists in the presence of AMPA and NMDA receptor blockers,28,29 thus suggesting that glutamate synaptic transmission is not necessary for stimulus-evoked serotonin release in the DRN. Of course, electrical stimulation may itself directly depolarize the dendrites, thus obviating the need for glutamate receptor activation. Thus, at this point, it is difficult to fully evaluate this hypothesis on the evidence published to date.
A second alternative emerges from studies showing that serotonergic cell groups can be interconnected. The DRN in particular has been shown to receive serotonergic inputs from the caudal Raphe nuclei.54,55 Thus, it is possible that serotonin release in the DRN may not originate from DRN serotonergic neurons but rather from extrinsic serotonergic afferents (Figure 1C).54,55 This would explain both the presence of serotonin release in the DRN as well as the difficulty observing robust involvement of 5-HT1A autoreceptors in the regulation of firing rate.
CONCLUSIONS
Historically, 5-HT1A receptor-mediated autoinhibition in the DRN has been thought to function as a homeostatic feedback mechanism controlling the pacemaker-like firing, the so-called firing rate, of serotonergic neurons. However, evidence in support of this hypothesis is limited, and there is considerable evidence, especially from in vitro brain slice work, that is not consonant with this idea. As outlined above, we believe that accumulating evidence hints at alternative roles for 5-HT1A receptor-mediated autoinhibition in the DRN beyond the homeostatic control of firing rate, although alternate theories will require further investigation. For example, it is possible that 5-HT1A autoinhibition plays a role in regulating glutamate signaling to serotonin neurons or mediates inputs from distal serotonergic cell groups or yet other possible functions. Such effects would be hard to identify in brain slices, or even in anesthetized animals, which could explain the difficulties assigning a functional role to 5-HT1A receptor autoinhibition. Fortunately, sophisticated tools are quickly becoming available to address synaptic organization experimentally and for manipulating the in vivo activity of serotonergic neurons. We anticipate that advances along these fronts will open new avenues for continuing to test the hypotheses discussed above and for uncovering new hypotheses regarding the functional role of 5-HT1A receptor-mediated autoinhibition in the serotonergic system.
Acknowledgments
Funding
Work in the Andrade laboratory is supported by NIH grants MH43985 and MH100850.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, Fox S, Maeno H, Deneris ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J. Neurosci. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Denstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 1964;62:55. [PubMed] [Google Scholar]
- 4.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–708. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- 6.Aghajanian GK, Vandermaelen CP. Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res. 1982;238:463–469. doi: 10.1016/0006-8993(82)90124-x. [DOI] [PubMed] [Google Scholar]
- 7.Lanfumey L, Jacobs BL. Developmental analysis of raphe dorsalis unit activity in the rat. Brain Res. 1982;242:317–320. doi: 10.1016/0006-8993(82)90315-8. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs BL, Heym J, Trulson ME. Behavioral and physiological correlates of brain serotoninergic unit activity. J. Physiol. 1981;77:431–436. [PubMed] [Google Scholar]
- 9.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse BD, Devilbiss D, Seiple S, Markowitz R. Sensorimotor-related discharge of simultaneously recorded, single neurons in the dorsal raphe nucleus of the awake, unrestrained rat. Brain Res. 2004;1000:183–191. doi: 10.1016/j.brainres.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Dalphin N, Hyland BI. Association with reward negatively modulates short latency phasic conditioned responses of dorsal raphe nucleus neurons in freely moving rats. J. Neurosci. 2013;33:5065–5078. doi: 10.1523/JNEUROSCI.5679-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki K, Miyazaki KW, Doya K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J. Neurosci. 2011;31:469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagne-Clavel J, Oliveras JL, Martin G. Single-unit recordings at dorsal raphe nucleus in the awake-anesthetized rat: spontaneous activity and responses to cutaneous innocuous and noxious stimulations. Pain. 1995;60:303–310. doi: 10.1016/0304-3959(94)00129-3. [DOI] [PubMed] [Google Scholar]
- 14.Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J. Neurophysiol. 2009;102:3026–3037. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- 15.Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- 16.Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. I. Role of adrenergic system. Eur. J. Pharmacol. 1976;39:341–355. doi: 10.1016/0014-2999(76)90144-8. [DOI] [PubMed] [Google Scholar]
- 17.Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by alpha1-adrenoceptors in brain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- 18.Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J. Physiol. 1994;478:437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heym J, Steinfels GF, Jacobs BL. Chloral hydrate anesthesia alters the responsiveness of central serotonergic neurons in the cat. Brain Res. 1984;291:63–72. doi: 10.1016/0006-8993(84)90651-6. [DOI] [PubMed] [Google Scholar]
- 20.Heym J, Trulson ME, Jacobs BL. Effects of adrenergic drugs on raphe unit activity in freely moving cats. Eur. J. Pharmacol. 1981;74:117–125. doi: 10.1016/0014-2999(81)90521-5. [DOI] [PubMed] [Google Scholar]
- 21.Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 22.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 23.Andrade R, Beck SG. Cellular effects of serotonin in the CNS. In: Muller CP, Jacobs BL, editors. The Behavioural Neurobiology of Serotonin. Amsterdam: Elsevier; 2010. [Google Scholar]
- 24.Bunin MA, Prioleau C, Mailman RB, Wightman RM. Release and uptake rates of 5-hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. J. Neurochem. 1998;70:1077–1087. doi: 10.1046/j.1471-4159.1998.70031077.x. [DOI] [PubMed] [Google Scholar]
- 25.Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J. Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor JJ, Kruk ZL. Fast cyclic voltammetry can be used to measure stimulated endogenous 5-hydroxytryptamine release in untreated rat brain slices. J. Neurosci. Methods. 1991;38:25–33. doi: 10.1016/0165-0270(91)90150-x. [DOI] [PubMed] [Google Scholar]
- 27.Stamford JA, Palij P, Davidson C, Jorm CM, Millar J. Simultaneous “real-time” electrochemical and electrophysiological recording in brain slices with a single carbon-fibre microelectrode. J. Neurosci. Methods. 1993;50:279–290. doi: 10.1016/0165-0270(93)90035-p. [DOI] [PubMed] [Google Scholar]
- 28.Pan ZZ, Colmers WF, Williams JT. 5-HT-mediated synaptic potentials in the dorsal raphe nucleus: interactions with excitatory amino acid and GABA neurotransmission. J. Neurophysiol. 1989;62:481–486. doi: 10.1152/jn.1989.62.2.481. [DOI] [PubMed] [Google Scholar]
- 29.Pan ZZ, Williams JT. Differential actions of cocaine and amphetamine on dorsal raphe neurons in vitro. J. Pharmacol. Exp. Ther. 1989;251:56–62. [PubMed] [Google Scholar]
- 30.Aghajanian GK. Chemical-feedback regulation of serotonin-containing neurons in brain. Ann. N.Y. Acad. Sci. 1972;193:86–94. doi: 10.1111/j.1749-6632.1972.tb27826.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: evidence for collateral inhibition. Brain Res. 1977;132:186–193. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang RY, Aghajanian GK. Collateral inhibition of serotonergic neurones in the rat dorsal raphe nucleus: pharmacological evidence. Neuropharmacology. 1978;17:819–825. doi: 10.1016/0028-3908(78)90070-9. [DOI] [PubMed] [Google Scholar]
- 33.Chazal G, Ohara PT. Vesicle-containing dendrites in the nucleus raphe dorsalis of the cat. A serial section electron microscopic analysis. J. Neurocytol. 1986;15:777–787. doi: 10.1007/BF01625194. [DOI] [PubMed] [Google Scholar]
- 34.Chazal G, Ralston HJ., III Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites, and axon terminals. J. Comp. Neurol. 1987;259:317–329. doi: 10.1002/cne.902590302. [DOI] [PubMed] [Google Scholar]
- 35.Pecci SJ, Brusco A, Peressini S, Oliva D. A new case for a presynaptic role of dendrites: an immunocytochemical study of the n. raphé dorsalis. Neurochem. Res. 1986;11:997–1009. doi: 10.1007/BF00965589. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, Haskins JT, Jones D, Mansell HL, Reilly Y. WAY100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur. J. Pharmacol. 1993;237:283–291. doi: 10.1016/0014-2999(93)90280-u. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 38.Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, Jacobs BL. WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J. Pharmacol. Exp. Ther. 1996;278:752–762. [PubMed] [Google Scholar]
- 39.Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mundey MK, Fletcher A, Marsden CA. Effect of the putative 5-HT1A antagonists WAY100135 and SDZ 216–525 on 5-HT neuronal firing in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 1994;33:61–66. doi: 10.1016/0028-3908(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 41.Richer M, Hen R, Blier P. Modification of serotonin neuron properties in mice lacking 5-HT1A receptors. Eur. J. Pharmacol. 2002;435:195–203. doi: 10.1016/s0014-2999(01)01607-7. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JY, Amoroso MW, Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife. 2015;4:06346. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craven R, Grahame-Smith D, Newberry N. WAY-100635 and GR127935: effects on 5-hydroxytryptaminecontaining neurones. Eur. J. Pharmacol. 1994;271:R1–R3. doi: 10.1016/0014-2999(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DA, Gartside SE, Ingram CD. 5-HT1A receptor-mediated autoinhibition does not function at physiological firing rates: evidence from in vitro electrophysiological studies in the rat dorsal raphe nucleus. Neuropharmacology. 2002;43:959–965. doi: 10.1016/s0028-3908(02)00116-8. [DOI] [PubMed] [Google Scholar]
- 45.Liu RJ, Lambe EK, Aghajanian GK. Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 2005;21:945–958. doi: 10.1111/j.1460-9568.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- 46.Evans AK, Reinders N, Ashford KA, Christie IN, Wakerley JB, Lowry CA. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur. J. Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Mlinar B, Tatini F, Ballini C, Nencioni S, Della Corte L, Corradetti R. Differential autoinhibition of 5-hydroxytryptamine neurons by 5-hydroxytryptamine in the dorsal raphe nucleus. NeuroReport. 2005;16:1351–1355. doi: 10.1097/01.wnr.0000175249.25535.bf. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Whorton MR, MacKinnon R. Quantitative analysis of mammalian GIRK2 channel regulation by G proteins, the signaling lipid PIP2 and Na+ in a reconstituted system. eLife. 2014;3:e03671. doi: 10.7554/eLife.03671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves G(alpha)q family subunits, phospholipase C, and a readily diffusible messenger. J. Biol. Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 51.de Kock CP, Cornelisse LN, Burnashev N, Lodder JC, Timmerman AJ, Couey JJ, Mansvelder HD, Brussaard AB. NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing. J. Physiol. 2006;577:891–905. doi: 10.1113/jphysiol.2006.115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colgan LA, Cavolo SL, Commons KG, Levitan ES. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J. Neurosci. 2012;32:15737–15746. doi: 10.1523/JNEUROSCI.0020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colgan LA, Putzier I, Levitan ES. Activity-dependent vesicular monoamine transporter-mediated depletion of the nucleus supports somatic release by serotonin neurons. J. Neurosci. 2009;29:15878–15887. doi: 10.1523/JNEUROSCI.4210-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J. Comp. Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]