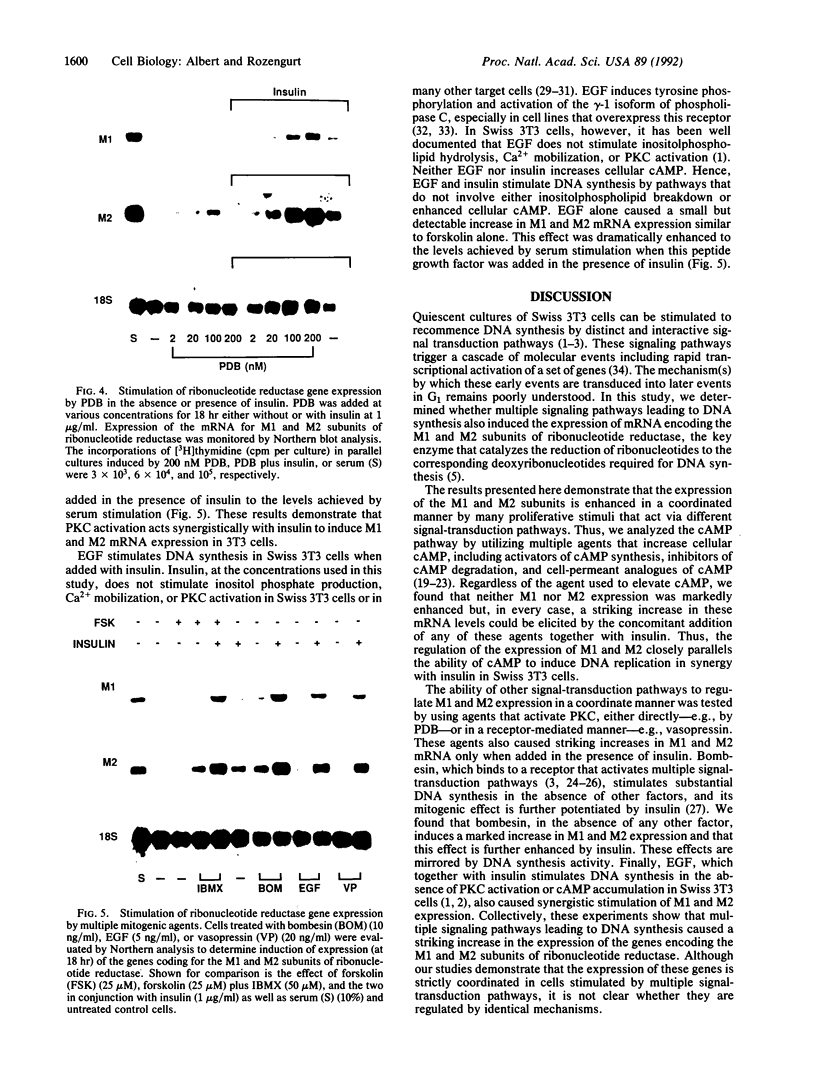
Abstract
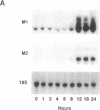
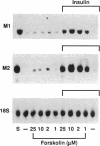
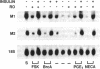
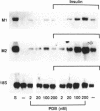
Ribonucleoside-diphosphate reductase (ribonucleotide reductase, EC 1.17.4.1) is the enzyme responsible for the in vivo production of deoxyribonucleotides for DNA synthesis and is essential for cell proliferation. We examined the signal transduction pathways leading to expression of the M1 and M2 subunits of this enzyme in Swiss 3T3 mouse fibroblasts by Northern blot analysis. Stimulation of quiescent cells resulted in coordinate expression of both subunits, beginning at 8 hr after serum addition, in late G1 phase, and peaking at 18-24 hr. Serum increased M2 message to 30 to 50 times that of quiescent cells, in contrast with M1 message, which was increased 10 times. Agents that elevated cAMP, including forskolin, and the cAMP analogue 8-bromo-cAMP modestly stimulated gene expression. Each of these agents was synergistic with insulin, and these combinations induced expression equivalent to that induced by serum stimulation. Likewise, agents that activate protein kinase C such as phorbol 12,13-dibutyrate, bombesin, and vasopressin were also synergistic with insulin with respect to ribonucleotide reductase gene expression, as was epidermal growth factor, which stimulates receptor tyrosine kinase activity. The time course for induction of mRNA expression by each of these agents alone or in combination was identical to that for induction stimulated by serum. Finally, the synergistic effects apparent in Northern analysis of ribonucleotide reductase gene expression were mirrored in parallel determinations of DNA synthesis. Thus, the combinatorial nature of signal transduction pathways resulting in proliferation of Swiss 3T3 cells is expressed at the level of ribonucleotide reductase gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. A., Gudas L. J., Nodzenski E. Deoxyribonucleotide metabolism and cyclic AMP resistance in hydroxyurea-resistant S49 T-lymphoma cells. J Cell Physiol. 1987 Feb;130(2):262–269. doi: 10.1002/jcp.1041300212. [DOI] [PubMed] [Google Scholar]
- Albert D. A., Gudas L. J. Ribonucleotide reductase activity and deoxyribonucleoside triphosphate metabolism during the cell cycle of S49 wild-type and mutant mouse T-lymphoma cells. J Biol Chem. 1985 Jan 10;260(1):679–684. [PubMed] [Google Scholar]
- Björklund S., Skog S., Tribukait B., Thelander L. S-phase-specific expression of mammalian ribonucleotide reductase R1 and R2 subunit mRNAs. Biochemistry. 1990 Jun 12;29(23):5452–5458. doi: 10.1021/bi00475a007. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Haupt D. M., Stumpo D. J. Insulin activation of protein kinase C: a reassessment. J Biol Chem. 1991 Jun 15;266(17):10946–10952. [PubMed] [Google Scholar]
- Bravo R. Growth factor-responsive genes in fibroblasts. Cell Growth Differ. 1990 Jun;1(6):305–309. [PubMed] [Google Scholar]
- Brissenden J. E., Caras I., Thelander L., Francke U. The structural gene for the M1 subunit of ribonucleotide reductase maps to chromosome 11, band p15, in human and to chromosome 7 in mouse. Exp Cell Res. 1988 Jan;174(1):302–308. doi: 10.1016/0014-4827(88)90165-6. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Levinson B. B., Fabry M., Williams S. R., Martin D. W., Jr Cloned mouse ribonucleotide reductase subunit M1 cDNA reveals amino acid sequence homology with Escherichia coli and herpesvirus ribonucleotide reductases. J Biol Chem. 1985 Jun 10;260(11):7015–7022. [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Gräslund A., Skog S., Thelander L., Tribukait B. Cell cycle-dependent regulation of mammalian ribonucleotide reductase. The S phase-correlated increase in subunit M2 is regulated by de novo protein synthesis. J Biol Chem. 1984 Oct 10;259(19):11695–11700. [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Erusalimsky J. D., Friedberg I., Rozengurt E. Bombesin, diacylglycerols, and phorbol esters rapidly stimulate the phosphorylation of an Mr = 80,000 protein kinase C substrate in permeabilized 3T3 cells. Effect of guanine nucleotides. J Biol Chem. 1988 Dec 15;263(35):19188–19194. [PubMed] [Google Scholar]
- Escribano J., Rozengurt E. Cyclic AMP increasing agents rapidly stimulate vimentin phosphorylation in quiescent cultures of Swiss 3T3 cells. J Cell Physiol. 1988 Nov;137(2):223–234. doi: 10.1002/jcp.1041370204. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Rozengurt E. Arachidonic acid release by bombesin. A novel postreceptor target for heterologous mitogenic desensitization. J Biol Chem. 1990 Nov 15;265(32):19973–19979. [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Wedegaertner P. B., Kim J. W., Rhee S. G., Carpenter G., Kim J. J. Selectivity of phospholipase C phosphorylation by the epidermal growth factor receptor, the insulin receptor, and their cytoplasmic domains. Proc Natl Acad Sci U S A. 1990 Jan;87(1):424–428. doi: 10.1073/pnas.87.1.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Adenosine receptor activation in quiescent Swiss 3T3 cells. Enhancement of cAMP levels, DNA synthesis and cell division. Exp Cell Res. 1982 May;139(1):71–78. doi: 10.1016/0014-4827(82)90319-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rozengurt E., Collins M. K., Keehan M. Mitogenic effect of prostaglandin E1 in Swiss 3T3 cells: role of cyclic AMP. J Cell Physiol. 1983 Sep;116(3):379–384. doi: 10.1002/jcp.1041160316. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Erusalimsky J., Mehmet H., Morris C., Nånberg E., Sinnett-Smith J. Signal transduction in mitogenesis: further evidence for multiple pathways. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):945–954. doi: 10.1101/sqb.1988.053.01.109. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Neuropeptides as cellular growth factors: role of multiple signalling pathways. Eur J Clin Invest. 1991 Apr;21(2):123–134. doi: 10.1111/j.1365-2362.1991.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Synergistic stimulation of DNA synthesis by cyclic AMP derivatives and growth factors in mouse 3T3 cells. J Cell Physiol. 1982 Aug;112(2):243–250. doi: 10.1002/jcp.1041120213. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Feng T. L., Barton D. E., Thelander L., Lewis W. H., Srinivasan P. R., Francke U. Ribonucleotide reductase M2 subunit sequences mapped to four different chromosomal sites in humans and mice: functional locus identified by its amplification in hydroxyurea-resistant cell lines. Genomics. 1987 Sep;1(1):77–86. doi: 10.1016/0888-7543(87)90108-x. [DOI] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]