Abstract
Context
Characterization of stress exposure requires an understanding seasonal variability in stress biomarkers.
Objective
To compare acute and chronic stress biomarkers between two seasons in a cohort of rural, Hispanic mothers.
Methods
Stress questionnaires and cortisol measurements (hair, blood, saliva) were collected in the summer and fall.
Results
Cortisol biomarkers were significantly different and stress questionnaires were significantly correlated between seasons.
Discussion
The variability in cortisol and relative stability of questionnaires between seasons may indicate that cortisol responds to subtle stressors not addressed in questionnaires.
Conclusions
There are significant differences in stress biomarkers in our cohort between seasons.
Keywords: biomonitoring, exposure modeling, child exposure/health
Introduction
Characterizing the complex patterns and impacts of environmental stress exposure is important to understanding its role in disease susceptibility. Stress is one of the most ubiquitous environmental exposures, however, many studies have found that it disproportionately impacts low-income and minority communities(Cohen and Janicki-Deverts 2012, Chen and Miller 2013), and may be a mediating factor in the relationship between income and health(Chen, Fisher et al. 2003, Schreier and Chen 2013). One particular group that may be at increased risk of high stress exposure is Hispanic mothers living in rural, agricultural communities. Many of these women are separated from family, experience a barrier in communication, have higher rates of discrimination and difficult working conditions (Snipes, Thompson et al. 2007). Women in this community are frequently employed in agricultural settings, leading to inherent changes in work and financial stress between seasons. Depressive symptoms have been related to seasonal changes in farmworker communities(Grzywacz, Quandt et al. 2010). As seasons change, employment opportunities and worker tasks also shift, leading to changes in resource availability and potentially social stress.
In order to fully characterize the stress conditions impacting this community, it is necessary to understand the seasonality of stress across a variety of assessment techniques for both acute and chronic stress. Stress is commonly evaluated through questionnaires or by measuring the biomarker cortisol, in blood, saliva, hair and urine (Pearson Murphy 2002, Levine, Zagoory-Sharon et al. 2007, Adam and Kumari 2009, Staufenbiel, Penninx et al. 2013). Cortisol is released into the blood in a pulsatile manner in response to environmental stress (Young, Carlson et al. 2001, Keenan 2004, Young, Abelson et al. 2004, McMaster, Jangani et al. 2011)and transferred to saliva and hair and excreted in urine. Saliva samples allow for the assessment of diurnal cortisol patterns as well as acute fluctuations in stress. In most healthy individuals, cortisol is diurnally regulated to peak about 30 minutes after waking (Kunz-Ebrecht, Kirchbaum et al. 2004) and then slowly decline throughout the day, decreasing by about 50% by bedtime(Adam 2000, Stone, Schwartz et al. 2001, Hellhammer, Fries et al. 2007). Variations of this diurnal pattern have been related to stress and disease states(Bhattacharyya, Molloy et al. 2008, Chida and Steptoe 2009, Suglia, Staudenmayer et al. 2010). However, some studies have suggested that healthy diurnal regulation may vary by individual(Stone, Schwartz et al. 2001) or even be related to race and ethnicity(DeSantis, Adam et al. 2007). Furthermore, saliva cortisol patterns may also be affected by season(Persson, Garde et al. 2008). Hair cortisol is a more stable measure of stress and higher concentrations have been correlated with unemployment(Dettenborn, Tietze et al. 2010), negative life events(Staufenbiel, Koenders et al. 2014) and work stress(Steinisch, Yusuf et al. 2014). Given the increased susceptibility of Hispanic mothers to social stressors, it is especially important to characterize their cortisol patterns in the context of seasonal changes.
In this paper, we compare chronic and acute stress measures between two seasons, the summer and fall, in a cohort of Hispanic mothers living in an underserved rural setting. Between these seasons agricultural community members may be transitioning from thinning crops to harvesting apples and pears. To assess how these changes may impact stress levels, both biological measures of cortisol in blood, saliva and hair as well as questionnaire measures of stress are compared within and between two different agricultural seasons. Due to the intricate relationship between agricultural communities and season(Grzywacz, Quandt et al. 2010), we expect that stress measures may be different between agricultural seasons. However, within a season, we expect stress to be correlated.
Methods
Field Sample Collections
Description of Cohort
Study participants were recruited from the larger University of Washington Center for Children's Environmental Health Risk Research (CHC) Cohort established in the Yakima Valley, Washington and described in Thompson et al. 2014(Thompson, Griffith et al. 2014). The founding CHC Cohort recruited families with children aged 2-6 in 2005. This study is set in one of Washington State's primary agricultural regions, the Yakima Valley. Roughly half the study participants are farmworkers and almost all the participants speak Spanish as the primary language in the home. In 2011, 27 non-pregnant women of childbearing age (18-49 years) were recruited to participate in this nested stress assessment study.
Sampling Design
To measure acute and chronic stress assessments, we collected biomarkers and questions during two different seasons approximately ten weeks apart. Acute stress is measured by within season salivary and blood cortisol measurements. Chronic stress is measured by hair cortisol and stress questionnaire assessment. During each season, saliva samples were collected five times per day for two consecutive days, one blood sample and one hair sample were collected and participants responded to the 10 item Perceived Stress Scale(PSS) (Cohen, Kamarck et al. 1983) and Mexican Immigrant Farmworker Stress Scale(MIFSS)(Snipes, Thompson et al. 2007), available in both Spanish and English (Figure 1). For each sampling day, saliva samples were taken upon awakening, 30 minutes post awakening, 11:00 a.m., 4:00 p.m. and before bed. To measure chronic stress and long-term variability, a second set of samples was collected approximately ten weeks later. Summer sampling occurred between July and August of 2011, and fall sampling was in October 2011. Data collection procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center's Institutional Review Board (File IR 5946).
Figure 1.
Study design for stress assessments. Each seasonal sampling period consisted of two consecutive sampling days. Seasonal sampling periods were spaced approximately 10 weeks apart between late summer and early fall. On each sampling day five saliva samples were collected. In each season a total of 10 saliva samples, one blood sample and the PSS-10 and the Mexican Immigrant Farmworker Stress Scale (MIFSS) were collected.
Saliva collection
Saliva samples were collected using the SalivaBio Oral Swab (SOS) (Salimetrics, State College, Pennsylvania, USA). Participants placed the SOS under the tongue for one minute and then discharged it directly back into the tube. At the time of sampling, participants recorded the time and whether or they consumed caffeine or alcohol prior to taking the sample. Participants were instructed to avoid alcohol for 12 hours before sample collection, major meals within 1 hour of sample collection, dairy products within 20 minutes of sample collection and foods with high sugar, drinks with high caffeine content, and fruit juices immediately before sample collection. Participants stored samples in the refrigerator for up to two days, until they were collected by the study staff.
Blood Collection
Blood draws were performed by a trained phlebotomist during each seasonal sampling period. As directed by the study protocol, the exact time and conditions of the blood draw were recorded. Blood was collected in pre-labeled 10 mL EDTA tubes and immediately inverted 10 times to mix the blood with the EDTA anticoagulant. Samples were refrigerated until processing.
Hair Collection
Hair samples approximately 0.5 cm diameter were cut as close to the scalp of the posterior vertex of the head as possible using ceramic scissors, similar to other published studies(Kirschbaum, Tietze et al. 2009, Dettenborn, Tietze et al. 2010). A rubber band was used to secure the scalp end of the sample and tape was used to secure the non-scalp end of the hair sample. Hair samples were stored in 50 mL graduated plastic centrifuge tubes at −10°C until laboratory preparation and analysis.
Questionnaire Collection
Participants responded to the PSS-10(Cohen, Kamarck et al. 1983) and the Mexican Immigrant Farmworker Stress Scale (MIFSS)(Snipes, Thompson et al. 2007). Questionnaires were available in English and Spanish and administered in the participant's choice of language. Questionnaires were administered by trained interviewers. At the time of the first interview income, number of children, age and other socio-demographic characteristics were recorded.
Laboratory Methods
Saliva and Plasma Cortisol Assay Methods
Samples were centrifuged and stored in an −80 °C freezer until thawed for the assay procedure. Cortisol concentrations in both plasma and saliva samples were quantified using a cortisol enzyme-linked immunosorbent assay (ELISA) (Salimetrics, State College, PA). The limit of detection (LOD) was 0.01 μg/dl. Half the LOD was used to obtain a value for samples below the LOD (0.005 μg/dl). Intra-assay coefficients of variability were less than 20%.
Hair Analysis
Once in the laboratory, hair samples were weighed and measured. The 2.5 cm closest to the scalp were cut, weighed and vortexed once with isopropanol (LC-MS grade) for 1 minute and then immediately aspirated dry. Any residual solvent was evaporated in an 80° C vacuum oven for 1 hr and then cooled overnight while in oven. Samples were minced into approximately 2-3 mm segments, placed in 2 mL vials and then weighed to confirm that each has sufficient mass for cortisol quantification (25–35 mg). Samples were pulverized with two stainless steel balls (sizes 3/16 and 7/32 inch) in a ball mill for 30 minutes. Cortisol was extracted from pulverized samples with 1 mL aliquots of methanol with internal standards (9,11,12,12-D4-Cortisol, and 1,2-D2-cortisone, each 10 ng/mL; Cambridge Isotope Laboratories, and CDN Isotopes) ) on a tube rotator for 4 hours and then centrifuged (at 11500 rpm for 2 min) to make a hair pellet at the bottom of the extract. The extract was removed taking care not to disturb the pellet and the extract were evaporated to dryness under nitrogen gas and then reconstituted in 50 μL methanol. Instrument-ready samples were stored at −20°C until analysis. Analysis was on a Agilent 1260/ 6460 LC-MS-MS system using a Phenomenex Synergi Fusion-RP column (150 × 2 mm, 4μm; 5 μl injection) and matching guard column. Gradient elution with mobile phases (A) water and (B) acetonitrile started with 30%B for 2min, 30-60%B over 3min, 60%B for 1min, and then 30%B for 4min (10 minute total run time). Jet Stream electrospray ionization source in positive mode was used. The quantitative and confirmation transitions are listed in Supplemental Table 1 and are similar to Singh and Bruton (Singh and Bruton 2008). Calibration standards are prepared in the range of 0.1 to 100 ppb and run with each LC sequence.
Statistical Methods
Analyses using the questionnaire data were completed by calculating a total score for both the PSS and the MIFSS. As done in Cohen et al. 1983(Cohen, Kamarck et al. 1983), the PSS negatively phrased questions was reversed such that the responses could be summed. For the MIFSS, responses were scored on a scale of 1 to 5, with scores of 5 indicating greater stress. For this questionnaire all questions were phrased similarly and no reversal was necessary.
In order to characterize daily cortisol fluctuation we calculated the diurnal index, area under the curve (AUC) and AM change from the five daily saliva cortisol samples, similar to other studies (Adam and Kumari 2009, Suglia, Staudenmayer et al. 2010, Nater, Hoppmann et al. 2013, Ross, Murphy et al. 2014). The diurnal index was calculated by subtracting the bedtime saliva cortisol concentration from the 30-minutes post waking peak. The AUC was calculated using all five daily saliva samples and adjusted to account for variable waking hours and represents the total cortisol production during waking hours. The AM change is the difference between the waking and 30 minutes post-waking saliva cortisol concentrations. These metrics allow us to characterize the dynamic, diurnal cortisol patterns across sampling days. For individual saliva samples and the AUC, log transformations were used in further correlation analyses and figures.
In order to test the relationships between samples, we used Pearson's one sided correlation test(with alpha=0.05) to compare questionnaire responses, saliva samples, AM changes, AUCs and diurnal indices taken on consecutive days to those taken ten weeks apart. Because blood cortisol samples were collected at the participant's convenience, the time of day was not standard across individuals. To adjust for this, we used the average salivary cortisol diurnal index for each season as a slope for change in cortisol throughout the day. We used this slope to calculate blood cortisol at waking for each participant. This allowed us to standardize blood cortisol concentrations in terms of the hours since waking for blood collection times for each participant during each season. Diurnally adjusted blood samples and hair samples were also compared using the Wilcoxon rank test. The potential effects of caffeine and alcohol consumption on cortisol biomarkers were considered using a linear mixed effects model with caffeine and season as the random effects fit by the Lme package using the REML critereon in R, version 3.2.1.
Results
Study Cohort
Study participants were generally low-income, Hispanic women with multiple children (Supplemental Table 2). Each participant was asked to provide 20 saliva samples over the course of the study. Overall, participants provided 97% samples we requested for a total of 524 saliva samples. Of the 27 women who participated in the summer sampling period, 26 returned for the fall sampling period, allowing us to collect a total of 53 blood samples and 53 hair samples.
Stress Biomarkers
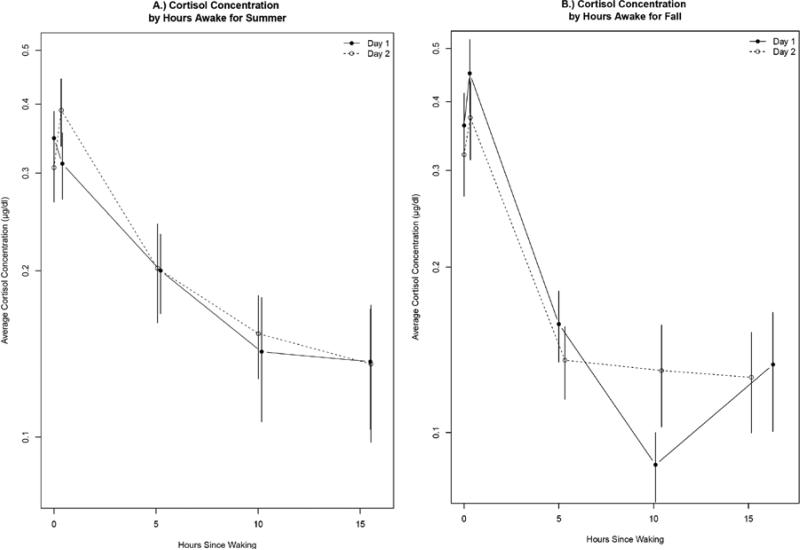
Similar to other reports(Stone, Schwartz et al. 2001), salivary cortisol changed throughout the day, peaking in the morning and declining in the evening in most participants (Figure 2). Some salivary cortisol samples were correlated within a season (primarily in the fall), however, samples collected at similar times of day on days ten weeks apart were not correlated, suggesting some seasonal changes. The correlations for between and within season salivary cortisol concentrations are shown in Table 1. Within the summer, the 30 minutes post-waking saliva samples were correlated (r= 0.53, p<0.05, Figure 2) and within the fall, all five daily saliva samples were correlated between consecutive days (r >0.55, p< 0.05, Figure 2). We characterized the diurnal patterns by calculating the Area Under the Curve, Diurnal Index and AM Change. In both seasons, the AUC (summer r=0.54 p<0.05, fall r=0.87, p <0.05) and Diurnal indexes (summer r=0.51, fall r=0.75, p<0.05) were correlated between days; however the correlations were much strong in the fall than the summer. The AM change was only correlated in the fall (r=0.65, p<0.05). Overall, fall samples were far more similar than summer samples, suggesting some differences in stress patterns between the seasons. This is substantiated by the lack of significant correlations between the AUC and Diurnal Indexes between seasons and the negative correlation of the AM change between seasons (−0.61, p<0.05). The within and between season relationships in the AUC, Diurnal Index and Am Change are shown in Supplemental Figure S1. Blood cortisol concentrations averaged 10.7 and 13.3 μg/dl in the summer and fall respectively. Blood cortisol samples collected ten weeks apart were significantly different when corrected for time of day (Wilcoxon rank sum test, p<0.05).
Figure 2.
Mean Salivary Cortisol concentrations by hours awake for Summer (A) and Fall (B). For both seasons, day 1 is shown by the solid line and day 2 is shown by the dashed line. Error bars are constructed using the standard error of geometric mean. For summer samples only the 30 minutes post-waking samples were significantly correlated and in the fall all samples were significantly correlated (Pearson Correlation, p<0.05).
Table 1.
Between and within season correlations for salivary Cortisol concentrations collected on two sets of consecutive days in the summer and fall. Samples were collected at waking, 30-minutes post waking, 11am, 4pm and bedtime. We calculated the area under the curve (AUC), AMChange (difference between 30 minutes post waking and waking samples) and the diurnal Index (DI, difference between 30-minutes post waking and bedtime samples). Results are presented as the Pearson's correlation coefficient, 95% confidence intervals and the p value.
| Within the Summer | Correlation [r] | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| AUC | 0.54 | 0.19 | 0.76 | 0.005 |
| AM Change | 0.32 | −0.06 | 0.63 | 0.099 |
| DI | 0.51 | 0.16 | 0.74 | 0.007 |
| Waking | 0.11 | −0.28 | 0.47 | 0.587 |
| 30 min post-waking | 0.53 | 0.19 | 0.76 | 0.004 |
| 11:00 AM | 0.37 | −0.01 | 0.66 | 0.054 |
| 4:00 PM | 0.32 | −0.09 | 0.64 | 0.118 |
| Bedtime | 0.35 | −0.03 | 0.65 | 0.071 |
| Within the Fall | ||||
| AUC | 0.87 | 0.72 | 0.94 | <0.001 |
| AM Change | 0.65 | 0.33 | 0.83 | 0.001 |
| DI | 0.75 | 0.52 | 0.88 | <0.001 |
| Waking | 0.91 | 0.80 | 0.96 | <0.001 |
| 30 min post-waking | 0.83 | 0.65 | 0.92 | <0.001 |
| 11:00 AM | 0.61 | 0.28 | 0.81 | 0.001 |
| 4:00 PM | 0.55 | 0.20 | 0.77 | 0.004 |
| Bedtime | 0.71 | 0.43 | 0.86 | <0.001 |
| Between Season | ||||
| AUC | −0.28 | −0.61 | 0.13 | 0.179 |
| AM Change | −0.61 | −0.81 | −0.29 | 0.001 |
| DI | 0.27 | −0.13 | 0.60 | 0.180 |
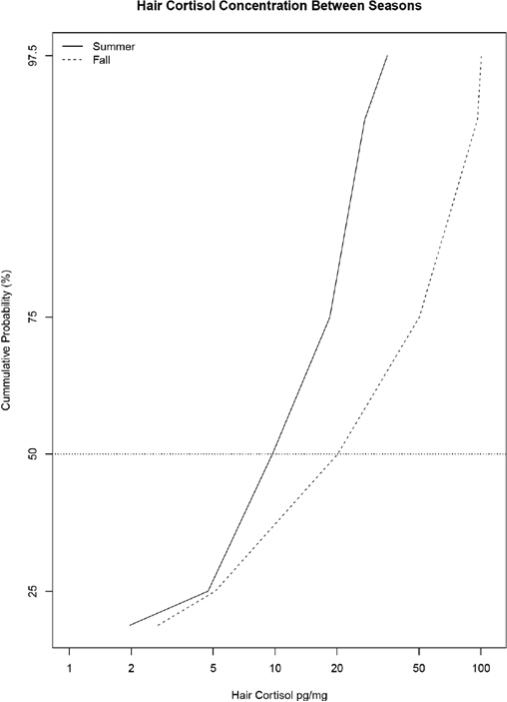
The average percent recovery for hair cortisol internal standards was 99.06 with a standard deviation of 0.22. The geometric mean of hair cortisol concentrations were 13.03 (geometric standard deviation: 1.85 pg/mg) during the summer and 19.40 (geometric standard deviation: 3.48 pg/mg) during the fall. As hair grows, cortisol is incrementally incorporated into the shaft, therefore our segmental analysis of the most recent 2.5 centimeters of growth allow us to retrospectively assess cortisol in the previous 2.5 months. Therefore, the summer sample reflects stress from mid-May through the beginning of August and the fall sample reflects stress from mid-August through October. Hair cortisol concentrations were significantly different by season (Wilcoxon rank sum test, p<0.05). This difference was also evident using a linear mixed effects model adjusted for caffeine consumption, season and hair cortisol that demonstrated that hair cortisol levels were significantly affected by season, however, caffeine consumption was not. Saliva, blood and hair cortisol levels were not impacted by caffeine or alcohol consumption in this cohort.
Questionnaires
The average total PSS was approximately 16.19 (standard deviation: 5.7) in the summer and 14.65 (standard deviation: 6.07) in the fall. Contributions of each question to the total PSS score were relatively equal, with on average, no single question comprising more than 14% or less than 7% of the total score. This concept is also shown in a stacked barplot (Supplemental, Figure S2). The responses from MIFSS are shown in Supplemental Table S3. The PSS totals were correlated between seasons (r=0.59, p<0.05). The MIFSS totals were also correlated between seasons (r=0.77, p<0.05). The PSS totals were significantly correlated to the MIFSS totals in the summer (r=0.56, p<0.05), but not in the fall. When the averages across both seasons were compared there was a moderately significant correlation between the PSS and the MIFSS (r=0.55, p<0.05). Income and number of children were not correlated with the average stress questionnaire responses for either the PSS or the MIFSS.. The PSS scores did not correlate to saliva, blood or hair cortisol measurements. The average of all four 30-minute post-waking saliva cortisol samples was, but significantly correlated to the average MIFSS scores (r=0.46, p>0.05).
Discussion
This study provides new insights on seasonal differences in acute and chronic stress assessment methods in a uniquely susceptible population of Hispanic Mothers. Similar to our expectations, biological measures of acute stress were correlated within seasons and biological measures of chronic stress were significantly different between seasons. Questionnaire stress assessments, on the other hand, were more stable between seasons and showed significant positive correlations between seasons. Other studies have seen similar differences in salivary cortisol samples collected months(Ross, Murphy et al. 2014) or seasons(Persson, Garde et al. 2008) apart. However, to our knowledge, this is the first report of seasonal differences in hair cortisol concentrations. The differences in biological measures of stress, compared to the stability of questionnaire measures of stress suggest that biological samples may be capturing subtle changes in the stress response that are not accounted for in stress questionnaires. Cortisol samples may be responding to seasonal changes, such as the start of the school year, potential job changes for the participants or someone else in their family or even more natural factors like changes in weather or daylight. The MIFSS asks questions specific to stress caused by childcare availability, having too much or too little work and lack of financial resources(Snipes, Thompson et al. 2007). Because these responses were not different between seasons, it is possible that the biomarkers are accessing more subtle stress responses than the questionnaires. Additionally, cortisol is intricately tied to the diurnal cycle(Nater, Hoppmann et al. 2013, Ross, Murphy et al. 2014). Circadian cycle changes related to schedule changes or the amount of daylight may be impacting cortisol. This cohort is located in Washington State's Yakima Valley. In the summer (August 1st) there are almost 15 hours of daylight in Yakima Valley and by the end of October there are only 10 hours of daylight. The substantial loss of daylight between the two seasons, coupled with the change in weather, may impact cortisol and could be contributing to the seasonal differences observed in this study.
The MIFSS is a stress questionnaire designed using focus groups of Mexican immigrant farmworkers living in agricultural Yakima, Washington(Snipes, Thompson et al. 2007). Because of the similarities between our study cohort and the community that created this questionnaire, the types of questions are exceptionally relevant to our cohort. However, it previously had not been compared to the more globally used PSS. In this study, PSS responses were significantly correlated to newer MIFSS responses and the MIFSS responses were correlated between seasons, providing evidence of its ability to assess chronic stress and its stability over time. The MIFSS asks questions regarding specific life stressors that are relevant to Hispanic mothers, such as concerns over discrimination, the language barrier, access to healthcare, access to childcare and other life factors. The significant correlation between the PSS and MIFSS allows us to trace the etiology of stress to specific conditions and events. In this study, the top three stressors as identified by the MIFSS were about paying bills, not being able to speak English and feelings of desperation over family being far away. These questions are also the three highest scoring questions in the original publication of the MIFSS from 2007 (Snipes, Thompson et al. 2007). This demonstrates reliability of the MIFSS and the consistency of community stressors across years.
It is particularly relevant to assess stress in this population as demographic characteristics may increase the risk of social stress exposure. The women enrolled in this study have, on average, 3.6 children, putting them at risk for experiencing a diversity of unique midlife stressors and stress combinations(Scott, Whitehead et al. 2013). Furthermore, socioeconomic status is an important factor in health disparities and has been associated chronic stress (Chen and Miller 2013, Barrington, Stafford et al. 2014). The sum of the PSS responses in this study (average of 16.91, 14.65 in the summer and fall, respectively) were lower than responses in women (average of 17.4) (Cohen and Janicki-Deverts 2012), low-income populations(average of 17.77) (Cohen and Janicki-Deverts 2012) or Hispanic populations (average or 17.77) (Cohen and Janicki-Deverts 2012). The low levels of stress are unique and may be related to support factors not assessed in this study. This idea is substantiated by other studies in this cohort have demonstrated that our study participants have a positive outlooks exhibited by their strong commitment to their communities and future generations (Hohl, Gonzalez et al. 2014).
Conclusions
This study shows that there are significant biological differences in stress in Hispanic immigrant mothers living in an agricultural community between seasons. These differences were not reflected in stress questionnaires. This study highlights the importance of seasonality in cortisol biomarker assessment and provides evidence that in this population, the PSS and MIFSS stress questionnaires are stable between seasons. While the results of this study provide some insight on the seasonality of cortisol in a vulnerable population, further work examining these trends in a larger population (n>27) would strengthen our conclusions. As the stress assessment technologies and methodologies improve, it will be possible to integrate questionnaire and biological stress assessments. For example, currently smartphone applications exist for monitoring mental health in real time(Donker, Petrie et al. 2013), as these types of applications expand, it will be possible to ask individuals about their instantaneous stress levels, thus linking acute biological and questionnaire measures of stress. Because we found seasonal differences in cortisol measurements, but not in stress questionnaires, further research improving the integration of stress assessment methods would help substantiate these findings.
Supplementary Material
Figure 3.
Segmental hair cortisol concentrations were significantly different between seasons (Wilcoxon rank sum test, p<0.05). The cumulative probability on the Y-axis shows that at the 50th percentile (horizontal line), hair cortisol concentrations were lower in the summer than in the fall. There is also an increase in the range of hair cortisol samples in the fall, relative to the summer. This is evident by the similarity in hair cortisol concentrations at lower percentiles between the two seasons, but large difference in hair cortisol concentrations at higher percentiles between the two seasons.
Acknowledgements
This work was supported through the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (Contract No. HHSN267200700015C and HHSN267200700023C), the National Institute Of Environmental Health Sciences (Award Numbers 5 P01 ES00960, 5 P30 ES0070331) and the USEPA (Grant Number RD83451401). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, or the USEPA.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Adam E, Gunna MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2000;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Barrington WE, Stafford M, Hamer M, Beresford SA, Koepsell T, Steptoe A. Neighborhood socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health Place. 2014;27:120–126. doi: 10.1016/j.healthplace.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya MR, Molloy GJ, Steptoe A. Depression is associated with flatter cortisol rhythms in patients with coronary artery disease. J Psychosom Res. 2008;65(2):107–113. doi: 10.1016/j.jpsychores.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65(6):984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who's Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 20091. Journal of Applied Social Psychology. 2012;42(6):1320–1334. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Donker T, Petrie K, Proudfoot J, Clarke J, Birch MR, Christensen H. Smartphones for smarter delivery of mental health programs: a systematic review. J Med Internet Res. 2013;15(11):e247. doi: 10.2196/jmir.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz JG, Quandt SA, Chen H, Isom S, Kiang L, Vallejos Q, Arcury TA. Depressive Symptoms among Latino Farmworkers across the Agricultural Season: Structural and Situational Influences. Cultural diversity & ethnic minority psychology. 2010;16(3):335–343. doi: 10.1037/a0019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait componenets. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hohl SD, Gonzalez C, Carosso E, Ibarra G, Thompson B. “I did it for us and I would do it again”: perspectives of rural latinos on providing biospecimens for research. Am J Public Health. 2014;104(5):911–916. doi: 10.2105/AJPH.2013.301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, R. Ferdinand, Veldhuis Johannes D. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287:E656–E661. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht S, Kirchbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiology & Behavior. 2007;90(1):43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- McMaster A, Jangani M, Sommer P, Han N, Brass A, Beesley S, Lu W, Berry A, Loudon A, Donn R, Ray D. Ultradian Cortisol Pulsatility Encodes a Distinct, Biologically Important Signal. Plos One. 2011;6(1):1–9. doi: 10.1371/journal.pone.0015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Hoppmann CA, Scott SB. Diurnal profiles of salivary cortisol and alpha-amylase change across the adult lifespan: Evidence from repeated daily life assessments. Psychoneuroendocrinology. 2013;38(12):3167–3171. doi: 10.1016/j.psyneuen.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson Murphy BE. Urinary Free Cortisol Determinations: What They Measure. The Endocrinologist. 2002;12(2):143–150. [Google Scholar]
- Persson R, Garde AH, Hansen AM, Osterberg K, Larsson B, Orbaek P, Karlson B. Seasonal variation in human salivary cortisol concentration. Chronobiol Int. 2008;25(6):923–937. doi: 10.1080/07420520802553648. [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier HM, Chen E. Socioeconomic status and the health of youth: a multilevel, multidomain approach to conceptualizing pathways. Psychol Bull. 2013;139(3):606–654. doi: 10.1037/a0029416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SB, Whitehead BR, Bergeman CS, Pitzer L. Combinations of stressors in midlife: examining role and domain stressors using regression trees and random forests. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):464–475. doi: 10.1093/geronb/gbs166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Bruton JL. Quantiative Analysis of Cortisol and Cortisone in Urine by LC-MS/MS. Thermo Scientific- Application Note 427. 2008 [Google Scholar]
- Snipes S, Thompson B, O'Connor K, Godina R, Ibarra G. Anthropological and Psychological Merge: Design of a Stress Measure for Mexican Farmworkers. Cult Med Psychiatry. 2007;31(3):359–388. doi: 10.1007/s11013-007-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel SM, Koenders MA, Giltay EJ, Elzinga BM, Manenschijn L, Hoencamp E, van Rossum EF, Spijker AT. Recent negative life events increase hair cortisol concentrations in patients with bipolar disorder. Stress. 2014:1–27. doi: 10.3109/10253890.2014.968549. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Steinisch M, Yusuf R, Li J, Stalder T, Bosch JA, Rahman O, Strümpell C, Ashraf H, Fischer JE, Loerbroks A. Work stress and hair cortisol levels among workers in a Bangladeshi ready-made garment factory – Results from a cross-sectional study. Psychoneuroendocrinology. 2014;50(0):20–27. doi: 10.1016/j.psyneuen.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26(3):295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ. Cumulative Stress and Cortisol Disruption among Black and Hispanic Pregnant Women in an Urban Cohort. Psychol Trauma. 2010;2(4):326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychological Trauma: Theory, Research, Practice, and Policy. 2010;2(4):326. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, Faustman EM. Variability in the take-home pathway: Farmworkers and non-farmworkers and their children. J Expo Sci Environ Epidemiol. 2014 doi: 10.1038/jes.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Young EA, Carlson NE, Brow MB. Twenty-Four-Hour ACTH and Cortisol Pulsatility in Depressed Women. Neuropsychopharmacology. 2001;25(2):267–287. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.