Abstract
Huntington’s disease (HD) is a dominantly inherited neurodegenerative disorder characterized by a constellation of motor, cognitive, and psychiatric features. Striatal medium spiny neurons, one of the most affected populations, are dependent on brain-derived neurotrophic factor (BDNF) anterogradely transported from the cortex for proper function and survival. Recent studies suggest both receptors for BDNF, TrkB and p75 neurotrophin receptor (p75), are improperly regulated in the striata of HD patients and mouse models of HD. While BDNF–TrkB signaling almost exclusively promotes survival and metabolic function, p75 signaling is able to induce survival or apoptosis depending on the available ligand and associated co-receptor. We investigated the role of p75 in the Q175 knock-in mouse model of HD by examining the levels and activation of downstream signaling molecules, and subsequently examining Hdh+/Q175;p75−/− mice to determine if p75 represents a promising therapeutic target. In Hdh+/Q175;p75+/+ mice, we observed enhanced survival signaling as evidenced by an increase in phosphorylation and activation of Akt and the p65 subunit of NFκB in the striatum at 5 months of age and an increase in XIAP expression compared to Hdh+/+;p75+/+ mice; this increase was lost in Hdh+/Q175;p75−/− mice. Hdh+/Q175;p75−/− mice also showed a decrease in Bcl-XL expression by immunoblotting compared to Hdh+/Q175;p75+/+ and Hdh+/+;p75+/+ littermates. Consistent with diminished survival signaling, DARPP-32 expression decreased both by immunoblotting and by immunohistochemistry in Hdh+/Q175;p75−/− mice compared to Hdh+/+;p75+/+, Hdh+/Q175;p75+/+, and Hdh+/+;p75−/− littermates. Additionally, striatal volume declined to a greater extent in Hdh+/Q175;p75−/− when compared to Hdh+/Q175;p75+/+ littermates at 12 months, indicating a more aggressive onset of degeneration. These data suggest that p75 signaling plays an early role in augmenting pro-survival signaling in the striatum and that disruption of p75 signaling at a pre-symptomatic age may exacerbate pathologic changes in Hdh+/Q175 mice.
Keywords: Huntington’s, BDNF, p75, Q175
INTRODUCTION
Huntington’s disease (HD) is an adult-onset, progressive, and invariably fatal neurodegenerative disease characterized by a constellation of motor, cognitive, and psychiatric features. HD is dominantly inherited and caused by a CAG repeat expansion in the huntingtin gene (Group, 1993). There are only limited symptomatic treatment options and no disease-modifying therapies. Striatal medium spiny neurons (MSNs) are one of the earliest and most affected populations of neurons (Walker, 2007). MSNs are dependent on cortically derived neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), anterogradely transported to the striatum for normal development, function, and maintenance (Mizuno et al., 1994; Baquet et al., 2004; Baydyuk et al., 2013).
Huntingtin protein (HTT) regulates cortical BDNF transcription, with mutated HTT (mHTT) being impaired in its ability to regulate BDNF (Zuccato et al., 2001). The BDNF gene encodes as many as eight promoters, with promoters I, II, and IV being most highly transcriptionally active and well-studied (West et al., 2014). BDNF transcription from promoter II depends largely on the activity of the repressor element 1/neuron-restrictive silencer element (RE1/NRSE) present in this promoter of the BDNF gene. This sequence recruits RE1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF), which actively silences transcription (Timmusk et al., 1999). HTT sequesters REST in the cytoplasm, preventing it from binding to the RE1/NRSE, and subsequently leading to higher levels of BDNF transcription from promoter II. mHTT is less able to sequester REST in the cytoplasm, leading to reduced transcription of BDNF from promoter II (Zuccato et al., 2003). Consistent with these studies, in several mouse models of HD and in post-mortem HD brains, lower levels of cortical BDNF expression were reported (Zuccato et al., 2008). In addition, HTT may be involved in the anterograde trafficking of BDNF from the cortex to the striatum (Gauthier et al., 2004), which is also consistent with the lower levels of BDNF observed in the striatum of several animal models of HD and in post-mortem HD brains (Ferrer et al., 2000; Her and Goldstein, 2008). BDNF protein levels can be difficult to measure due to the lack of antibodies of sufficient specificity and sensitivity. To overcome this difficulty, Ma et al. crossed the Q175 mouse line to a line expressing BDNF with an HA epitope tag. Using highly sensitive and specific HA antibodies, a decrease in BDNF protein levels in the striatum of Q175 mice at 6 months of age was observed (Ma et al., 2015). A decrease in BDNF levels and signaling in the striatum is proposed to contribute to the pathogenesis of HD (Zuccato and Cattaneo, 2007).
There are two known BDNF receptors, Tropomyosin receptor kinase B (TrkB) and p75 neurotrophin receptor (p75). Both TrkB and p75 are expressed in the striatum. Signaling downstream of TrkB activation almost exclusively promotes survival and metabolic function, and disruption of TrkB signaling is likely detrimental to MSN functional integrity (Gupta et al., 2013; Baydyuk and Xu, 2014). While TrkB levels and trafficking were studied in multiple animal models of HD and in post-mortem HD brains, p75 has been examined only recently. p75 mRNA levels were reported to be elevated in post-mortem HD striata (Zuccato et al., 2008), and striatal p75 protein levels were reported as elevated in two mouse models of HD (Brito et al., 2013). Unlike TrkB, signaling downstream of p75 is highly context-dependent and may support neuronal survival or contribute to neuronal dysfunction and death (Kraemer et al., 2014). p75 can be activated by all four neurotrophins, pro-forms of neurotrophins, and other ligands (Hempstead, 2002; Nykjaer et al., 2005).
As a member of the TNF family of death receptors, p75 is capable of inducing apoptosis when complexed with pro-apoptotic co-receptors or adaptor proteins, such as sortilin and NRIF (Nykjaer et al., 2004). p75 can also interact with Trk receptors, however, and these interactions are crucial for maximal survival signaling in several cell types (Ceni et al., 2010; Kommaddi et al., 2011; Matusica et al., 2013; Negrini et al., 2013). p75 stabilizes activated Trk receptors on the membrane, extending survival signaling (Makkerh et al., 2005). p75 is also capable of promoting survival signaling independently of Trk receptors through activation of nuclear factor kappa B (NFκB) (Carter et al., 1996). Based on the ability of p75 to complement Trk signaling and to promote survival signaling independently of Trk activation, we hypothesized that p75 signaling augments survival signaling in the striata of HD mouse models.
To explore the role of p75 in HD, we crossed a germline p75 knock-out mouse into the Q175 mouse model of HD. The Q175 mouse is a knock-in model containing approximately 200 glutamine repeats in the closely related murine homolog of htt (hdh). These mice develop behavioral phenotypes and pathological changes beginning at 6–12 months of age (Smith et al., 2014). In order to investigate signaling changes and the function of p75 prior to the onset of visible pathology, we examined mice at 5 months of age to determine whether p75 had a compensatory protective function for MSNs and again at 12 months of age to determine the pathological consequences of the loss of p75. We examined the effect of p75 deletion on pro-survival signaling in the following genotypes Hdh+/+;p75+/+, Hdh+/Q175;p75+/+, Hdh+/Q175;p75−/−, and Hdh+/+;p75−/−. Our data suggested that p75 plays an early role in augmenting survival signaling in the striatum and that disruption of p75 signaling at a pre-symptomatic age may exacerbate pathologic changes in Hdh+/Q175 mice.
EXPERIMENTAL PROCEDURES
Mice
Heterozygous Q175 (Hdh+/Q175) knock-in mice (C57Bl/6 background) and wild-type (Hdh+/+) littermate controls were obtained from Jackson Laboratories (Stock #027410, Bar Harbor, ME, USA). CAG repeat numbers were in the range of 176–201 (reported by Jackson Laboratories). For initial experiments, mice arrived in one cohort at 5 months of age, with an equal number of males and females used for biochemical analyses. A separate cohort of Hdh+/Q175 mice were used for breeding to produce all additional animals. p75 deletion was achieved by crossing Hdh+/Q175 mice with p75−/− mice in a mixed background (C57Bl/6 and 129/Sv) (Bogenmann et al., 2011) for two successive generations to produce the necessary genotypes. All mice examined were littermates. For the initial Hdh+/Q175 and Hdh+/+ experiments (Figs. 1 and 2), eight mice of each genotype were examined. For the Hdh+/Q175;p75−/− experiments, 10–12 mice of each genotype were used for western blotting, five to seven mice of each genotype were used for 7-month IHC, and four mice of each genotype were used for 12-month volume measurements. In all figures, the number of mice analyzed is indicated within each graph. Mice were housed with access to food and water ad libitum under a 12:12-h light/dark cycle. All animal procedures were performed in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan.
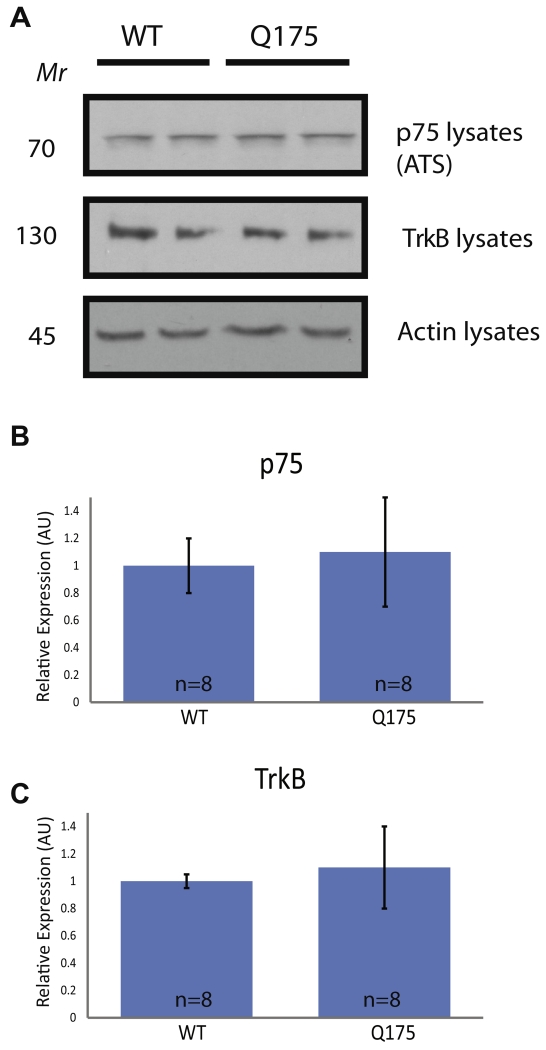
Fig. 1.
Analysis of TrkB and p75 in the striatum of Hdh+/Q175 mice at 5 months. A half of the striata from 5-month-old mice were used for whole-cell lysates (WCLs). (A) Western blots for p75 and TrkB from the IPs (top two panels) and from actin are shown. (B, C) Quantifications of (A) are graphed as mean ± SEM. Actin levels were used as a loading control, and protein levels are normalized to Hdh+/+ animals processed on the same blots. No significant differences in p75 or TrkB levels between Hdh+/+ and Hdh+/Q175 mice were observed (t-test; p > 0.05).
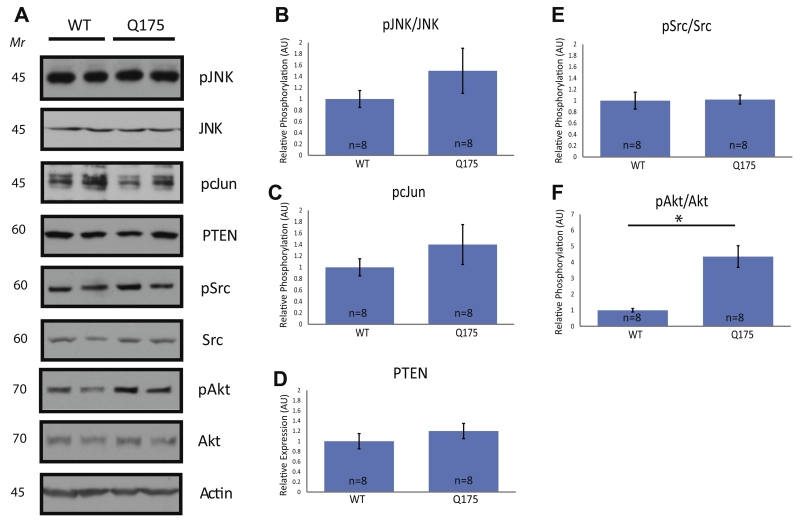
Fig. 2.
Biochemical analysis of apoptotic and survival signaling in the striatum of Hdh+/Q175 mice at 5 months. WCLs of the striata from 5-month-old mice of the stated genotypes were analyzed by immunoblotting. (A) Western blots were run on WCLs using the stated antibodies. (B–F) Quantifications of (A) were performed as in Fig. 1. No significant differences were seen between Hdh+/+ and Hdh+/Q175 mice in JNK, cJun, or Src activation, or in PTEN levels. A significant increase in Akt activation was observed. (t-test; p < 0.05).
Striata dissections
Mice were anesthetized by isoflurane, euthanized by decapitation, and striata were rapidly isolated using a Zivic instruments mouse brain slicer. Striata were immersed in 300 μL of immunoprecipitation buffer (10% glycerol, protease inhibitors, sodium vanadate in Tris buffered saline (TBS), pH 6.8) with each hemisphere of the striatum treated individually. Tissues were homogenized rapidly (Tissuelyser II, Qiagen) and detergent-extracted (Addition of nonidet p-40 to 1%) by incubation for 30 min, with rotation, at 4 °C. To produce whole-cell lysates (WCLs), one striatal hemisphere from each animal was mixed with 300 μL 2× sodium dodecyl sulfate (SDS) sample buffer (20% glycerol, 4% SDS, 1% β-mercaptoethanol (β-ME), and bromophenol blue in TBS, pH 6.8), boiled for 6 min, resolved by 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes.
Immunoblotting and antibodies
Membranes were incubated with blocking solution (3% bovine serum albumin (BSA) or 4% milk) in TBS-T (Tris buffered saline, pH 7.4, with 0.1% Tween-20) and incubated overnight with the appropriate primary antibodies in blocking solution. The blots were washed, incubated with appropriate HRP-linked secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA), washed again, and visualized using an enhanced chemiluminescent substrate (Advansta and ThermoScientific, Waltham, MA, USA). The following primary antibodies were used: anti-p75 (1:2000 Promega, Madison, WI, USA, catalog G323A), anti-p75 (1:2000 Millipore, Billerica, MA, USA, catalog 07-476), anti-p75 (1:2000 Advanced Targeting Systems, San Diego, CA, USA, AB-N01AP), anti-TrkB (1:1000 BD biosciences, San Jose, CA, USA, catalog 610101), anti-actin (1:2000 Iowa Hybridoma Bank, Iowa City, Iowa, USA, catalog JLA 20-9), anti-Bcl-XL (1:4000 Abcam, Cambridge, United Kingdom, catalog ab 178844), anti-XIAP (1:1000 Abcam, catalog ab28151), anti-DARPP-32 (1:2000 Abcam, catalog ab40801), anti-phospho-cJun (1:1000 catalog 9261), anti-phospho-JNK (1:1000 catalog 9251), anti-phospho-Akt (1:1000 Y243/241, catalog 4058), anti-phospho-Src (1:1000 Y416, catalog 2101), and all non-phosphorylated versions of these proteins (1:1000 Cell Signaling Technologies, Danvers, MA, USA). All immunoblots were quantified using ImageJ software (NIH) and graphed as mean ± SEM.
Perfusion and immunohistochemistry (IHC)
Seven- to eight- and 12–13-month-old mice were terminally anesthetized with isofluorane and perfused transcardially with heparinized saline (0.1%) followed by 4% paraformaldehyde (PFA) in phosphate buffer. Brains were removed and post-fixed in 4% PFA for 1 h before cryoprotection in 30% sucrose. Brains were frozen in isopentane chilled over dry ice and stored at −80 °C until use. Coronal sections were cut at 60 μm on a cryostat (CM1950, Leica), and stored in cryoprotection solution at −20 °C until use. For IHC, sections were rinsed in phosphate-buffered saline (PBS) and endogenous peroxidases were quenched in 1% hydrogen peroxide for 10 min. After rinses, sections were incubated in 2.5% normal donkey serum (Jackson labs) and MOM (Vector, for NeuN-staining) in 0.3% Triton X-100 in PBS. Sections were then incubated for 15–18 h at RT with either anti-NeuN (1:10,000; Millipore) or anti-DARPP-32 (1:300,000; Abcam). After washing, sections were incubated with a biotinylated secondary antibody (anti-mouse or rabbit, 1:500; Jackson ImmunoResearch). Staining of tissue-bound antibodies was visualized using a standard peroxidase-based method (Vectastain Elite, Vector Labs) with the 3,3-diaminobenzadine chromogen (DAB, Sigma, St. Louis, MO, USA).
Image analysis and cell counting
Immunohistochemical images were collected using a Zeiss Axiovert 200M inverted microscope at 10×. All conditions were imaged using identical microscope settings and entire striata were tiled together for analysis. From a randomized starting point, every sixth slice (60-μm thickness) containing the striata was imaged. An average of six slices per animal were imaged, and these results were averaged to provide one value per animal. For volume measurements, contours were drawn around the striatum on every sixth slice, and volume was estimated using the Cavalieri volume estimation method. An experimenter blinded to genotype made all measurements.
Statistics
All results are expressed as mean ± SEM. Experimental data from Q175;p75 mice were analyzed by one-way analyses of variance (ANOVAs) followed by post hoc Tukey–Kramer minimum significant differences comparison. Experimental data from Q175 mice were analyzed by Student t-tests. A value of p < 0.05 was accepted as denoting statistically significant differences. To justify parametric testing, all data sets were tested for normality using the Shapiro–Wilk test for normality. Sampling was random, and all samples are independent. Homogeneity of variance was determined using the Brown–Forsythe test.
RESULTS
p75 and TrkB levels unchanged at 5 months in the striatum of Q175 mice
Although the levels of p75 were reported to be increased in some animal models of HD, levels of p75 have not been examined in Hdh+/Q175 mice before 12 months of age. We examined p75 protein levels in striata of Hdh+/+ and Hdh+/Q175 mice from WCLs at 5 months of age (Fig. 1). This age was chosen because it is a pre-symptomatic time point and may provide insight into pathophysiological changes occurring before onset of overt pathology. p75 levels from WCLs were not altered (Fig. 1A, C). TrkB levels were examined previously in these mice at 6, 12, and 16 months, showing an increase in TrkB in the striatum at 12 months and no change in TrkB levels at 6 or 16 months in the striatum (Smith et al., 2014). In agreement with this finding, no significant difference in TrkB protein levels was observed in the striata of Hdh+/+ and Hdh+/Q175 mice at 5 months of age (Fig. 1A, C). These levels, however, only reflect the total amount of TrkB protein in striata and do not represent activation or surface levels. Taken together, in the striata of 5-month-old Hdh+/Q175 mice, there was no significant change in striatal levels of either p75 or TrkB when compared to Hdh+/+ mice.
Apoptotic signaling pathways were not altered in the striatum of Hdh+/Q175 mice at 5 months
In some cellular contexts, signaling through p75 leads to activation of apoptotic signaling pathways, caspase activation, and programed cell death (Kraemer et al., 2014). To determine if apoptotic signaling was enhanced at 5 months in Hdh+/Q175 mice as compared to Hdh+/+ mice, we examined the level of activation of c-Jun N-terminal kinase (JNK) and cJun, as well as expression levels of phosphatase and tensin homolog (PTEN). Activation of JNK leads to phosphorylation and activation of cJun, which then leads to transcription of pro-apoptotic genes (Putcha et al., 2001; Freeman et al., 2004). Activation of JNK also leads to activation of pro-death members of the Bcl-2 family, such as Bim and Bax (Bogoyevitch and Kobe, 2006). PTEN inhibits the PI3K/Akt signaling pathway (Datta et al., 1999). PTEN levels and JNK and cJun phosphorylation are increased by pro-apoptotic p75 activation (Kraemer et al., 2014). WCLs of Hdh+/+ and Hdh+/Q175 mice were examined by p-JNK, JNK, and p-cJun immunoblotting. Phosphorylation of cJun leads rapidly to its own transcriptional upregulation and therefore, normalizing to total cJun levels can lead to an underrepresentation of the extent of cJun activation (Eilers et al., 1998). The 46-kDa isoform of JNK was analyzed as it was significantly more abundant in our samples than the 54-kDa isoform. When comparing Hdh+/Q175 mice to Hdh+/+ littermates, there were no differences in striatal JNK or cJun phosphorylation (Fig. 2A, B, C). Likewise, PTEN immunoblotting did not reveal any significant differences between Hdh+/Q175 mice and Hdh+/+ mice (Fig. 2A, D). Given that the activation of JNK and cJun and the levels of PTEN were unchanged, there does not appear to be an increase in p75-related apoptotic signaling in the striatum of Hdh+/Q175 mice at 5 months of age.
Akt activation was increased in the striatum of Hdh+/Q175 mice at 5 months
While p75 signaling can lead to apoptosis, it can also lead to survival when activated independently or when paired with the correct co-receptors and downstream effectors. To examine whether survival pathways were affected in Hdh+/Q175 striata at 5 months of age, we examined phosphorylation of Src on Tyr416 and Akt on Ser473. Src can be phosphorylated on Tyr416 following TrkB activation and this phosphorylation event induces Src catalytic activity, enhancing cell survival (Huang and McNamara, 2010). By immunoblotting, there was no change in Src activation in the striata of Hdh+/Q175 mice as compared to Hdh+/+ mice (Fig. 2A, E). Akt activation, which can be monitored via Akt phosphorylation at Ser473, promotes survival by inactivating Bad and Caspase 9, among other pro-apoptotic proteins (Datta et al., 1999). We examined Akt phosphorylation because it can be activated by TrkB and p75 independently (Datta et al., 1999; Kraemer et al., 2014). Phosphorylation of Akt at Ser473 was significantly increased in the striata of Hdh+/Q175 mice when compared to Hdh+/+ littermates (Fig. 2A, F). This increase in the activation of Akt in the striata of Hdh+/Q175 mice at 5 months of age suggests augmented survival signaling.
Analysis of p75 and TrkB in Hdh+/Q175;p75 mice
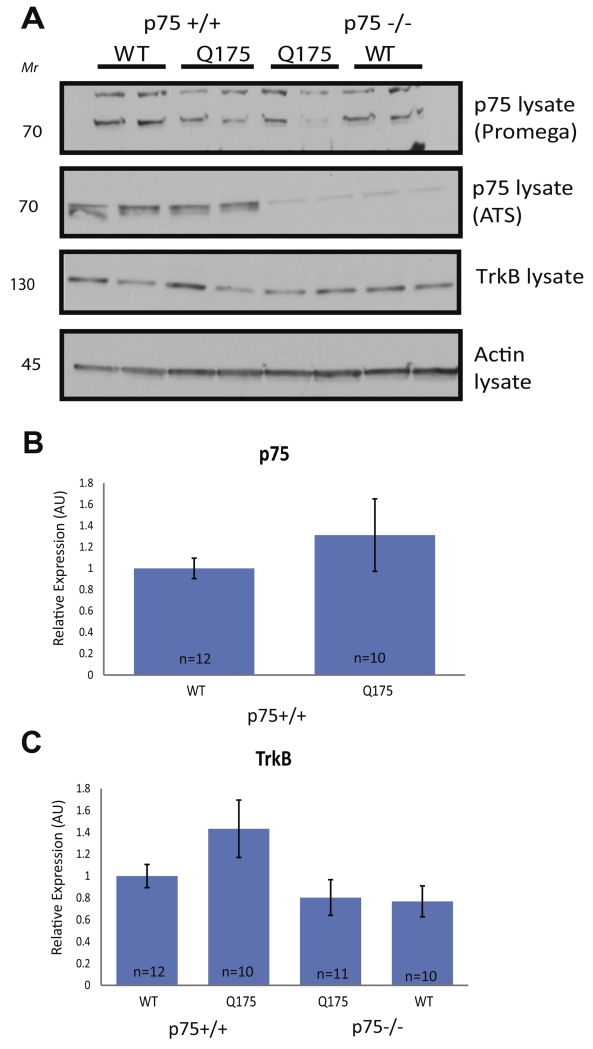
After surveying survival and apoptotic signaling pathways in Hdh+/Q175 mice and Hdh+/+ littermates, we evaluated the functional role of p75 in the observed pro-survival signaling. To address this question, Hdh+/Q175 mice were crossed into a germ-line knock-out of p75 (Bogenmann et al., 2011), and the following genotypes were examined at 5 months of age: Hdh+/+;p75+/+, Hdh+/Q175;p75+/+, Hdh+/Q175;p75−/−, and Hdh+/+;p75−/−. In this mixed background, we again examined p75 levels by direct immunoblotting (WCL). In attempting to probe the WCLs for p75 using a commonly reported p75 antibody (Promega), our results yielded a non-specific band at the same size as p75 that was present equally in p75+/+ and p75−/− extracts (Fig. 3A panel 1). To combat this issue, we used an alternative antibody (Advanced Targeting Systems, ATS) to measure levels of p75 in WCLs (Fig. 3A panel 2). At 5 months, no significant change in levels of p75 in the striatum of Hdh+/Q175;p75+/+ mice when compared to Hdh+/+;p75+/+/+ littermates was detected (Fig. 3A, B). There was no p75 protein detected in WCLs from Hdh+/+;p75−/− or Hdh+/Q175;p75−/− mice as expected (Fig. 3A panel 2). The levels of TrkB were also examined in these four genotypes by immunoblotting WCLs for TrkB. As seen in Fig. 1, there was no change in the levels of TrkB in Hdh+/Q175;p75+/+ mice compared to Hdh+/+;p75+/+mice. While there appeared to be a slight decrease in TrkB in both Hdh+/Q175;p75−/− and Hdh+/+;p75−/− mice, this difference was not statistically significant (Fig. 3A, C).
Fig. 3.
Examination of p75 and TrkB association and expression in the striatum of Hdh+/Q175;p75−/− mice at 5 months. A half of the striata from 5-month-old mice of the stated genotypes were used to produce WCLs. (A) Western blots for WCLs using the stated antibodies. The WCLs were tested for p75 using two separate antibodies, as listed. (B, C) Quantifications of (A). Actin levels were used as a loading control, and protein levels were normalized to WT animals processed on the same gel. Graphs are mean ± SEM. No significant change in p75 levels was observed in Q175 mice compared to WT littermates. A significant decrease was observed in WCL TrkB levels in both Hdh+/Q175;p75−/− and Hdh+/+;p75−/− compared to WT littermates. (ANOVA; p < 0.05).
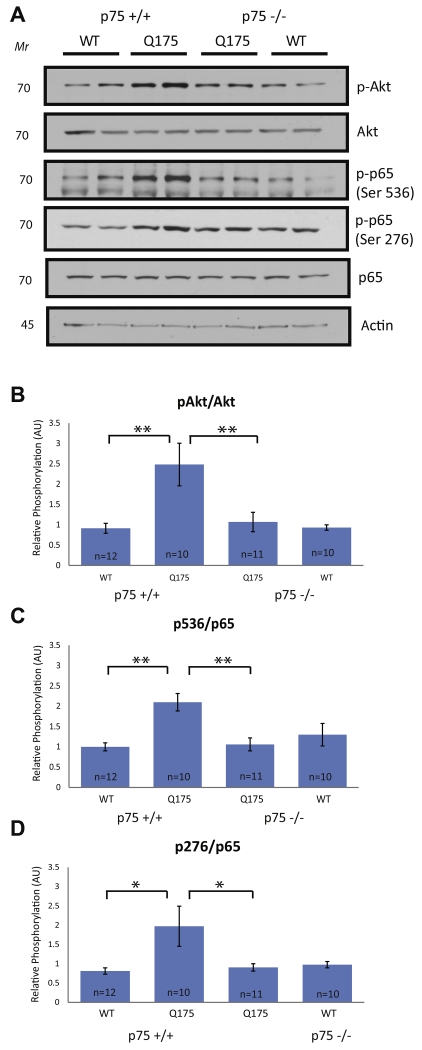
Increased survival signaling in Hdh+/Q175 mice is abolished by p75 deletion
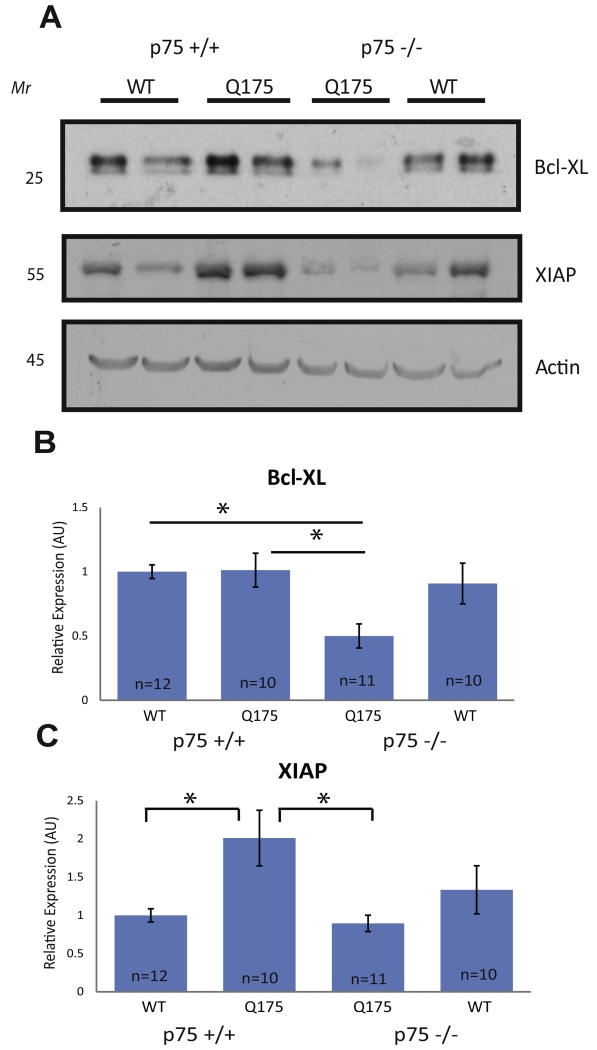
To determine mechanistically whether p75 was important for the increase in survival signaling observed in Hdh+/Q175 mice at 5 months of age, Akt activation was examined as in Fig. 2. Similar to the increase seen in Hdh+/Q175 mice, Akt activation was also increased in Hdh+/Q175;p75+/+ mice compared to Hdh+/+;p75+/+ control littermates (Fig. 4B). This increase was completely abolished in Hdh+/Q175;p75−/− mice (Fig. 4A, B). Because Akt activation was increased in Hdh+/Q175 mice (Fig. 2A, F), we decided to also examine activation of NFκB. NFκB activation is capable of promoting survival downstream of p75 activation in multiple cell types (Mattson et al., 1997; Middleton et al., 2000; Vicario et al., 2015), but does so independently of TrkB activity (Carter et al., 1996). The p65 subunit of NFκB can be phosphorylated on several different serine residues, all leading to slightly different transcriptional outcomes. We examined phosphorylation of p65 at Ser276 and at Ser536 because these two serines are important for more potent and longer NFκB-dependent transcriptional activation (Neumann and Naumann, 2007). p65 subunit phosphorylation at both residues was increased in Hdh+/Q175;p75+/+ mice compared to Hdh+/+;p75+/+ mice. This increase was completely abolished in Hdh+/Q175;p75−/− mice (Fig. 4A, C, D). B-cell lymphomaextra large (Bcl-XL) and X-linked inhibitor of apoptosis (XIAP) are inhibitors of apoptosis known to be transcriptionally up-regulated by activation of NFκB (Stehlik et al., 1998; Chao et al., 2011). Interestingly, levels of both Bcl-XL and XIAP were significantly lower in Hdh+/Q175;p75−/− mice when compared to Hdh+/Q175;p75+/+ mice (Fig. 5). We also examined the expression of Bim, a pro-apoptotic BH3-only Bcl2 family member that is induced during programed cell death (Putcha et al., 2001). We did not see any alteration in the expression of Bim between Hdh+/+;p75+/+, Hdh+/Q175;p75+/+, Hdh+/Q175;p75−/−, or Hdh+/+;p75−/− mice (data not shown). Taken together, these data suggest that at pre-symptomatic ages in Hdh+/Q175 mice p75 upregulates pro-survival signaling pathways, and that loss of this p75-mediated augmentation leads to decreased survival signaling.
Fig. 4.
Activation of Akt and NFkB is increased in the striatum of Hdh+/Q175;p75+/+ mice and not Hdh+/Q175;p75−/− mice at 5 months. WCLs from 5-month-old mice of the stated genotypes were examined by immunoblotting. (A) Western blots were run on WCLs using the stated antibodies. (B–D) Immunoblots were normalized and quantified as in Fig. 1. A significant increase in phosphorylation of Akt at Ser473 and NFkB at Ser276 and Ser536 was observed in Hdh+/Q175;p75+/− mice, and this increase was abolished in Hdh+/Q175;p75−/− mice (ANOVA; p < 0.05).
Fig. 5.
XIAP and Bcl-XL expression decreased in the striatum of Hdh+/Q175;p75−/− mice compared to Hdh+/Q175;p75+/+ mice at 5 months. (A) WCLs from a half of the striata of the stated genotypes at 5 months of age were tested using the listed antibodies. (B, C) Quantifications of (A). A significant decrease in XIAP and Bcl-XL expression was observed in Hdh+/Q175;p75−/− mice compared to Hdh+/Q175;p75+/+ littermates (ANOVA; p < 0.05).
Striatal dysfunction is accelerated in Hdh+/Q175; p75−/− mice
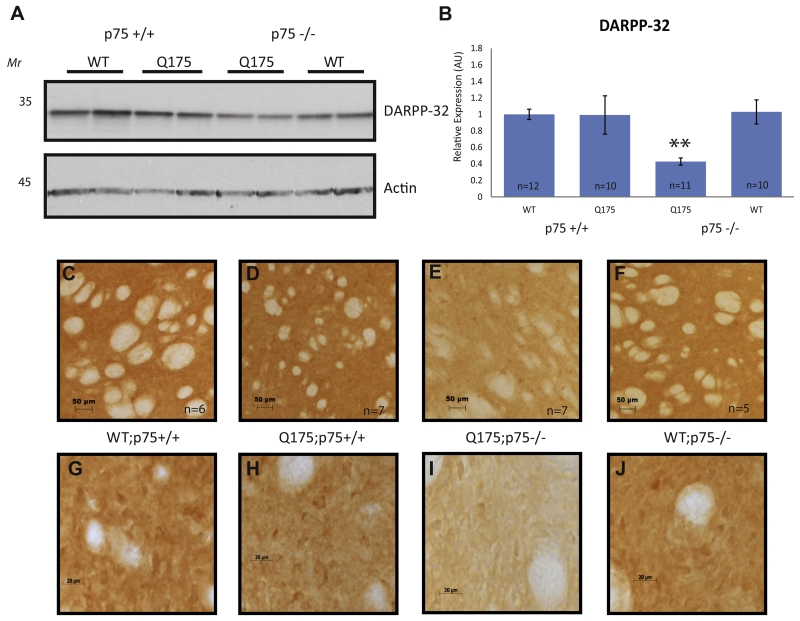
Striatal dysfunction and eventual degeneration is a hallmark in patients with HD and in some animal models of HD. Healthy MSNs express high levels of dopamine and cAMP-regulated phosphoprotein, MW 32 kDa (DARPP-32), which plays a critical role in the processing of dopamine signaling in the striatum. Decreased expression of DARPP-32 is a pathological marker of several diseases affecting striatal function, including HD (Bibb et al., 2000; Dellen et al., 2000). A previous examination of DARPP-32 levels in Hdh+/Q175 mice showed DARPP-32 to be significantly reduced in Hdh+/Q175 mice compared to Hdh+/+ littermates at 12 months of age, but not at earlier time points (Smith et al., 2014). In agreement with prior work, we did not observe a change in DARPP-32 expression in Hdh+/Q175;p75+/+ mice at 5 months by western blot, nor at 7 months by IHC, when compared to Hdh+/+;p75+/+ littermates (Fig. 6). Interestingly, DARPP-32 expression decreased significantly in Hdh+/Q175;p75−/− mice both by western blot at 5 months and by IHC at 7 months compared to Hdh+/+;p75+/+, Hdh+/Q175;p75+/+, and Hdh+/+;p75−/−, suggesting an earlier onset of striatal dysfunction (Fig. 6).
Fig. 6.
DARPP-32 expression decreased in the striatum of Hdh+/Q175;p75−/− mice compared to Hdh+/+;p75+/+ mice. (A) WCLs from a half of the striata of the stated genotypes at 5 months of age were probed for the listed antibodies. (B). Quantification of (A). (C–F) Immunohistochemistry was performed on the striata of 7-month-old mice of the stated genotype for DARPP-32 as described in the methods at 10×. (G–J) Images at 64×. Decreased levels of DARPP-32 were observed with WB and IHC in the striatum of Hdh+/Q175;p75−/− mice compared to Hdh+/+;p75+/+, Hdh+/Q175×;p75+/+, and Hdh+/+;p75−/− littermates.
Striatal volume decreased in Hdh+/Q175;p75−/− mice at 12 months of age
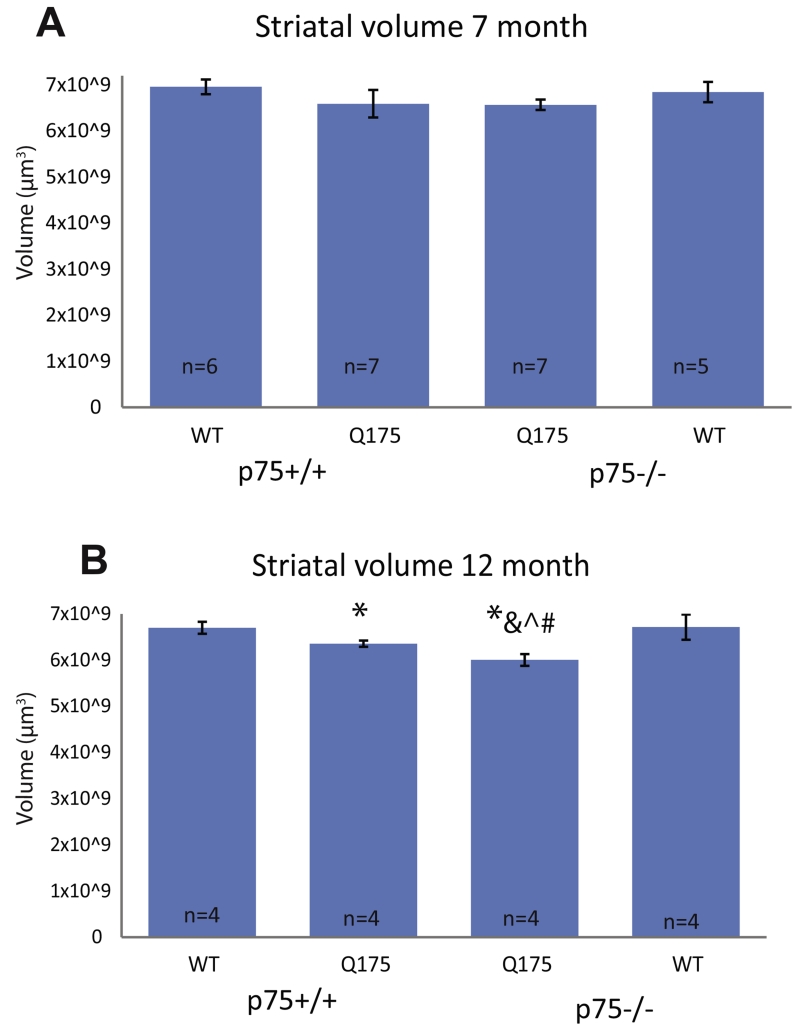
We evaluated striatal volume using NeuN IHC at 7 and 12 months of age. Differences in striatal volume have been reported at 12 months of age in Hdh+/Q175 mice, but not earlier (Smith et al., 2014). No significant difference was observed in striatal volume in any genotype examined at 7 months of age (Fig. 7). As the density of NeuN+ cells also does not change (data not shown), overt neuronal cell loss does not seem to have begun in these mice at this time point. At 12 months of age, however, striatal volume is significantly decreased in Hdh+/Q175;p75+/+ mice compared to Hdh+/+;p75+/+, as reported previously. Importantly, striatal volume is further decreased in Hdh+/Q175;p75−/− mice compared to both Hdh+/+;p75+/+ and Hdh+/Q175;p75+/+ mice. This decrease demonstrates a more severe degeneration in the striatum of Hdh+/Q175;p75−/− mice compared to Hdh+/Q175;p75+/+ littermates.
Fig. 7.
Striatal volume is decreased in the striatum at 12 months. NeuN immunohistochemistry was performed on the striata of 7–8- and 12–13-month-old mice of the stated genotypes as described in the methods. (A) Striatal volume quantifications of 7–8-month-old mice. No significant differences were observed in either neuronal density or striatal volume in any genotype. (B) Striatal volume quantifications of 12–13-month-old mice. * denotes significant decrease compared to Hdh+/+;p75+/+ littermates. & denotes significant decrease compared to Hdh+/Q175;p75+/+ littermates, # denotes significant decrease compared to Hdh+/+;p75−/− littermates, and ^ denotes significant decrease compared to the same genotype at 7 months (ANOVA; p > 0.05).
DISCUSSION
In this study, we examined the extent to which p75 is involved in the early striatal response to mutant Hdh and effects of the loss of early p75 expression on later stage neuronal survival. Hdh+/Q175;p75+/+ mice showed increased activation of Akt, increased phosphorylation of the p65 subunit of NFκB at two sites, and increased XIAP expression at 5 months of age when compared to Hdh+/+;p75+/+ littermates. These increases were abolished in Hdh+/Q175;p75−/− mice, suggesting that p75 is essential for this enhanced survival signaling. Additionally, Hdh+/Q175;p75−/− mice showed a decrease in Bcl-XL expression compared to Hdh+/Q175;p75+/+ and Hdh+/+;p75+/+ littermates, as well as a decrease in DARPP-32 expression, consistent with a loss of survival signaling and an earlier onset of dysfunction in Hdh+/Q175;p75−/− mice. Finally, Hdh+/Q175;p75−/− showed decreased striatal volume at 12 months of age compared to both Hdh+/+;p75+/+ and Hdh+/Q175;p75+/+ littermates. These data suggest a role for p75 in augmenting survival signaling in the striata of Hdh+/Q175 mice, and that the loss of this early pro-survival signaling leads to earlier onset of striatal dysfunction/degeneration.
While these data support a role for p75 in augmenting survival signaling prior to onset of dysfunction in the striata of Hdh+/Q175 mice, it is possible that the role of p75 changes with disease progression, acts in a region-specific manner, and/or differs with animal model. Plotkin et al. demonstrated that the inhibition of p75 rescued the loss of synaptic plasticity seen in the striatum of symptomatic BACHD mice (2014). The BACHD model used in this study differs substantially from the Hdh+/Q175 mice used in this study, both in symptom onset and disease course, and this paper was examining a specific type of synaptic plasticity, while we focused on biochemical and histological changes. In addition, Brito et al. reported rescued learning and memory deficits in Q111 mice by decreasing the level of p75 expression in the hippocampus (2014). These two studies suggest a potentially pathogenic role for p75 in HD at symptomatic disease stages and/or possibly in a regionally specific, or animal model-specific fashion. To address whether p75 signaling can switch from survival-promoting to dysfunctional later in disease progression, or whether p75 functions differently in different populations of cells, conditional p75 mice should be utilized in future experiments. Crossing conditional p75 mice into varied Cre recombinase-expressing strains will allow for cell-type-specific and temporal-specific deletion. It is important to acknowledge that, unlike our results, other studies have reported an increase in p75 protein and mRNA levels in the striatum of mouse models of HD and HD post-mortem brains, respectively (Zuccato et al., 2008; Brito et al., 2013). The mouse models used in these prior studies were a truncation model (R6/1) and a knock-in model (Q111). The R6/1 model differs considerably from the heterozygous Hdh+/Q175 mice. R6/1 is more rapidly progressive, and the truncated mutation is transgenically expressed rather than being expressed from the endogenous locus (Brooks et al., 2011; Pouladi et al., 2013). We were unable to achieve specific labeling with the p75 antibody used in the Q111 and human post-mortem studies, and therefore a direct comparison with these studies is difficult (Brito et al., 2013, 2014). Interestingly, in the same Q175 model of HD used in this study, p75 levels were reported to be decreased at 12 months of age in both the heterozygous and homozygous mutants when compared to WT animals (Ma et al., 2015). p75 levels in the striatum, therefore, may vary depending on which HD mouse model is investigated.
Considering these data along with the growing literature involving p75 and HD, it is clear that p75 signaling in HD mouse models is more complicated than initially appreciated. There is interest in developing p75 antagonists as a disease-modifying treatment for HD. Antagonizing p75 prior to dysfunction may be counter-productive, however, and developing p75-specific antagonists may only be helpful later in the course of the disease. An effective early intervention could take advantage of augmenting and/or prolonging this prosurvival signaling via p75. Additionally, it will be important to determine which cell type(s) express(es) p75 in the striatum of Hdh+/Q175 mice. The simplest model would be cell-autonomous signaling in MSNs leading to increased activation of Akt and NFκB and followed by an increase/normalization in survival targets of Akt and NFκB, such as XIAP and Bcl-XL. This process, however, may not necessarily be cell-autonomous. Other non-neuronal cell types present in the striatum, such as astrocytes, microglia, and oligodendrocytes could express p75 and provide some type of extrinsic support for the MSNs. Some expression of p75 in MSNs has been reported previously (Brito et al., 2014). A recent study, however, indicates that the majority of p75 in the striatum is expressed in immature oligodendrocytes, suggesting at least some p75 signaling is non-cell autonomous (Ma et al., 2015). Additionally, this enhanced survival signaling may be through p75 alone, through p75/TrkB complexes, or through p75 complexed with a different receptor. Ultimately, if this potential compensatory mechanism can be better understood, it may be possible to find novel targets to augment the brain’s natural defenses, prolonging normal function, and delaying, or even preventing, the onset of dysfunction.
Acknowledgments
We thank Sara Tallaksen-Greene, Francis Prael, Josh Haight and Rose Mauch for their expert technical assistance. We also thank Christopher Donnelly, Jennifer Shadrach, Nicole Gabreski and Dr. Robert Thompson for scientific discussions and critical reading of the manuscript. This research was supported by a Rackham Regents Fellowship (A.B.W.), University of Michigan School of Dentistry incentive funding (B.A.P.) and the Cure Huntington’s Disease Initiative (CHDI) contract A-8683.
Abbreviations
- Bcl-XL
B-cell lymphoma-extra large
- BDNF
brain-derived neurotrophic factor
- DARPP-32
dopamine and cAMP-regulated phosphoprotein, MW 32 kDa
- HD
Huntington’s disease
- HTT
Huntingtin protein
- IHC
immunohistochemistry
- JNK
c-Jun N-terminal kinase
- mHTT
mutated HTT
- MSN
medium spiny neuron
- NFκB
nuclear factor kappa B
- p75
p75 neurotrophin receptor
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PTEN
phosphatase and tensin homolog
- RE1/NRSE
repressor element 1/neuron-restrictive silencer element
- SDS
sodium dodecyl sulfate
- TBS
Tris-buffered saline
- TrkB
Tropomyosin receptor kinase receptor B
- WCL
whole-cell lysate
- XIAP
X-linked inhibitor of apoptosis
REFERENCES
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydyuk Ms, Xu B. BDNF signaling and survival of striatal neurons. Front Cell Neurosci. 2014;8:254. doi: 10.3389/fncel.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydyuk M, Xie Y, Tessarollo L, Xu B. Midbrain-derived neurotrophins support survival of immature striatal projection neurons. J Neurosci. 2013;33:3363–3369. doi: 10.1523/JNEUROSCI.3687-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, et al. Severe deficiencies in dopamine signaling in presymptomatic Huntington’s disease mice. Proc Nat Acad Sci USA. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenmann E, Thomas PS, Li Q, Kim J, Yang L-T, Pierchala B, Kaartinen V. Generation of mice with a conditional allele for the p75NTR neurotrophin receptor gene. Genesis. 2011;49:862–869. doi: 10.1002/dvg.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito V, Puigdellivol M, Giralt A, del Toro D, Alberch J, Gines S. Imbalance of p75NTR/TrkB protein expression in Huntington’s disease: implication for neuroprotective therapies. Cell Death Dis. 2013;4:e595. doi: 10.1038/cddis.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito V, Giralt A, Enriquez-Barreto L, Puigdell, et al. Neurotrophin receptor p75NTR mediates Huntington’s disease-associated synaptic and memory dysfunction. J Clin Invest. 2014;124:4411–4428. doi: 10.1172/JCI74809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Jones L, Dunnett SB. Comparative analysis of pathology and behavioural phenotypes in mouse models of Huntington’s disease. Brain Res Bull. 2011;88:81–93. doi: 10.1016/j.brainresbull.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhäuser N, Böhm-Matthaei R, Baeuerle PA, Barde Y-A. Selective activation of NF-κB by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- Ceni C, Kommaddi RP, Thomas R, Vereker E, Liu X, McPherson PS, Ritter B, Barker PA. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J Cell Sci. 2010;123:2299–2307. doi: 10.1242/jcs.062612. [DOI] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Lee EHY. Brain-derived neurotrophic factor enhances Bcl-XL expression through protein kinase casein kinase 2-activated and nuclear factor kappa B-mediated pathway in rat hippocampus. Brain Pathol. 2011;21:150–162. doi: 10.1111/j.1750-3639.2010.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Dellen AV, John Welch, Dixon Ruth M, Patricia Cordery, Denis York, Styles Peter, Colin Blakemore, HannanAnthony J. N-Acetylaspartate and DARPP-32 levels decrease in the corpus striatum of Huntington’s disease mice. Neuroreport. 2000;11:3751–3757. doi: 10.1097/00001756-200011270-00032. [DOI] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- Freeman RS, Burch RL, Crowder RJ, Lomb DJ, Schoell MC, Straub JA, Xie L. NGF deprivation-induced gene expression: after ten years, where do we stand? Prog Brain Res. 2004;146:111–126. doi: 10.1016/S0079-6123(03)46008-1. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompieree JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Group, H.S.D.C.R A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci. 2013;14:10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Her L-S, Goldstein LSB. Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant Huntingtin. J Neurosci. 2008;28:13662–13672. doi: 10.1523/JNEUROSCI.4144-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, McNamara JO. Mutual regulation of Src family kinases and the neurotrophin receptor TrkB. J Biol Chem. 2010;285:8207–8217. doi: 10.1074/jbc.M109.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommaddi RP, Thomas R, Ceni C, Daigneault K, Barker PA. Trk-dependent ADAM17 activation facilitates neurotropin survival signaling. FASEB J. 2011;25:2061–2070. doi: 10.1096/fj.10-173740. [DOI] [PubMed] [Google Scholar]
- Kraemer BR, Yoon SO, Carter BD. The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handb Exp Pharmacol. 2014;220:121–164. doi: 10.1007/978-3-642-45106-5_6. [DOI] [PubMed] [Google Scholar]
- Ma Q, Yang J, Li T, Milner TA, Hempstead BL. Selective reduction of striatal mature BDNF without induction of proBDNF in the zQ175 mouse model of Huntington’s disease. Neurobiol Dis. 2015;82:466–477. doi: 10.1016/j.nbd.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkerh JPS, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. P75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6:936–941. doi: 10.1038/sj.embor.7400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Matusica D, Skeldal S, Sykes AM, Palstra N, Sharma A, Coulson EJ. An intracellular domain fragment of the p75 neurotrophin receptor (p75(NTR)) enhances tropomyosin receptor kinase A (TrkA) receptor function. J Biol Chem. 2013;288:11144–11154. doi: 10.1074/jbc.M112.436469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffrey E, Hay RT, Davies AM. Cytokine-induced nuclear factor-κ B activation promotes the survival of developing neurons. J Cell Biol. 2000;148:325–332. doi: 10.1083/jcb.148.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Negrini S, D’Alessandro R, Meldolesi J. NGF signaling in PC12 cells: the cooperation of p75 (NTR) with TrkA is needed for the activation of both mTORC2 and the PI3K signaling cascade. Biol Open. 2013;2:855–866. doi: 10.1242/bio.20135116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. P75NTR – live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Plotkin Joshua L, Day M, Peterson Jayms D, Xie Z, Kress Geraldine J, Rafalovich I, et al. Impaired TrkB receptor signaling underlies corticostriatal dysfunction in Huntington’s disease. Neuron. 2014;83:178–188. doi: 10.1016/j.neuron.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouladi MA, Morton J, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14:708–721. doi: 10.1038/nrn3570. [DOI] [PubMed] [Google Scholar]
- Putcha G, Moulder K, Golden J, Bouillet P, Adams J, Strasser A, Johnson E. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Smith GA, Rocha EM, McLean JR, Hayes MA, Izen SC, Isacson O, Hallett PJ. Progressive axonal transport and synaptic protein changes correlate with behavioral and neuropathological abnormalities in the heterozygous Q175 KI mouse model of Huntington’s disease. Hum Mol Genet. 2014;23:4510–4527. doi: 10.1093/hmg/ddu166. [DOI] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-κB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor α-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Lendahl U, Metsis M. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. J Biol Chem. 1999;274:1078–1084. [PubMed] [Google Scholar]
- Vicario A, Kisiswa L, Tann JY, Kelly CE, Ibanez CF. Neuron-type-specific signaling by the p75NTR death receptor is regulated by differential proteolytic cleavage. J Cell Sci. 2015;128:1507–1517. doi: 10.1242/jcs.161745. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- West A, Pruunsild P, Timmusk T. Neurotrophins: transcription and translation. Handb Exp Pharmacol. 2014;220:67–100. doi: 10.1007/978-3-642-45106-5_4. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of Huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225–238. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]