Abstract
The interaction of CD28, which is constitutively expressed on T cells, with B7.1/B7.2 expressed on APCs is critical for T cell activation. CD28 is also expressed on murine and human plasma cells but its function on these cells remains unclear. There are two types of plasma cells: short-lived ones that appear in the secondary lymphoid tissue shortly after Ag exposure, and long-lived plasma cells that mainly reside in the bone marrow. We demonstrate that CD28-deficient murine short- and long-lived plasma cells produce significantly higher levels of Abs than do their wild-type counterparts. This was owing to both increased frequencies of plasma cells as well as increased Ab production per plasma cell. Plasma cells also express the ligand for CD28, B7.1, and B7.2. Surprisingly, deficiency of B7.1 and B7.2 in B cells also led to higher Ab levels, analogous to Cd28−/− plasma cells. Collectively, our results suggest that the CD28–B7 interaction operates as a key modulator of plasma cell function.
Plasma cells are the terminally differentiated effector cells of the humoral arm of adaptive immunity. Plasma cells can be short-lived, with a half-life as short as 2 wk, or long-lived and persist for a lifetime (1). Upon encounter with cognate T-dependent or -independent Ags, marginal zone and follicular B cells proliferate and differentiate into extrafollicular foci of short-lived plasma cells. Exposure to T-dependent but not T-independent Ags induces a subset of the Ag-primed B cells to migrate into the B cell follicle and form germinal centers (2). In the germinal centers, B cells undergo affinity maturation, somatic hypermutation, and rigorous selection of high-affinity B cell clones (3). Some of the selected germinal center B cells enter the memory pool whereas others migrate into the bone marrow and mature into long-lived plasma cells that persist for a lifetime. Plasma cells are heterogeneous in isotype and function (4, 5) and hence are pivotal in providing immediate and long-term immunity. However, the mechanisms involved in the regulation of plasma cell function are not fully understood.
CD28 is a 44-kDa surface protein constitutively expressed on naive T cells and upregulated on activated T cells. The role of CD28 on T cell function is well established (6, 7). CD28 binds to B7.1 (CD80) and B7.2 (CD86) on activated APCs and provides the critical second signal for optimal activation of naive T cells that have encountered cognate Ag. T cells that respond to Ag in the absence of CD28 costimulation are rendered anergic. CD28 mediates its costimulatory functions by lowering the activation threshold, promoting survival, differentiation, and proliferation of activated T cells (6, 8–12). Predictably, mice deficient in CD28 or its ligands B7-1 and B7-2 are impaired in T cell immune response (6). Consequently, T-dependent B cell responses such as germinal center formation and Ig class switching are compromised (13, 14).
Interestingly, CD28 expression is induced on human and murine plasma cells during their terminal differentiation (15, 16). The role of CD28 expression on plasma cells has mainly been examined in transformed plasma cell lines and in multiple myeloma patients where it is associated with enhanced survival and poor disease prognosis (17–20). Consistent with these observations, a recent study demonstrated that in response to a model Ag, CD28 is crucial for the survival of IgG1+ plasma cells (21). However, the role of CD28 expression on survival and function of plasma cells bearing non-IgG1 Abs or plasma cells induced in response to live virus infections remains unknown. In this study, we first confirmed that CD28 is expressed on the surface of short- and long-lived plasma cells. Next, we investigated whether CD28 has an intrinsic role in the function and survival of normal plasma cells generated in response to model Ags and influenza virus infection. Our data show that most short-lived and long-lived plasma cells lacking CD28 produced higher levels of Ab than did the wild-type (WT) controls. Moreover, short-lived plasma cells deficient in CD28 upregulated the expression of plasma cell survival receptors, including B cell maturation Ag (BCMA), TNFR type II (TNFR II), transmembrane activator and CAML interactor (TACI), BAFF-R, CD25, and IFN-αR. We also analyzed mice that lack the ligands for CD28, B7.1, and B7.2. We found that B7.1/B7.2-deficient short-lived and long-lived plasma cells exhibit enhanced Ab production analogous to CD28-deficient plasma cells. Taken together, our data provide evidence that the CD28–B7 interaction operates as a crucial regulator of plasma cell function.
Materials and Methods
Mice, immunization, and Ags
C57BL/6 mice were purchased from Charles River and The Jackson Laboratory. B6.129S2-Cd28tm1Mak (Cd28−/−), B6.129S4-Cd80tm1Shr Cd86tm2shr/J (Cd80/Cd86−/−), B6.129S7-Rag1tm/Mom/J (Rag1−/−), B6.SJL.Ptprca Pepcb/BoyJ (Ly5.1), and B6.129S2-Igh-6tm1Cgn/J (μMT) mice were purchased from The Jackson Laboratory. All the mice were housed under specific pathogen-free conditions at the Emory Vaccine Center of Emory University School of Medicine. Mice were immunized i.p. with type II T-independent Ag, 4-hydroxy-3-nitrophenyl acetyl (NP)-Ficoll (50 μg), or T-dependent Ag NP-chicken γ-globulin (CGG; 50 μg) in aluminum hydroxide (Alum), with the NP reconstitution ratio ranging from 20 to 25. For inducing influenza virus-specific immune responses mice were anesthetized and immunized i.m. with 1400 hemagglutination units of whole, formalin-inactivated influenza A virus, A/FM/1/47, or infected intranasally with 0.1 × LD50 of influenza virus A, A/PR/8/34, as we described (22). Serum samples, spleens, and bone marrow cells from femurs were collected at designated time points. All animal studies had approval of the Emory University’s Institutional Animal Care and Use Committee.
Reagents, Abs, and flow cytometry
NP-Ficoll, NP-CGG, and NP-BSA were purchased from Biosearch Technologies (Novato, CA). Imject Alum was purchased from Pierce (Rockford, IL) and used as per the manufacturer’s specifications. Rat anti-mouse IgG/IgM and unlabeled mouse IgG/IgM were purchased from SouthernBiotech (Birmingham, AL). AffiniPure F(ab′)2 fragment goat anti-mouse IgM was purchased from Jackson ImmunoResearch (West Grove, PA). Fluoro-chrome or biotin-conjugated B220, CD19, CD138, CD28, CD4, CD25, CD45.2, CD45.1, CD80, CD86, GL-7, IgM, TNFR II, and TACI were purchased from BD Biosciences (San Jose, CA). Peanut agglutinin (PNA)-biotin was purchased from Sigma-Aldrich (St. Louis, MO), and the CaspGlow caspase-12 staining kit was purchased from MBL International (Woburn, MA). BCMA was purchased from R&D Systems, and BAFF-R was from Santa Cruz Biotechnology (Santa Cruz, CA). Tetra-methylbenzidine substrate solution was purchased from eBioscience (San Diego, CA), and the alkaline phosphatase substrate kit was purchased from Vector Laboratories (Burlingame, CA). For flow cytometry, single-cell suspensions of splenocytes or bone marrow lymphocytes were prepared from immunized and naive animals, stained, and analyzed as previously described (23).
Ab and Ab-secreting cell detection
For ELISA assays, flat-bottom 96-well immunoplates (Nunc) were coated with 20 μg NP-BSA or NP-CGG and the standard wells were coated with 2 μg/ml rat anti-mouse IgG or IgM. The coated plates were incubated overnight and then washed and blocked with 4% BSA in PBS/0.05% Tween 20. Sera samples and standard mouse IgG/IgM were serially diluted and added onto the plates and incubated at room temperature for 2 h. We then washed the plates and added either biotin-conjugated anti-mouse IgG or anti-mouse IgM and incubated the plates at room temperature for 2 h. Thereafter, the plates were washed and incubated with streptavidin-HRP for 45 min. Next, we washed the plates and added HRP-substrate and monitored the detection of Ag-specific bound Abs and the reaction was stopped with 2 N H2SO4. ELISA plates were read with a Synergy 2 plate reader (BioTek) at 450 nm. A standard curve was generated from the OD450 of serially diluted mouse standard IgG/IgM (the Ig concentrations of the standards are known). We then used this standard curve to interpolate the concentration of NP-specific IgG and IgM from the OD450 of the sera samples using Prism software (GraphPad Software, La Jolla, CA). The Ag-specific IgM/IgG concentrations are relative to the standard curve. ELISPOT assays were done as described in Chappell and Jacob (23).
Cell sorting and adoptive transfer
For sorting resting B cells, RBC-cleared splenocytes were stained with a biotin-labeled Ab mixture, followed by anti-biotin microbeads (mouse B cell isolation kit; Miltenyi Biotec). Labeled cells were then placed onto an LS column in a magnetic field and unbound cells were collected. Isolated cell purities ranged from 95 to 99%. For plasma cell sorting, CD138+ cells were first enriched using magnetic beads. Briefly, RBC-cleared splenocytes from NP-Ficoll immune mice were stained with PE-CD138, followed by anti-PE microbeads (Miltenyi Biotec). Labeled cells were then passed through the LS column in a magnetic field and cells bound to the columns were eluted outside the magnetic field. Eluted cells were further stained with allophycocyanin-B220 prior to cell sorting by flow cytometry. Sorted CD138+B220− plasma cells had purities that ranged from 85 to 98%. For adoptive transfer, sorted cells were pelleted and resuspended in 200 μl PBS. Cell suspensions were then administered to the tail vein of Rag1−/− or μMT recipients.
Generation of bone marrow chimeras
WT female B6.SJL (Ly5.1) mice were lethally irradiated (whole body) with two doses of 550 cGy from a [137Cs] source administered at 3-h intervals. Bone marrow cells (2 × 106) from Cd28−/− (Ly5.2) or WT (Ly5.2) and μMT (Ly5.2) mice were resuspended at a ratio of 40:60 and injected into the tail vein of the irradiated mice. These mice were rested for 6 wk to allow reconstitution of their immune system, which was confirmed by flow cytometry of peripheral blood cells. The reconstituted mice were immunized i.p. with 50 μg NP-CGG in Alum, and the extent of chimerism was assessed by flow cytometry at the time of harvest.
Serum hemagglutination inhibition and microneutralization assays
For live virus infection, we infected mice intranasally with 25 μl of PBS containing 0.1 to 100 × LD50 of mouse-adapted live virus under anesthesia. We collected serum samples at designated time points. To prevent nonspecific virus binding, we treated sera with receptor-destroying enzyme (RDE) II (Denka Seiken, Tokyo, Japan) overnight and carried out influenza virus-specific hemagglutination inhibition (HAI) and micro-neutralization assays as described (22). Briefly, serial dilutions of RDE II-treated sera were mixed with influenza viruses freshly grown in MDCK cells at a dose of 8 hemagglutination units/50 μl. Mixtures of virus and serum dilutions were incubated for 15 min, followed by addition of 50 μl 0.5% chicken RBCs (Innovative Research, Southfield, MI). The reciprocal of the highest serum dilution inhibiting hemagglutination was taken as the HAI titer. For microneutralization, we serially diluted RDE II-treated sera in 96-well plates and mixed with viruses freshly grown in MDCK cells at a dose of 2 × 103 (50% tissue culture-infective dose/ml). The plates were incubated at 37°C for 2 h and then MDCK cells were added and incubated overnight. Infection of MDCK cells by virus was assessed by the presence of influenza virus nucleoprotein. Briefly, cells were fixed with 80% acetone and incubated with biotinylated anti-nucleoprotein Ab (Chemicon International), followed by streptavidin-HRP (SouthernBiotech). Bound HRP was visualized using 1× tetramethylbenzidine substrate solution (eBioscience) and the developed color was assessed using the Bio-Rad microplate reader 550. The reciprocal of the highest serum dilution that generated >50% specific signal was considered to be the neutralization titer: 50% specific signal = (OD450 virus control − OD450 cell control)/2 + OD450 cell control.
Statistical analysis
A Student two-tailed t test was used to compare Cd28−/− or B7.1/B7.2−/− with WT controls. When the variances of the two groups were significantly different as determined by the F test, the Welch correction was used on the t test. For comparing two genotypes over multiple time points we used the two-way ANOVA. Only significant p values are shown on graphs.
Results
Splenic and bone marrow plasma cells express CD28
CD28 is expressed on human plasma cells, and its expression is regulated by Pax5 (15, 16). We first determined whether murine plasma cells produced CD28 in response to T-dependent and T-independent Ags. Briefly, we immunized C57BL/6 mice i.m. with whole inactivated influenza A virus, A/FM/1/47, or i.p. with a T-dependent Ag (NP-CGG) or T-independent Ag (NP-Ficoll). We then examined CD28 expression at the peak of splenic plasma cell responses (day 7 after immunization) and in the bone marrow at a memory time point (day 28) by flow cytometry. B cells did not express CD28, whereas both splenic and bone marrow plasma cells induced by A/FM/1/47 immunization expressed CD28 (Fig. 1A–C). Immunization with NP-CGG also demonstrated CD28 expression on short-lived splenic and long-lived bone marrow plasma cells (data not shown). Similarly, mice immunized with T-independent Ag, NP-Ficoll (Fig. 1D), expressed CD28 on their splenic plasma cells. These data confirm that normal murine short-lived splenic and long-lived bone marrow plasma cells express CD28 on their surface irrespective of how they are induced.
FIGURE 1.
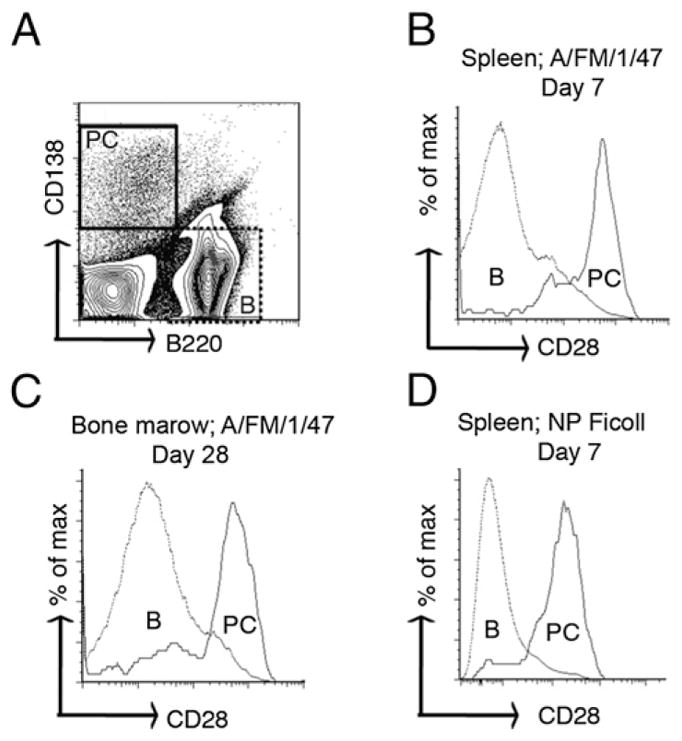
CD28 is expressed on plasma cells. Cohorts of C57BL/6 mice were immunized with either 1400 hemagglutinin units of influenza A virus (A/FM/1/47) i.m. or 50 μg NP-Ficoll i.p. At days 7 and 28 following immunization, spleen and bone marrow lymphocytes were isolated and stained with fluorescent-labeled Abs against B220, CD138, and CD28. The stained cells were analyzed by flow cytometry. (A) Dot plot showing the phenotypic distinction of B220+CD138− B cells (shown as B) and B220− CD138+ plasma cells (shown as PC) harvested from spleen 7 d after A/FM/1/47 immunization. (B–D) Histogram plots showing the CD28 expression profile of (B) splenic short-lived plasma cells and B cells 7 d postimmunization with A/FM/1/47, (C) bone marrow long-lived plasma cells and B cells 28 d following immunization with A/FM/1/47, and (D) splenic T-independent short-lived plasma cells and B cells 7 d postimmunization with NP-Ficoll. The data represent at least three independent experiments done with five mice per group.
T-independent Ab responses are modulated in the absence of CD28 on short-lived plasma cells
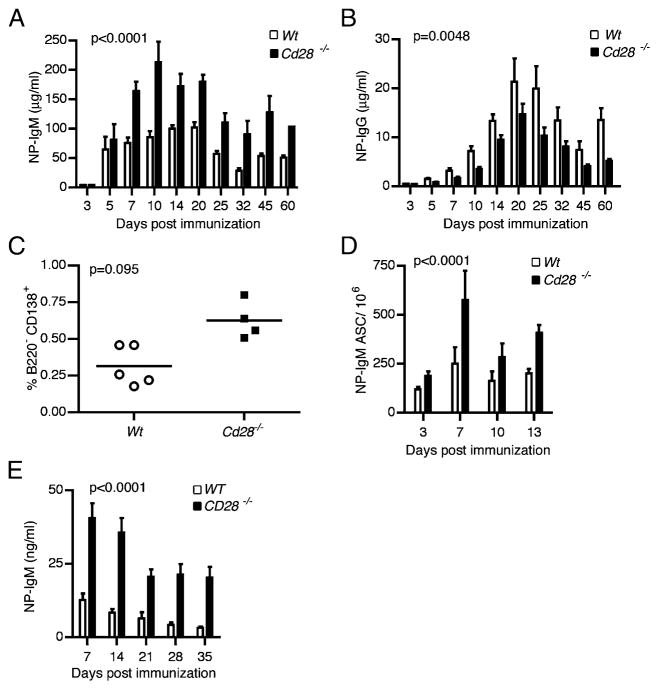
It is well established that CD28 is a crucial costimulator for T cell activation (9, 11, 12). Recent studies suggest that CD28 expression on plasma cells may promote their IgG production (16, 21). Hence, we reasoned that loss of CD28 would diminish plasma cell function and survival. To test this hypothesis, we compared the Ab responses of Cd28−/− mice and syngeneic C57BL/6 WT mice. Cd28−/− mice are impaired in germinal center formation and in development of long-lived plasma cells owing to dysfunctional T cells (13). To bypass the T cell dependency of Ab response, we immunized mice with type II T-independent Ag, NP-Ficoll, which delivers prolonged and persistent signaling to B cells in the absence of T cell help (24). We collected sera at different time points and measured the NP-specific IgM levels, the predominant isotype of T-independent responses, from day 3 through 60 post-immunization. Surprisingly, at all time points tested Cd28−/− mice exhibited significantly (p < 0.0001) higher serum NP-specific Ab levels than did their WT counterparts from day 7 through 60 postimmunization (Fig. 2A). During T-independent responses, IgG Abs are also produced but 10-fold lower than IgM Abs. Unlike the IgM Abs, Cd28−/− mice produced significantly (p = 0.0048) lower NP-specific IgG than did the WT controls from day 7 through 60 postimmunization (Fig. 2B).
FIGURE 2.
Ab responses are heightened in the absence of CD28. Cohorts of Cd28−/− mice or syngeneic WT C57BL/6 mice were immunized i.p. with 50 μg NP-Ficoll or PBS. Sera (n = 10), splenocytes (n = 10), and plasma cells (n = 10) were collected from each immunized mouse cohort and PBS control mice on the indicated time points. ELISA results show levels of NP-specific IgM (A) and IgG (B) in the sera from immunized mice. Nonspecific Ig titers from PBS controls have been subtracted from shown NP-specific titers. (C) Percentage of splenic B220−CD138+ plasma cells in Cd28−/− and WT mice was determined by flow cytometry on day 7 postimmunization. (D) Frequency of ASCs in the splenocytes from immunized Cd28−/− mice and WT controls was determined by ELISPOT on the indicated time points. (E) Bar graph showing serum NP-specific IgM levels from Rag1−/− adoptive hosts of Cd28−/− or WT splenic short-lived plasma cells isolated from splenocytes of the NP-Ficoll–immunized hosts. NP-IgM levels were determined by ELISA. Results are representative of at least three separate experiments using five mice per group. Statistical analyses to compare the IgM levels and number of ASCs in Cd28−/− versus WT control hosts were done by ANOVA; p values are shown on respective graphs.
We next examined the frequency of plasma cells in the immunized hosts by flow cytometry. Consistent with the high serum anti–NP-IgM levels, Cd28−/− mice had substantially higher frequencies of B220−CD138+ plasma cells in their spleens than did their WT counterparts (Fig. 2C). We further examined the IgM response by enumerating the Ag-specific plasma cells and again found significantly higher (p < 0.0001) frequencies of NP-specific IgM plasma cells in Cd28−/− hosts than in WT controls (Fig. 2D).
To ensure that the effect we saw was solely due to CD28 deficiency in the B cell lineage, we adoptively transferred 107 purified B cells from either Cd28−/− or WT mice into Rag1−/− mice; 2 wk later we immunized them with NP-Ficoll and subsequently measured NP-specific Ab levels at days 3–45. We found that recipients of Cd28−/− B cells made significantly higher Ab levels at days 7–45 postimmunization compared with WT control mice (data not shown). We next determined whether the increased Ab production was intrinsic to Cd28−/− plasma cells by directly comparing Ab production from WT and Cd28−/− short-lived plasma cells. Briefly, we immunized WT or Cd28−/− mice with NP-Ficoll, and 7 d later, we sorted total B220−CD138+ plasma cells by flow cytometry and adoptively transferred equal numbers of either Cd28−/− or WT plasma cells into Rag1−/− recipients. We then collected serum samples every week to assess NP-specific Ab response in the recipients (Fig. 2E). Adoptive hosts that received Cd28−/− plasma cells produced significantly more (p < 0.0001) NP-specific IgM Abs than did WT controls at all time points tested. These data demonstrate that increased IgM production in Cd28−/− mice is an intrinsic property of plasma cells, and they suggest that Cd28−/− splenic IgM plasma cells may selectively express higher levels of survival markers or produce more Ig on a per cell basis, or both.
Heightened Ab response of Cd28−/− short-lived plasma cells is likely due to prolonged survival
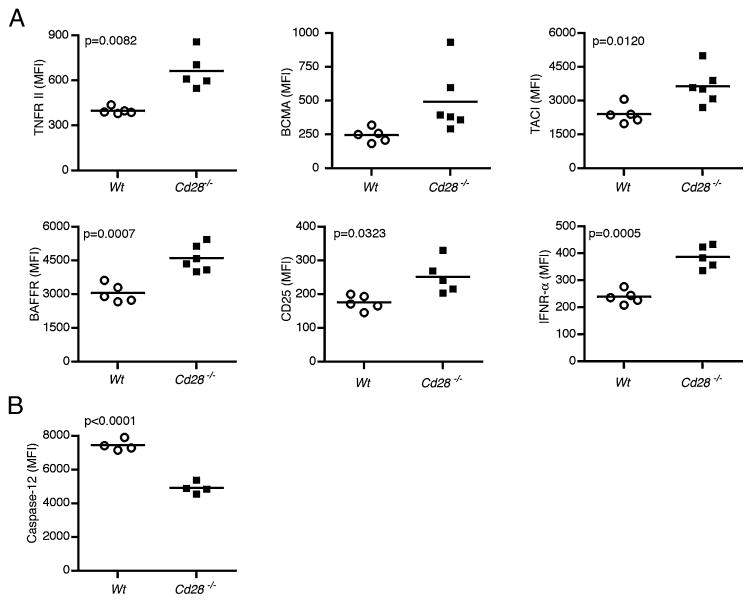
Our observation that Cd28−/− mice exhibited increased Ab production over time (Fig. 2A) as compared with WT mice suggested that Cd28−/− splenic plasma cells possess features that confer survival advantage. Hence, we examined the level of expression of surface proteins associated with survival of plasma cells, mature B cells, or lymphocytes in general (25–29). We assessed the expression of TNFR II and its homologs, TACI, BCMA, and BAFF-R. We also examined CD25 and IFN-αR in Cd28−/− and WT hosts 7 d after NP-Ficoll immunization by flow cytometry. Interestingly, in Cd28−/− splenic plasma cells, we observed significantly higher levels of TNFR II (p = 0.0039), TACI (p = 0.0120), BAFF-R (p = 0.0007), IFN-αR (p = 0.0125), and CD25 (p = 0.0323) (Fig. 3A). We also observed a trend of higher BCMA levels in Cd28−/− plasma cells (Fig. 3A), although the difference was not statistically significant. Expression of other plasma cell survival factors, including IL-6R (29), CD44 (30), and IL-10 receptors (31, 32), was not significantly different between Cd28−/− and WT short-lived splenic plasma cells (data not shown). Collectively, our data suggest that Cd28−/− short-lived splenic plasma cells may survive longer than do their WT counterparts owing to their higher levels of known plasma cell survival factors.
FIGURE 3.
Cd28−/− plasma cells express higher levels of survival factor receptors and lower levels of apoptotic caspase-12. Cohorts of Cd28−/− (n = 20) and WT control (n = 20) mice were immunized with 50 μg NP-Ficoll or PBS. Splenocytes were harvested on days 7 and 13 following immunization and stained with fluorescent-labeled Abs against survival and apoptosis markers. Stained cells were examined by flow cytometry. (A) Graphs showing the expression levels of TNFR II, BCMA, TACI, BAFF-R, CD25, and IFNα-R on splenic short-lived plasma cells 7 d following immunization, shown as mean MFI. (B) Graphic representation of intracellular active caspase-12 levels in splenic short-lived plasma cells that were measured 13 d after immunization, shown as MFI. Data are representative of at least two experiments done with four to six mice per group. A two-tailed Student t test with a Welch correction was done to compare MFIs of Cd28−/− versus WT hosts. Significant values (p < 0.05) are shown on corresponding plot.
To cope with the production of copious amounts of Ig that ensues upon plasma cell differentiation, differentiating B cells induce the unfolded protein response pathway (33, 34). This pathway enhances the efficiency of protein processing, thus preventing endoplasmic reticulum (ER) stress. However, toward the end of the short-lived plasma cell lifespan, ER stress increases and this leads to the induction of ER-associated apoptotic caspase-12 (35). Because we observed enhanced expression of survival factor receptors on Cd28−/− plasma cells, we reasoned that Cd28−/− plasma cells might be less susceptible to ER-associated apoptosis than are WT plasma cells. To test this idea, we immunized WT or Cd28−/− mice with NP-Ficoll and late in the response, at day 13, we assessed the expression of active caspase-12 in short-lived plasma cells by intracellular flow cytometry. Interestingly, we observed significantly reduced (p < 0.0001) levels of active caspase-12 protein expression in the Cd28−/− plasma cells in comparison with the WT plasma cells (Fig. 3B). The concurrent increase in expression of survival factor receptors and decrease in apoptotic caspase-12 strongly suggest that Cd28−/− plasma cells have a survival advantage over WT short-lived plasma cells.
Loss of CD28 expression on long-lived bone marrow plasma cells leads to increased Ag-specific IgM and IgG Ab production
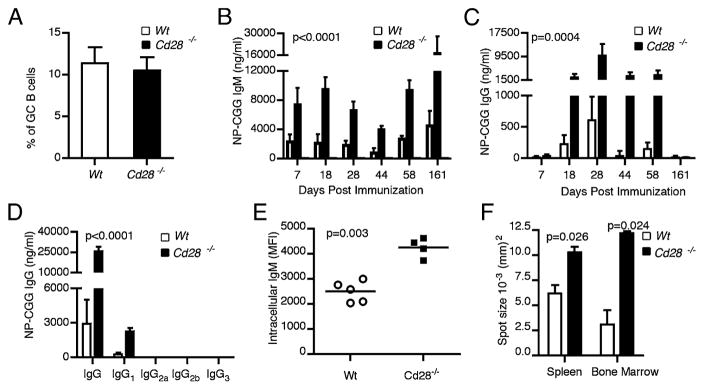
Our data thus far demonstrate that the lack of CD28 expression on short-lived splenic plasma cells leads to increased Ab levels and likely confers survival advantage. Next, we investigated whether CD28 expression affects the function and survival of long-lived bone marrow plasma cells. To test this, we used μMT mice as adoptive transfer hosts because these mice have intact, CD28-sufficient T cells yet lack B cells. We purified B cells from the spleens of Cd28−/− or control WT C57BL/6 hosts and adoptively transferred them into the B cell-deficient μMT hosts. Because long-lived plasma cells originate from germinal centers, we were concerned about the possibility that CD28-deficient B cells may be impaired in germinal center formation. Hence, we first tested whether Cd28−/− B cells, upon adoptive transfer into T cell-sufficient μMT recipients, would undergo normal germinal center differentiation in response to the T-dependent Ag, NP-CGG. CD28 deficiency in B cells did not impair germinal center formation, as μMT recipients with WT as well as Cd28−/− B cells exhibited comparable numbers of splenic germinal center B cells (B220+GL-7+PNA+) (Fig. 4A). Next, we examined the serum NP-CGG–specific IgM and IgG Ab levels in the two cohorts of adoptive hosts up to 6 mo postimmunization. We observed significantly (p < 0.0001) higher IgM titers from hosts that received Cd28−/− B cells at all time points tested (Fig. 4B).
FIGURE 4.
Cd28−/− long-lived plasma cells produce more Abs than do WT plasma cells. Two cohorts of B cell-deficient μMT hosts were reconstituted with 107 splenic B cells from either Cd28−/− (n = 20) or WT C57BL/6 control (n = 20) hosts. Three to 14 d after transfer the adoptive hosts were immunized i.p. with 50 μg NP-CGG/Alum or PBS and the B cell responses were assessed. (A) Frequency of B220+GL-7+PNA+ germinal center B cells in the μMT adoptive hosts of WT or Cd28−/− B cells, 14 d after NP-CGG/Alum immunization. (B–D) ELISA results showing the concentration of NP-CGG–specific IgM (B) and IgG (C, D) in the serum of μMT adoptive hosts of Cd28−/− or WT B cells, on indicated time points postimmunization. ANOVA was done to compare Ab levels in Cd28−/− versus WT adoptive hosts. (E) Flow cytometry of intracellular IgM 6 mo after NP-CGG/Alum immunization shown as MFI. (F) ELISPOT results showing the mean spot sizes of ASCs from spleen and bone marrow of Cd28−/− versus WT adoptive hosts. Data are representative of two experiments done with four to five mice per group. Statistical comparison of intracellular IgM levels in WT versus Cd28−/− hosts were done by the two-tailed Student t test.
NP-CGG immunization elicits a T-dependent response associated with isotype switching and hence IgG production; therefore, we examined the effect of CD28 deficiency on the serum level of NP-specific IgG and its subclasses by ELISA. Analogous to the IgM response, there was a significant (p = 0.0004) increase in the Ag-specific serum IgG levels in μMT recipients with Cd28−/− B cells than in the WT controls from day 18 through 58 post-immunization (Fig. 4C). Consistent with this increase, there was substantially (p < 0.0001) more IgG1 in these mice when assessed at 28 d postimmunization (Fig. 4D). We also assessed NP-CGG–specific IgG2a, IgG2b, and IgG3 levels, but all of these were below the threshold of detection. In this system in which we adoptively transferred B cells into B cell-deficient μMT hosts, we observed substantially higher levels of IgM than IgG in response to NP-CGG immunization. Nonetheless, both the IgG and IgM responses were significantly higher in the Cd28−/− hosts in comparison with the WT controls, suggesting that CD28 expression modulated both IgG and IgM production by plasma cells responding to protein Ag.
One of the reasons for enhanced Ab production in adoptive hosts with Cd28−/− B cells could be that individual CD28-deficient plasma cells may produce more Ig than do WT plasma cells. We tested this using intracellular staining for IgM on long-lived plasma cells 6 mo postimmunization. Our data shows that Cd28−/− plasma cells contained substantially higher levels (p = 0.003) of IgM per cell than did WT plasma cells (Fig. 4E). To corroborate this observation, we conducted ELISPOT assays on the spleen and bone marrow cells harvested from the adoptive hosts 6 mo post-immunization. We compared the mean spot sizes of the plasma cells from Cd28−/− hosts and WT hosts. Ab-secreting cells (ASCs) from the spleens and bone marrow of Cd28−/− hosts had significantly larger spot sizes (p = 0.02) than did WT controls (Fig. 4F). Taken together, our results show that Cd28−/− plasma cells produce more Abs on a per cell basis than do WT bone marrow plasma cells.
Ag-specific Cd28−/− long-lived bone marrow plasma cells are retained at higher frequencies than WT plasma cells
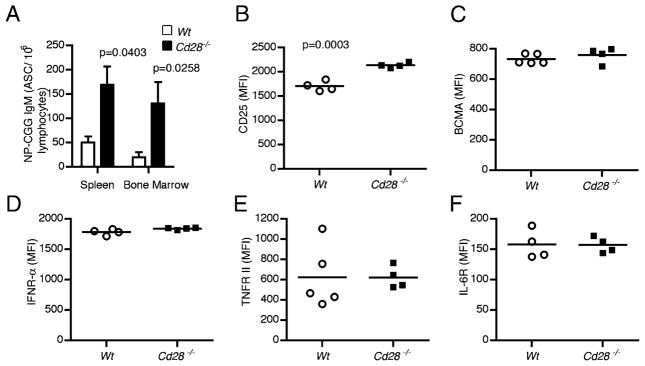
In view of the increased Ab levels in recipient mice with Cd28−/− B cells at late time points (Fig. 4A), we examined whether the long-lived Cd28−/− plasma cells were present in higher numbers than WT plasma cells at later time points. Because the Ab response in these hosts was predominantly IgM, we enumerated Ag-specific IgM secreting long-lived plasma cells 6 mo post-immunization of μMT adoptive hosts that received either Cd28−/− or WT B cells by ELISPOT assay. We observed significantly higher numbers of NP-CGG–specific IgM plasma cells in the bone marrow (p = 0.0258) and spleen (p = 0.0403) of Cd28−/− adoptive hosts than in WT controls (Fig. 5A). This suggested that Cd28−/− plasma cells survive longer; hence, we tested whether Cd28−/− long-lived plasma cells also exhibited enhanced expression of survival factor receptors. We isolated bone marrow cells from the immunized Cd28−/− and WT adoptive hosts 6 mo post-immunization and assessed the expression of CD25, BCMA, TNFR II, IFN-αR, and IL-6R by flow cytometry. A comparison of the mean fluorescence intensities (MFIs) of these receptors shows that the Cd28−/− long-lived bone marrow plasma cells expressed higher levels of CD25 (p = 0.0003) (Fig. 5B). Conversely, BCMA, IFNR-α, TNFR II, and IL-6R levels were comparable to WT (Fig. 5C–F). Thus, although Cd28−/− long-lived plasma cells existed at higher frequencies than did WT plasma cells, they did not exhibit enhanced expression of most survival factor receptors, with the exception of CD25.
FIGURE 5.
Cd28−/− plasma cells outlast WT plasma cells. Two groups of B cell-deficient μMT hosts were reconstituted with 107 splenic B cells from either Cd28−/− (n = 10) or WT C57BL/6 control (n = 10) hosts. Three to 14 d following the transfer, the adoptive hosts were immunized i.p. with 50 μg NP-CGG/Alum or PBS, and 6 mo later the long-lived plasma cells from the bone marrow were examined. (A) Frequency of NP-CGG–specific ASCs in the bone marrow and splenic lymphocytes of μMT adoptive hosts of Cd28−/− or WT control B cells were determined by ELISPOT. (B–F) Graphs showing the expression of CD25 (B), BCMA (C), IFN-αR (D), TNFR (E), and IL-6R (F) in bone marrow plasma cells of μMT adoptive hosts of Cd28−/− or WT B cells was examined by flow cytometry and shown as MFI. Data represent two independent experiments with five mice per group. Statistical comparisons of Cd28−/− and WT plasma cells were done by the two-tailed Student t test; significant differences (p < 0.05) are shown.
CD28-deficient plasma cells from mixed bone marrow chimeras also exhibit increased Ab production and higher plasma cell frequency
Contrary to our findings, a recent paper by Rozanski et al. (21) reported that CD28-deficient plasma cells exhibited decreased survival and produced significantly lower levels of IgG1 Abs. A possible explanation for this discrepancy could be that we used adoptive transfer of mature CD28-deficient B cells into μMT mice for our experiments whereas Rozanski et al. used mixed bone marrow chimeras. To ascertain whether our observations were because of this difference, we generated mixed bone marrow chimeras. Briefly, we lethally irradiated WT B6.SJL (Ly5.1) mice and reconstituted them with bone marrow cells from Cd28−/− (Ly5.2) or WT (Ly5.2) and μMT (Ly5.2) mice to generate mixed bone marrow chimeras. The reconstitution was done at a 40:60 (Cd28−/−/WT:μMT) ratio. Six weeks later, we confirmed reconstitution by flow cytometry (Ly5.1 versus Ly5.2) and then immunized the chimeras with NP-CGG/Alum. We then monitored the IgG response to the T-dependent Ag over a course of 6 wk. We observed a significant upregulation (p = 0.0002) of IgG from Cd28−/− mixed bone marrow chimeric hosts in comparison with the WT counterparts (Fig. 6A). This is consistent with our previous findings in which we reconstituted μMT mice with mature B cells (Fig. 4).
FIGURE 6.
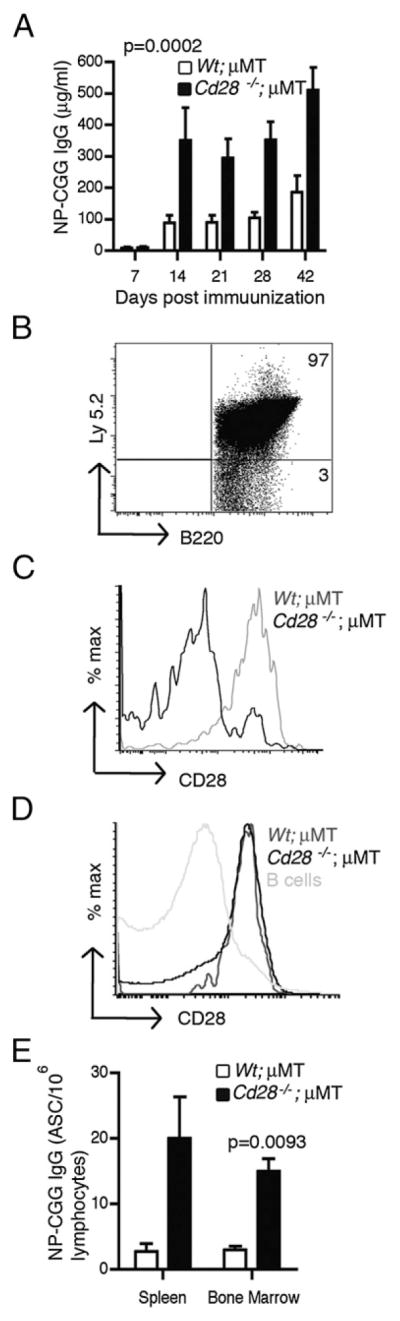
CD28 deficiency in plasma cells from mixed bone marrow chimeras enhances Ab production and increases plasma cell frequency. Two cohorts of B6.SJL (Ly5.1) mice were lethally irradiated and reconstituted with 1.2 × 106 bone marrow cells from μMT and 8 × 105 bone marrow cells from either Cd28−/− (Ly5.2) or WT (Ly 5.2) mice. Six weeks after reconstitution, the chimeras were immunized i.p. with 50 μg NP-CGG in Alum and their B cell responses were examined for 6 wk. (A) ELISA results showing serum NP-CGG–specific IgG levels from Cd28−/−;μMT and WT;μMT chimeric hosts on the indicated time points. (B) Flow cytometry dot plot showing donor-derived Ly5.2 expression in splenic B cells from the chimeric hosts. (C and D) Histogram plots showing CD28 expression in plasma cells (C) and CD4 T cells (D) from the spleens of Cd28−/−;μMT and WT;μMT chimeras. CD28 expression was examined by flow cytometry. (E) Graph showing the mean frequency of NP-CGG/IgG ASCs in spleens and bone marrows from Cd28−/−;μMT and WT;μMT chimeras, enumerated via ELISPOT. Experiments were done with five mice per group. Statistical comparisons between Cd28−/−;μMT and WT;μMT chimeras were done using the two-tailed Student t test and ANOVA. Error bars represent SEM.
To confirm that the responses we observed are mediated by donor-derived Cd28−/− and WT plasma cells, respectively, we examined the phenotype of lymphocytes in the spleen and bone marrow of these hosts by flow cytometry at 6 wk postimmunization. As expected, >95% of the B cells originated from the donors, thus expressing Ly5.2 (Fig. 6B), and 88 ± 0.95% of the plasma cells from the Cd28−/−;μMT chimeras lacked CD28 expression, whereas 80 ± 1.5% of the plasma cells from WT;μMT chimeras expressed CD28 (Fig. 6C). To assess whether these cohorts had comparable levels of CD28-sufficient CD4 T cell help, we examined CD28 expression on CD4 T cells from the spleens of these chimeric hosts. We found that CD4 T cells from these cohorts expressed comparable levels of CD28 (Fig. 6D), with 78 ± 0.75% of the CD4 T cells from Cd28−/−;μMT hosts expressing CD28 and 81 ± 0.5% of the CD4 T cells from WT;μMT hosts expressing CD28, whereas the B cell controls did not express CD28. Next, we enumerated the plasma cells from the spleens and bone marrows of these hosts by ELISPOT assay 6 wk after immunization. Consistent with our previous observations, Cd28−/−;μMT chimeras had higher frequency of NP-CGG–specific IgG-secreting plasma cells both in the spleen and in the bone marrow (p = 0.0093) in comparison with the WT counterparts (Fig. 6E). Collectively, these observations demonstrate that the enhanced function of CD28-deficient plasma cells is observed in mixed bone marrow chimeras as well.
Interaction between CD28 and B7 molecules is required for regulation of splenic and bone marrow plasma cells
The only known ligands for the CD28 receptor are B7-1 and B7-2 molecules, which are expressed not only on APCs but also on plasma cells (36). To confirm this, we tested whether normal splenic and bone marrow plasma cells express B7-1 or B7-2. We found that both plasma cell populations expressed high levels of B7-1 and modest levels of B7-2 (data not shown). Given the observed upregulation of plasma cell function in the absence of CD28, we reasoned that engagement of CD28 was needed for negative regulation of plasma cells. Because plasma cells also express the ligands for CD28, we hypothesized that the loss of B7-1/B7-2 expression may likewise result in enhanced plasma cell function. Thus, we first determined whether lack of B7.1/B7.2 on short-lived plasma cells would similarly affect their function during a T-independent Ab response. We immunized B7.1/B7.2−/− mice or syngeneic WT C57BL/6 mice with 50 μg NP-Ficoll and we followed serum NP-specific IgM levels of the immunized hosts for 2 mo. As anticipated, the B7.1/B7.2−/− mice displayed significantly (p < 0.0001) higher levels of NP-specific Abs than did the WT controls on days 14–60 postimmunization (Fig. 7A). Taken together, these data suggest that the B7.1/B7.2−/− short-lived splenic plasma cells, analogous to their Cd28−/− counterparts, survive longer than do their WT counterparts and thus sustain higher Ab titers.
FIGURE 7.
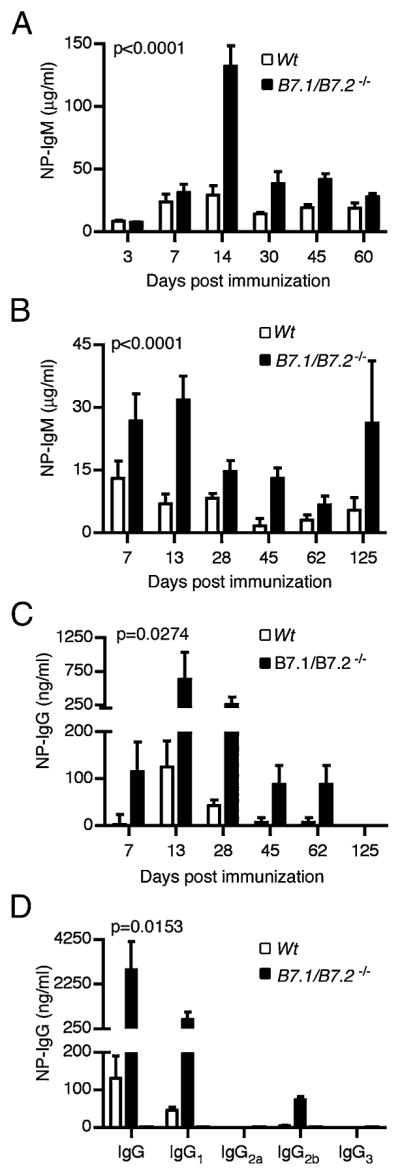
B7.1/B7.2 deficiency increases Ab production in short-lived and long-lived plasma cells. To generate short-lived plasma cells, B7.1/B7.2−/− or syngeneic C57BL/6 WT controls were immunized i.p. with 50 μg NP-Ficoll or PBS control. Serum was collected from the immunized mice weekly for 2 mo to monitor serum Ab levels via ELISA assays. (A) Serum NP-IgM concentrations from B7.1/B7.2−/− or WT mice immunized with NP-Ficoll. (B–D) To generate long-lived plasma cells, two groups of B cell-deficient μMT mice were reconstituted with 107 splenic B cells from B7.1/B7.2−/− or WT control mice. The adoptive hosts were immunized with 50 μg NP-CGG/Alum or PBS control, and the B cell responses were monitored for 4 mo. Serum NP-CGG–specific IgM (B) and IgG (C, D) levels from μMT adoptive hosts of B7.1/B7.2−/− or WT B cells at the indicated time points after NP-CGG/Alum immunization are shown. Experiment was done with five mice per group. Error bars represent SEM.
Next, we investigated whether the loss of B7.1/B7.2 expression would likewise enhance the function of long-lived plasma cells in the bone marrow. We transferred equal numbers of B7.1/B7.2−/− B cells or C57BL/6 WT B cells into cohorts of μMT hosts that were subsequently immunized with NP-CGG plus Alum. Then we determined their serum NP-CGG–specific IgM and IgG levels by ELISA. As expected, the B7.1/B7.2−/− adoptive hosts had significantly (p < 0.0001) higher IgM titers than did the WT controls (Fig. 7B). We next examined the effect of B7.1/B7.2 expression on the IgG response and found that loss of B7.1 and B7.2 led to a significant (p = 0.0274) increase in NP-specific IgG from day 7 through 62 postimmunization (Fig. 7C). We also assessed the level of IgG subclasses on day 28 postimmunization. Consistent with the total NP-specific IgG response, we observed a significant increase (p = 0.0153) in IgG1 and IgG2b in the B7.1/B7.2−/− adoptive hosts in comparison with WT controls (Fig. 7D). In summary, the loss of either CD28 or its ligands B7.1/B7.2 leads to the enhancement of Ab production by plasma cells.
CD28 deficiency on plasma cells enhances production of neutralizing Abs to influenza virus
Our observations using model Ags suggest that CD28 expression on plasma cells regulates their Ab production and frequency. Next, we investigated whether the quality or functionality of Abs produced by Cd28−/− plasma cells were comparable to WT plasma cells. To test this, we studied the immune response to influenza virus infection. Briefly, we adoptively transferred into Rag1−/− mice WT T cells and either Cd28−/− or WT B cells. Two weeks later, we infected the recipient mice with a sublethal dose of A/PR/8/34 influenza virus. We collected sera on the indicated time course and monitored the HAI and virus-neutralization titers. On day 7 postinfection the HAI titers were comparable between the two cohorts; however, by day 14–60 postinfection there was a 2-to 4-fold increase in the HAI titers from the Cd28−/− adoptive hosts in comparison with WT controls (Fig. 8A). Likewise, we found a 2- to 4-fold increase in the neutralization titers from Cd28−/− B cell adoptive hosts in comparison with WT B cell hosts (Fig. 8B). The increase in HAI and neutralization titers from recipient mice with Cd28−/− B cells corroborates our observations of heightened Ab responses from Cd28−/− plasma cells in response to hapten-carrier Ags. Collectively, our data suggest that during an immune response to influenza virus infection, lack of CD28 expression on plasma cells enhances virus-specific neutralizing Ab production.
FIGURE 8.
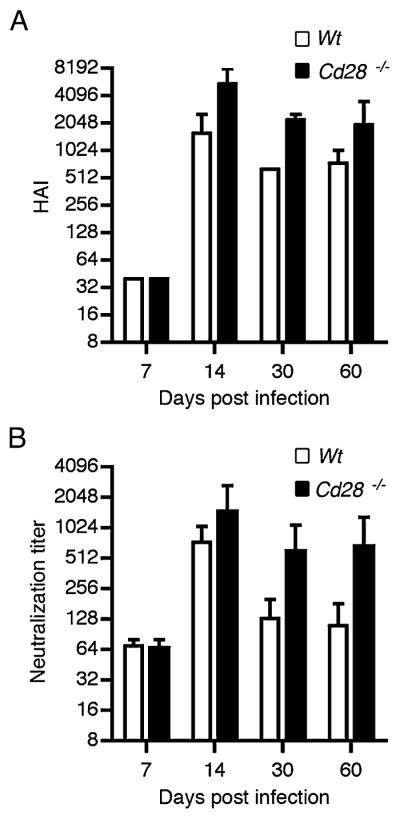
CD28 deficiency on plasma cells increases influenza virus hemagglutination titers. Splenic T cells from WT C57BL/6 mice and B cells from either Cd28−/− or WT control mice were adoptively transferred into cohorts of Rag1−/− mice via the tail vein. Two weeks later the recipient mice were intranasally infected with 0.1 × LD50 A/PR/8/34 influenza virus or PBS as control. Serum samples were collected 7, 14, 30, and 60 d after infection. Sera were treated with RDE II, heat inactivated, and examined for HAI titers (A) and neutralization titers (B). Experiment was done with six mice per group. Error bars represent SEM.
Discussion
The CD28/B7 costimulatory pathway is well known for its critical role in T cell activation. It is also important in shaping the type of T cell response (37, 38) and for optimal memory responses (39–41). In this study, we show that the CD28–B7 interaction plays a novel role as a modulator of plasma cell survival and function.
Our data demonstrate that in the absence of CD28 most of the plasma cells generated in response to NP-Ficoll, NP-CGG, or influenza virus exhibit enhanced function and survival. This was strictly an intrinsic effect of CD28 on the B cell lineage, as the Cd28−/− B cells transferred into Rag1−/− (Fig. 2E) or μMT recipients (Fig. 4) or Cd28−/−;μMT mixed bone marrow chimeras (Fig. 6) produced more Ag-specific Ig than did WT B cells upon immunization with NP-Ficoll or NP-CGG, respectively. Additionally, upon infection with influenza virus Rag1−/− adoptive hosts of Cd28−/− B cells exhibited an increase in their HAI and neutralization titers in comparison with WT hosts. The elevated Ab function of Cd28−/− plasma cells was most likely resulting from a higher rate of Ig production and enhanced frequency of plasma cells (Figs. 4E, 4F, 5A). Consistent with these observations, Cd28−/− short-lived plasma cells upregulated TNFR family proteins and CD25, proteins involved in survival of B cells and T cells (Figs. 3, 5). Similar to CD28 deficiency, the lack of B7.1/B7.2 resulted in increased Ab production (Fig. 7), suggesting that the CD28–B7 interaction on plasma cells ensures normal Ab production and disruption of this interaction leads to enhanced Ab production.
Our data disagree with a recent study (21), which concluded that CD28 is a positive regulator of plasma cells. That study showed that overall, Cd28−/− and Cd80/86−/− mice have lower numbers of long-lived plasma cells in the bone marrow and concluded that loss of CD28–B7 interaction on plasma cells led to diminished plasma cell survival. However, previous studies have demonstrated that mice deficient in CD28 or CD80/CD86 are unable to form productive germinal centers (13, 14), which are the major source of long-lived plasma cells. Therefore, it is not surprising that these mice have diminished bone marrow plasma cells. The authors also showed diminished Ag-specific IgG1+ plasma cells in the bone marrow of immunized Cd28−/−:μMT bone marrow chimeric mice, but it is unclear whether the total numbers of Ag-specific, non-IgG1+ plasma cells in the bone marrow were diminished. In our experiments using Cd28−/−:μMT bone marrow chimeras, we did not observe decreased Ab levels but instead we saw increased plasma cells numbers and Ab production.
The CD28/B7 costimulatory pathway is also important in the generation and maintenance of regulatory T cells (42). Mice deficient in CD28 or B7.1/B7.2 have a lower frequency of regulatory T cells. In the absence of regulatory T cells both T cell responses as well as T-dependent Ab responses are amplified in some cases, leading to the development of autoimmune disease (43–45). Additionally, recent studies suggest that regulatory T cells can directly modulate B cell responses (46) and plasma cell generation (47). Our data obtained from Cd28−/−, B7.1/B7.2−/−, and Rag1−/− mice could be impacted by the deficiency of regulatory T cells in these hosts; however, we observe comparable results in experiments done in μMT hosts and mixed bone marrow chimeras, which are intact in their regulatory T cell compartment. Thus, to the best of our knowledge, regulatory T cells do not appear to be a factor in the CD28–B7-mediated regulation of plasma cell function we are reporting in this study.
The molecular mechanism by which CD28 regulates plasma cell responses remains unclear. At present, most of the studies in the literature have examined the effect of CD28 expression on transformed plasma cell lines, which may not reflect the physiological role of CD28 in normal, Ag-induced plasma cells. Some of these studies have shown that CD28 expression on malignant plasma cells from multiple myeloma patients correlates with tumor expansion and treatment failure (17–19). Such observations led to the preposition that CD28 is a survival factor for multiple myeloma plasma cells (19). On the contrary, Zhang and colleagues (48) showed that addition of agonistic Ab against CD28 induces apoptosis of multiple myeloma cell lines in vitro.
Our data clearly show that the CD28–B7 interaction is important for normal functioning of plasma cells and that disruption of this interaction, either due to CD28 or B7 deficiency, leads to enhanced plasma cell persistence and Ab production. CD28, CD80, CD86, and CTLA-4 exist in two forms, membrane bound and soluble, owing to alternative mRNA splicing (49–52). Interestingly, these soluble forms are elevated in the sera of autoimmune patients including Ab-mediated autoimmune disorders such as systemic lupus erythematosus (53, 54) and rheumatoid arthritis (55). Although these soluble molecules clearly play a role in modulating T cell responses, they may also exhibit direct effects on plasma cells by disrupting the CD28–B7 interaction and relieving the negative regulation of plasma cells. Such disruption may induce autoreactive plasma cells to survive longer and produce more Ig per plasma cell, thus exacerbating the autoimmunity. Neutralizing these soluble molecules could possibly be explored as a therapeutic strategy to downmodulate Ab-mediated autoimmunity.
The precise role of CD28–B7 interaction on plasma cells in a nonautoimmune setting is not known. It is unclear why plasma cells would need an extra level of regulation because the bone marrow plasma cells are long-lived and continue to produce Abs for a lifetime. Although further studies are warranted to fully understand this, it may not be inappropriate to consider that perhaps the soluble molecules are a way to enhance Ab production by pre-existing plasma cells. Production of soluble CD28, B7, or CTLA-4 molecules may be a mechanism by which the host could significantly increase the amount of Ab produced from the same number of existing plasma cells. This could be particularly useful during times of stress or inflammation and be a means to enhance the overall serological immunity in the host. Studies to test this are in progress. The use of soluble molecules could be a way to enhance overall Ab production.
Longevity of plasma cells is critical for maintaining long-term immune memory and conferring protection from pathogenic challenge, making it a critical feature of vaccination. Constant secretion of Ag-specific Abs by plasma cells is the first line of defense of the adaptive immunity prior to the reactivation of memory cells; circulating Abs efficiently lower the pathogenic threat. Conversely, the sustained Ab production and persistence of plasma cells make them a menace when directed against self-Ags in autoimmunity. In this study, we show that in the absence of CD28 most plasma cells upregulate their Ab production capacity and enhance their yield over time. Thus, interruption of the CD28–B7 interaction on plasma cells can enhance Ab production, whereas facilitating this interaction may restrict Ab production and plasma cell survival. Collectively, our observations indicate that the CD28/B7 pathway could be a potential target for modulation of plasma cell responses in both vaccination and autoimmune disease therapies.
Acknowledgments
This work was supported by American Cancer Society Grant RSG-05-144-01 (to J.J.).
We thank Dr. Kiran Gill, Wenxin Pang, and Youliang Wang of the Emory Vaccine Center Flow Cytometry Core for exceptional technical support.
Abbreviations used in this article
- Alum
aluminum hydroxide
- ASC
Ab-secreting cell
- BCMA
B cell maturation Ag
- CGG
chicken γ-globulin
- ER
endoplasmic reticulum
- HAI
hemagglutination inhibition
- MFI
mean fluorescence intensity
- NP
4-hydroxy-3-nitrophenyl acetyl
- PNA
peanut agglutinin
- RDE
receptor-destroying enzyme
- TACI
transmembrane activator and CAML interactor
- TNFR II
TNFR type II
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 3.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 4.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 5.Dogan I, Bertocci B, Vilmont V, Delbos F, Mégret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 6.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas PJ, Negishi I, Nakayama K, Fields LE, Loh DY. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J Immunol. 1995;154:5757–5768. [PubMed] [Google Scholar]
- 10.Shahinian A, Pfeffer K, Lee KP, Kündig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 11.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 12.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- 14.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 15.Kozbor D, Moretta A, Messner HA, Moretta L, Croce CM. Tp44 molecules involved in antigen-independent T cell activation are expressed on human plasma cells. J Immunol. 1987;138:4128–4132. [PubMed] [Google Scholar]
- 16.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge-Morineau N, Wijdenes J, Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597–2603. [PubMed] [Google Scholar]
- 18.Robillard N, Jego G, Pellat-Deceunynck C, Pineau D, Puthier D, Mellerin MP, Barillé S, Rapp MJ, Harousseau JL, Amiot M, Bataille R. CD28, a marker associated with tumoral expansion in multiple myeloma. Clin Cancer Res. 1998;4:1521–1526. [PubMed] [Google Scholar]
- 19.Bahlis NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA, Terebelo HR, Byrne GE, Jr, Levine BL, Boise LH, Lee KP. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair JR, Carlson LM, Koorella C, Rozanski CH, Byrne GE, Bergsagel PL, Shaughnessy JP, Jr, Boise LH, Chanan-Khan A, Lee KP. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol. 2011;187:1243–1253. doi: 10.4049/jimmunol.1100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chappell CP, Jacob J. Identification of memory B cells using a novel transgenic mouse model. J Immunol. 2006;176:4706–4715. doi: 10.4049/jimmunol.176.8.4706. [DOI] [PubMed] [Google Scholar]
- 24.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor BP, V, Raman S, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 28.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 29.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 30.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 31.Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J Biol Chem. 2002;277:49047–49054. doi: 10.1074/jbc.M205011200. [DOI] [PubMed] [Google Scholar]
- 34.van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Auner HW, Beham-Schmid C, Dillon N, Sabbattini P. The life span of short-lived plasma cells is partly determined by a block on activation of apoptotic caspases acting in combination with endoplasmic reticulum stress. Blood. 2010;116:3445–3455. doi: 10.1182/blood-2009-10-250423. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 37.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 38.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- 39.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 40.Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. Therapeutic memory T cells require costimulation for effective clearance of a persistent viral infection. J Virol. 2009;83:8905–8915. doi: 10.1128/JVI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 42.Boden E, Tang Q, Bour-Jordan H, Bluestone JA. The role of CD28 and CTLA4 in the function and homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:55–63. doi: 10.1002/0470871628.ch5. discussion 63–66, 106–114. [DOI] [PubMed] [Google Scholar]
- 43.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 45.Curotto de Lafaille MA, Lafaille JJ. CD4+ regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–778. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 46.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 47.Jang E, Cho WS, Cho ML, Park HJ, Oh HJ, Kang SM, Paik DJ, Youn J. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011;186:1546–1553. doi: 10.4049/jimmunol.1002942. [DOI] [PubMed] [Google Scholar]
- 48.Qiu YH, Sun ZW, Shi Q, Su CH, Chen YJ, Shi YJ, Tao R, Ge Y, Zhang XG. Apoptosis of multiple myeloma cells induced by agonist monoclonal antibody against human CD28. Cell Immunol. 2005;236:154–160. doi: 10.1016/j.cellimm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Magistrelli G, Jeannin P, Elson G, Gauchat JF, Nguyen TN, Bonnefoy JY, Delneste Y. Identification of three alternatively spliced variants of human CD28 mRNA. Biochem Biophys Res Commun. 1999;259:34–37. doi: 10.1006/bbrc.1999.0725. [DOI] [PubMed] [Google Scholar]
- 50.Magistrelli G, Jeannin P, Herbault N, Benoit De Coignac A, Gauchat JF, Bonnefoy JY, Delneste Y. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 51.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 52.Jeannin P, Magistrelli G, Aubry JP, Caron G, Gauchat JF, Renno T, Herbault N, Goetsch L, Blaecke A, Dietrich PY, et al. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity. 2000;13:303–312. doi: 10.1016/s1074-7613(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 53.Hebbar M, Jeannin P, Magistrelli G, Hatron PY, Hachulla E, Devulder B, Bonnefoy JY, Delneste Y. Detection of circulating soluble CD28 in patients with systemic lupus erythematosus, primary Sjögren’s syndrome and systemic sclerosis. Clin Exp Immunol. 2004;136:388–392. doi: 10.1111/j.1365-2249.2004.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:989–994. doi: 10.1093/rheumatology/keh663. [DOI] [PubMed] [Google Scholar]
- 55.Hock BD, O’Donnell JL, Taylor K, Steinkasserer A, McKenzie JL, Rothwell AG, Summers KL. Levels of the soluble forms of CD80, CD86, and CD83 are elevated in the synovial fluid of rheumatoid arthritis patients. Tissue Antigens. 2006;67:57–60. doi: 10.1111/j.1399-0039.2005.00524.x. [DOI] [PubMed] [Google Scholar]