Abstract
Visceral adipose inflammation mediated by innate and adaptive immune alterations plays a critical role in diet-induced obesity and insulin resistance (IR). The dietary supplement α-lipoic acid (αLA) has been shown to ameliorate inflammatory processes in macrophages, however the relative significance of these effects in the context of visceral adipose inflammation and IR remain unknown. In this study we investigated its effects via both intraperitoneal and oral administration in lean and obese transgenic mice expressing yellow fluorescent protein (YFP) under control of a monocyte specific promoter (c-fmsYFP+). αLA significantly improved indices of insulin-resistance concomitant with a decrease in total (YFP+CD11b+) and activated (YFP+CD11b+CD11c+) visceral adipose tissue macrophages. Histologically, the visceral adipose tissue of obese mice receiving αLA had fewer “crown-like structures,” a hallmark of adipose inflammation in murine obesity. Monocyte adhesion assessed by intravital microscopy of cremasteric venules was attenuated by αLA. In cultured WT and toll-like receptor 4 (TLR4) null primary mouse macrophages, αLA significantly decreased basal CCR-2, MCP-1 and TNF-α expression levels. LPS treatment resulted in increased TNFα, MCP-1, and IL-6 expression while αLA partially abrogated the LPS effect on MCP-1 and TNFα; Interestingly, CCR-2 was not coordinately regulated. AαLA prevented LPS-induced nuclear factor kappa B (NFκB) activation in the same cultured macrophages. These data suggest that αLA may modulate visceral adipose inflammation, a critical determinant of IR via TLR4 and NF-κB pathways.
Keywords: Visceral adipose inflammation, Crown-like structures, Antioxidant, c-fms
Introduction
A large body of evidence links inflammation to the development of insulin resistance (IR) in type 2 diabetes mellitus and obesity [1, 2]. Adipose tissue in both disorders is typ-ified by a dramatic remodeling of adipose and stereotypic alterations in the numbers and function of macrophage and dendritic cells in visceral fat [2–4]. Inflammation in visceral adipose tissue is thought to play a central role, with the degree of inflammation being demonstrated to inversely correlate with insulin sensitivity [5–8]. Conversely, treatment of inflammation has been shown to reverse insulin resistance and improve indices of whole body glucose homeostasis. The mechanisms by which a dysregulated immune axis may contribute to IR is multifactorial but a key pathway is through the release of pro-inflammatory cytokines (TNFα and IL-6), and immunoattractant chemokines that further contribute to an adaptive immune response [9–16]. Mice that lack TNFα and CCR-2, the receptor for monocyte chemoattractant protein 1 (MCP-1/CCL-2), have improved insulin sensitivity and glucose metabolism when compared to adiposity-matched controls [13, 17]. Recent studies show that the Toll-like receptor 4 (TLR4) may play a central role in the link among insulin resistance, inflammation, and obesity; TLR4 deficiency prevented insulin resistance and obesity-mediated activation of IκB kinase (IKKb) and c-Jun NH2-terminal kinase (JNK), suggesting that TLR4 is a key modulator in the cross-talk between inflammatory and metabolic pathways [18–22].
Alpha lipoic acid (αLA) is a disulfide derivative of octanoic acid that forms an intra-molecular disulfide bond that is readily reduced to dihydrolipoic acid intra-cellularly [23]. Anorectic effects in rodents have been reported for αLA as well as improved hypertriglyceridemia in Zucker diabetic fatty rats [24]. αLA has been reported to increase insulin sensitivity in the skeletal muscle via AMPK activation in obese rats [25] though other researchers have failed to confirm this finding [24]. αLA has been shown to reduce NF-κB activation in human monocytic cells and reduce inflammation [26]. However the impact of αLA on innate immune inflammation and whether these effects lead to coordinate change in indices of IR/glucose homeostasis have not been investigated. We employed a novel model of diet-induced obesity/IR using transgenic reporter mice that express YFP under control of a c-fms reporter (c-fmsYFP+) that allows monocyte specific expression of the reporter and unparalleled ability to track these cells in visceral adipose tissue. In carefully performed pair-fed experiments, we demonstrate that αLA improves key metrics of innate immune activation and IR.
Materials and Methods
Animals
Oral αLA Regimen
c-fmsYFP+ transgenic mice were generated at the Transgenic Animal Service of Queensland, Brisbane, Queensland, Australia by injection of the transgenes into pronuclei of (C57BL/6 × CBA)F1 (BCBF1) fertilized eggs [27]. The Committee on Use and Care of Animals from the Ohio State University (OSU) approved all experimental procedures. c-fmsYFP+ mice of the FVB/N strain were bred and genotyped at OSU and housed in cages individually. YFP+ males at 6 weeks of age were fed a HFD for 8 weeks (42% energy from fat—Harlan Teklad TD88137) prior to randomized to three dietary groups (5 mice/group): ad libitum, αLA-fed, and pair-fed to αLA. Pair-feeding was employed as αLA is well known to modulate central appetite pathways and reduce food intake. Food intake was monitored every other day by weighing the remaining chow; pair-fed mice were then offered the same amount of food as consumed by the αLA-mice. αLA (2 mg/ml; Sigma Aldrich T5625) was administered in the drinking water (ultrapure 18.2 MΩ) at a pH of 8.0 with NaOH. Pair-fed mice were given ultrapure water at a pH of 8.0. αLA was administered for 8 weeks before mice were sacrificed. Mice consumed approximately 6 mg of αLA per day over the course of the study, which was a dose of 167 mg/kg at time zero, and 157 mg/kg after 8 weeks due to weight gain.
Determining an appropriate oral αLA dose was challenging as there were no published data on plasma αLA levels in mice after oral administration. Thus, we used a dose previously shown to be effective in mice on disease processes, that did not have adverse side effects, and which was reasonable in regards to what plasma levels might be expected. Yi and Maeda [28] used approximately 200 mg/ kg via addition to the chow and showed significant abrogation of atherosclerotic lesion development. Additionally, if one considers 50 mg/kg injections in mice resulted in modest plasma levels of 7.6 ± 1.4 μg/ml [29], the oral dose of ~160 mg/kg used in this study is expected to result in <7 μg/ml plasma concentrations due to bioavailability limits and gastrointestinal metabolism.
IP αLA Regimen
Males at 6 weeks of age were fed a HFD for 8 weeks, before being randomized to αLA or vehicle control groups. A solution of sterile αLA in saline (4 mg/ml) was injected intraperitoneally at a dose of 10 mg/kg once daily, 6 days a week. Mice were weighed daily to ensure accurate dosing. αLA dosing in the mouse was done with attention to the concentration reasonably obtained in human plasma by use of conventional dietary supplements. Healthy human subjects given an average oral dose of 8.25 mg/kg (600 mg) of R-αLA sodium salt dissolved in water were shown to have a mean maximum plasma concentration (Cmax) of 16.03 μg/ml [30]. However, others have reported a much lower oral bioavailability, with 600 mg of racemic αLA via solid supplement resulting in a Cmax of 2.85 [31] and 2.7 μg/ml [32]. These data suggest that potassium or sodium salt of lipoic acid have higher oral bioavailability, as was administered in this study. Additionally, a human oral dose of 1,200 mg resulted in plasma Cmax levels of 3.8 ± 2.6 to 10.3 ± 3.8 μg/ml with area under the curve (AUC) levels from 443.1 ± 283.9 to 848.8 ± 360.5 which was similar to a subcutaneous injection of 50 mg/kg αLA in mice which yielded 7.6 ± 1.4 μg/ml and 223 ± 20 AUC [29]. The dose of 10 mg/kg (daily) and 50 mg/kg (acute) used in this report are reasonable if not conservative in this context.
Serum Cytokine Analysis
Blood was collected via heart puncture under CO2 anesthesia and allowed to coagulate at room temperature followed by centrifugation. Serum cytokine levels were analyzed using BDTM Cytometric Bead Array, Mouse inflammation kit according to the manufacturer instructions.
Epididymal Fat Pad Digestion and Quantification of ATMs
Epididymal fat pads from c-fmsYFP+ mice at the end of the treatment phase were excised, minced, washed in 1× PBS, and digested with sterile collagenase type II from Clos-tridium histolyticum (1 mg/ml) in DMEM (10% FBS) at 37 °C with shaking (140 rpm) as detailed previously [4, 33]. The digesta was filtered through a 100 μm nylon cell strainer before centrifugation (300×g, 10 min). The resulting pellet was defined as the stromal vascular fraction (SVF). Viable adipose tissue mononuclear cells were isolated from SVF using Lympholyte M (Cedarlane Laboratories Ltd, Burlington, NC). Approximately 106 cells were incubated with mouse FcR blocking reagent (Miltenyi Biotec Inc., Auburn CA) in FACS buffer (1× PBS, 5% FBS) for 10 min at 4 °C followed by staining with F4/80-PE-Cy5, CD11b-PE, and isotype control antibodies (Bio-legend, San Diego, CA). Cells were washed in FACS buffer 3 times and measured (BD FACS LSR IITM flow cytometer, Becton–Dickinson, San Jose, CA). Data were analyzed using BD FACS Diva 6.0.1 software (Becton–Dickinson, San Jose, CA). Gates were set using the appropriate isotype controls.
Live Confocal Microscopy of Unfixed Adipose Tissue
Epididymal fat was removed using sterile techniques and carefully cut into ~3–4 mm pieces. After rinsing with 1× PBS the tissue was incubated with Griffonia simplicifolia isolectin GS-IB4 conjugated to AlexaFluor 488 (10 μg/ml—Molecular Probes), BODIPY 558/568 (5 μM—Molecular Probes), and Hoechst 33342 (40 μM—Molecular Probes) for 1 h in 1× PBS supplemented with 1 mM CaCl2. Isolectin has been shown to be an endothelial cell specific stain in the adipose [34]. Tissue was visualized on a Zeiss laser scanning microscope 510 under 40× water immersion.
Monocyte-Vascular Adhesion as Assessed by Intravital Microscopy
Mice were given αLA IP (50 mg/kg body weight) 24 h before the start of the experiment. TNFα was injected IP (1 μg/kg; 0.9% saline with 1.0% BSA) 4 h before visualization. Under ketamine/xylazine anesthesia, the testicular cremaster muscle was exposed using a dissecting microscope (2×; Nikon SMZ 645, Japan). The cremaster muscle was bathed with Ringers Lactate at 37 °C and monocyte-endothelial interaction was assessed in 15–25 vessels using a Nikon Eclipse FN1 microscope (Nikon, Japan) with a 40×/0.80 W water immersed objective at a 2.0 mm working distance. In all experiments video images were captured and digitalized to 12-bit TIF images using Metamorph software (version 7.1.2.0, Metamorph, Downingtown, USA). Rolling YFP+ cells were counted per minute for different vessel diameters and vessel segments. All YFP+ cells, per 100 μm of vessel length, that were immobile for at least 30 s were interpreted as adherent cells [35]. Calculations to determine the number of rolling and adherent cells according to vessel diameter were performed using Opti Test (Version 1.4.1.0).
Bone Marrow Derived Macrophage Culture and Differentiation
Bone marrow was isolated from WT or TLR4 deficient mice and grown in DMEM media supplemented with 10% FBS in the presence of L-cell conditioned media for 5 days. The differentiated macrophages were pretreated with αLA (100 μg/ml) 45 min before LPS (0.5 μg/ml) addition. Post absorption, αLA is rapidly cleared from circulation via renal excretion and tissue uptake. While much is excreted, tissues especially the liver, heart, skeletal muscle, and possibly the brain, accumulate αLA and extensively catabolize to a dozen or more metabolites depending on species [36, 37]. There is also evidence the αLA is rapidly reduced by cells in vitro to DHLA and subsequently excreted [37]. Thus, determining in vitro doses that might be physiologically relevant was challenging. 100 μg/ml (~0.5 mM) αLA was previously shown to prevent LPS-induced TNFα expression in mouse monocytes in vitro and was found to be an optimal dose for Akt phosphorylation [38]. This does is higher than would be obtained in mouse serum, thus it most likely super-physiological but is no more than tenfold higher than what is possible in serum. Also, relative to published in vitro studies using αLA the dose used herein is conservative [39, 40].
Quantitative-Real-Time PCR Detection of Macrophage Activation Status
RNA was isolated using Absolutely RNA®, StratageneTM according to the manufacturer’s instructions including DNase digestion. RNA quality and quantity were assessed by agarose gel electrophoresis and a NanodropTM spectrophotometer. cDNA was reverse transcribed using 800 ng of total RNA according the manufacturer’s instructions (Invitrogen Life Technologies—M-MLV reverse transcriptase) using random primers. PCR was performed using SYBR Green I master mix (Roche) on a Roche Lightcycler 480. All real-time reactions had the following profile conditions: 10 min hot start at 95 °C followed by 45 cycles of 94 °C 10 s, 60 °C 20 s, 72 °C 20 s. Reference and target gene dilution standards were run in triplicate for each primer set to calculate PCR efficiency using the above profile. The concentration ratios were determined after PCR efficiency correction by relative quantification analysis using Lightcycler 480 software. All target genes were expressed as fold increase compared to control. Melting/dissociation curves were run on each plate to assure the production of one amplicon of the same melting temperature for each primer set. Real time primers (listed below) were designed to span genomic introns, thus avoiding amplification of genomic DNA possibly present in the RNA samples. “No template,” cDNA negative controls were included for each gene set in all PCR reactions to detect contamination. Primers used were: TNFα For 5′-caacggcatggatctcaaagac- 3′, Rev 5′-agatagcaaatcggctgacggt-3′; CCR2 For 5′-ttgg gtcatgatccctatgtgg-3′, Rev 5′-ccttcctaatcctgtgaccctt-3′; IL-6 For 5′-attaacacatgttctctgggaaatcgt-3′ Rev 5′-tatatccagtttgg tagcatccatca-3′ MCP-1 For 5′-gcagcaggtgtcccaaagaa-3′ Rev 5′-atttacgggtcaacttcacattcaa-3′ Macrophage galactose N-acetyl-galactosamine receptor-specific lectin 1 (Mgl1) For 5′-tggatgggaccgactttgagaa-3′; Mgl1 Rev 5′-gggac cacctgtagtgatgtg-3′; Glyceraldehyde-3-phosphate dehydro-genase (GAPDH) For 5′-gtgaagcaggcatctgaggg-3′; GAPDH Rev 5′-cgaaggtggaagagtgggagt-3′
Chemiluminescent Electrophoretic Mobility Shift Assay
Nuclear extracts for use in EMSAs were performed using the previously published protocol [41, 42]. Oligonucleo-tides probes (NFκB sense 5′-AGTTGAGGGGACTT TCCCAGGC-3′, NFκB antisense 5′-GCC TGG GAA AGT CCC CTC AAC T-3′) were biotinylated using Biotin 3′ End DNA Labeling Kit (Pierce, Rockford IL #89818) according to manufacturer’s instructions. LightshiftTM Chemiluminescent EMSA kit (Peirce, Rockford IL #20148) was used to perform the binding reaction and chemiluminescent detection. Briefly, 5 μg of nuclear extract was incubated at room temperature in a binding reaction which included a final concentration of: 1× binding buffer, 50 ng/μl Poly (dI·dC), 0.05% NP-40, 2.5% glycerol, and 5 mM MgCl2 for 15 min prior to addition of the biotinylated probes. The complex was run on a pre-electrophoresed 6% polyacrylamide gel in 0.5× TBE (pH 8.4) at 100 V for approximately 45 min followed by wet-transfer in 0.5× TBE to Amersham HybondTM –N+ membrane (GE Healthcare) at 380 mA for 30 min. The transferred DNA was cross-linked to the membrane before continuing with protocol according to manufacturer’s instructions. X-ray film was exposed to membrane and developed.
Results
Alpha Lipoic Acid (αla) Administration Improved Markers of Systemic and Local Insulin Sensitivity and Triacylglycerol Metabolism
Oral αLA Regimen
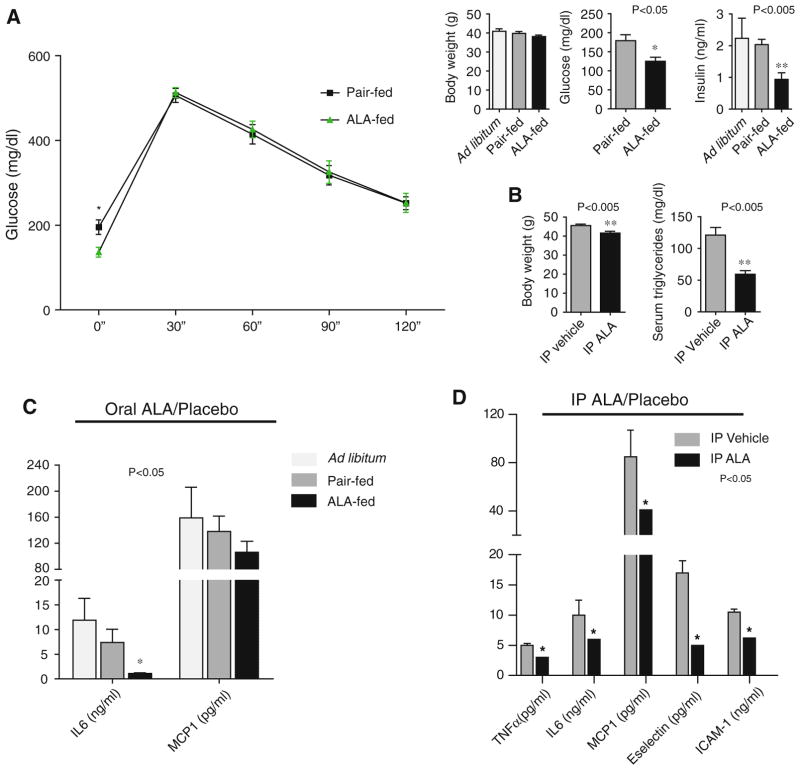
At the end of 8 weeks of HFD feeding, c-fmsYFP+ trans-genic mice were markedly insulin resistant demonstrating evidence of fasting hyperglycemia and hyperinsulinemia. Body weight increased approximately 16 g. Following this period mice were assigned to ad libitum treatment (αLA 2 mg/ml drinking water), or pair-fed groups. c-fmsYFP+ mice administered αLA-fed mice exhibited improved fasting glucose approaching a high normal level (125 ± 10.6 mg/dl) compared to the pair-fed group (179 ± 15.7 mg/dl) (Fig. 1a). Fasting insulin levels were also significantly lower in αLA-fed mice (0.96 ± 0.21 ng/ml) than the pair-fed group (2.03 ± 0.17 ng/ml) and the ad libitum group (2.23 ± 0.63 ng/ml). Food intake suppression was modest and decreased over time with non-significantly different body weights across groups after 8 weeks of feeding (Ad lib 40.9 ± 1.4 g; Pair-fed 39.8 ± 0.9 g; αLA-fed 38.2 ± 0.8 g). Cholesterol and triglyceride levels were unchanged with αLA feeding. αLA feeding resulted in a significant decrease in serum IL-6 levels (Ad lib 11.9 ± 4.3 ng/ml; Pair-fed 7.4 ± 2.6 ng/ml; αLA-fed 1.1 ± 0.2 ng/ml), an insignificant but measured decrease in MCP-1, and no change in circulating IL-12, TNFα, IFNγ, and IL-10 cytokines (Fig. 1c).
Fig. 1.
Effect of α-lipoic acid (αLA) on measures of glucose metabolism. a Plasma glucose was measured every 30 min after intra-peritoneal injection of 2 mg/g body weight dextrose after an overnight fast. Fasting glucose and insulin in the αLA-fed mice was significantly lower than the pair-fed control group; *P < 0.05; ** P <0.005 αLA-fed group compared to pair-fed (N = 5/group). However, glucose clearance post-bolus was not improved in the αLA group. b IP αLA resulted in significant weight loss, a more dramatic effect on glucose clearance (not shown), and decreased fasting serum triglycerides compared to IP vehicle; **P < 0.005. Serum cytokines in HFD fed mice receiving either αLA or placebo by drinking water (c) or via intraperitoneal injection (d). Oral αLA resulted in a significant decrease in circulating IL6 levels and a nonsignificant decrease in MCP1; there were no changes in serum IL-12, TNFα, IFNγ, and IL-10 cytokine values within the detection the range of the assay. IP αLA administration resulted in a significant decrease in circulating cytokines involved with vascular adhesion and inflammation
IP αLA Regimen
In contrast to the weight-neutral effects of oral αLA, an IP regimen did have significant weight loss effects. In this experiment as with the oral regimen, after 8 weeks of HFD feeding, mice were randomized to αLA and vehicle control groups. IP αLA resulted in a significant decrease in body weight of 3.9 ± 0.8 g (41.6 ± 0.93 g in IP αLA vs. 45.5 ± 0.73 g in vehicle injected controls). There was no change in plasma total cholesterol but circulating triglycerides were significantly lower in the IP αLA group (59.25 ± 5.7 mg/dl) compared to IP vehicle controls (121 ± 12 mg/dl). IP αLA resulted in significant decreases in serum IL-6, MCP-1, and TNFα. IP αLA also resulted in a significant decrease in serum E-selectin and ICAM-1, markers of monocyte vascular adhesion (Fig. 1d).
In light of its weight neutral effects we proceeded to investigate oral supplementation of αLA at ~160 mg/kg. IP-αLA induced weight loss would have rendered dissociation of weight-loss effects from weight-loss independent effects difficult. Thus continued experimentation focused on the effects of dietary αLA as the effects on food intake could be more easily addressed and because αLA is most commonly consumed as a dietary supplement.
Dietary αLA Attenuated Visceral Adipose Tissue Macrophage (VATM) Infiltration and In Vivo Macrophage Activation
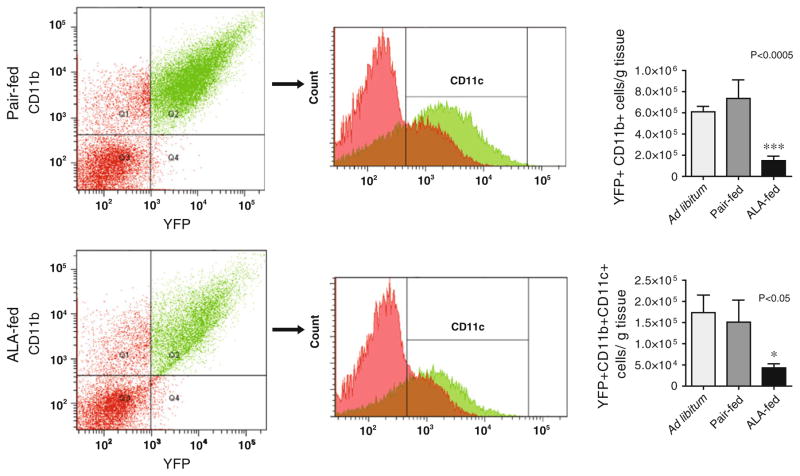
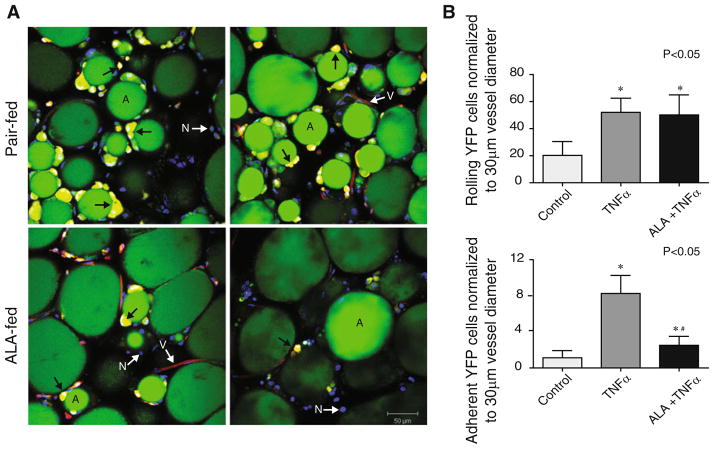
VATM content of the epididymal adipose of c-fmsYFP+ mice was quantified using yellow fluorescent protein expression (CD115) in combination with surface staining for CD11b and F4/80, markers for monocytes and mature macrophages, respectively. We found that dietary αLA treatment dramatically decreased the number of YFP+ CD11b+ macrophages per gram of epididymal fat from 6.1 × 105 ± 0.5 cells per gram in the ad libitum and 7.4 × 105 ± 1.7 in the pair fed group to 1.5 × 105 ± 0.4 cells per gram in αLA-fed mice (Fig. 2). This phenomenon was visualized histologically using confocal microscopy of unfixed epididymal adipose collected, stained, and visualized consecutively upon sacrifice. Representative images are provided in Fig. 3a. Assessment of confocal images in the pair-fed mice demonstrate a pattern of crown like structures (CLS) indicative of dead or dying adipocytes [43] and macrophage infiltration. The prevalence of CLS was dramatically decreased in the visceral adipose of the αLA-fed group compared to pair-fed and ad libitum (Fig. 3a). We then examined CD11c+ cells in the stromal vascular fraction of adipose tissue derived from YFP+ animals. αLA-feed significantly attenuated the number of CD11c+ inflammatory macrophages in the visceral adipose with the ad libitum group containing 1.7 × 105 ± 0.4 cells per gram, the pair-fed group 1.5 × 105 ± 0.4 cells per gram and the αLA-fed group only 0.4 × 105 ± 0.09 cells per gram. To further examine the effect of αLA on monocyte/endothelial interactions and monocyte activation pathways, we tested the effects of αLA on TNFα mediated monocyte adhesion using a model of acute inflammation and additionally tested the effects of αLA on monocyte activation in vitro.
Fig. 2.
Macrophage content analysis of the epididymal adipose by flow cytometry. The ad libitum and pair-fed groups had significantly more CD11b+ YFP+ cells per gram of adipose tissue than the αLA fed group, ***P < 0.005. Oral αLA significantly decreased the number of CD11c positive, inflammatory macrophages, per gram of adipose, *P <0.05 versus pair-fed
Fig. 3.
a Representative confocal microscopy images of live epidid-ymal adipose. YFP+ (yellow/indicated by arrow) cell infiltration and the prevalence of multiple “crown-like” structures surrounding adipocytes (green/indicated by the letter A) in the pair-fed and αLA-fed groups. Blood vessels are shown in red (indicated by white arrow V) due to endothelial staining with Griffonia simplicifolia isolectin GS-IB4 conjugated to AlexaFluor 488, most of the YFP expressing cells are outside of the vasculature. The adiposomes are shown in green (indicated by the letter A) due to BODIPY 558/568 staining and nuclei are blue (indicated by white arrow N) due to Hoechst 33342 staining. b Acute αLA IP pretreatment reduced YFP+ cell adhesion in cremasteric vessels after injection of TNFα; *P < 0.05 versus control, #P < 0.05 versus TNFα
αLA Pretreatment Prevented TNFα-Mediated Vascular Adhesion In Vivo
Intravital microscopy of the cremasteric vasculature showed that the number of free-flowing, rolling YFP+ cells was increased by TNFα (Fig. 3b). While αLA did not have an effect of rolling monocytes, αLA pretreatment significantly prevented TNFα-mediated YFP+ cell adherence to vessel walls suggesting that αLA may modulate adhesion molecules involved in firm monocyte adhesion but not those involved in rolling. To further examine the effect of αLA on mechanisms by which αLA may modulate excess monocyte infiltration into the adipose we examined the effects of αLA on MCP-1 and CCR-2 in response to a prototypical TLR4 agonist, LPS.
αLA Attenuates MCP-1 Expression on Macrophages via TLR4 Mechanisms
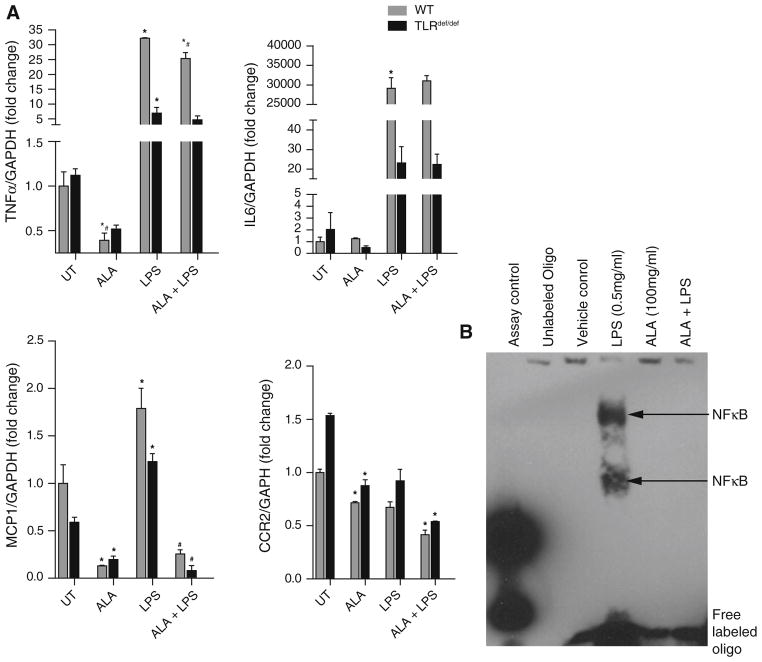
Bone marrow-derived macrophages (BMDM) from wild-type Balb/c mice and toll-like receptor 4 (TLR4) null mice were pretreated with αLA or vehicle control then activated with lipopolysaccharide (LPS), a TLR4 ligand [44]. αLA significantly decreased MCP-1 and TNFα gene expression in response to LPS stimulation but had no effect on IL-6 activation (Fig. 4a). αLA significantly decreased basal TNFα expression in both TLR4 WT and deficient macrophages. The degree of attenuation of LPS-mediated TNFα expression by αLA alone was small but comparable to that seen with TLR4def/def. The TNFα and IL-6 response to LPS in TLR4def/def macrophages was significantly blunted compared to the WT cells. MCP-1 induction by LPS was small compared to TNFα and IL6. LPS-mediated MCP-1 expression, however, was potently inhibited by αLA pretreatment. Interestingly, LPS activation had no effect on CCR2 expression, though αLA significantly down-regulated CCR-2 expression in the presence and absence of LPS; suggesting that non-TLR4 pathways are probably involved in CCR2 down-regulation in response to αLA. αLA increased Mgl1 gene expression, a marker of alternative macrophage activation, while LPS decreased expression (data not shown). These data suggest that αLA may function as an anti-inflammatory agent by preventing inflammatory gene expression. Since nuclear factor kappa B (NF-κB) plays a pivotal role in cellular inflammatory processes we decided to assay the effect of αLA on NF-κB-DNA binding in these TLR WT macrophages. An electrophoretic mobility shift assay showed LPS treatment dramatically increased the binding of NF-κB to the oligo-nucleotide probe. αLA (100 μg/ml) pretreatment of WT BMDM lead to the inhibition of LPS-mediated (0.5 μg/ml) NF-κB activation as measured by binding to probe DNA.
Fig. 4.
αLA pretreatment (100 μg/μl) attenuated expression of inflammatory and homing molecules in cultured bone marrow derived macrophages activated with LPS (0.5 μg/μl), *P < 0.05 compared to respective untreated (UT) control; #P < 0.05 compared to respective LPS treatment group. TNFα, MCP-1, and CCR2 gene expression is significantly down-regulated by αLA in cultured murine macrophages
Discussion
Visceral adipose inflammation is believed to play an etio-logic role in the development of insulin resistance (IR) in obesity and is typified by early, and often dramatic, increases in innate immune cells such as macrophages. With accumulation of neutral lipids in the adiposome, adipocytes hypertrophy and a subset undergo “necrosis-like” cell death resulting in the recruitment of macrophages [45, 46]. The relationship between alterations in innate and adaptive immune cell numbers, their effect on the adipose, and their contribution to the eventual development of obesity is largely attributed to the production and systemic introduction of inflammatory mediators. The pathophysiological processes that develop due to obesity have various putative etiological origins involving multiple tissues, including immune-modulated inflammation of the visceral adipose.
In this study we assessed the effects of αLA on the development of insulin resistance and adipose inflammation in a novel model of murine diet-induced obesity and IR. We demonstrate in pair-feeding experiments that oral αLA exerts beneficial weight loss-independent effects on insulin sensitivity, visceral adipose inflammation, vascular adhesion, and whole body inflammatory cytokine markers. Oral αLA reduced VATM content and activation in insulin resistant, obese mice. αLA also prevented LPS-mediated NF-κB activation and decreased the expression of inflammatory/migratory genes in un-stimulated and LPS-stimulated macrophages. These results suggest that αLA has a potent effect on macrophage activation status and thus may modulate the innate immune response in chronic inflammation in the visceral adipose tissue, a hallmark of type 2 diabetes and the metabolic syndrome.
Though oral αLA was less effective than IP αLA at lowering blood glucose and normalizing markers of insulin resistivity, at the dose used orally, it lacked weight loss effects and obviously prevented its confounding influence in the assessment of results. αLA decreases hypothalamic AMPK activity and causes profound weight loss in rodents by reducing food intake and enhancing energy expenditure [47]. Pair-fed experiments suggest a minimal effect of oral αLA at ~160 mg/kg on food intake and body weight over 8 weeks in mice. It should be noted that the first week of oral αLA did result in decreased food intake and some weight loss, however, over the subsequent weeks the effect subsided and weights normalized then increased to levels comparable to controls. The reduction in fasting glucose and insulin by oral αLA did not translate into improved glucose bolus clearance, suggesting that αLA did not abrogate the effects of obesity on glucose metabolism in the long term. However, αLA may selectively regulate fasting indices of glucose homeostasis which have been shown to be sensitive to anti-inflammatory measures [48]. Our data suggest that αLA has a moderate effect in already obese mice. The effect may be more dramatic in mice that begin oral αLA prior to a HFD or in mice that are not already overtly insulin resistant.
The marginal effects of post-prandial indices contrasted dramatically with the ability of αLA to reduce the number and activation status of macrophages in the epididymal adipose. Visceral adipose inflammation is a major area of study in obesity and IR, with visceral adipose inflammation being a putative pathophysiological phenomenon with the activation status of VATM being of particular interest. The phenotypic activation state of macrophages is a crucial determinant of functionality and the inflammatory state. Resident tissue macrophages in lean mice display a phenotype that is typical of cells committed to efferocytosis and scavenger functions and are referred to as M2 or “alternatively activated” macrophages expressing anti-inflammatory cytokines (Il10, Arginase1, Mgl1) [49] concomitantly with lower levels of M1 genes. High fat diet (HFD) and obesity in mice lead to a M1 or “classically activated” state which have been shown to express CD11c and secrete pro-inflammatory cytokines such as TNFα, IL-6, and IL-12 [4]. This phenotypic shift is now believed to play an important role in the genesis of IR [4]. The dramatic attenuation in VATM numbers and CD11c expression observed by αLA is consistent with an effect on activation status of adipose macrophages. Interestingly, the effects of αLA are similar to those reported in obese CCR2 null mice versus obese wild type (WT) mice [13]. Obese CCR2 null mice also showed a reduction in VATM with a modest decrease in whole body insulin resistance. Our data suggest that modulation of VATM activation and visceral infiltration by αLA in already obese mice decreases systemic markers of inflammation and improves markers of insulin sensitivity.
In separate experiments, we addressed the effects of αLA on the monocyte/macrophage activation state and extravasation by demonstrating that acute αLA administration reduced monocyte adhesion to the endothelium. Homing of macrophages to the visceral adipose may be modified by decreasing vascular adhesion, the first step in extravasation and homing to tissue. Our intravital microscopy data demonstrate a dramatic effect of αLA in reducing monocyte adhesion to venules and supports the hypothesis that αLA may reduce monocyte efflux from the vasculature to tissues due to inflammatory signals. Directly measuring monocyte/macrophage efflux to the epididymal adipose is a preferable method but was not technically possible at the time of these mouse experiments.
To further address the role of αLA on TLR4-mediated macrophage activation and signaling we examined the effects of αLA on bone marrow-derived macrophages from TLR4def/def and WT animals. Recent studies show that the Toll-like receptor 4 (TLR4) may play a central role in the link among insulin resistance, inflammation, and obesity. TLR4 deficiency prevented DIO-mediated insulin resistance and activation of IkB kinase (IKKb) and c-Jun NH2-terminal kinase (JNK), suggesting that TLR4 is a key modulator in the cross-talk between inflammatory and metabolic pathways [18–22]. Recently it was demonstrated that high fat meals can increase circulating LPS levels and may result from increased intestinal permeability to LPS [50, 51]. Additionally, DIO was shown to induce the expression and activation of TLR4 in the adipose of obese rats, while exercise reduced circulating LPS and TLR4 activation [21]. High levels of saturated NEFA, such as palmitate, when combined with hyperinsulinemia, have been shown to activate human monocytes via TLR4 agonism resulting in the production of pro-inflammatory cytokines [52]. Additionally, high glucose conditions may result in oxidative stress-mediated NF-κB activation and subsequent up-regulation of TNFα and MCP-1 [53, 54]. We demonstrated that αLA was an effective inhibitor of NF-κB activation in murine macrophages, a potent transcription factor regulating inflammatory genes including TNFα and IL6 in this cell. We show that αLA pretreatment is capable of preventing TLR4-mediated up-regulation of MCP-1. MCP-1 is the primary cytokine recruiting monocytes, memory T cells, and dendritic cells to sites of tissue injury and inflammation. It has been shown in endothelial cells that αLA prevented high-glucose induced MCP-1 expression via inhibition of reactive oxygen species-mediated NF-κB activation [54]. The cognate receptor for MCP-1, CCR2 is also down-regulated on monocytes by αLA (albeit via non-TLR4 pathways) suggesting that it may make monocytes less responsive to a MCP-1 chemotactic gradient. Interestingly, CCR2 expression was not induced by TLR4 activation, conversely LPS acted synergistically with αLA to further down-regulate CCR2 expression. αLA also potently decreased LPS-stimulated TNFα gene expression demonstrating that αLA can modulated macrophage activation post TLR4 ligation. These data suggest that αLA is a potent modulator of inflammation via TLR4, non-TLR4 and NF-κB pathways.
We acknowledge several important limitations of this study beginning with our focus on adipose-inflammation centric mechanisms. Our study did not investigate the effects of αLA on hepatic glucose generation or adipocyte function. Prior studies with αLA have clearly demonstrated an effect of αLA in improving serum lactate and pyruvate concentrations and improving glucose levels in lean and obese patients with type 2 diabetes [55]. Our experimental design did not clearly examine the effect of αLA on adipocytes themselves. We cannot say whether αLA decreased visceral adipocyte apoptosis and subsequent VATM infiltration or whether αLA prevented monocyte/macrophage activation and infiltration leading to a lower incidence of adipocyte apoptosis. It is also important to note that αLA may have different mechanisms of action based on duration of treatment, route of administration, and maximal plasma concentration thus comparing data between treatment protocols and in vivo and in vitro results is tenuous and must be taken within context. For example, while αLA potently down-regulated TNF, IL6, and MCP-1 gene expression in vitro, the in vivo effects were not nearly as dramatic in the oral αLA group with only IL-6 being significantly down-regulated and more comparable in the IP-αLA mice. It should also be noted that αLA can activate nuclear factor erythroid2-related factor (Nrf2), the principal transcriptional regulator of antioxidant response element (ARE)-mediated gene expression [56] and through these pathways could exert powerful effects on adipose inflammation [57, 58]. Though prior studies have demonstrated important effects on systemic IR and inflammation, a thorough examination of the long-term weight loss-independent effects of αLA on insulin sensitivity and macrophage activation pathways in already obese individuals was needed.
Acknowledgments
The primary author was supported by Award Number F32DK083903 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. The research was also supported in part by Grants to Dr Rajagopalan: R01ES015146, R21DK088522, and R21HL106487.
Abbreviations
- αLA
α-Lipoic acid
- c-fms
Proto-oncogene c-fms
- CCR-2
C-C chemokine receptor type 2
- FBS
Fetal bovine serum
- HFD
High fat diet
- JNK
c-Jun NH2-terminal kinase
- IFNγ
Interferon gamma
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- IP
Intraperitoneal
- IR
Insulin resistance
- LPS
Lipopolysaccharide
- MCP-1/CCL-2
Monocyte chemoattractant protein 1
- PBS
Phosphate buffered saline
- NFκB
Nuclear factor kappa B
- TBE
Tris-buffered Ethylenediaminetetraacetic acid
- TLR4
Toll-like receptor 4
- TNFα
Tumor necrosis factor alpha
- VATM
Visceral adipose tissue macrophages
- YFP
Yellow fluorescent protein
- SVF
Stromal vascular fraction
References
- 1.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3–L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, Wang F, Hull RL, Boyko EJ, Retzlaff BM, Walden CE, Knopp RH, Kahn SE. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997;190:227–235. doi: 10.1016/s0378-1119(96)00730-5. [DOI] [PubMed] [Google Scholar]
- 8.Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, Rosato FE, Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 10.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 15.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64:163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- 16.Sethi JK, Vidal-Puig AJ. Thematic review series: adipo-cyte biology adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 18.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 19.Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, Schaffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, Guadagnini D, Carvalheira JB, Saad MJ. Physical exercise reduces circulating lipopolysaccharide and Toll-like receptor 4 activation and improves insulin signaling in tissues of diet-induced obesity rats. Diabetes. 2011;60:784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 23.Merry BJ, Kirk AJ, Goyns MH. Dietary lipoic acid supplementation can mimic or block the effect of dietary restriction on life span. Mech Ageing Dev. 2008;129:341–348. doi: 10.1016/j.mad.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Butler JA, Hagen TM, Moreau R. Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys. 2009;485:63–71. doi: 10.1016/j.abb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Lee HA, Hughes DA. Alpha-lipoic acid modulates NF-kappaB activity in human monocytic cells by direct interaction with DNA. Exp Gerontol. 2002;37:401–410. doi: 10.1016/s0531-5565(01)00207-8. [DOI] [PubMed] [Google Scholar]
- 27.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 28.Yi X, Maeda N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- 29.Yadav V, Marracci GH, Munar MY, Cherala G, Stuber LE, Alvarez L, Shinto L, Koop DR, Bourdette DN. Pharmacokinetic study of lipoic acid in multiple sclerosis: comparing mice and human pharmacokinetic parameters. Mult Scler. 2010;16:387–397. doi: 10.1177/1352458509359722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- 31.Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:625–628. [PubMed] [Google Scholar]
- 32.Breithaupt-Grogler K, Niebch G, Schneider E, Erb K, Hermann R, Blume HH, Schug BS, Belz GG. Dose-proportionality of oral thioctic acid–coincidence of assessments via pooled plasma and individual data. Eur J Pharm Sci. 1999;8:57–65. doi: 10.1016/s0928-0987(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 33.Deiuliis JA, Shin J, Bae D, Azain MJ, Barb R, Lee K. Developmental, hormonal, and nutritional regulation of porcine adipose triglyceride lipase (ATGL) Lipids. 2008;43:215–225. doi: 10.1007/s11745-007-3146-1. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 35.Lim LH, Bochner BS, Wagner EM. Leukocyte recruitment in the airways: an intravital microscopic study of rat tracheal microcirculation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L959–L967. doi: 10.1152/ajplung.00261.2001. [DOI] [PubMed] [Google Scholar]
- 36.Harrison EH, McCormick DB. The metabolism of dl-(1,6–14C)lipoic acid in the rat. Arch Biochem Biophys. 1974;160:514–522. doi: 10.1016/0003-9861(74)90428-7. [DOI] [PubMed] [Google Scholar]
- 37.Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CK, Lee EY, Kim YG, Mun SH, Moon HB, Yoo B. Alpha-lipoic acid inhibits TNF-alpha induced NF-kappa B activation through blocking of MEKK1-MKK4-IKK signaling cascades. Int Immunopharmacol. 2008;8:362–370. doi: 10.1016/j.intimp.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Sung MJ, Kim W, Ahn SY, Cho CH, Koh GY, Moon SO, Kim DH, Lee S, Kang KP, Jang KY, Park SK. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ Res. 2005;97:880–890. doi: 10.1161/01.RES.0000186522.89544.4D. [DOI] [PubMed] [Google Scholar]
- 41.Cheshire JL, Baldwin AS., Jr Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaB beta degradation. Mol Cell Biol. 1997;17:6746–6754. doi: 10.1128/mcb.17.11.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Muroi M, Suzuki T. Role of protein kinase A in LPS-induced activation of NF-kappa B proteins of a mouse macrophage-like cell line, J774. Cell Signal. 1993;5:289–298. doi: 10.1016/0898-6568(93)90019-i. [DOI] [PubMed] [Google Scholar]
- 45.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, Defuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 46.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 48.Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, Berry EA. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27:2166–2171. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- 49.Charo IF. Macrophage polarization and insulin resistance: PPARgamma in control. Cell Metab. 2007;6:96–98. doi: 10.1016/j.cmet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 52.Bunn RC, Cockrell GE, Ou Y, Thrailkill KM, Lumpkin CK, Jr, Fowlkes JL. Palmitate and insulin synergistically induce IL-6 expression in human monocytes. Cardiovasc Diabetol. 2010;9:73. doi: 10.1186/1475-2840-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quan Y, Jiang CT, Xue B, Zhu SG, Wang X. High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol Sin. 2011;32:188–193. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang WS, Seo JW, Han NJ, Choi J, Lee KU, Ahn H, Lee SK, Park SK. High glucose-induced NF-kappaB activation occurs via tyrosine phosphorylation of IkappaBalpha in human glomerular endothelial cells: involvement of Syk tyrosine kinase. Am J Physiol Renal Physiol. 2008;294:F1065–F1075. doi: 10.1152/ajprenal.00381.2007. [DOI] [PubMed] [Google Scholar]
- 55.Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–287. doi: 10.2337/diacare.22.2.280. [DOI] [PubMed] [Google Scholar]
- 56.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 58.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]