Abstract
Within a two year period in the 1940’s, two Boston physicians published dramatically opposing views on the underlying nature of a syndrome now known as complex regional pain syndrome (CRPS). Evans suggested, in several papers in 1946–1947, that sympathetic reflexes maintain pain and dystrophy in affected limbs. Foisie, in 1947, suggested arterial vasospasms were key in the etiology of this pain syndrome. Evans’ hypothesis established the nomenclature for this syndrome for 60 years, and his term “reflex sympathetic dystrophy” guided clinical treatment and research activities over the same period. Foisie’s proposed nomenclature was unrecognized, and had virtually no impact on the field. Recent evidence suggests that Evans’ contribution to the field may have in fact lead clinicians and researchers astray all those years. This focus article on CRPS compares recent observations with these 2 earlier theories and asks the question -- what if we had adopted Foisie’s nomenclature from the beginning?
Keywords: Complex regional pain syndrome, CRPS, reflex sympathetic dystrophy, arterial vasospasm, nomenclature, etiology, pathophysiology, ischemia
Complex regional pain syndrome (CRPS; see Table 1 for characteristics and diagnostic criteria)35,61 is the current name for a syndrome that has been known over the years by many names, including causlagia, minor causalgia, algodystrophy, shoulderhand syndrome, and Sudeck’s atrophy. However, the most common and recently used name for CRPS was reflex sympathetic dystrophy.83,86 Although mechanistic differences may not exist, the term CRPS-I has been used to replace reflex sympathetic dystrophy and CRPS-II to replace causalgia, with the former diagnosis in cases where no nerve injury is detected, and the latter when nerve injury is confirmed.61 Recent discouragement with the sympathetic nervous system as a therapeutic target, 13,25,53 and an underlying pathology,3,66,84,90 had no doubt encouraged the taxonomic change to CRPS,83,86 and it has been argued that the former syndrome name may have misguided research and treatment since its inception.8 In a treatise on the concept that inspired William Shakespeare to pen the phrase “That which we call a rose by any other name would smell as sweet,” the question should be asked—“What’s in a (syndrome) name?” (Romeo and Juliet 2.2,43–44).81 That is, would history have been different if another name than reflex sympathetic dystrophy had been adopted for the predominant nomenclature of CRPS? To address this question, it would be instructive to review the thinking of the time, back in the mid-1940s, when the term reflex sympathetic dystrophy (and an alternative name) was coined.
Table 1.
International association for the Study of Pain (IASP) Diagnostic Criterion for CRPS-I and CRPS-II61
| CPRS-I (Reflex Sympathetic Dystrophy) | CRPS-II (Causalgia) |
|---|---|
Note: Criteria 2 to 4 must be satisfied. |
Note: All 3 criteria must be satisfied. |
In 1946–47, James A. Evans, a physician at the Lahey Clinic in Burlington, Massachusetts, reported on patients’ intense suffering combined with features characteristic of what he called “stimulation of the sympathetic, … namely rubor, pallor, or a mixture of both, sweating and atrophy….”20 In a series of 4 papers that described at first 3219,20 then a total of 57 patients,21,22 he observed these symptoms in association with fractures (21%), sprains (21%), vascular complications (19%), amputations (9%), joint/bone inflammation (5%), and lacerations (2%), or more minor injuries/conditions including bruises (9%) and static defects of the foot (7%). Pain was described as deep, boring, diffuse, but with trigger points, and manipulation or use aggravated patient suffering. Importantly, Evans reported that often pain in these patients was relieved by sympathetic blocks, as the limb warmed. Evans attributed the pain to abnormality in the sympathetic nervous system, and first used the term reflex sympathetic dystrophy.19–22 Evans suggested that excessive afferent nerve inputs associated with injury initiated central nervous system activity that spread through an “internuncial pool” of hyperactive spinal neurons that stimulated sympathetic neurons. Activity generated in postganglionic sympathetic efferents was then proposed to induce arterial spasms and ischemia that increased capillary filtration pressure with resultant edema and swelling. Evans’ conclusions were clearly influenced by Lorento de No,51 who discussed how “prolonged bombardment of pain impulses” triggers “pools of constant circling of activity across (spinal) synapses” and “a vicious circle” which causes sympathetic neurons to trigger vascular spasms that raise filtration pressure causing oedema. He was also inspired by Livingston,50 who had applied Lorento de No’s above ideas and MacKenzie’s52 theory of an “irritable focus” in spinal cord (in relation to referred pain) to the condition of causalgia, and who had adopted the techniques of Leriche49 by performing sympathectomies to break the vicious circle.
One year after Evans coined the term reflex sympathetic dystrophy, Philip S. Foisie, a surgeon at Boston City Hospital, concluded that a persisting low-grade arterial spasm after soft-tissue injury can lead to a syndrome with severe pain, extreme tenderness or allodynia, tissue swelling, muscle atrophy, bone osteoporosis, joint stiffness, and limited motion. Basically, the constellation of symptoms that Evans would diagnose as reflex sympathetic dystrophy, Foisie termed “traumatic arterial vasospasm.”23 According to Foisie, subacute, undetected arterial vasospasms led to decreases in nutritional blood supply that resulted in degenerative changes primarily in the musculoskeletal system. Foisie was careful to point out that these vasospasms were not in large vessels that produce tissue-threatening ischemia, but rather in small arterioles. As causalities of war, some of the injuries to Foisie’s patients were severe and may have included nerve as well as soft-tissue injury, but others were less severe and the pain was disproportionate to the injury. He described these symptoms in a single paper on wounded soldiers with fractures, soft-tissue injuries, minor gunshot and shell-fragment wounds, but not in extensive “wide-open” wounds.23 Importantly, he observed that the syndrome was even more likely in patients with compression injuries that resulted in increased tissue tension (ie, where microvascular pressure is reduced because of increased intracompartmental pressure). He also noted that swelling was often present early on, particularly following casting and immobilization, and concluded that edema should be expected as ischemia causes microvascular injury that increases plasma extravasation with increased permeability of capillary walls. Additionally, these patients would sometimes respond to a combination of sympathetic blocks, massage, and physical therapy to encourage the restoration of nutritional blood supply back to deep tissues. Foisie described 2 cases in which pain relief was obtained following upper dorsal (sympathetic) ganglionectomy or lumbar (sympathetic) ganglionectomy. Foisie was probably influenced by the work of Leriche,49 who had shown the sympathectomies were useful in the treatment of posttraumatic contractures and causalgia. He was likely also influenced by Homans39 (also from Boston), who discussed the possibility that “gross arterial spasm” and “diffuse peripheral spasm” contributed to minor causalgia.
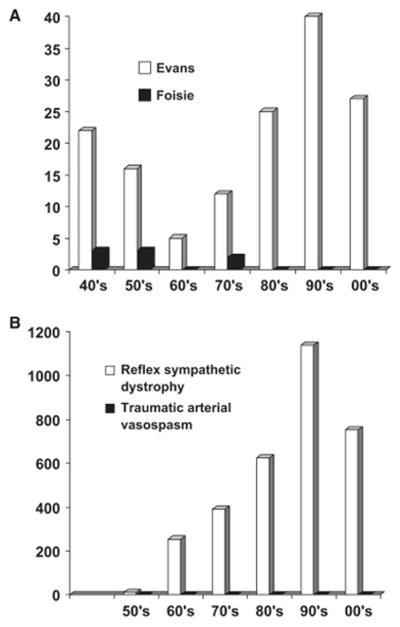
The most interesting point to note is that although Evans and Foisie were both suggesting that sympathetic blocks could be useful to relieve pain in their patients, they came to different conclusions as to what pathology produced the pain. Although, like Foisie, Evans identified arterial vasospasms and capillary permeability as key factors, it was their emphasis on sympathetic nervous system activity that differed dramatically. For Evans, increased activity in sympathetic efferents was crucial to the maintenance of the vicious circle, and spinal hyperactivity maintained the reflex sympathetic drive. For Foisie, it was the nature of injury itself that initiated the vasospasm (particularly in cases where injuries increased intracompartmental pressure), and sympathetic blocks were seen as a way to overcome the vasospasm. And as history has shown through the citation and key word record searches (see Fig 1), Evans’ conclusions were almost universally adopted, whereas Foisie’s were almost completely ignored. Indeed, the volume of references to the term reflex sympathetic dystrophy clearly show that this name dominated medical thinking throughout the latter half of the 20th century. In fact, the emphasis of the sympathetic contribution to reflex sympathetic dystrophy has been a large driver in both the predominant therapeutic practice and mechanistic research activity for the past 6 decades.
Fig. 1. Impact of Evans’ and Foisie’s papers on the field.
A) Histogram of the numbers of Science Citation Index citations found for the papers of Evans19–22 and Foisie,23 respectively by decade between 1940 and 2009. B) Histogram of the numbers of Medline citations found for the terms “reflex sympathetic dystrophy” and “traumatic arterial vasospasm” by decade between 1950 and 2009.
If Not Sympathetically Maintained Pain, Then What?
Now that the pendulum has swung away from the view that sympathetic nervous system is causal for the pain of CRPS, the question is whether it is about time to seriously consider Foisie’s hypothesis. From the obvious lack of attention that Foisie’s theory received, could it be said that while being preoccupied by the notions of reflex sympathetic dystrophy and vicious circles, and later by theories of sympathetically maintained pain and sympathetically independent pain (see below), Foisie’s hypothesis was the one that got away? What if rather than reflex sympathetic dystrophy and sympathetically maintained pain, traumatic arterial vasospasms, and ischemia-maintained pain were the catch phrases for this syndrome? Would a diagnosis of traumatic arterial vasospasm have changed the way the patient would have been treated, or would a syndrome so named have changed the course of research history? Given the lack of progress in the field during the reflex sympathetic dystrophy fixation, and recent research findings that support Foisie’s conclusion, it is likely that the answer is yes!
So what has been learned over the 60-odd years since Evans’ and Foisie’s respectively renowned and ignored hypotheses? First, it is becoming clear that sympathetic blocks do not always relieve pain in CRPS patients.13,25,53 Clearly, some patients have remarkable but often short-lived responses to sympathetic blocks. Other patients do not respond at all, and still others respond, as noted by both Evans and Foisie, only following multiple blocks. However, since many of these trials lack control subjects, gradual improvement over time may reflect the natural history of the syndrome, rather than the use of sympathetic nerve blocks. These findings lead to two possible conclusions: 1) that the pain of CRPS depends on the sympathetic nervous system in some patients but not others; or 2) that the sympathetic nervous system is not critical to pain in CRPS, but may influence pain by affecting other crucial factors. The first conclusion was adopted by proponents of the once-popular notions of sympathetically maintained70 and sympathetically independent24 pain (where the pain is proposed to be dependent on, or independent of, activity in sympathetic efferent fibers, respectively), whereas the latter conclusion has not, until recently, received much attention.
A second important finding is that numerous investigators have now reported that sympathetic activity is actually blunted, rather than enhanced, particularly in early-stage CRPS (when the limb is often hot and swollen).45,77,95 This findings leads to the important conclusion that CRPS cannot depend on enhanced activity in sympathetic efferent fibers, at least not at stages during which the activity is blunted. It also supports the theory that late-phase CRPS (when the limb is often cold and cyanotic) may depend on denervation hypersensitivity of sympathetic vasoconstrictors,27,53,89 a phenomenon that would be expected if sympathetic activity is blunted for prolonged periods.
Role of Ischemia-Reperfusion Injury
A third critical finding is that many CRPS patients have subtle but convincing evidence of persistent tissue ischemia in skin and particularly deep tissue. Thus, muscle tissue from an amputated CRPS limb was found to exhibit lipofuscin pigment, atrophic fibers, and severely thickened basal membrane layers of the capillaries.90 Although amputated tissue may represent extreme cases of CRPS, this conclusion is also supported by functional observations of increased density of perfused vessels, lower capillary filtration capacity, arteriovenous shunting, and ischemia of peripheral subcutaneous tissue in intact CRPS limbs.57,78 It has also been reported that CRPS limbs have high arterial flow, but low oxygen consumption, as well as high lactate flux—indicative of tissue ischemia, despite increased flow in large vessels.29 There is also an impairment of high-energy phosphate metabolism in muscle tissue of CRPS limbs,30,37 suggestive of mitochondrial oxygen deficiency. All of these observations suggest that ischemia in deep tissues contributes to CRPS. Others have also found evidence of microvascular injury in skin, including hypertrophic multilayered, and sometimes collapsed, endothelial cell walls around the arteries and arterials from the skin of amputated CRPS limbs,3 and reduced skin nutritive flow and capillary hemoglobin oxygenation in intact CRPS limbs.44,45,71
So it seems that Evans may have been wrong about sympathetic hyperactivity. However, if sympathetic hyperactivity does not cause the arterial vasospasms that both Foisie and Evans described, what does? Foisie provided a hint about a possible mechanism. The hint was that arterial vasospasms and painful symptoms were more likely (and more severe) after compression injuries that increased tissue tension (that is, lowered microvascular pressure because of increased intracompartmental pressure). Foisie suggested that traumatic arterial vasospasm takes place when increased tissue tension occurs after increased vascular permeability and accumulation of interstitial fluid. He further suggested that arterial vasospasm caused a “prolonged arterial inadequately” or an oxidative “malnutrition” which “… results in degenerative changes of the musculoskeletal system primarily.”23
Importantly, injuries that produce enough edema to increase intracompartmental pressure can result in microvascular changes and injury that depend both on the degree and length of time of the increased intracompartmental pressure. Minor inflammatory injuries cause microvascular disturbances that increase capillary filtration pressure and vascular permeability due to the formation of gaps in the endothelial cell lining of microvessels.58 However, as interstitial fluid increases, there is a compensatory increase in lymphatic flow, which prevents increases in intracompartmental pressure.62 More significant inflammation, after moderate soft tissue injury or fracture, produces plasma extravasation at a rate that cannot be compensated for by lymphatic flow, resulting in increased intracompartmental pressure.64,82 Intramuscular intracompartmental pressures that approach diastolic blood pressure (typically around 70 mmHg) for at least several hours produce a phenomenon known as acute compartment syndrome (although prolonged intracompartmental pressures above 40 to 45 mmHg are typically a warning that an acute compartment syndrome may occur, and indicate that aggressive treatment is warranted).32,97 Acute compartment syndrome results when the pressure from excessive interstitial fluid within a closed anatomical compartment compresses small blood vessels and blocks oxygen supply to the tissue for several hours or more.32
The consequences of a very prolonged acute compartment syndrome can be catastrophic or even fatal, as the ischemic muscle eventually degenerates, producing toxic metabolites that lead to systemic inflammatory response syndrome and multiple organ dysfunction, characteristic of crush syndrome or tourniquet shock.32,79 However, a critical study by Vollmar et al92 has shown that intracompartmental pressures much lower than those needed to produce acute compartment syndrome also have significant effects on microvascular function. Thus, intracompartmental pressures between 25 and 35 mmHg block arterial flow in smaller arterioles and venules. Furthermore, an even lower intracompartmental pressure of 12 mmHg produces a 50% reduction in the number of perfused capillaries, reducing nutritive blood flow to the involved tissue.92 Importantly, it was also found that subsequent reduction of intracompartmental pressure by as little as 4 mmHg restores capillary flow.92 Therefore, for a significant number of capillaries, capillary flow can be blocked (causing ischemia) and re-established (causing reperfusion) with very small changes in intracompartmental pressures, at levels that are much lower than required to cause acute compartment syndrome. If the ischemia associated with the capillary blockade occurs for a few hours, the tissue within the capillary bed will be subjected to an ischemia reperfusion (IR) injury, once the pressure is reduced and the tissue is reperfused. Importantly, it has been shown that after closed soft tissue injury of the rat hind limb, intracompartmental pressures are elevated between 13.6 and 25.4 mmHg in the anterior compartment and 7.9 and 16.1 mmHg in the posterior compartment for up to 72 hours post injury. Similar to tourniquet-induced IR injury, these increases lead to significant vascular permeability, edema formation, leukocyte rolling and adherence, and decreases in functional capillary density, but not acute compartment syndrome.75,88 More severe soft-tissue injury also produces arterial constriction and capillary perfusion failure lasting 5 days,6 and the microvasculature dysfunction associated with severe soft-tissue injury is alleviated with the free radical scavenger N-acetylcysteine.74 Soft-tissue injury in the tibial compartment has also been shown to produce similar microvascular dysfunction in the rat tibial periosteum,76 whereas closed fracture of the rat tibia produces microvascular dysfunction in adjacent skeletal muscle.101 Indeed, patients often exhibit high muscle intracompartmental pressures (over 40 mmHg for 24 hours) after tibial fractures. The increased intracompartmental pressures produce simultaneous reductions in perfusion pressure (reflecting low capillary flow), which correlates highly with later muscle deficits.64 Thus, it seems the mechanism on which Foisie was not able to elaborate in his patients may have been microvascular dysfunction following IR injury. IR injuries are so named because of the recognition that most of the damage that occurs following prolonged ischemia occurs after the tissue is reperfused, a discovery made three decades after Foisie’s paper.
Importantly, recent evidence indicates that IR injuries may cause some of the bewildering symptoms observed in CRPS patients. IR injuries initially produce increased capillary and postcapillary venule permeability leading to extensive plasma extravasation and edema,58 a very significant feature of early-stage CRPS.48,96 IR injuries are produced by the generation of oxygen free radicals and proinflammatory cytokines,41,87,100 which produce both microvascular dysfunction and additional IR injury in neighboring tissues, including muscle, bone, and skin.10 Recent evidence suggests that both oxygen-free radicals and proinflammatory cytokines are elevated in CRPS,18,38 and CRPS is alleviated by treatments aimed at reducing these mediators.40,67 CRPS often spreads beyond dermatomal borders, and affects both skin and various deep tissues.54 This parallels what happens with IR injuries, where all tissues (including muscle, bone, and skin) within the occluded area suffer the same consequences (although bone and skin are more resistant than muscle).10 While IR injuries clearly cannot account for motor abnormalities or altered sympathetic or sudomotor activity often seen in CRPS limbs, or the occasional spread of symptoms to the contralateral limb, it is possible that afferent input from injured muscle may be instrumental in producing central sensitization93 that could contribute to these phenomena.
It could be argued that many CRPS patients do not have the type of tissue damage that leads to IR injury. However, CRPS commonly follows factures, sprains, contusions and crush injuries, athroscopic surgery, overly tight casting, and other edematous soft-tissue injuries.4,26,73 A common feature of all these conditions is an early inflammatory response in soft tissue that has the potential to produce microvascular and ischemic changes in various tissues. While others have noted that CRPS depends on an exaggerated inflammatory response,30,37 most CRPS patients are seen by pain specialists many weeks, months, or years after their original injuries, and initial inflammatory symptoms may be long forgotten. If the soft-tissue injury and inflammation is severe enough to cause a temporary (hours long) increase in intracompartmental pressure that occludes capillary blood flow, a microvascular IR injury will occur. These microvascular injuries would then be expected to produce sustained hypoperfusion and reduced nutritive blood flow. Importantly, since this is most likely to occur in deep tissues, the ensuing ischemic changes may go undetected.
Role of Microvascular Dysfunction
IR injuries produce significant arterial vasospasms or enhanced vasoconstriction due to microvascular dysfunction associated with increased responsiveness of smooth muscle adrenoceptors to the sympathetic transmitter norepinephrine,72 and a reduced production of the endogenous vasodilator nitric oxide.47,94 Both Foisie and Evans described arterial vasospasms, and recent evidence indicates that CRPS limbs exhibit both reduced nitric-oxide33 and enhanced vasoconstrictor responsiveness to norepinephrine.2,7,16,89 Importantly, the main cause of arterial vasospasm or enhanced vasoconstriction following IR injury is the dramatic loss of nitric oxide (much of which is scavenged by increased oxygen-free radicals to produce nitrogen-free radicals).10,94 Thus, arterial vasospasms can be maintained after IR injury, even if sympathetic activity is blunted in CRPS patients, which itself would be a natural homeostatic response to the vasospasm.
Avery significant later consequence of IR injury is damage to capillaries. Free radicals and cytokines generated by IR injury trigger neutrophil recruitment and adhesion, capillary plugging, endothelial cell swelling, and the eventual collapse of the capillary lumen, leading to a phenomenon called capillary no-reflow.36,60 Capillary no-reflow results in arterial venous shunting,42 and a significant reduction of nutritive blood flow within the affected capillary. Importantly, arterial venous shunting and collapsed capillaries have been observed in the limbs of patients with CRPS.57,90 Furthermore, IR injuries initially produce enhanced vascular permeability that contributes to the damage causing vasospasms, but as vasospasms progress to no-reflow, plasma extravasation is diminished, as there is reduced blood flow within the microvasculature.59 This shift from initial high plasma extravasation to later capillary no-reflow, could explain why many CRPS patients start out with significant edema, but then progress to a state with less edema and more ischemia.9,11,45 Later capillary no-reflow may also explain why CRPS is less likely to respond to anti-sympathetic treatment with greater time following syndrome onset,1 as anti-sympathetic treatment likely relieves vasospasms, but not capillary no-reflow.
Recent evidence further links the fields of CRPS and IR injury by showing that after a hind paw IR injury, rodents develop vascular and painful symptoms similar to that exhibited by CRPS patients.14,46 Allodynia that develops following rat hind paw IR injury also depends on the generation of oxygen-free radicals and proinflammatory cytokines.46 Importantly, after hind paw IR injury, rats with vasoconstrictive hypersensitivity to norepinephrine (vasospasms) are more likely to develop allodynia, as well as painful responses to intraplantar norepinephrine.98 Similar to CRPS patients,17 sympathetic blockade using guanethidine is more effective at relieving allodynia in rats when performed early after hind paw IR injury.99 As well as vasospasms, rats with hind paw IR injuries develop no-reflow over a time course that parallels allodynia, and exhibit muscle ischemia and increased muscle lactate (as do CRPS patients29) that is directly correlated with the allodynia.46 Like CRPS patients,91 rats with hind pawIR injuries also display painful responses to exercise, and parallel increases in muscle lactate and allodynia after exercise.46
Novel Therapeutics and Conclusions
There is growing evidence that targeting pathologies associated with microvascular dysfunction and ischemia are effective against CRPS. Thus, CRPS is alleviated with free radical scavengers,31,67,104 and antitumor necrosis factor-a antibodies,40,56,80 which would mitigate the inflammatory and damaging effects of oxygen-free radicals and proinflammatory cytokines. CRPS has also been shown to be alleviated by nitric-oxide donors and other vaosodilators,34,55,63 or a-adrenergic antagonists,63,68 and topical or regional clonidine,15,28,43,69 which act by inhibition of the effects of sympathetic vasoconstrictors on vasculature smooth-muscle cells. Antioxidant vitamin C administration has also recently been shown to reduce the incidence of CRPS when administrated as a prophylactic treatment after fracture.5,102,103
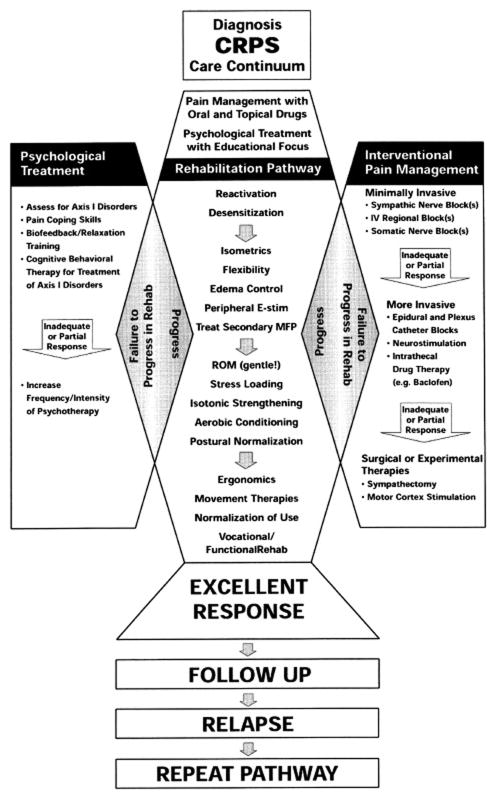
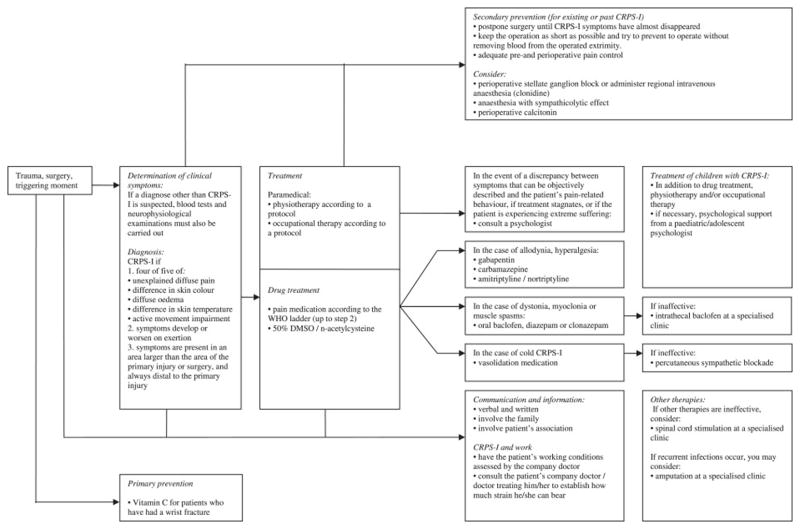
The very recent shift in thinking about the treatment of CRPS is reflected in the differences between a therapeutic algorithm for CRPS outlined by an expert panel that met in Cardiff in 200185 (Fig 2) versus the evidence-based guidelines for CRPS published by the Netherlands Society of Rehabilitation Specialists and the Netherlands Society of Anaesthesiologists in 200765 (Fig 3). The 2001 algorithm lists sympathetic nerve blocks as a first interventional pain-management procedure and lists surgical sympathectomy as a more invasive procedure to be performed in case of inadequate or partial response. The 2007 Dutch evidence-based guidelines list free radical scavengers, 50% DMSO, and n-acetylcysteine under drug treatments, as well as vasodilators for “cold CRPS” and vitamin C as primary prevention for wrist fractures. Importantly, these later guidelines do not list sympathetic blockade as a first-line treatment, and recommend “extreme caution” when considering surgical sympathectomy. Importantly, sympathetic blocks may still be useful in alleviating CRPS pain associated with microvascular dysfunction and deep tissue ischemia, but they should not imply that sympathetic abnormalities are causative.
Fig. 2. Consensus views of therapy for CRPS at the end of the 20th century.
Therapeutic algorithm (“Diagnosis CRPS Care Continuum” with emphasis on therapeutic options in response to patient’s clinical progress in the rehabilitation pathway. Based on the Interdisciplinary Clinical Pathway for CRPS prepared by an Expert Panel in Cardiff, Wales in 2001 (reprinted with permission from Stanton-Hicks et al, An updated interdisciplinary clinical pathway for CRPS: Report of an expert panel. Pain Practice, John Wiley and Sons85).
Fig. 3. Very recent views on therapy for CRPS.
Summary chart of the 2007 evidence-based guidelines for complex regional pain syndrome reported by the Netherlands Society of Rehabilitation Specialists and the Netherlands Society of Anesthesiologists.65 Reprinted with permission of the Dutch Institute for Health Care Improvement, Evidence-Based Guidelines Development programme (EBGD) of the Order of Medical Specialists.
While recent evidence points to the strong possibility that following IR injury, arterial vasospasms, no-reflow, and deep-tissue ischemia may be key underlying mechanisms of CRPS, one may ask the question: Why were these mechanisms overlooked for so long? Both Evans and Foisie described arterial vasospasms and ischemia as key features present in their patients. Perhaps the sympathetic reflex hypothesis derived by Evans was just too compelling to allow us to entertain other possibilities. And by concentrating on the sympathetic nervous system, everyone lost sight of the key role of vasospasms and ischemia. Evans’ hypothesis was based on the earlier theories of Livingston,50 a clinician-scientist who had a large influence on his contemporaries and future pain researchers. The ideas of Evans and Livingston clearly had an impression on John Bonica, key founder and second president of the International Association for the Study of Pain. Bonica11,12 was a strong proponent of the idea that reflex sympathetic dystrophy depended on a vicious circle and sympathetic efferent hyperactivity, and he was instrumental in the adoption of the nomenclature proposed by Evans. But more than people, it is ideas that shape research trends, and it seems that, at the time, Evans’ idea was more attractive than Foisie’s. This may have stemmed from a neuron-centric view of pain processing, with an overemphasis of the importance of nerve cells to the generation, as well as transmission, of pain signals. Hopefully, recent research findings outlined here will inspire pain researchers and clinicians to consider endothelial cells and the microvasculature as important potential generators of the noxious inputs critical for CRPS, and put to rest not only the name, reflex sympathetic dystrophy, but the misguided theories, research, and limited treatment it spawned. Perhaps more than 6 decades later, Foisie and his little-known paper will finally receive their due recognition, and patients with CRPS will benefit from the multitude of additional potential therapies new thinking will generate.
Perspective.
This article discusses two opposing historical views on the etiology of what is now known as CRPS, and how they affected nomenclature, research, and clinical therapy in subsequent decades. This focus article may help researchers and clinicians realize the importance of syndrome names, and how they may inadvertently misdirect research and treatment.
Acknowledgments
The author wishes to thank Dr. Gary J. Bennett for comments on the manuscript.
This work was supported by grants from CIHR, NSERC and the Louise and Alan Edwards Foundation.
References
- 1.AbuRahma AF, Robinson PA, Powell M, Bastug D, Boland JP. Sympathectomy for reflex sympathetic dystrophy: Factors affecting outcome. Ann Vasc Surg. 1994;8:372–379. doi: 10.1007/BF02133000. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman WE, Munir MA, Zhang J-M. Assessment of laser Doppler imaging for the diagnosis of complex regional pain syndrome I. J Neuropathic Pain Symp Pal. 2005;1:13–20. [Google Scholar]
- 3.Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Paré M, Davar G, Rice FL. Pathologic alterations of cutaneous innervations and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: A retrospective chart review of 134 patients. Pain. 1999;80:539–544. doi: 10.1016/S0304-3959(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 5.Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82:873. doi: 10.2106/00004623-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Amon M, Laschke MW, Harder Y, Vollmar B, Menger MD. Impact of severity of local soft-tissue trauma on long-term manifestation of microcirculatory and microlymphatic dysfunctions. J Trauma. 2006;61:924–932. doi: 10.1097/01.ta.0000195979.25659.fe. [DOI] [PubMed] [Google Scholar]
- 7.Arnold JMO, Teasell RW, Macleod AP, Brown JE, Carruthers SG. Increased venous alpha-adrenoceptors responsiveness in patients with reflex sympathetic dystrophy. Ann Intern Med. 1993;83:185–192. doi: 10.7326/0003-4819-118-8-199304150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bennett GJ. Epilogue. In: Harden RN, Baron R, Janig W, editors. Complex Regional Pain Syndrome, Progress in Pain Research and Management. Vol. 22. Seattle, WA: IASP Press; 2001. pp. 323–325. [Google Scholar]
- 9.Birklein F, Riedl B, Claus D, Neundorfer B. Pattern of autonomic dysfunction in time course of complex regional pain syndrome. Clin Auton Res. 1998;8:79–85. doi: 10.1007/BF02267817. [DOI] [PubMed] [Google Scholar]
- 10.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonica JJ. Causalgia and other reflex sympathetic dystrophies. In: Bonica JJ, editor. The Management of Pain. 2. Philadelphia, PA: Lea & Febiger; 1990. pp. 220–243. [Google Scholar]
- 12.Bonica JJ. The Management of Pain. 1. Philadelphia, PA: Lea & Febiger; 1953. [Google Scholar]
- 13.Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: A narrative and systematic review. Clin J Pain. 2002;18:216–233. doi: 10.1097/00002508-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-type I (CRPS-I;reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Davis KD, Treede RD, Raja SN, Meyer RA, Cambell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 16.Dayan L, Salman S, Norman D, Vatine JJ, Calif E, Jacob G. Exaggerated vasoconstriction in Complex Regional Pain Syndrome-1 is associated with impaired resistance artery endothelial function and local vascular reflexes. J Rheumatol. 2008;35:1339–1345. [PubMed] [Google Scholar]
- 17.Driessen JJ, van derWerken C, Nicolai JP, Crul JF. Clinical effects of regional intravenous guanethidine (Ismelin) in reflex sympathetic dystrophy. Acta Anaesthesiol Scand. 1983;27:505–509. doi: 10.1111/j.1399-6576.1983.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg E, Shtahl S, Geller R, Reznick AZ, Sharf O, Ravbinovich M, Erenreich A, Nagler RM. Serum and salivary oxidative analysis in Complex Regional Pain Syndrome. Pain. 2008;138:226–232. doi: 10.1016/j.pain.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Evans JA. Reflex sympathetic dystrophy. Surg Clin North America. 1946;26:780–790. [PubMed] [Google Scholar]
- 20.Evans JA. Reflex sympathetic dystrophy. Surg Gynecol Obstet. 1946;82:36–44. [PubMed] [Google Scholar]
- 21.Evans JA. Reflex sympathetic dystrophy: report of 57 cases. Ann Intern Med. 1947;26:417–426. doi: 10.7326/0003-4819-26-3-417. [DOI] [PubMed] [Google Scholar]
- 22.Evans JA. Sympathectomy for reflex sympathetic dystrophy: A case report of twenty-nine cases. JAMA. 1946;132:620–623. doi: 10.1001/jama.1946.02870460010003. [DOI] [PubMed] [Google Scholar]
- 23.Foisie PS. Traumatic arterial vasospasm. N Engl J Med. 1947;237:295–302. doi: 10.1056/NEJM194708282370901. [DOI] [PubMed] [Google Scholar]
- 24.Frost SA, Raja SN, Campbell JN, Meyer RA, Khan AA. Does hyperalgesia to cooling stimuli characterize patients with sympathetically maintained pain (reflex sympathetic dystrophy)? In: Dubner R, Gebhart GF, Bond MR, editors. Proceedings of the 5th World Congress on Pain, Pain Research and Clinical Management. Vol. 3. Amsterdam, The Netherlands: Elsevier; 1988. pp. 151–156. [Google Scholar]
- 25.Furlan AD, Lui PW, Mailis A. Chemical sympathectomy for neuropathic pain: Does it work? Case report and systematic literature review. Clin J Pain. 2001;17:327–336. doi: 10.1097/00002508-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in Complex Regional Pain Syndrome: A pilot survey. J Pain Symptom Manage. 2000;20:286–292. doi: 10.1016/s0885-3924(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the pathophysiology of complex regional pain syndrome: Focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717–724. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 28.Gintautas J, Housny W, Kraynack BJ. Successful treatment of reflex sympathetic dystrophy by bier block with lidocaine and clonidine. Proc West Pharmacol Soc. 1999;42:101. [PubMed] [Google Scholar]
- 29.Goris RJ. Conditions associated with impaired oxygen extraction. In: Guitierrez G, Vincent JL, editors. Tissue Oxygen Utilization. 1. Berlin, DE: Springer; 1991. pp. 350–369. [Google Scholar]
- 30.Goris RJ. Reflex sympathetic dystrophy: Model of a severe regional inflammatory response syndrome. World J Surg. 1998;22:197–202. doi: 10.1007/s002689900369. [DOI] [PubMed] [Google Scholar]
- 31.Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3:13–18. doi: 10.3109/10715768709069764. [DOI] [PubMed] [Google Scholar]
- 32.Gourgiotis S, Villias C, Germanos S, Foukas A, Ridolfini MP. Acute limb compartment syndrome: A review. J Surg Educ. 2007;64:178–186. doi: 10.1016/j.jsurg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groeneweg JG, Niehof S, Wesseldijk F, Huygen FJ, Zijlstra FJ. Vasodilative effect of isosorbide dinitrate ointment in complex regional pain syndrome type 1. Clin J Pain. 2008;24:89–92. doi: 10.1097/AJP.0b013e318156db3b. [DOI] [PubMed] [Google Scholar]
- 35.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 36.Harris AG, Steinbauer M, Leiderer R, Messmer K. Role of leukocyte plugging and edema in skeletal muscle ischemia reperfusion injury. Am J Physiol. 1997;273:H989–H996. doi: 10.1152/ajpheart.1997.273.2.H989. [DOI] [PubMed] [Google Scholar]
- 37.Heerschap A, den Hollander JA, Reynen H, Goris RJA. Metabolic changes in reflex sympathetic dystrophy: A 31P NMR spectroscopy study. Muscle Nerve. 1993;16:367–373. doi: 10.1002/mus.880160405. [DOI] [PubMed] [Google Scholar]
- 38.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;1:28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homans J. Minor causalgia following injuries and wounds. Ann Surg. 1941;113:932–941. doi: 10.1097/00000658-194106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. J Pain Sympt Manage. 2004;27:101–103. doi: 10.1016/j.jpainsymman.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Inauen W, Suzuki M, Granger DN. Mechanisms of cellular injury: Potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc Endothelium Lymphatics. 1989;5:143–155. [PubMed] [Google Scholar]
- 42.Kennedy TJ, Miller SH, Nellis SH, Buck D, Flaim SF, Graham WP, 3rd, Davis TS. Effects of transient ischemia on nutrient flow and arteriovenous shunting in canine hindlimb. Ann Surg. 1981;193:255–263. doi: 10.1097/00000658-198103000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkpatrick AF, Derasari M. Transdermal clonidine: Treating reflex sympathetic dystrophy. RegAnesth. 1993;18:140–141. [PubMed] [Google Scholar]
- 44.Koban M, Leis S, Schultze-Mosgau S, Birklein F. Tissue hypoxia in complex regional pain syndrome. Pain. 2003;104:149–157. doi: 10.1016/s0304-3959(02)00484-0. [DOI] [PubMed] [Google Scholar]
- 45.Kurvers HA, Jacobs MJ, Beuk RJ, Van den Wildenberg FA, Kitslaar PJ, Slaaf DW, Reneman RS. Reflex sympathetic dystrophy: Evolution of microcirculatory disturbances in time. Pain. 1995;60:333–340. doi: 10.1016/0304-3959(94)00133-y. [DOI] [PubMed] [Google Scholar]
- 46.Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran JV, Huygen FJ, Bennett GJ, Coderre TJ. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol Pain. 2008;4:49. doi: 10.1186/1744-8069-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefer AM, Tsao PS, Lefer DJ, Ma XL. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5:2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- 48.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 49.Leriche R. La chirurgie de la douleur. 1. Paris, FR: Masson et Cie; 1937. [Google Scholar]
- 50.Livingston WK. Pain Mechanisms. 1. New York, NY: Macmillan; 1943. [Google Scholar]
- 51.Lorento de Nó R. Analysis of the activity of chains of internuncial neurons. J Neurophysiol. 1938;1:207–244. [Google Scholar]
- 52.MacKenzie J. Some points bearing on the association of sensory disorders and visceral disease. Brain. 1893;16:321–354. [Google Scholar]
- 53.Mailis A, Furlan A. Sympathectomy for neuropathic pain. Cochrane Database Syst Rev. 2003:CD002918. doi: 10.1002/14651858.CD002918. [DOI] [PubMed] [Google Scholar]
- 54.Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in Complex Regional Pain Syndrome, Type I (Reflex Sympathetic Dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- 55.Manahan AP, Burkman KA, Malesker MA, Benecke GW. Clinical observation on the use of topical nitroglycerin in the management of severe shoulder-hand syndrome. Nebr Med J. 1993;78:87–89. [PubMed] [Google Scholar]
- 56.Manning DC. New and emerging pharmacological targets for neuropathic pain. Curr Pain Headache Rep. 2004;8:192–198. doi: 10.1007/s11916-004-0051-7. [DOI] [PubMed] [Google Scholar]
- 57.Matsumura H, Jimbo Y, Watanabe K. Haemodynamic changes in early phase reflex sympathetic dystrophy. Scand J Plast Reconstr Surg Hand Surg. 1996;30:133–138. doi: 10.3109/02844319609056395. [DOI] [PubMed] [Google Scholar]
- 58.McDonald DM, Thurston G, Baluk P. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation. 1999;6:7–22. [PubMed] [Google Scholar]
- 59.Menger MD, Pelikan S, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: significance of “reflow paradox”. Am J Physiol. 1992;263:H1901–H1906. doi: 10.1152/ajpheart.1992.263.6.H1901. [DOI] [PubMed] [Google Scholar]
- 60.Menger MD, Rücker M, Vollmar B. Capillary dysfunction in striated muscle ischemia/reperfusion: On the mechanisms of capillary “no-reflow”. Shock. 1997;8:26–31. [PubMed] [Google Scholar]
- 61.Merskey H, Bogduk N, editors. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 62.Modi S, Stanton AW, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: A critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5:183–202. doi: 10.1089/lrb.2007.5306. [DOI] [PubMed] [Google Scholar]
- 63.Muizelaar JP, Kleyer M, Hertogs IA, DeLange DC. Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia):Management with the calcium channel blocker nifedipine and/or the alpha-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg. 1997;99:26–30. doi: 10.1016/s0303-8467(96)00594-x. [DOI] [PubMed] [Google Scholar]
- 64.Müller M, Disch AC, Zabel N, Haas NP, Schaser KD. Initial intramuscular perfusion pressure predicts early skeletal muscle function following isolated tibial fractures. J Orthop Surg Res. 2008;3:14. doi: 10.1186/1749-799X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Netherlands Society of Rehabilitation Specialists and the Netherlands Society of Anaesthesiologists. [Accessed March, 2009];Guidelines for Complex Regional Pain Syndrome type. 1 Available at: http://www.posttraumatischedystrofie.nl/pdf/CRPS_I_Guidelines.pdf. [Google Scholar]
- 66.Oaklander AI, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;120:235–243. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 67.Perez RS, Zuurmond WW, Bezemer PD, Kuik DJ, van Loenen AC, de Lange JJ, Zuidhof AJ. The treatment of complex regional pain syndrome type I with free radical scavengers: A randomized controlled study. Pain. 2003;102:297–307. doi: 10.1016/S0304-3959(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 68.Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: A diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698. doi: 10.1097/00000542-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 69.Reuben SS, Sklar J. Intravenous regional anesthesia with clonidine in the management of complex regional pain syndrome of the knee. J Clin Anesth. 2002;14:87–91. doi: 10.1016/s0952-8180(01)00346-4. [DOI] [PubMed] [Google Scholar]
- 70.Roberts W. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- 71.Rosen L, Ostergren J, Fagrell B, Stranden E. Skin microvascular circulation in the sympathetic dystrophies evaluated by videophotometric capillaroscopy and laser Doppler fluxmetry. Eur J Clin Invest. 1988;18:305–308. doi: 10.1111/j.1365-2362.1988.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 72.Sapienza P, Edwards JD, Mingoli A, McGregor PE, Cavallari N, Agrawal DK. Ischemia-induced peripheral arterial vasospasm role of alpha1- and alpha2-adrenoceptors. J Surg Res. 1996;62:192–196. doi: 10.1006/jsre.1996.0194. [DOI] [PubMed] [Google Scholar]
- 73.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 74.Schaser KD, Bail HJ, Schewior L, Stover JF, Melcher I, Haas NP, Mittlmeier T. Acute effects of N-acetylcysteine on skeletal muscle microcirculation following closed soft tissue trauma in rats. J Orthop Res. 2005;23:231–241. doi: 10.1016/j.orthres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Schaser KD, Vollmar B, Menger MD, Schewior L, Kroppenstedt SN, Raschke M, Lübbe AS, Haas NP, Mittlmeier T. In vivo analysis of microcirculation following closed soft-tissue injury. J Orthop Res. 1999;17:678–685. doi: 10.1002/jor.1100170509. [DOI] [PubMed] [Google Scholar]
- 76.Schaser KD, Zhang L, Haas NP, Mittlmeier T, Duda G, Bail HJ. Temporal profile of microvascular disturbances in rat tibial periosteum following closed soft tissue trauma. Langenbecks Arch Surg. 2003;388:323–330. doi: 10.1007/s00423-003-0411-5. [DOI] [PubMed] [Google Scholar]
- 77.Schürmann M, Gradl G, Zaspel J, Kayser M, Löhr P, Andress HJ. Peripheral sympathetic function as a predictor of complex regional pain syndrome type I (CRPS I) in patients with radial fracture. Auton Neurosci. 2000;86:127–134. doi: 10.1016/S1566-0702(00)00250-2. [DOI] [PubMed] [Google Scholar]
- 78.Schurmann M, Zaspel J, Grandl G, Wipfel A, Christ F. Assessment of the peripheral microcirculation using computer-assisted venous congestion plethysmography in post-traumatic complex regional pain syndrome type I. J Vasc Res. 2001;38:453–461. doi: 10.1159/000051078. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz JT, Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71:392–400. [PubMed] [Google Scholar]
- 80.Schwartzman RJ, Chevlen E, Bengtson K. Thalidomide has activity in treating complex regional pain syndrome. Arch Inten Med. 2003;163:1487–1488. doi: 10.1001/archinte.163.12.1487. [DOI] [PubMed] [Google Scholar]
- 81.Romeo Shakespeare W, Juliet . In: Shakespeare: The Complete Works. 2. Harrison GB, editor. New York, NY: Harcourt Brace & Company, Ltd; 1952. pp. 474–510. [Google Scholar]
- 82.Smith JB, Pedersen NC, Morris B. The role of the lymphatic system in inflammatory responses. SerHaematol. 1970;3:17–61. [PubMed] [Google Scholar]
- 83.Stanton-Hicks MD. Complex regional pain syndrome: A new name for reflex sympathetic dystrophy and causalgia. Curr Pain Headache Rep. 1997;1:34–40. [Google Scholar]
- 84.Stanton-Hicks MD. Reflex sympathetic dystrophy: A sympathetically mediated pain syndrome or not? Curr Rev Pain. 2000;4:268–275. doi: 10.1007/s11916-000-0103-6. [DOI] [PubMed] [Google Scholar]
- 85.Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, Hassenbusch SJ, Lubenow TR, Oakley JC, Racz GB, Raj PP, Rauck RL, Rezai AR. An updated interdisciplinary clinical pathway for CRPS: Report of an expert panel. Pain Pract. 2002;2:1–16. doi: 10.1046/j.1533-2500.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- 86.Stanton-Hicks MD, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain. 1995;63:127–133. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 87.Sternbergh WC, Tuttle TM, Makhoul RG, Bear HD, Sobel M, Fowler AA. Postischemic extremities exhibit immediate release of tumor necrosis factor. J Vasc Surg. 1994;20:474–481. doi: 10.1016/0741-5214(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 88.Szczesny G, Veihelmann A, Nolte D, Messmer K. Changes in the local blood and lymph microcirculation in response to direct mechanical trauma applied to leg: In vivo study in an animal model. J Trauma. 2001;51:508–517. doi: 10.1097/00005373-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Teasell RW, Arnold JM. Alpha-1 adrenoceptor hyperresponsiveness in three neuropathic pain states: Complex regional pain syndrome 1, diabetic peripheral neuropathic pain and central pain states following spinal cord injury. Pain Res Manage. 2004;9:89–97. doi: 10.1155/2004/150503. [DOI] [PubMed] [Google Scholar]
- 90.van der Laan L, ter Laak HJ, Gabreels-Festen A, Gabreels F, Goris RJA. Complex regional pain syndrome type I (RSD): Pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–25. doi: 10.1212/wnl.51.1.20. [DOI] [PubMed] [Google Scholar]
- 91.Veldman PH, Reyen HM, Arnt IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: Prospective study of 829 patients. Lancet. 1993;342:1012–1016. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- 92.Vollmar B, Westermann S, Menger MD. Microvascular response to compartment syndrome-like external pressure elevation: An in vivo fluorescence microscopic study in the hamster striated muscle. J Trauma. 1999;46:91–96. doi: 10.1097/00005373-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 93.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol (Lond) 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang WZ, Anderson G, Fleming JT, Peter FW, Franken RJ, Acland RD, Barker J. Lack of nitric oxide contributes to vasospasm during ischemia/reperfusion injury. Plast Reconstr Surg. 1997;99:1099–1108. doi: 10.1097/00006534-199704000-00028. [DOI] [PubMed] [Google Scholar]
- 95.Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): Complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613–620. doi: 10.1001/archneur.56.5.613. [DOI] [PubMed] [Google Scholar]
- 96.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 97.Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43–51. doi: 10.1097/00003086-197511000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Xanthos DN, Bennett GJ, Coderre TJ. Norepinephrine-induced nociception and vasoconstrictor hypersensitivity in rats with chronic post-ischemia pain. Pain. 2008;137:640–651. doi: 10.1016/j.pain.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xanthos DN, Coderre TJ. Sympathetic vasoconstrictor antagonism and vasodilatation relieve mechanical allodynia in rats with chronic postischemia pain. J Pain. 2008;9:423–433. doi: 10.1016/j.jpain.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L, Bail H, Mittlmeier T, Haas NP, Schaser KD. Immediate microcirculatory derangements in skeletal muscle and periosteum after closed tibial fracture. J Trauma. 2003;54:979–985. doi: 10.1097/01.TA.0000025796.74054.5B. [DOI] [PubMed] [Google Scholar]
- 102.Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am. 2007;89:1424–1431. doi: 10.2106/JBJS.F.01147. [DOI] [PubMed] [Google Scholar]
- 103.Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: A randomised trial. Lancet. 1999;354:2025–2028. doi: 10.1016/S0140-6736(99)03059-7. [DOI] [PubMed] [Google Scholar]
- 104.Zuurmond WW, Langendijk PN, Bezemer PD, Brink HE, de Lange JJ, van Loenen AC. Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiol Scand. 1996;40:364–367. doi: 10.1111/j.1399-6576.1996.tb04446.x. [DOI] [PubMed] [Google Scholar]