Abstract
Objective
Reactive oxygen species (ROS) are known to regulate platelet activation; however, the mechanisms of ROS production during platelet activation remain unclear. Platelets express different isoforms of nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidases (NOXs). Here we investigated the role of NOX1 and NOX2 in ROS generation and platelet activation using NOX1 and NOX2 knockout mice.
Approach and Results
NOX1−/Y platelets showed selective defects in G protein-coupled receptor (GPCR)-mediated platelet activation induced by thrombin and thromboxane A2 analog U46619, but were not affected in platelet activation induced by collagen-related peptide (CRP), a glycoprotein VI (GPVI) agonist. In contrast, NOX2−/− platelets showed potent inhibition of CRP-induced platelet activation, and also showed partial inhibition of thrombin-induced platelet activation. Consistently, production of ROS was inhibited in NOX1−/Y platelets stimulated with thrombin, but not CRP, whereas NOX2−/− platelets showed reduced ROS generation induced by CRP or thrombin. Reduced ROS generation in NOX1/2 deficient platelets is associated with impaired activation of Syk and phospholipase Cγ2 (PLCγ2), but minimally affected mitogen-activated protein kinase pathways. Interestingly, laser-induced arterial thrombosis was impaired but the bleeding time was not affected in NOX2−/− mice. WT thrombocytopenic mice injected with NOX2−/− platelets also showed defective arterial thrombosis, suggesting an important role for platelet NOX2 in thrombosis in vivo but not hemostasis.
Conclusions
NOX1 and NOX2 play differential roles in different platelet activation pathways and in thrombosis. ROS generated by these enzymes promotes platelet activation via the Syk/PLCγ/calcium signaling pathway.
INTRODUCTION
Platelets play a crucial role in hemostasis and arterial thrombosis. The initial step of thrombus formation is mediated by interaction of platelets with activated endothelial cells and/or subendothelial matrix proteins - von Willebrand factor (vWF) and collagen.1 Adherent and activated platelets secrete the contents of their α-granules, including P-selectin, and also release dense granular molecules, such as adenosine diphosphate (ADP), that activates other platelets and facilitates platelet aggregation. Mounting evidence shows that reactive oxygen species (ROS) produced during vascular injury can regulate platelet activation.2–4 Although it is thought that endothelial cells, phagocytic leukocytes, vascular smooth muscle cells, and fibroblasts are the major source of ROS following vascular injury,5 several studies demonstrated that collagen-activated platelets can produce superoxide anion (O2−), hydroxyl radicals (OH•), and hydrogen peroxide (H2O2).6–8 Nevertheless, the mechanism of platelet ROS production and regulatory role of platelet ROS during thrombosis and hemostasis remain poorly understood.
Nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidases (NOXs) transfer electrons across biological membranes to generate superoxide anion (O2−).9 NOX1, NOX2, NOX4, and NOX5 are expressed in intravascular cells in humans. Unlike the other three NOXs, NOX5 is not present in rodents. The enzymatic activity of NOX1 and NOX2 is regulated during cell activation,9 whereas it is controversial whether NOX4 is constitutively active10, 11 or its activity is regulated.12 Both NOX1 and NOX2 become active when they form a complex with multiple regulatory subunits.9 NOX2 forms a multi-protein complex with p22phox, p40phox,p47phox, p67phox and the small GTPase Rac. NOX1 also interacts with similar proteins, including p22phox, NOXO1 (similar to p47phox), NOXA1 (similar to p67phox), and Rac. Human platelets express NOX1 and NOX2.13 Previous studies have shown that 2-acetylphenothaizine (2-APT or ML171), a NOX1 inhibitor,14 attenuated collagen-induced integrin αIIbβ3 activation, Syk phosphorylation, and in vitro platelet thrombus formation under shear conditions.13 Treatment of human platelets with ROS scavengers or non-specific NOX inhibitors significantly reduces thrombin-induced intracellular ROS generation and platelet aggregation.15, 16 Interestingly, platelets from patients with X-linked chronic granulomatous disease (X-CGD) that are genetically deficient in NOX2 (gp91phox), showed defects in ROS generation and CD40 ligand expression induced by thrombin, collagen, and arachidonic acid.17 Thus, it remains controversial whether platelet NOX activity and its role in stimulating platelet activation are limited to agonists that activate glycoprotein VI (GPVI)/ immunoreceptor tyrosine-based activation motif (ITAM) pathway.13, 18 Although previous studies demonstrated that ROS regulate platelet activation,2, 4, 15, 19 it remains to be determined whether different NOX isoforms may serve as the mechanisms responsible for platelet ROS generation and whether they play important roles in platelet activation, thrombosis and hemostasis.
Using NOX1 and NOX2 knockout (KO) mice, we show the distinct features of NOX1 and NOX2 in differentially regulating platelet activation. NOX1 is selectively important in GPCR-mediated platelet activation, whereas NOX2 is important in adhesion receptor GPVI-dependent platelet activation as well as in GPCR-induced platelet activation. Furthermore, we demonstrate that platelet NOX2 is critical for platelet thrombus formation at the site of laser-induced arteriolar injury in vivo.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Platelet NOX expression in NOX1- and NOX2-knockout mice
NOX1−/Y and NOX2−/− platelets were used to investigate the roles of NOX1 and NOX2 in platelet function. Polymerase chain reaction (PCR) analyses demonstrated the lack of NOX1 or NOX2 cDNA in platelets from NOX1−/Y or NOX2−/− mice respectively (Figure 1A). Western blot analyses confirmed the lack of protein expression of NOX1 or NOX2 in NOX1−/Y or NOX2−/− platelets respectively (Figure 1B). To determine whether NOX3 or NOX4 are expressed in platelets, RNA was isolated from mouse and human platelets and the presence of NOX3 and NOX4 mRNA was probed via RT-PCR. NOX3 RNA was not detected in platelets (Figure 1C). This was expected, as NOX3 has a highly restrictive tissue distribution and is mainly expressed in the inner ear.20 However, NOX4 RNA was readily detectable (Figure 1C) and to evaluate whether NOX4 expression was altered in NOX1−/Y and NOX2−/− platelets, lysates from these respective platelets were probed with NOX4 antibody. NOX4 was indeed detected but there was no difference in expression between WT and NOX1−/Y and WT and NOX2−/− (Figure 1D).
Figure 1. Expression of NOX1 and NOX2 in murine platelets.
(A) PCR analysis of NOX1−/Y and NOX2−/− mice. (B) Immunoblotting of lysates of littermate control, NOX1−/Y WT (C57BL/6), and NOX2−/− platelets. (C) RT-PCR for NOX3 and NOX4 using RNA isolated from mouse and human platelets, with RNA from human umbilical vein cells used as control. (D) Western blot of NOX4 in human umbilical vein cells and in WT versus NOX1−/Y and NOX2−/− mouse platelets with tubulin loading control.
The selective effects of NOX1 knockout on platelet activation induced via G protein-coupled receptors
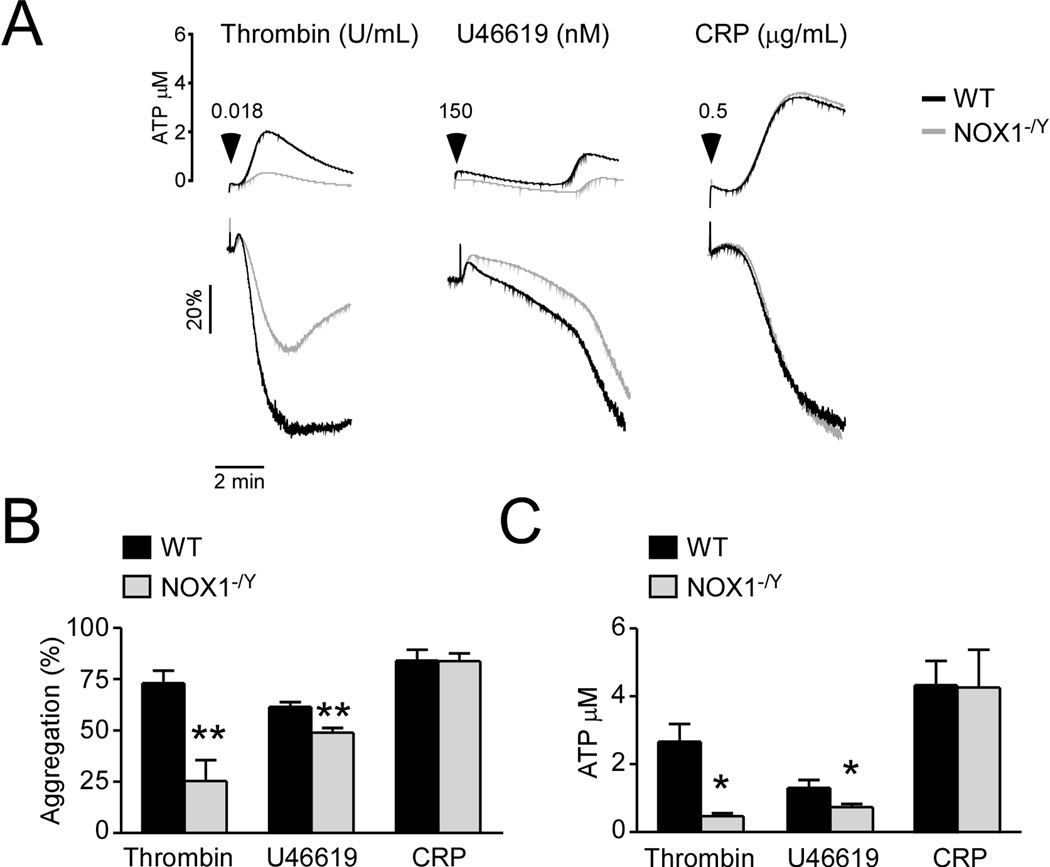
To determine whether NOX1 regulates platelet activation, NOX1−/Y platelets were compared with wild type mouse platelets in platelet aggregation and secretion induced by various platelet agonists. NOX1−/Y platelets were defective in platelet aggregation and ATP secretion induced by thrombin and thromboxane A2 analog U46619 (Figure 2). These data suggest that NOX1 is important in platelet activation induced by GPCR agonists. Interestingly, NOX1−/Y platelets showed no difference from the wild type platelets in platelet aggregation and secretion induced by GPVI agonist collagen-related peptide (CRP) (Figure 2). These data suggest that NOX1 plays an important role for platelet activation induced by GPCR agonists but is not required for GPVI/ITAM signaling.
Figure 2. NOX1 selectively plays an important role platelet aggregation and secretion induced by GPCR agonists.
WT control, and NOX1−/Y platelets, (3 × 108/mL) were stimulated with 0.018 U/mL thrombin, 150 nM U46619 or 0.5 µg/mL CRP. (A) Platelet aggregation and adenosine triphosphate (ATP) secretion traces. (B) Quantification of platelet aggregation. (C) Quantification of platelet secretion. ** and * represents P<0.01 and P<0.05, respectively as determined by Student’s t-test (n=3).
The selective role of NOX1 in GPCR-induced platelet ROS generation and calcium mobilization and activation of Syk and PLCγ
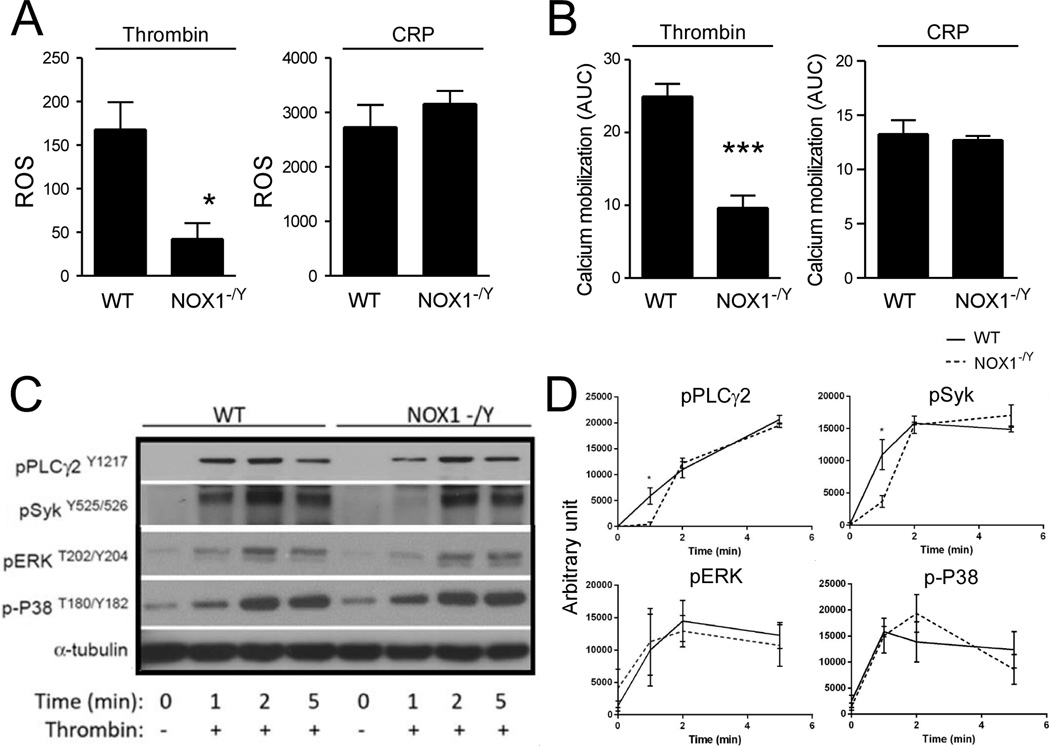
To determine whether NOX1 also selectively mediates GPCR-dependent platelet ROS production, we measured platelet ROS (H2O2) generation with flow cytometry using fluorescent DCF as a probe. NOX1−/Y platelets showed significant defects in thrombin-induced intracellular ROS production relative to WT controls (Figure 3A). In contrast, NOX1−/Y platelets were not different from wild type platelets in CRP-induced intracellular ROS production (Figure 3A), suggesting that NOX1 is selectively important in the GPCR-mediated platelet ROS production.
Figure 3. The selective role of NOX1 in GPCR-induced platelet ROS generation, calcium mobilization, and phosphorylation of PLCγ2 and Syk.
(A–B) Control and NOX1−/Y platelets were incubated with either (A) H2DCFDA or (B) FLIPR calcium dye and stimulated with 0.025 U/mL thrombin or 0.5 µg/mL CRP. (A) DCF fluorescence was measured using flow cytometry and is quantified as mean + SEM (n = 3). (B) Ca2+ mobilization was monitored via fluorescence using the FLIPR calcium assay. Data are shown as mean + SEM. The area under the curve was integrated to calculate the total increase in Ca2+-mobilization over time (n=3). (C) Western blot of phosphorylated PLCγ2, Syk, ERK and P38 in control and NOX1−/Y platelets stimulated with thrombin (0.025 U/mL) in a lumi-aggregometer over time. (D) Quantification (n=3) of western blots from (C). For all data, *** and * represents P<0.001 and P<0.05, respectively, as determined by Student’s t-test.
To provide insight as to how NOX1 regulates platelet activation, we examined whether NOX1 is important for thrombin- and CRP-induced elevation of intracellular calcium concentration, which is a central step downstream of phospholipase C isoforms during both Gαq and GPVI/ITAM signaling. NOX1−/Y platelets were defective in thrombin, but not in CRP-induced Ca2+-mobilization (Figure 3B), consistent with its selective role in GPCR-mediated ROS production and platelet aggregation. Unexpectedly, although GPCRs classically mediate calcium signaling via G protein-PLCβ interaction,1 we also observed that thrombin-induced activation of Syk and its downstream signaling mediator PLCγ. NOX1−/Y platelets showed a significant decrease in the phosphorlyation of Syk and its downstream target PLCγ. However, this defect was only at the early time point (1 minute) during platelet activation (Figure 3C,D), but there was no difference between NOX1−/Y and wild type platelets at later time points. Additionally, NOX1-deficient platelets showed no changes in the mitogen-activated protein kinase signaling pathway, also known to be important for promoting platelet activation.21, 22 Since PLCγ is known to be an activator of intracellular calcium mobilization,1 these results suggest that one pathway by which NOX1-dependent ROS production promotes platelet activation is the Syk-PLCγ pathway. However, these data do not exclude other pathways that may also be important for the role of NOX1 in GPCR-mediated platelet activation.
The effects of NOX2 knockout on platelet aggregation and secretion
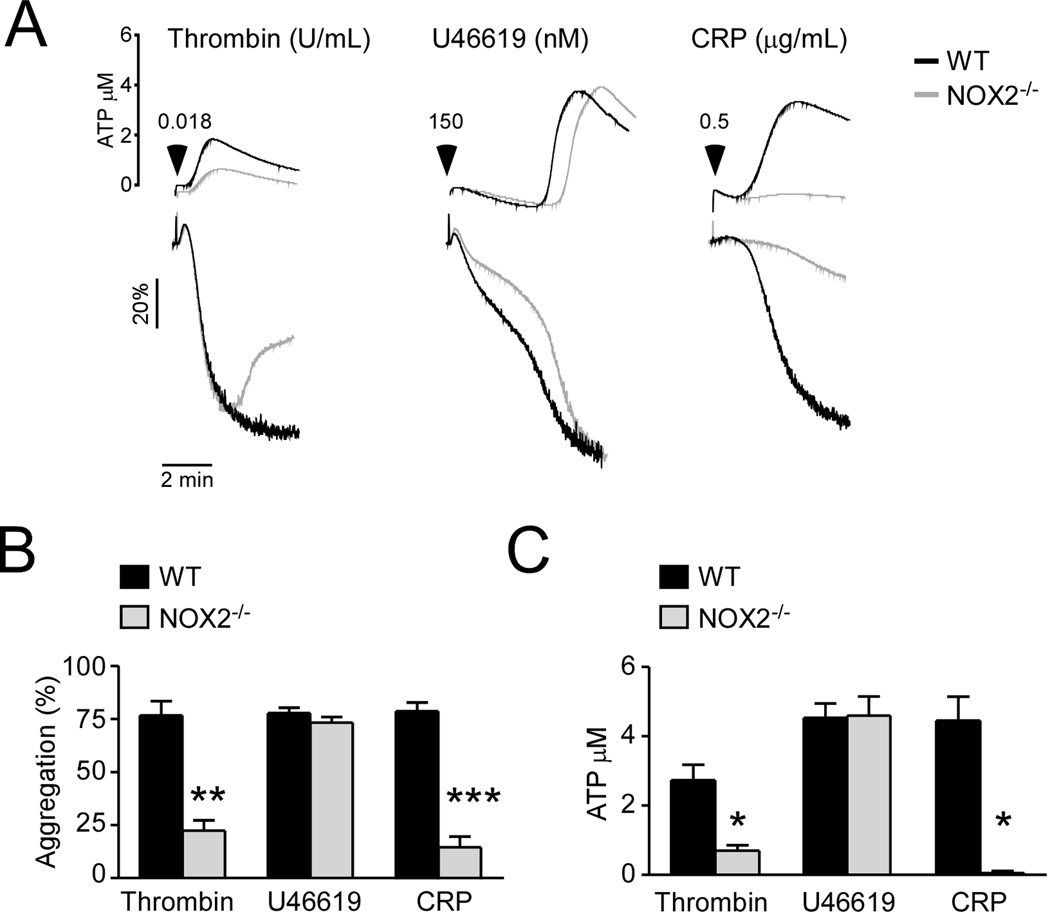
We further determined the role of NOX2 in platelet activation. Unlike NOX1, NOX2−/− platelets were significantly defective in CRP-induced platelet aggregation and ATP secretion (Figure 4). NOX2−/− platelets also showed partial inhibition of platelet activation induced by thrombin but minimal inhibition in U46619-induced platelet aggregation and ATP secretion (Figure 4). These data clearly indicate that NOX2 plays an important role in regulating the GPVI/ITAM-dependent platelet activation pathway and in thrombin-induced platelet activation, but its role is distinct from that of NOX1.
Figure 4. NOX2 promotes GPVI/ITAM-dependent and low dose thrombin-induced platelet aggregation and secretion.
WT control, and NOX2−/− platelets, (3 × 108/mL) were stimulated with 0.018 U/mL thrombin, 150 nM U46619 or 0.5 µg/mL CRP. (A) Platelet aggregation and ATP secretion traces. (B) Quantification of platelet aggregation. (C) Quantification of platelet secretion. ***, ** and * represents P<0.001, P<0.01 and P<0.05, respectively as determined by Student’s t-test (n=3).
The role of NOX2 in GPVI- and thrombin-induced platelet ROS generation, calcium mobilization and activation of Syk and PLCγ
Consistent with the above functional data, NOX2−/− platelets showed reduced ROS production and calcium mobilization during CRP-induced GPVI/ITAM-dependent platelet activation (Figure 5A,B). Similarly, NOX2−/− platelets also showed reduced intracellular ROS production and calcium mobilization in response to low dose thrombin (Figure 5A,B), indicating that, unlike NOX1, NOX2 is important for both GPVI-induced and thrombin-induced platelet ROS production. Furthermore, in contrast to NOX1−/Y platelets, NOX2−/− platelets also showed a significant and sustained reduction in the phosphorylation of Syk and PLCγ2 during platelet activation. The MAPK signaling pathways were not affected (Figure 5D–F). These data suggest that NOX2-dependent ROS generation plays a key role in promoting activation of the Syk-PLCγ pathway, leading to calcium mobilization. Together, the above data clearly indicate that NOX1 and NOX2 play distinct roles in ROS production induced via different platelet activation pathways. NOX1 selectively mediates GPCR-dependent ROS production and platelet activation whereas NOX2 plays a key role in mediating GPVI/ITAM- as well as thrombin receptor-mediated platelet activation. The above data also suggest that ROS generated via these different NOX isoforms may promote platelet activation by activating the Syk-PLCγ-calcium mobilization signaling pathway.
Figure 5. The role of NOX2 in thrombin and GPVI-induced platelet ROS generation, calcium mobilization, and signaling.
(A–B) Control and NOX2−/− platelets were incubated with either (A) H2DCFDA or (B) FLIPR calcium dye and stimulated with 0.025 U/mL thrombin or 0.5 µg/mL CRP. (A) DCF fluorescence was measured using flow cytometry and is quantified as mean + SEM (n=3). (B) Calcium mobilization was monitored via fluorescence using the FLIPR calcium assay. Data are shown as mean + SEM. The area under the curve was integrated to calculate the total increase in calcium mobilization over time (n=3). (C–D) Western blot of phosphorylated PLCγ2, Syk, ERK and P38 in control and NOX2−/− platelets stimulated with thrombin (0.025 U/mL) or collagen (4 µg/mL) in a lumi-aggregometer over time. (E) Quantification (n=3) of western blots from (C). (F) Quantification (n=3) of western blots from (D). For all data, ***, ** and * represents P<0.001, P<0.01 and P<0.05, respectively, as determined by Student’s t-test.
NOX2, but NOX1, is required for platelet thrombus formation following laser-induced arteriolar injury in live mice
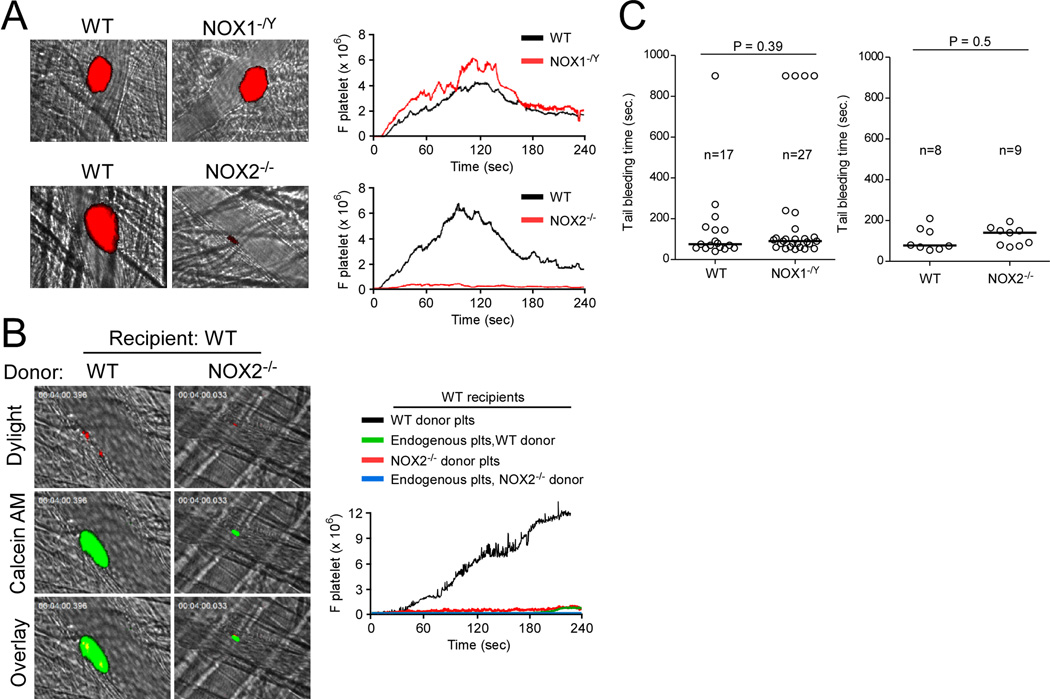
Since both NOX1 and NOX2 are involved in modulating platelet activation, we further investigated to which extent the observed defects of NOX1−/Y and NOX2−/− platelets influence thrombotic events in vivo. Vascular injury was induced by laser ablation on cremaster arterioles, and adherent and accumulating platelets were visualized by infusion of a Dylight 649-conjugated anti-mouse CD42c antibody. Interestingly, compared to WT control mice, NOX1−/Y mice did not show significant defects in platelet adhesion and accumulation at the site of arteriolar injury (Figure 6A, Figure IIA), either using laser-induced arterial thrombosis model or FeCl3-induced carotid artery thrombosis model. In sharp contrast, NOX2−/− mice had a severe and significant defect in adhesion and accumulation of platelets at the site of laser-induced arteriolar wall injury (Figure 6A, Figure IIB). These results indicate that NOX2, but not NOX1, is required for platelet thrombus formation in this laser-induced arteriolar injury model in vivo.
Figure 6. The effects of NOX2 or NOX1 deficiency on in vivo thrombosis and hemostasis.
Laser-induced arterial thrombosis and intravital microscopy was performed as described in Methods. (A) Representative images of platelet thrombi (red) after vascular injury. The median integrated fluorescence intensities of anti-CD42c antibodies (F platelet) were obtained from 28–30 thrombi in 3 WT, 3 NOX1−/Y or 3 NOX2−/− mice and are presented as a function of time. (B) Representative images of endogenous platelets (red) and calcein-AM labeled platelets (green) shown after vascular injury. The median integrated fluorescence intensities of Dylight 649-conjugated anti-CD42c antibodies (residual WT endogenous platelets) and calcein-AM labeled platelets (donor WT or NOX2−/− platelets) were obtained from 17–24 thrombi in 5 mice per group and are presented as a function of time. (C) Tail bleeding time was determined by cutting 5 mm of the tail of WT, NOX1−/Y or NOX2−/− mice. The tail was immediately immersed in 37°C 150 mM NaCl, and time to stable cessation of bleeding was recorded. The bleeding time of each mouse is shown and the horizontal bars represent the median bleeding time.
Platelet NOX2 is required for platelet thrombus formation at the site of laser-induced arteriolar wall injury in vivo
To further determine the role of platelet NOX2 in thrombus formation, we infused calcein AM-labeled WT or NOX2−/− platelets into thrombocytopenic WT recipient mice. 23 Compared to the infused WT platelets, the infused NOX2−/− platelets displayed a marked reduction in the size of platelet thrombi following vascular injury (Figure 6B, Figure IIC), indicating that platelet-derived NOX2 is important for thrombosis. These results support our conclusion that platelet NOX2 is indeed important for thrombus formation following vascular injury in vivo.
NOX1 and NOX2 are dispensable for hemostasis in mice
We next wanted to determine whether deficiency in either NOX1 or NOX2 affects hemostatic function in vivo. As measured by tail bleeding time after tail amputation, NOX1−/Y and NOX2−/− mice did not display prolonged tail bleeding time in comparison with their respective WT controls (Figure 6C). These results suggest that the functions of NOX1 and NOX2 are not essential for hemostasis in mice.
DISCUSSION
In this study, we used a genetic approach to elucidate the mechanisms of ROS production in different platelet activation pathways. We show that NOX1 and NOX2 play differential roles in different platelet activation pathways. NOX1 is selectively important in GPCR-dependent intracellular ROS generation but dispensable for GPVI-ITAM-dependent ROS generation. In contrast, NOX2 is important for ROS generation induced by both the GPCR and GPVI/ITAM signaling pathways. Consistent with the differential roles of NOX1 and NOX2 in ROS production in different platelet activation pathways, we further demonstrate that NOX1 plays a selective role in GPCR-induced platelet aggregation and secretion, whereas NOX2 plays important roles in both the GPCR- and ITAM-dependent platelet aggregation and secretion. Importantly, NOX2 is critical for thrombosis in vivo, but dispensable for hemostasis. Thus, it would be interesting to further study whether NOX-dependent ROS generation may have the potential as a target for developing anti-thrombotics with reduced bleeding complications.
We demonstrate the role of NOX1 in platelet activation using the method of targeted gene deletion in mice. Previously, NOX1 was shown to be expressed in human platelets and mouse megakaryocytes.13, 24 Whereas some previous reports suggested the role of NOX1 in platelet activation, the implications of these investigations have been limited by the reliance on the pharmacological effect of a small molecule NOX inhibitor, 2-acetylphenothaize (2-APT or ML171), which may inhibit both NOX1 and NOX2 and possibly other molecules at concentrations used (ML171 have an IC50 of 250nM for NOX1 and 5 µM for NOX2).14 ML171 was shown to inhibit GPVI-induced platelet superoxide production, aggregation, thrombus formation and activation of αIIbβ3 in previous studies,13,25 suggesting that NOX1 is important in the GPVI-ITAM pathway. In contrast, our data demonstrate that NOX1 is selectively important in GPCR-mediated platelet activation induced by thrombin and thromboxane A2 analog, but is not required for the GPVI-dependent platelet activation induced by CRP. To resolve this contradiction, we also tested the effect of ML171 on thrombin-induced platelet aggregation. To our surprise, ML171 enhanced platelet aggregation and secretion in platelets (Figure III). This effect of ML171 is likely to be non-specific because ML171 also enhanced thrombin-induced platelet aggregation in NOX1−/Y platelets. We suggest that the difference between our conclusions and previous studies are caused by nonspecific effects of these inhibitors, but we do not exclude the possibility of differences between human and mouse platelets. Regardless, our data clearly indicate that NOX1 is not required for CRP-induced ROS production and platelet activation but is selectively important in low dose GPCR agonist-induced ROS production and platelet activation in mice.
Previous work has provided evidence that NOX2 is important for platelet activation. For example, platelets from patients with X-linked chronic granulomatous disease (X-CGD), who are genetically deficient in NOX2, were shown to be defective in ROS generation, platelet secretion, and platelet activation.17 However, it remains controversial as to whether NOX2 plays a general role in platelet activation or whether its role is limited to the GPVI-dependent signaling pathway.13, 18 The function of NOX2 in platelet activation was also based on conclusions using non-specific inhibitors, which prevented conclusive identification of isoform-specific roles in platelets. Here we utilized NOX2-deficient platelets. Not only did we convincingly show that NOX2 plays a role in GPVI-induced platelet activation, but we also demonstrated that NOX2 is important for thrombin-induced platelet activation. Therefore, our data demonstrate that NOX2 indeed plays a more general role in facilitating platelet activation, in agreement with the phenotype observed with X-CGD platelets.
Our data not only demonstrate differential roles of NOX1 and NOX2 in different platelet activation pathways, but also suggest a shared ROS-dependent Syk-PLCγ pathway mediating downstream signaling. We show that both NOX1 and NOX2 deficiency is associated with defective calcium elevation. The protein tyrosine kinase Syk is known to phosphorylate and activate PLCγ, and thus promote calcium elevation induced via the ITAM signaling pathway.26, 27 We show that knockout of NOX2 in platelets caused a sustained reduction in CRP-induced activation of Syk-PLCγ signaling, indicating that NOX2-mediated ROS generation activates this Syk-PLCγ-calcium signaling pathway to promote platelet activation. To our surprise, although it is recognized that GPCR signaling leads to G protein activation of PLCβ, we show that thrombin potently induces phosphorylation of Syk and PLCγ. Interestingly, NOX2−/− platelets also showed a sustained reduction in thrombin-induced phosphorylation of Syk and PLCγ. In contrast, NOX1−/Y platelets showed only a transient decrease in phosphorylation of Syk and PLCγ, during the initial phase of platelet activation. Thus, it appears that NOX1 and NOX2 share a ROS-dependent Syk-PLCγ pathway during GPCR-mediated platelet activation. Previous studies have demonstrated that ROS inactivates tyrosine phosphatases, thus facilitates tyrosine phosphorylation and activation of ITAM pathway28, 29, which we speculate as a mechanism for ROS generated by NOX2 and NOX1 to facilitate activation of Syk/PLCγ and calcium mobilization in platelets. However, NOX1-dependent ROS is only important during an early period, whereas NOX2-dependent ROS is important during the full course of GPCR-mediated platelet activation. It remains unclear whether the transient role of NOX1 in the activation of the Syk-PLCγ pathway is fully responsible for mediating calcium elevation. We do not exclude the possibility that NOX1-dependent ROS may have another downstream signaling mechanism (e.g. PLCβ).
As the inhibitory effects of NOX1 and NOX2 knockout were diminished at high concentrations of agonists, future studies are needed to further determine whether the roles of individual NOX isoforms can be partially compensated by other NOX isoforms or whether there are additional mechanisms of ROS production in platelets. In this regard, we detected the presence of NOX4 in addition to NOX1 and NOX2. It is interesting to determine whether NOX4 also play roles in platelet activation in the future. It is important to note that the conclusions we made are derived from data using a mouse model, which may or may not be different from humans. To conclusively identify the roles of different NOX isoforms in humans, future studies are needed when necessary tools become available.
We conclude that NOX2 is important in thrombosis in vivo. We further conclude that platelet NOX2 is important in thrombosis in vivo. These conclusions are based on data showing defective thrombosis in NOX2 knockout mice and in wild type mice transfused with NOX2−/− platelets. These in vivo data are also consistent with our in vitro data that NOX2 plays a role in platelet activation induced by both the GPCR and GPVI/ITAM pathways. It is still puzzling why NOX1 is not required for in vivo thrombosis, despite its in vitro role in thrombin-induced platelet activation. This is unlikely caused by the lack of importance of thrombin in laser-induced thrombosis because previous data shows that thrombin is required in laser-induced platelet thrombus formation30, 31, and our data show that knockout of NOX1 also had no significant effect in the different FeCl3-induced thrombosis model (data not shown). One explanation for this observation is the possible in vivo compensation of lack of NOX1 by ROS generated by other cells such as leukocytes and endothelial cells in GPCR-dependent platelet activation. Also, although the mechanism for the dominant role of NOX2 in the in vivo model remains to be further investigated, one of the reasons is a greater role for the ITAM-dependent ROS generation (which is NOX2-dependent), in this in vivo model. Alternatively, the role of NOX1 in the GPCR-mediated platelet activation pathway may be partially compensated by NOX2 which also plays a role in GPCR pathway. It is important to note that although we did not show the requirement for the NOX1 in the laser-induced thrombosis model, we do not exclude the possibility NOX1 may be important under different experimental or pathological conditions.
Antiplatelet drugs are used extensively as a means to treat and prevent thrombosis. However, the current antiplatelet drugs cause adverse bleeding. This may derive from the fact that they pharmacologically target cyclooxygenase, ADP receptors and integrins, which are important for both hemostasis and thrombosis32. In this respect, it would be ideal to identify novel molecular targets for developing antithrombotic agents that will not result in significant bleeding side effects33. In this study, we demonstrate that NOX2 is dispensable for hemostasis in vivo, as suggested by mouse tail-bleeding time, but is required for arteriole thrombosis in vivo. Thus, it is tempting to speculate that NOX2 may represent a potentially novel molecular target for the development of an antithrombotic that may have reduced adverse outcomes on bleeding. Therefore, further studies on the roles and mechanisms of actions of NOX isoforms, particularly NOX2, in platelet activation, thrombosis and hemostasis is warranted.
Supplementary Material
KEY POINTS.
NOX1 and NOX2 play differential roles during platelet activation. NOX1 plays an important role in GPCR-mediated platelet activation, whereas NOX2 is important in the thrombin and GPVI-dependent ITAM signaling pathways.
SIGNFICANCE.
NOX1 and NOX2 are expressed in platelets and they play differential roles in platelet activation and thrombosis. Knockout (KO) of NOX1 causes a defect in G-protein coupled receptor (GPCR)-mediated platelet activation, whereas knockout of NOX2 causes a defect in not only GPCR- but also GPVI-mediated platelet activation. Interestingly, although NOX1 KO mice have no defect in thrombosis and hemostasis in vivo, NOX2 KO mice are defective in thrombosis in vivo but their hemostatic function remains intact. Thus, it is interesting to further study the potential of targeting NOX pathways for anti-thrombotic intervention.
Acknowledgments
M.K.D., B.E. and K.K. performed experiments, analyzed and interpreted data, and wrote the manuscript. A.S., B.S. and Z.X. performed experiments. M.U-F. and K-H.K. provided transgenic mice. X.D. and J.C. designed research, analyzed data, and wrote the manuscript. We thank Dr. Karl-Heinz Krause for providing NOX1 knockout mice.
SOURCES OF FUNDING
This work is supported in part by grants from NIH (HL109439 to J.C.; HL116976 and HL112293 to M.U.F; and HL062350, HL125356 and HL080264 to X.D.).
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- ATP
adenosine triphosphate
- CRP
collagen related peptide
- GP
glycoprotein
- GPCR
G-protein coupled receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- NOX
nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidases
- PL
phospholipase
- ROS
reactive oxygen species
- WT
wild type
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begonja AJ, Teichmann L, Geiger J, Gambaryan S, Walter U. Platelet regulation by no/cgmp signaling and nad(p)h oxidase-generated ros. Blood Cells Mol Dis. 2006;36:166–170. doi: 10.1016/j.bcmd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Muller G, Morawietz H. Nitric oxide, nad(p)h oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–1731. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- 4.Violi F, Pignatelli P. Platelet oxidative stress and thrombosis. Thromb Res. 2012;129:378–381. doi: 10.1016/j.thromres.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Jiang F, Zhang Y, Dusting GJ. Nadph oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 6.Caccese D, Pratico D, Ghiselli A, Natoli S, Pignatelli P, Sanguigni V, Iuliano L, Violi F. Superoxide anion and hydroxyl radical release by collagen-induced platelet aggregation--role of arachidonic acid metabolism. Thromb Haemost. 2000;83:485–490. [PubMed] [Google Scholar]
- 7.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood. 1998;91:484–490. [PubMed] [Google Scholar]
- 8.Pratico D, Pasin M, Barry OP, Ghiselli A, Sabatino G, Iuliano L, FitzGerald GA, Violi F. Iron-dependent human platelet activation and hydroxyl radical formation: Involvement of protein kinase c. Circulation. 1999;99:3118–3124. doi: 10.1161/01.cir.99.24.3118. [DOI] [PubMed] [Google Scholar]
- 9.Lassegue B, Griendling KK. Nadph oxidases: Functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. Nox4 activity is determined by mrna levels and reveals a unique pattern of ros generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vara D, Campanella M, Pula G. The novel nox inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a gpvi-dependent manner. Br J Pharmacol. 2013;168:212–224. doi: 10.1111/j.1476-5381.2012.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific nadph oxidase-1 (nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet nad(p)h-oxidase-generated ros production regulates alphaiibbeta3-integrin activation independent of the no/cgmp pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
- 16.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U. Nad(p)h oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 17.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. Gp91phox-dependent expression of platelet cd40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 18.Arthur JF, Qiao J, Shen Y, Davis AK, Dunne E, Berndt MC, Gardiner EE, Andrews RK. Itam receptor-mediated generation of reactive oxygen species in human platelets occurs via syk-dependent and syk-independent pathways. J Thromb Haemost. 2012;10:1133–1141. doi: 10.1111/j.1538-7836.2012.04734.x. [DOI] [PubMed] [Google Scholar]
- 19.Loiko EN, Samal AB, Shulyakovskaya SM. H2o2-induced platelet aggregation and increase in intracellular ca2+ concentration are blocked by inhibitors of intracellular signaling. Biochemistry (Mosc) 2003;68:1210–1216. doi: 10.1023/b:biry.0000009135.85883.65. [DOI] [PubMed] [Google Scholar]
- 20.Bedard K, Krause KH. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin αIIbβ3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 22.Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel rac1-mapk-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113:893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieswandt B, Bergmeier W, Rackebrandt K, Gessner JE, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- 24.McCrann DJ, Eliades A, Makitalo M, Matsuno K, Ravid K. Differential expression of nadph oxidases in megakaryocytes and their role in polyploidy. Blood. 2009;114:1243–1249. doi: 10.1182/blood-2008-12-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh TG, Berndt MC, Carrim N, Cowman J, Kenny D, Metharom P. The role of nox1 and nox2 in gpvi-dependent platelet activation and thrombus formation. Redox Biol. 2014;2:178–186. doi: 10.1016/j.redox.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, Tybulewicz VL, Watson SP. The fc receptor gamma-chain and the tyrosine kinase syk are essential for activation of mouse platelets by collagen. Embo J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmeier W, Stefanini L. Platelet itam signaling. Current opinion in hematology. 2013;20:445–450. doi: 10.1097/MOH.0b013e3283642267. [DOI] [PubMed] [Google Scholar]
- 28.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 29.Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible pecam-1 tyrosine phosphorylation and shp-2 binding. Am J Physiol Heart Circ Physiol. 2003;285:H2336–H2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- 30.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vwf independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estevez B, Kim K, Delaney MK, Stojanovic-Terpo A, Shen B, Ruan C, Cho J, Ruggeri ZM, Du X. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood. 2016;127:626–636. doi: 10.1182/blood-2015-04-638387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estevez B, Shen B, Du X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler Thromb Vasc Biol. 2015;35:24–29. doi: 10.1161/ATVBAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen B, Zhao X, O'Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503:131–135. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.