Abstract
Extensive evidence highlights the role of inflammatory processes in Major Depressive Disorder (MDD). However, most studies have examined a consistent set of inflammatory cytokines and there is evidence that other immune-derived products may play a role in MDD. In this article, we present data from three complimentary studies that support the role of a novel cytokine, interleukin-33 (IL-33), in depression risk. First, we show that a two-SNP haplotype in the IL-33 gene (rs11792633 and rs7044343) moderated the link between women's history of childhood abuse and their history of recurrent MDD (rMDD), such that the link between childhood abuse and rMDD was stronger among women with fewer copies of the protective IL-33 CT haplotype. Second, linking these findings to differences in circulating cytokine levels, we show in a separate sample that those with a history of rMDD had higher peripheral levels of IL-33 and IL-1β compared to women with a single MDD episode or no history of MDD. Third, providing initial evidence of brain regions underlying these effects using archival rat brain tissue, we show that an acute stressor increased IL-33 expression in the paraventricular nucleus of the hypothalamus and, to a lesser extent, the prefrontal cortex, key brain regions underlying stress response and emotion regulation. These findings provide converging support for the potential role of IL-33 in risk for recurrent MDD.
Keywords: Cytokines, inflammation, major depressive disorder, interleukin-33, interleukin -1β
A rapidly growing body of research suggests that immune-derived signaling factors, including proinflammatory cytokines, play an important role in the etiology and pathophysiology of major depressive disorder (MDD; Capuron et al., 2002; Dantzer et al., 2008; Levine et al., 1999; Musselman et al., 2001; Owen et al., 2001; Raison et al., 2006). This said, not every MDD patient exhibits increased inflammation, suggesting that the link between inflammation and MDD may only be apparent among specific subgroups of depressed individuals such as those with pre-existing vulnerabilities, including cognitive and genetic vulnerabilities or histories of early life stress (Raison et al., 2006; Slavich & Irwin, 2014).
Although a number of classic cytokines have emerged from human studies of MDD (e.g., IL-1β, IL-6, IFN), a wide range of other promising immune-derived products have yet to be explored (Raison et al., 2006). In the present report, we focused on a well-characterized member of the IL-1 cytokine family, IL-1β, and a newly identified, multifunctional ligand within the IL-1 superfamily, IL-33 (Baekkevold et al., 2003; Figure 1). The first interleukin to be described, IL-1β, constitutes one of the most potent molecules of the innate immune system. It is secreted from activated monocytes and macrophages and it initiates local and systemic inflammatory effects by binding to its receptor complex, which is a heterodimer consisting of IL-1 receptor type 1 (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP; Maes, Song, & Yirmiya, 2012; Sims & Smith, 2010). IL-1β drives peripheral inflammation and neuroinflammation by further stimulating its own production as well as up-regulating the production of other inflammatory cytokines both peripherally and in the central nervous system (CNS; Sone et al., 1994; Xiao, Xia, Ferin, & Wardlaw, 1999). As such, IL-1β was shown to be involved in multiple inflammation-associated conditions, including psoriasis, multiple sclerosis, Crohn's disease, asthma, and ulcerative colitis (Sims & Smith, 2010). Notably, there is also evidence for its involvement in MDD, though some studies fail to confirm this association (Dowlati et al., 2010; Maes et al., 2012). Specifically, extensive research suggests that individuals with current MDD evidenced increased circulating levels of IL-1β as well as increased stimulated production of IL-1β (Heiser, Lanquillon, Krieg, & Vedder, 2008; M Maes et al., 1991; Piletz et al., 2009; Suarez, Lewis, Krishnan, & Young, 2004). However, recent meta-analytic findings that excluded studies of induced cytokine production failed to support the association between circulating levels of IL-1β and current MDD (Dowlati et al., 2010).
Figure 1.
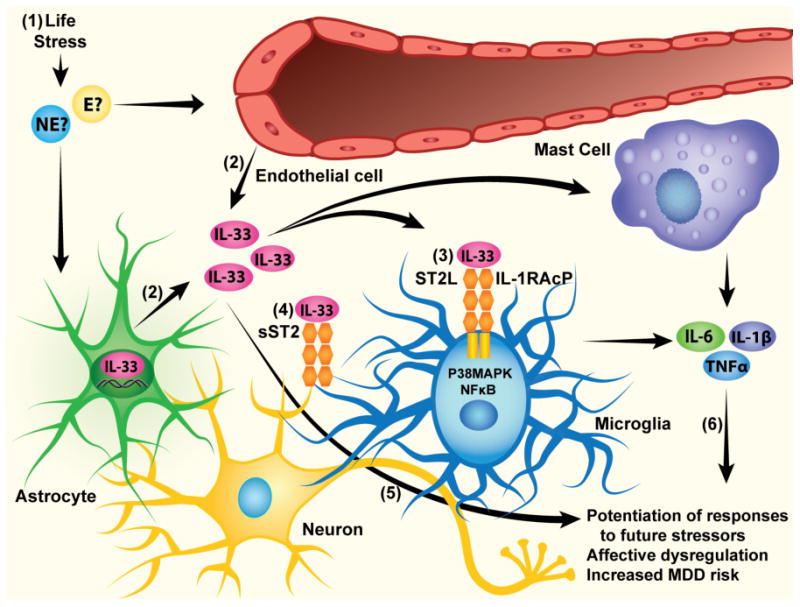
Cellular mechanisms of IL-33 and their relation to MDD risk. (1) Early life stress precipitates increased expression of neuroimmune genes, potentially via norephinephrine and epinephrine release, (2) which includes secretion of IL-33 by endothelial cells and astrocytes. (3) IL-33 is known to interact with a heterodimeric complex comprised of ST2 and IL-1RAcP, which are coupled to both NF-κB and P38 MAPK signaling pathways, ultimately driving the expression of other cytokines and chemokines. (4) It should be noted that ST2 can also be released from cells in soluble form, where it serves to inactivate extracellular IL-33 by serving as a decoy receptor. (5) IL-33 may impact MDD risk directly by modulating neuronal circuits responsible for affect regulation, or (6) indirectly by promoting the expression of other key cytokines/chemokines known to moderate MDD risk.
IL-33 is abundantly expressed in the central nervous system (CNS), with the brain and spinal cord being the organs with the highest mRNA levels of IL-33 in the body (Schmitz et al., 2005). IL-33 is constitutively expressed in endothelial cells, microglia, and astrocytes, whereas its receptors are mainly located on microglia and astrocytes (Moussion, Ortega, & Girard, 2008a; Yasuoka et al., 2011). Inflammation-inducing stimuli, such as pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS) and double-stranded RNA (dsRNA), as well as PAMPs with added adenosine triphosphate (ATP), increase IL-33 mRNA levels in astrocytes (Hudson et al., 2008). Moreover, IL-33 secreted from cells treated with PAMPs + ATP induce the production of proinflammatory cytokines in mast cells (Hudson et al., 2008). Additionally, IL-33 mRNA expression is upregulated following subarachnoid hemorrhage in neurons and astrocytes (Huang et al., 2015). Therefore, IL-33 activity in the brain may constitute a critical factor in CNS inflammatory processes. Upon release, IL-33 interacts with a heterodimeric receptor complex comprised of the ST2 receptor and IL-1 RAcP (Kakkar & Lee, 2008b; Liew, Pitman, & McInnes, 2010). IL-33/ST-2 binding activates nuclear factor kappa B (NF-κB) and p38 mitogen-activated protein kinases (MAPK), culminating in altered expression of pro-inflammatory cytokines, chemokines, and Th2-associated cytokines (Allakhverdi, Smith, Comeau, & Delespesse, 2007; Mirchandani, Salmond, & Liew, 2012). Specifically, IL-33 induces the production of pro-inflammatory mediators in microglia and mast cells in a dose-dependent manner (Moulin et al., 2007; Yasuoka et al., 2011). Mature IL-33 protein also associates with chromatin material within the nucleus, serving as a transcriptional regulator, and has been proposed as a novel “alarmin”, that primes host defense during impending threats (Moussion, Ortega, & Girard, 2008b).
IL-33 is a multimodal cytokine with diverse functions in different systems and plays a significant role in multiple diseases, including cardiovascular disease (Kakkar & Lee, 2008b), rheumatoid arthritis (Xu, Zhang, Zhang, & Ye, 2013), respiratory allergy and asthma (Makrinioti, Toussaint, Jackson, Walton, & Johnston, 2014), and atopic dermatitis (Cevikbas & Steinhoff, 2012). There is also evidence for the involvement of IL-33 in brain pathology, including Alzheimer's disease (Chapuis, Hot, Hansmannel, Kerdraon, Ferreira, Hubans, & Maurage, 2009). Moreover, there is evidence from one study of increased circulating IL-33 levels among individuals diagnosed with bipolar disorder (Barbosa et al., 2014). Thus, IL-33 appears to be embedded within key signaling pathways that are known to link cytokine signaling with MDD (Allakhverdi et al., 2007; Kakkar & Lee, 2008a), yet its association with MDD has not yet been directly examined.
Based on this apparent functional role of IL-33, we queried the human brain database of the Allen Brain Atlas (Allen Human Brain Atlas [Internet], 2015) to determine whether IL-33 might be expressed in anatomical regions known to be critical for affective function, including the paraventricular nucleus of the hypothalamus (PVN), superior, middle, and inferior frontal gyri, amygdala, and cingulate gyrus. Our analyses revealed that IL-33 expression was significantly increased in the PVN (12.5 fold change), cingulate gyrus (11.2 fold change), amygdala (37.5 fold change), and inferior frontal gyrus (8.8 fold change), relative to the occipital pole, a structure largely unrelated to affective processes. In contrast, IL-33 was not differentially expressed in the superior or middle frontal gyri. Additionally, IL-33 was higher on this list of differential expression than any of the other more commonly examined cytokine-related genes (e.g., IL-1β, IL-6) in all of our areas of interest, except for superior and middle frontal gyri. Together, these findings suggest that IL-33 is uniquely positioned, from both signaling and anatomical perspectives, to emerge as an important link in the cytokine theory of MDD. In this investigation, we used existing data from three separate samples to ask questions about the plausible role of IL-33 in depression using a parallel, as opposed to a serial, approach.
Study 1
In Study 1, we tested a gene × environment (G×E) model of risk for MDD. One of the strongest environmental influences on MDD in humans is stress, which strongly regulates the expression of inflammatory molecules (de Kloet, Joels, & Holsboer, 2005; Iwata, Ota, & Duman, 2013). In rodent models, stress exposure initiates time-dependent and site-selective changes in inflammatory cytokines, suggesting a key role for central cytokines as mediators of the downstream consequences of stress (Hueston & Deak, 2014). These findings are consistent with human studies showing that circulating pro-inflammatory cytokine concentrations increase in response to psychosocial stressors (Carroll et al., 2011; Slavich, Way, Eisenberger, & Taylor, 2010) and that psychosocial stress moderates the link between inflammation and depression (Häfner et al., 2011). Although different types of early life stress, ranging from severe maltreatment to less severe variations in caretaking environment, are associated with increased risk for MDD risk, they vary in the magnitude of the adverse impact on the circuits involved in MDD development (Essex et al., 2011; Heim & Binder, 2012; Slavich & Irwin, 2014). Of the various forms of early life stress, childhood abuse is one of the most robust predictors of long-term risk for depression (Goodman & Brand, 2000; Springer, Sheridan, Kuo, & Carnes, 2003), has lasting biological effects that increase susceptibility for developing future MDD (Fumagalli, Molteni, Racagni, & Riva, 2007; Kessler, Davis, & Kendler, 1997), and meta-analyses of previous G × E studies has suggested that it may be more strongly related future MDD in the context of genetic risk than other, more proximal stressors (e.g.,Karg, Burmeister, Shedden, & Sen, 2011). Childhood abuse could be conceptualized as a form of targeted rejection, a class of negative interpersonal events that has been strongly linked to cytokine expression in other research (for a review, see Slavich & Irwin, 2014). This said, not all children exposed to maltreatment go on to develop depression (Cicchetti, 2013) suggesting the presence of important moderating factors, including genetic influences, that may help to determine which individuals are at a greater risk for depression following childhood abuse (Moffitt, Caspi, & Rutter, 2006). Thus, our goal in Study 1 was to determine whether genes that code for IL-33 and IL-1β moderate the impact of childhood abuse on risk for MDD. Supporting the role of inflammatory factors, there is evidence that early life stress leads to increased inflammation during adulthood in animals and humans (Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Fagundes & Way, 2014). Therefore, we hypothesized that variation in the IL-33 and IL-1β genes would moderate the impact of childhood abuse on participants' risk for MDD. Because recurrent MDD (rMDD) is characterized by distinct genetic, neurobiological and hormonal profiles compared to a single episode of MDD (sMDD; Admon et al., 2014; Holsen et al., 2013; Lewinsohn, Allen, Seeley, & Gotlib, 1999), carries greater risk for adverse and chronic consequences (Burcusa & Lacono, 2007), and is less responsive to antidepressant medication (Kaymaz, van Os, Loonen, & Nolen, 2008), we examined sMDD and rMDD separately.
Although research characterizing functional haplotypes in the IL-33 gene is limited, there is evidence that two (rs11792633 and rs7044343) or three (rs1157505, rs11792633, and rs7044343) SNPs in the promoter/enhancer region of the IL-33 gene form a CT or a CCT haplotype. To date, these haplotypes have been studied in relation to Alzheimer's disease (AD) but not depression. For example, both haplotypes were overrepresented in participants who suffered from Alzheimer's disease and did not carry APOE e4 allele (Chapuis, Hot, Hansmannel, Kerdraon, Ferreira, Hubans, & Maurage, 2009; J.-T. Yu et al., 2012). Chapuis et al. reported decreased mRNA expression of IL-33 in the brains of individuals with AD, compared to controls. Presence of the CCT haplotype was also associated with higher cerebral amyloid angiopathy, a pathological hallmark of AD. Consistent with this, in vitro studies have shown that overexpression of IL-33 led to decreased expression of Aβ (Fryer et al., 2003). Together, these findings indirectly suggest that CCT and CT haplotypes are associated with decreased expression of IL-33, which may confer increased risk for the development of AD or other neuropathological conditions. Given this, we hypothesized that the presence of the IL-33 CT/CCT haplotype would buffer the impact of childhood abuse on MDD risk. Similarly, building from evidence that two SNPs in the promoter/enhancer region of the IL-1β gene (rs1143627, rs16944) form an AG haplotype that is associated with decreased IL-1β protein secretion (Borkowska et al., 2011; Hall et al., 2004), we predicted that the presence of the IL-1β AG haplotype would also buffer the impact of childhood abuse on MDD risk.
Method
Participants
A total of 241 women were recruited from the community based on their histories of MDD: rMDD (n = 76), sMDD (n = 40), and no MDD (n = 125). Of the participants (average age = 40.11, SD = 6.79), 4.1% (n = 10) were diagnosed with current MDD, 8.71% (n = 21) with a current anxiety disorder, and 3.3% (n = 8) with comorbid current MDD and anxiety disorder. Exclusion criteria for all groups included the presence of schizophrenia, organic mental disorder, a history of alcohol or substance dependence within the last six months, or lifetime history of bipolar disorder. The majority of the participants (87.9%) was Caucasian, and the median annual family income was $50,001-55,000. Women in the three MDD groups did not differ significantly in age, ethnicity, BMI, or lifetime tobacco use. However, women in the sMDD and rMDD groups, compared to women in the no MDD group, reported lower annual income, greater rates of childhood abuse, and higher levels of current depressive symptoms (Table 1). The project was approved by the university's institutional review board.
Table 1. Descriptive statistics for Sample 1.
| No MDD | sMDD | rMDD | F/χ2 | |
|---|---|---|---|---|
| (n = 125) | (n = 40) | (n =76) | ||
| Age (M and SD) | 40.85 (6.32) | 39.38 (7.14) | 39.49 (7.31) | 1.28 |
| Ethnicity (%Caucasian) | 93.6a | 80.0b | 82.9a,b | 7.99* |
| Median Annual Income | 60,001-65,000a | 35,001-40,000b | 35,001-40,000b | 11.67** |
| BMI (M and SD) | 28.65 (7.02) | 29.36 (6.92) | 29.98 (9.07) | 0.71 |
| Lifetime tobacco use (% smoked ≥100 ea) | 28.0a | 47.5b | 44.7b | 8.21* |
| IL-1 β AG haplotype: | ||||
| (% within MDD) | ||||
| 0 copies | 10.4 | 7.5 | 13.2 | 4.52 |
| 1 copy | 44.8 | 30 | 38.2 | |
| 2 copies | 44.8 | 62.5 | 48.7 | |
| IL-33 haplotype: | ||||
| 0 copies | 12.8 | 12.5 | 17.1 | 1.20 |
| 1 copy | 41.6 | 37.5 | 40.8 | |
| 2 copies | 45.6 | 50 | 42.1 | |
| CTQ (% abused) | 15.2a | 35.0b | 59.2c | 41.97** |
| BDI-II (M and SD) | 3.74 (4.94)a | 11.79 (10.41)b | 15.39 (10.81)c | 58.30** |
Note. MDD = major depressive disorder. sMDD = single episode of MDD.
rMDD = recurrent MDD. BMI = Body Mass Index, BDI-II = Beck's Depression Inventory-II, CTQ = Childhood Trauma Questionnaire. Means with different superscripts differ significantly.
p < .05.
p < .001
Measures
Lifetime histories of psychiatric disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 2002). A subset of 20 SCID-I interviews was coded by a second interviewer and inter-rater reliability for diagnoses of MDD was excellent (κ = 1.00).
Participants' current symptoms of depression were assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996). In this sample, this measure showed excellent internal consistency (α = .91).
Histories of abuse were assessed using the Childhood Trauma Questionnaire (CTQ; Bernstein and Fink, 1997). In this sample, the CTQ exhibited good internal consistency (emotional abuse: α = .86, physical abuse: α = .85, sexual abuse: α = .96). Using the established cutoffs on the CTQ, moderate levels of abuse were defined as an emotional abuse (EA) subscale score greater than 12, a physical abuse (PA) subscale score greater than 9, and/or sexual abuse (SA) subscale score greater than 7. Of the women in our sample, 20 reported EA only (8.3%), 6 reported PA only (2.5%), 13 reported SA only (5.4%), 9 reported EA and PA (3.7%), 10 reported EA and SA (4.1%), 3 reported PA and SA (1.2%), and 17 reported all three types of abuse (7.1%). Consistent with prior research using the CTQ (e.g., Bradley et al., 2014), women were coded as having a history of no or mild abuse (n = 163) or having a history of moderate to severe levels of abuse (n = 78).
Women's DNA was collected and isolated from buccal cells using established methods (Freeman et al., 1997). The samples were genotyped for 3 SNPs in the promoter/ enhancer region of the IL-33 gene that have previously been shown to form a two or three-SNP haplotype (Chapuis, Hot, Hansmannel, Kerdraon, Ferreira, Hubans, & Maurage, 2009; J.-T. Yu et al., 2012) and 2 SNPs in in the promoter/enhancer region of the IL-1β gene (Borkowska et al., 2011). All polymorphisms were genotyped using the fluorogenic 5′ nuclease (Taqman, Applied Biosystems, Foster City, CA) method involving reagents (VIC(tm) and FAM(tm) labeled probes and TaqMan® Universal PCR Master Mix without AMPerase® UNG obtained from Applied Biosystems (ABI). Genotype determination was performed using primers purchased from ABI or Integrated DNA Technologies (Coralville, IA). Genotypes were obtained using an ABI Prism 7300 Sequence Detection System using both absolute quantification and allelic discrimination modes (Livak, Flood, Marmaro, Guisti, & Deetz, 1995). The markers did not differ from Hardy-Weinberg equilibrium using default parameters in Haploview (Barrett, Fry, Maller, & Daly, 2005). In order to (a) maximize the amount of information provided by the multiple markers, and (b) circumvent loss of power due to multiple testing, we utilized all of the available SNP data to identify haplotype blocks (i.e., the combinations of SNP markers that are statistically associated). Haploview was used to visualize haplotype blocks (Barrett et al., 2005, see Supplementary Figures 1S and 2S). Haplotypes for both chromosomes were then confirmed and extracted using PHASE [Version 2.1] (Stephens & Donnelly, 2003), requiring that the probability of a haplotype be greater than or equal to 0.80. PHASE haplotypes were used to construct diplotypes (i.e., combination of haplotypes across the pair of homologous chromosomes) that were used in the regression analyses. Similarly to Yu et al. (2012) we found a two-SNP haplotype in IL-33 in our sample (CT; rs11792633, rs7044343). A two-SNP haplotype was also found in IL-1β (AG; rs1143627, rs16944), similarly to previous research (Borkowska et al., 2011). Marker-to-marker statistics for IL-33 were as follows: rs1157505- rs11792633: linkage disequilibrium (D') = 0.61, r2 = .246; rs1157505 - rs7044343: D' = 0.54, r2 = .14; rs11792633 - rs7044343: D' = 0.98, r2 = .697. The marker-to-marker statistics for IL-1β was: rs1143627 - rs16944 D' = 1, r2 = .981. Consistent with previous research (Borkowska et al., 2011; Jöris et al., 2013; Sweeney, 2006; J.-T. Yu et al., 2012), the IL-33 and IL-1β haplotypes were coded in an additive manner reflecting the number of copies of the CT or AG haplotypes present (0, 1, or 2 copies).
Procedure
Participants were recruited from the community via a variety of means (e.g., TV, newspaper and bus ads, flyers). After providing consent, participants were administered the SCID-I, completed questionnaires, and provided a DNA sample.
Results
The demographic and clinical characteristics of the sample are presented in Table 1. Numbers and percentages of participants stratified according to history of childhood abuse, MDD, and genotype are presented in the Supplementary Information (Table S1).
Prior to conducting our primary gene × environment (G×E) analyses, we tested for potential gene-environment correlations (rGE). Women's histories of childhood abuse were not significantly related to the number of copies they carried of the IL-33 CT haplotype, χ2 (2) = 0.80, p = .67, or IL-1β AG haplotype, χ2 (2) = 3.75, p = .15. We then tested the G × E models predicting women's history of MDD (rMDD and sMDD). For these analyses, we used multinomial regressions with the criterion variable reflecting membership in one of three depression groups: rMDD, sMDD, or no MDD. Similarly to previous research, (Borkowska et al., 2011; Jöris et al., 2013; Sweeney, 2006; J.-T. Yu et al., 2012), we examined additive genetic effects for each haplotype and entered them in our analyses as continuous variables (reflecting 0, 1, and 2 copies of the haplotype). Focusing first on IL-33, we found a significant main effect of childhood abuse showing that women with a history of abuse, compared to women with no abuse history, were more likely to be in the rMDD group than the sMDD group, β = 2.70, Wald = 7.04 p = .01, or the no MDD group, β = 3.80, Wald = 18.81, p < .001, with no difference between the sMDD and no MDD groups, β = –1.10, Wald = 0.97, p = .33. In contrast, the main effect of the IL-33 CT haplotype did not significantly differentiate any of the three MDD groups (ps > .77). Importantly, the childhood abuse × IL-33 haplotype interactions were significant in differentiating the rMDD group from both the sMDD group, β = –1.25, Wald = 3.73, p = .05, and the no MDD group, β = 1.25, Wald = 5.03, p = .03, though it was not significant when comparing the sMDD and no MDD groups, β = –0.003, Wald = 0.001, p = .99. Examining the form of these interactions, we found that, among women with no history of childhood abuse, IL-33 haplotype did not significantly differentiate women in any of the three MDD groups (all ps > .48). In contrast, among women with a history of abuse, those with fewer copies of the protective IL-33 CT haplotype were more likely to be in the rMDD group than in the sMDD group, β = -1.02, Wald = 3.72, p = .05, or the no MDD group, β = –1.04, Wald = 4.84, p = .03. Among women with a history of abuse, IL-33 haplotype did not differentiate women in the sMDD and no MDD groups, β = –0.23, Wald = 0.01, p = .97. Thus, among women with a history of childhood abuse, the presence of more copies of the protective CT haplotype was associated with reduced risk for rMDD. Importantly, these results were maintained when we excluded participants with current MDD diagnosis from the analyses (n = 223), when we limited our sample to Caucasians (n = 212), and when we included participants' income, BMI, lifetime smoking, or race/ethnicity as covariates (full results are available in Supplementary Information). Similar results were obtained when we used a recessive model for our analyses (2 copies of CT vs. 1 or 0 copies of CT; full results are available in Supplementary Information).
Similar analyses were conducted to examine the effects of IL-1β haplotype, childhood abuse, and their interaction on participants' history of sMDD and rMDD. However, the the main effects of the IL-1β GA haplotype and the childhood abuse × IL-1β haplotype interactions were not significant (all ps > .16).
Study 2
The findings of Study 1 suggested that a haplotype in IL-33 that is hypothesized to be associated with decreased function of IL-33 served as a protective factor against rMDD risk among women with a history of childhood abuse. To the extent that IL-33 plays a role in risk for rMDD, we would expect to see elevations in peripheral IL-33 protein levels among individuals with rMDD. As before, we examined women with rMDD versus sMDD separately to determine if cytokine levels tracked severity of disorder. To ensure that any differences observed were not due simply to current depression, we focused on women with a history of past rMDD or sMDD that had fully remitted. As before, we also examined circulating levels of the more commonly examined IL-1β. We hypothesized that peripheral IL-33 and IL-1β levels would be the highest among women with rMDD, followed by women with sMDD episode, then women with no history of MDD (no MDD).
Method
Participants
A total of 40 women were recruited from the community and the groups (rMDD, n = 10; sMDD, n = 10; no MDD, n = 20) were matched on age, ethnicity, income, body mass index (BMI), body temperature (< 38 °C), and lifetime tobacco use (Table 2). None of the participants had current MDD, substance abuse, psychotic, obsessive-compulsive, or eating disorders. Ten percent (rMDD n = 2; sMDD n = 1; no MDD n = 1) had a current anxiety disorder (e.g. Panic Disorder). The average age was 33.32 years old (SD = 5.01). Approximately half (52.50%) of the sample was Caucasian and the rest (47.50%) was African American. The median annual family income was $20,001-25,000. The project was approved by the university's institutional review board.
Table 2. Descriptive statistics for the Sample 2.
| No MDD | sMDD | rMDD | F/χ2 | |
|---|---|---|---|---|
| (n = 20) | (n = 10) | (n = 10) | ||
| Age (M and SD) | 33.40 (4.55) | 35.30 (6.31) | 31.20 (4.29) | 1.70 |
| Ethnicity (% Caucasian) | 50 | 60 | 50 | 0.30 |
| Median Annual Income | 20,001-25,000 | 15,001-20,000 | 5,001-10,000 | 1.30 |
| BMI (M and SD) | 32.41 (9.07) | 36.59 (7.81) | 28.42 (9.78) | 2.08 |
| Body temperature, °C (M and SD) | 98.16 (0.60) | 97.87 (0.84) | 98.23 (0.82) | 0.75 |
| Lifetime tobacco use(% smoked ≥100 ea) | 40 | 60 | 60 | 1.60 |
| BDI-II (M and SD) | 6.79 (6.52) | 5.40 (5.64) | 10.09 (6.62) | 2.03 |
Note. MDD = major depressive disorder. sMDD = single episode of MDD.
rMDD = recurrent MDD, BMI = Body Mass Index, BDI-II = Beck's Depression Inventory-II.
None of the means or percentages in the table differed significantly.
Measures
Lifetime histories of psychiatric disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 2002).
Participants' current symptoms of depression were assessed via Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996). In this sample, this measure showed good internal consistency (α =. 80).
Whole blood was collected by certified phlebotomists into BD Vacutainer Blood Collection Sets into 4.0 mL tubes, coated with Ethylenediaminetetraacetic acid (EDTA). The majority of samples (60%) were collected between 11:00 -13:00 and the rest were collected between 17:00-19:00. No data regarding participants' food intake before the blood draw was collected. Plasma was seperated by centrifugation (1000 × g for 10 minutes at 4 °C ) and stored at –80 °C. Multiplex enzyme immunoassay (ELISA) array was conducted by Ray Biotech Inc. (Norcross, GA) with each sample ran in quadruplicates. The average inter- and intra assay coefficients of variation were 3.5% and 9%, respectively. The detection limits were 8.2 pg/mL for IL-33 and 1.5 pg/mL for IL-1β. Values for IL-33 and IL-1β concentrations were not normally distributed and were square-root transformed (after adding a constant of 2 to each value). Two outliers (>2.5 SDs) were recalculated to be at 2.5 SDs from the sample mean, similarly to previous research (Slavich et al., 2010). Body temperature was measured via an infrared thermometer (Exergen, Watertown, MA).
Procedure
Participants were recruited from the community via a variety of means (e.g., TV, newspaper and bus ads, flyers). After providing consent, participants were administered the SCID-I, completed questionnaires, and provided a venous blood sample.
Results
Demographic and clinical characteristics of this sample are presented in Table 2. Preliminary analyses were conducted to examine whether the groups differed on the time of day during which plasma was collected (11:00-13:00 vs. 17:00-19:00) and this was nonsignificant, χ2 (2) = 1.56, p = .46. Additionally, the results were maintained when time of day was included in the analyses. For our primary analyses, we conducted two separate one-way ANOVAs with depression history (rMDD, sMDD, no MDD) as the between-subjects factor and IL-33 or IL-1β concentrations as dependent variable. Results revealed significant group differences in circulating IL-33, F (2,39) = 3.70, p = .03, ηp2 = .17, and IL-1β, F (2,39) = 4.12, p = .02, ηp2 = .18, levels. Importantly, the results for both IL-33, F (2,39) = 3.58, p = .04, ηp2 = .18, and IL-1β, F (2,39) = 4.19, p = .02, ηp2 = .21, were maintained when we entered current depressive symptoms (BDI-II), participants' annual income, BMI, body temperature, and cigarette smoking history as a covariates suggesting that the group differences were not due simply to differences in current mood, income, BMI, body temperature, or smoking history.1 Post hoc analyses revealed that women in the rMDD group had significantly higher circulating levels of IL-33 (M = 32.06, SE = 7.48) than women in the sMDD (M = 6.20, SE = 7.48, p = .02) and the no MDD (M = 10.23, SE = 5.29, p = .02) groups, with the latter two groups not differing significantly (Figure 2A). Similarly, women in the rMDD group had significantly higher IL-1β levels than women in the sMDD (M = 1.79, SE = 0.70, p = .03) and the no MDD (M = 1.73, SE = 0.50, p = .01) groups, again with the latter two groups not differing significantly (Figure 2B).
Figure 2.
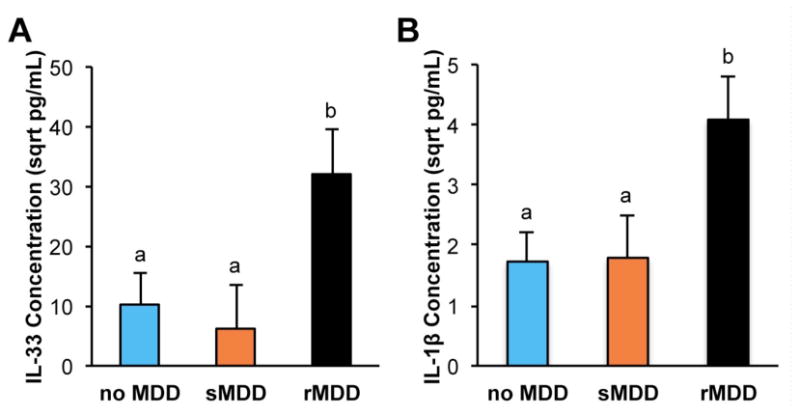
Peripheral levels of IL-33 and IL-1β in human plasma samples as a function of MDD history. Error bars represent standard error of the mean (SEM). Circulating IL-33 (A) and IL-1β (B) levels were significantly higher among women with a history of rMDD, compared to women with a history of sMDD or no MDD. Groups that differ significantly are denoted by different superscripts.
Study 3
The results of Study 1 suggest that a haplotype in IL-33 may moderate the impact of childhood abuse on risk for rMDD and the results of Study 2 suggest that women with a history of rMDD exhibit higher circulating levels of IL-33 than women with a history of sMDD or no history of MDD. A key remaining question is whether IL-33 gene expression in brain regions involved in stress reactivity and affect regulation is increased following exposure to an acute stressor. To address these questions, we examined IL-33 mRNA levels in archival brain tissue from rats exposed to an acute stress challenge, focusing on brain areas relevant to hypothalamic pituitary adrenal (HPA) axis functioning and affect regulation, including the rat analogues of the human PFC and PVN. We could not examine IL-33 expression in the amygdala and cingulate cortex, since the archival samples of those areas were not available. Extensive cytokine expression data from that study have been published (Hueston & Deak, 2014), showing a significant, time-dependent increase in IL-1β in those areas following footshock. However, previous analyses did not examine the role of IL-33. We predicted that IL-33 expression would be similarly increased in the PFC and PVN following a stress challenge.
Method
Participants
Adult male Sprague Dawley (Specified Pathogen Free; SPF; Harlan, Indiana) rats (n = 8 per group; n = 40) weighing 325-425g were pair housed in standard transparent Plexiglas cages at 22 ± 1°C with a 14:10 hour light cycle (lights on 06:00-20:00 h). All animals had ad libitum access to food and water, and were handled briefly for 3-5 minutes for 2 days prior to experimentation. All animal procedures were approved by the Institutional Animal Care and Use Committee at Binghamton University.
Measures and procedure
The footshock chamber measured 30.5 (L) × 26.5 (W) × 33 (H) cm (Habitest Chamber, Model H10-11R-TC-SF, Coulbourn Instruments, Allentown, PA, USA). The sidewalls of the chamber were constructed of stainless steel except the front doors that were constructed of clear Plexiglas. The chambers were adapted to deliver scrambled shocks through the grid floor (18 bars spaced 1.1 cm apart with a diameter of 4.0 mm) from a shock generator (LABLINC Model H01-01, and Precision Animal Shocker Model H13-15, Coulbourn Instruments, Allentown, PA, USA). The sound-attenuating chambers were illuminated by a 20-W white light bulb and background noise was provided by individual ventilation fans. Shock chambers and waste collection trays were cleaned with a wet paper towel after each session. Brain tissue was harvested at baseline (0 minutes), immediately upon removal from stressor exposure (15, 60, and 120 minutes), or after 2 hours of recovery in the home cage following 120 minutes of stress (240 minute group).
Animals were exposed to inescapable footshocks (1.0 mA, 5 s each, 90 s variable ITI) for 0, 15, 60, or 120 minutes (approximately 0, 10, 40, or 80 shocks respectively), or remained in their home cages. An additional group of rats was exposed to 120 min of shock and were returned to their home cages for 120 min (240 min time point) and served as a post-stress recovery sample. Immediately following the footshock paradigm, rats were killed via rapid decapitation (unanesthesized). Brains were removed, sliced at 2 mm using a brain block, and stored in RNAlater (Cat No: 76106; Qiagen, Valencia, CA) at 4 °C for 48 h. Rat analogues of human PFC and PVN were identified using a rat brain atlas (Watson & Paxinos, 2005), dissected under a microscope, and stored at –20 °C. Tissue samples preparation, RNA extraction, cDNA synthesis and amplification were performed using previously established methods (Hueston & Deak, 2014). Relative gene expression was quantified using the 2− ΔΔCT method. Primer sequences for IL-33 (NM_001014166.1) was forward: CCTGAGCACATACAACGACCA; reverse: TTCTTCCCATCCACACCGTC. β-Actin was used as a housekeeping gene, and a lack of significant changes in gene expression was confirmed for both the PVN and PFC (Hueston & Deak, 2014). Figure 3A provides a schematic timeline for the experiment and Figures 3B and C illustrate locations for the PFC and PVN analogues in the rat brain.
Figure 3.
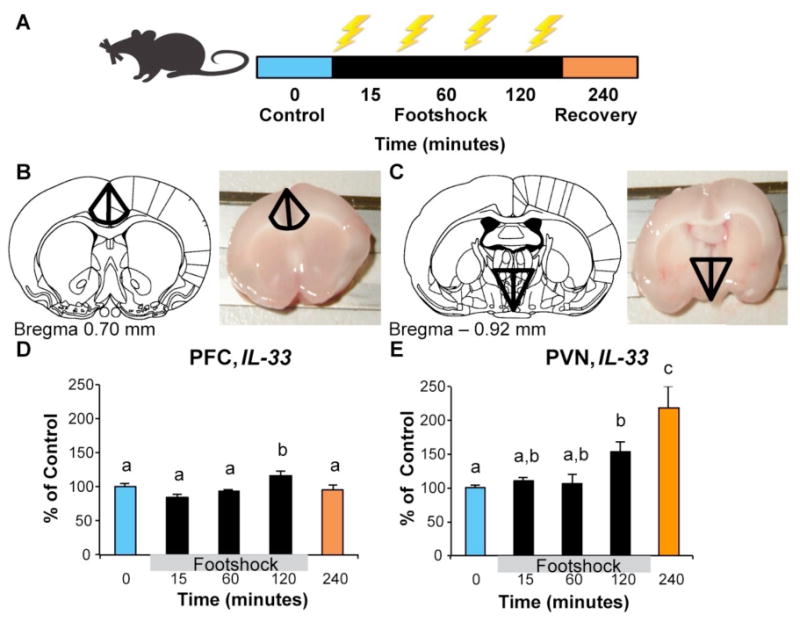
Gene expression analyses using archival rat brain tissue. Error bars represent standard error of the mean (SEM). A) A schematic diagram of the footshock procedure. Adult male were exposed to intermittent footshock (1.0 mA, 5 secs each, 90 sec variable inter-trial interval) for 0 (Control), 15, 60, or 120 min and tissue was harvested immediately as previously described. A separate group of rats was exposed to 120 min of intermittent footshock and were then returned to their home cages for 120 min (designated as the 240 min group) for assessment of a post-stress recovery time point. B&C) Diagrams of the tissue collection locations for the PFC and PVN respectively. D) IL-33 expression was modestly elevated at 120 minutes in the rat PFC following footshock exposure. E) IL-33 expression was significantly elevated at 120 minutes after the onset of footshock, and continued to escalate 2 hours after stress termination (during recovery from stress in the 240 minutes group) in the rat PVN. Groups that differed significantly are denoted by different superscripts.
Results
We conducted a 5 (Group: 0, 15, 60, 120, and 240 min exposure) × 2 (Brain region: PFC, PVN) repeated measures ANOVA with IL-33 mRNA level as the dependent variable. We found a significant main effect of region, F (1,26) = 28.03, p < 0.001, ηp2 = .52, and a significant group × region interaction, F (4,26) = 8.52, p < 0.001, ηp2 = .57. Next, we tested for group differences in each brain region separately. We found a significant group differences in IL-33 expression in both the PFC, F (4,37) = 4.45, p < 0.01, ηp2 = .35 and PVN, F (4,31) = 9.53, p < 0.001, ηp2 = .58. As shown in Figure 3D, post hoc analyses showed a significant elevation in IL-33 expression in the PFC at 120 min, p = 0.046, Cohen's d = .90. Within the PVN (Figure 3E), IL-33 expression was significantly elevated at 120 min, p = 0.03, d = 1.94, and continued to escalate during the post-stress recovery period (240 min), p < 0.01, d = 2.20.
Discussion
Our choice to investigate IL-33 in the context of depression and stress was guided by previous research suggesting that IL-33 (i) orchestrates the release of other inflammatory cytokines by immune cells peripherally and in the CNS (Hudson et al., 2008; Mirchandani et al., 2012; Moulin et al., 2007), (ii) is differentially expressed in brain areas relevant to emotional and stress reactivity (Allen Human Brain Atlas [Internet], 2015) and (iii) is elevated peripherally in individuals suffering from bipolar depression (Barbosa et al., 2014). To provide some context and basis of comparison for our investigation of IL-33, we also examined IL-1β, a well-characterized cytokine that has been extensively examined in the context of MDD and stress (Iwata et al., 2013).
We found that a haplotype in the promoter/enhancer region of the IL-33 gene (Chapuis, Hot, Hansmannel, Kerdraon, Ferreira, Hubans, & Maurage, 2009; J.-T. Yu et al., 2012) moderated the link between women's histories of childhood abuse and rMDD. Specifically, among women with a history of childhood abuse, those with more copies of the protective IL-33 CT haplotype were at reduced risk for rMDD compared to abused women with fewer copies of this protective haplotype. In contrast, no moderating effects were observed for variation in IL-1β. In addition, we found that circulating levels of IL-33 and IL-1β were higher among women with a history of rMDD, compared to women with sMDD or no history of MDD. Finally, utilizing archival rat brain tissue samples that had previously been used to show the time course of IL-1β expression following footshock (Hueston & Deak, 2014), we found that IL-33 exhibited a time-dependent increase in expression within the PVN and, to a lesser extent, the PFC, key sites of integration where neural circuits activated by stress converge.
The finding that the IL-33 CT haplotype moderated the link between women's history of childhood abuse and recurrent MDD builds upon a large body of evidence suggesting that exposure to stress may sensitize neurochemical responses to future stressors, increasing risk for the development of MDD (de Kloet et al., 2005). Our focus on childhood abuse builds upon research showing that preadolescence represents a unique developmental window during which exposure to stressors may result in lasting dysregulation of stress response systems (Bremne & Vermetten, 2001). Previous research suggests that the detrimental effects of early life stress may be further modified by genetic variations (for a review, see Clarke, Nymberg, & Schumann, 2012). Specifically, early life stress may render individuals who carry more “reactive” genotypes more susceptible to developing depression in response to future stressful life events. Conversely, carrying protective variants may buffer against the development of this dysregulation of neurochemical processes impacting future stress response. The mechanisms underlying these effects likely involve long-term effects on allostatic processes (Essex et al., 2011) and epigenetic changes in gene expression (Nestler, 2014).
Although we found that naturally occurring variability in the IL-33 gene was associated with rMDD among participants with a history of childhood abuse, the mechanisms by which this haplotype affects IL-33 protein signaling to impact rMDD risk remain unclear. Previous research found that fewer copies of the CCT haplotype in IL-33 was associated with increased risk for Alzheimer's disease, suggesting that expression of the CCT haplotype is protective against AD in individuals who do not also co-express allelic variations in the APOE4 gene (i.e., the primary allelic variation associated with AD risk; Chapuis, Hot, Hansmannel, Kerdraon, Ferreira, Hubans, Maurage, et al., 2009). Indeed, it is noteworthy that the IL-33 haplotype was not predictive of AD among individuals who did co-express allelic variations in APOE4, suggesting that the role of IL-33 haplotypes in AD progression may define a unique genetic vulnerability to AD that is independent of APOE4 allelic variations. Importantly, the mechanism hypothesized by Chapuis et al (2009) was that reduced functional release of IL-33 (due to the haplotype) led to reduced clearance of Aβ amyloid plaques, which comprise the principal cellular pathology associated with AD progression. This proposed mechanism is consistent with earlier in vitro reports showing that over-expression of IL-33 led to enhanced Aβ clearance (Fryer et al., 2003). In this way, although IL-33 haplotype has not yet been definitively characterized in regards to IL-33 expression, loss of IL-33 function is thought to present as increased risk for the development of AD.
Consistent with this loss of function interpretation, we show in the present studies that mature IL-33 protein is significantly elevated in plasma of women with a history of recurrent depression and childhood abuse (Study 2), and in rodent models after exposure to an intense, acute stressor (Study 3). Importantly, haplotypes in the IL-33 gene that have previously been suggested to reduce IL-33 function exert a protective effect against the rMDD in women with a history of childhood abuse (Study 1). Thus, in both cases, haplotypes in IL-33 may have conferred loss of function mutations in IL-33 signaling which produced phenotypes exactly as you would predict based on the hypothesized role of IL-33 in the respective conditions (i.e., increased risk for AD, yet decreased risk for MDD). Though seemingly paradoxical, it can be noted that a similar profile of risk/benefit has been shown for IL-1β, which may also attenuate AD-associated pathology through decreased Aβ protein and plaque formation (Shaftel et al., 2007). Together, these findings suggest that neuroinflammation plays an important role in steady-state formation of plaques an ultimately the progression of AD, and further supports our approach of examining both IL-33 and IL-1β, in the present studies, as they may exert parallel (or perhaps redundant) effects in MDD. Nevertheless, future studies will be necessary to clarify the cellular mechanisms by which these haplotypes confer their detrimental and/or protective effects on AD, MDD and other neuropathological conditions in which IL-33 may be important.
This is the first study to examine the association of IL-33 with MDD. Although the precise mechanisms of how IL-33 may moderate the impact of early life stress on depression risk are unknown, the combined findings suggest that initial exposure to childhood abuse might leave lasting effects on the expression of IL-33 that emerge during or shortly after stress cessation, alter immune functioning, and increase later stress reactivity. Although the precise reasons for our failure to detect significant G×E effects for IL-1β are not clear, we should also note that, although some studies demonstrated a link between IL-1β and MDD (Yu, Chen, Hong, Chen, & Tsai, 2003), others failed to do so (Wang et al., 2013). Possible reasons for this variability in findings could be differences in studied samples, ways of assessing MDD, and types of MDD included in the studies. One of the plausible explanations for the lack of expected moderation of the link between childhood abuse and rMDD by IL-1β could lie in the type or the timing of stressor we used. More specifically, depression is a heterogeneous disorder and there is evidence for cytokine disruption in only a subset of depressed individuals (Miller & Cole, 2012; Slavich & Irwin, 2014), or with specific depressive symptoms (Baune et al., 2012; Jokela, Virtanen, Batty, & Kivimäki, 2015). In addition, although we focused on childhood abuse in our study, there is evidence from other research that other specific forms of negative life events may impact cytokine levels (e.g., targeted rejection events; for a review, see Slavich & Irwin, 2014). Future research is therefore needed to further explore the potential moderating effects of IL-1β on depression risk.
The observed increase in circulating concentrations of IL-33 and IL-1β extends the existing body of research highlighting the involvement of pro-inflammatory cytokines in MDD (e.g. Dantzer et al., 2008) and suggests that these links may be stronger for rMDD than sMDD. Finally, the observed increase in IL-33 expression in the PVN and, to a lesser extent, the PFC following footshock suggests that, similarly to other inflammatory cytokines (i.e. IL-1β), IL-33 also participates in neuroimmune response to acute stressors. Combined with an earlier report of IL-1β expression in this sample (Hueston & Deak, 2014), the results of the current investigation point to potential differences in temporal patterns of IL-33 and IL-1β expression levels following acute stress. Specifically, whereas IL-1β expression was the highest in the PVN at 60 minutes post footshock onset, IL-33 expression peaked at 120 minutes in the PVN after stress cessation (240 min after footshock onset), suggesting that IL-33 may sustain the inflammatory signal for prolonged periods after cessation of the stressful event. This temporal pattern of IL-33 expression contrasts sharply with the expression of IL-1β, which returns to baseline levels shortly after termination of stress (Hueston & Deak, 2014). In this way, the cascading nature of the neuroimmune response to stress is revealed by a rapid, early peak in IL-1β that is then followed by a peak in IL-33 expression. These intriguing findings provide the first working timeline establishing the expression patterns of IL-33 after acute stress, and suggest that IL-33 may play a role in delayed and/or long-term consequences of stress exposure. Future studies are necessary to further understand (i) the spatial distribution of IL-33 reactivity in brain, (ii) whether the observed changes in mRNA are translated to bioactive protein, (iii) the functional role of IL-33 in rodent models of depression, and (iv) the relation between IL-33 and other cytokines implicated in this cascade. Furthermore, identification of the mechanisms by which stressors modulate the expression of IL-33 and determining whether these findings translate in human participants remains critical. Though little is currently known about IL-33 regulation by stress, it is interesting to note that adrenergic receptor activation is positively coupled to IL-33 expression (Yanagawa, Matsumoto, & Togashi, 2011), suggesting it may follow a similar regulatory pathway as IL-1β during stressful circumstances (Deak et al., 2015).
Strengths of this investigation include the consistency of findings across levels of analysis and species, which allowed examination of cytokine gene expression in specific brain regions in addition to the periphery. Another strength was the focus on differentiating rMDD from sMDD, with our findings suggesting that IL-33 plays a more central role in rMDD than sMDD. Despite these strengths, there were limitations, which highlight important directions for future research. First, the cross-sectional designs of Studies 1 and 2 preclude conclusions regarding causality. Second, although our focus on women increased the homogeneity of our sample, it may also limit the generalizability of our findings to men. Third, we focused on a self-report measure of childhood abuse, which may have been subject to recall or response bias. This said, this measure has shown excellent reliability and validity (Bernstein et al., 1997), and is commonly used in studies testing G×E models of risk (e.g. Bradley et al., 2014), facilitating comparisons between studies. Fourth, our sample size in Study 1 was relatively small and replication in larger samples is needed. Additionally, given the relatively small sample of women reporting a history of abuse, combined with the frequent comorbidity of abuse types, we could not examine the effects of emotional, physical, and sexual abuse separately. Future studies are needed, therefore, to determine whether the IL-33 haplotype is more likely to moderate the impact of certain types of events more than others on depression risk. Fifth, although the majority of the sample (87.9%) in Study 1 is Caucasian, approximately half of the participants (52.52%) in Study 2 were Caucasian and the rest were African American and it is always possible that population stratification affects circulating IL-33 levels. Sixth, since the brain samples that were available for analyses in Study 3 were of male rats only, future research could examine whether the findings replicate in brain samples of female rats in addition to male and female postmortem brains in humans. Additionally, future research is needed to investigate IL-33 involvement in acute stress response in other brain areas relevant for MDD, including the amygdala and hippocampus. Seventh, although the three studies presented here provide converging evidence for the role of IL-33, conclusions must remain tentative pending replication of each study in independent samples. Finally, there is the possibility in any genetic association study that unmeasured genetic or nongenetic third variable may account for the associations reported. This said, the consistency of findings across the G×E model of risk, human cytokine expression, and gene expression in rat brain provide promising initial evidence for the role of IL-33 and warrants its inclusion in future studies of cytokine influences along with more established cytokines.
In summary, the current investigation highlights the involvement of inflammation in stress and depression and supports the role of a novel cytokine, IL-33. Although each of the individual studies in this investigation has limitations and requires replication, our overall a priori hypothesis about the involvement of IL-33 in MDD and stress response was consistently supported. Our findings extend previous research on the influence of IL-33 peripherally and in the CNS by (i) suggesting that a haplotype in IL-33 may impact long-term risk for rMDD among those exposed to childhood abuse, (ii) establishing the association between circulating IL-33 levels and rMDD history, and (iii) supporting the involvement of IL-33 in neuroimmune response to stress. Thus, IL-33 may provide a promising new target of intervention for at-risk individuals. Future studies could employ animal models to identify specific IL-33 signaling pathways that mediate its effects on MDD and design interventions that would target those pathways in at-risk populations (e.g. those with a history of childhood abuse and 0 copies of IL-33 haplotype), providing another critical step towards personalized strategies for therapeutic intervention.
Supplementary Material
Acknowledgments
This project was supported by Nancy B. Forest and L. Michael Honaker Master's Grant for Research, APAGS/APA grant to A.Y. Kudinova, National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant MH098060 awarded to B.E. Gibb, National Science Foundation grant 0822129 awarded to T. Deak, and 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs to J. E. McGeary, and National Institute on Alcohol Abuse and Alcoholism grant AA021113 awarded to Dr. Palmer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health or the Department of Veterans Affairs. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Katie Burkhouse, Mary Woody, Sydney Meadows, Michael Van Wie Ariel Ravid, Devra Alper, Cope Feurer, Eric Funk, and Effua Sosoo for their help in conducting assessments for this project.
Footnotes
Details of these analyses are available from the first author.
Conflict of Interest: The authors declare no conflict of interest.
GSS: Previous research that explored the association between inflammation and depression mainly focused on a consistent set of cytokines and other immune-derived molecules, including recently discovered interleukin-33 (IL-33), have yet to be examined. This investigation provides initial evidence for the association between IL-33, stress reactivity, and recurrent depression.
References
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli Da. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biological Psychiatry. 2014;(49):67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. Journal of Immunology (Baltimore, Md: 1950) 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. doi:179/4/2051. [pii] [DOI] [PubMed] [Google Scholar]
- Allen Institute for Brain Science. [September 30, 2015];Allen Human Brain Atlas. 2015 [Internet] http://human.brain-map.org/
- Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. The American Journal of Pathology. 2003;163(1):69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa IG, Morato IB, de Miranda AS, Bauer ME, Soares JC, Teixeira AL. A preliminary report of increased plasma levels of IL-33 in bipolar disorder: Further evidence of pro-inflammatory status. Journal of Affective Disorders. 2014;157:41–44. doi: 10.1016/j.jad.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, Berger K. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Translational Psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report. San Antonio, TX: The Psychological Corporation; 1997. Retrieved from http://www.pearsonclinical.com/psychology/products/100000446/childhood-trauma-questionnaire-a-retrospective-self-report-ctq.html. [Google Scholar]
- Borkowska P, Kucia K, Rzezniczek S, Paul-Samojedny M, Kowalczyk M, Owczarek A, Kowalski J. Interleukin-1beta promoter (-31T/C and -511C/T) polymorphisms in major recurrent depression. Journal of Molecular Neuroscience. 2011;44(1):12–6. doi: 10.1007/s12031-011-9507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair H, Liu W. Influence of Child Abuse on Adult Depression. Archives of General Psychiatry. 2014;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremne JD, Vermetten E. Stress and development: behavioral and biological consequences. Development and Psychopathology. 2001;13(3):473–89. doi: 10.1017/s0954579401003042. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11523844. [DOI] [PubMed] [Google Scholar]
- Burcusa S, Lacono W. Risk for Recurrence in Depression. Clinical Psychology Review. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. Retrieved from http://www.sciencedirect.com/science/article/pii/S027273580700058X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud a, Neveu PJ, Miller aH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular Psychiatry. 2002;7(5):468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low Ca, Prather Aa, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity. 2011;25(2):232–8. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevikbas F, Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. The Journal of Investigative Dermatology. 2012;132(5):1326–9. doi: 10.1038/jid.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, Hubans C, Lambert JC. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Molecular Psychiatry. 2009;14(11):1004–16. doi: 10.1038/mp.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, Hubans C, Maurage C. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Molecular Psychiatry. 2009;14(11):1004–1016. doi: 10.1038/mp.2009.10. Retrieved from http://www.nature.com/mp/journal/vaop/ncurrent/full/mp200910a.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Resilient functioning in maltreated children: Past, present, and future perspectives. J Child Psychol Psychiatry. 2013;54(4):402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Nymberg C, Schumann G. Genetic and Environmental Determinants of Stress Responding. Alcohol Research. 2012;34(4):484–494. doi: 10.35946/arcr.v34.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. Retrieved from http://dx.doi.org/10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Deak T, Quinn M, Cidlowski Ja, Victoria NC, Murphy AZ, Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015:1–14. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff Ea, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Way B. Early-Life Stress and Adult Inflammation. Current Directions in Psychological Science. 2014;23(4):277–283. doi: 10.1177/0963721414535603. [DOI] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics. 1997;27(3):251–258. doi: 10.1023/A:1025614231190. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(21):7889–96. doi: 10.1523/JNEUROSCI.23-21-07889.2003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12944519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Progress in Neurobiology. 2007;81(4):197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Goodman S, Brand S. Depression and early adverse experiences. In: Gotlib IH, Hammen C, editors. Handbook of Depression. 2nd. New York: Guilford Press; 2000. pp. 249–274. [Google Scholar]
- Häfner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W, Ladwig KH. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain, Behavior, and Immunity. 2011;25(8):1701–7. doi: 10.1016/j.bbi.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Hall SK, Perregaux DG, Gabel Ca, Woodworth T, Durham LK, Huizinga TWF, Seymour AB. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis and Rheumatism. 2004;50(6):1976–83. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heiser P, Lanquillon S, Krieg JC, Vedder H. Differential modulation of cytokine production in major depressive disorder by cortisol and dexamethasone. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2008;18(12):860–70. doi: 10.1016/j.euroneuro.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski a, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:732–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LT, Li H, Sun Q, Liu M, Li WD, Li S, Hang CH. IL-33 expression in the cerebral cortex following experimental subarachnoid hemorrhage in rats. Cellular and Molecular Neurobiology. 2015;35(4):493–501. doi: 10.1007/s10571-014-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson Ca, Christophi GP, Gruber RC, Wilmore JR, Lawrence Da, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. Journal of Leukocyte Biology. 2008;84(3):631–43. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston CM, Deak T. The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiology and Behavior. 2014;124:77–91. doi: 10.1016/j.physbeh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, Behavior, and Immunity. 2013;31:105–14. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatry. 2015;1 doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- Jöris MM, Lankester AC, von dem Borne PA, Kuball J, Bierings M, Cornelissen JJ, Oudshoorn M. The impact of frequent HLA haplotypes in high linkage disequilibrium on donor search and clinical outcome after unrelated haematopoietic SCT. Bone Marrow Transplantation. 2013;48(4):483–90. doi: 10.1038/bmt.2012.189. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nature Reviews Drug Discovery. 2008a;7(10):827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nature Reviews Drug Discovery. 2008b;7(10):827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymaz N, van Os J, Loonen AJM, Nolen W. Evidence That Patients With Single Versus Recurrent Depressive Episodes Are Differentially Sensitive to Treatment Discontinuation: A Meta-Analysis of Placebo-Controlled Randomized Trials. Journal of Clinical Psychiatry. 2008;69(9):1424–1437. doi: 10.4088/jcp.v69n0910. Retrieved from http://ectweb.pbworks.com/f/Evidence+That+Patients+With+Single+Versus+Recurrent.pdf. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27(5):1101–1119. doi: 10.1017/S0033291797005588. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KNR, Rapoport A, Rebey M, Barak V. Cerebrospinal Cytokine Levels in Patients with Acute Depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. Retrieved from http://www.karger.com/DOI/10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108(3):483–489. doi: 10.1037/0021-843X.108.3.483. [DOI] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nature Reviews Immunology. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- Livak K, Flood S, Marmaro J, Guisti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods and Applications. 1995;4:357–362. doi: 10.1101/gr.4.6.357. Retrieved from http://genome.cshlp.org/content/4/6/357.short. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Suy E, Vandervorst C, DeJonckheere C, Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatrica Scandinavica. 1991;84(4):379–86. doi: 10.1111/j.1600-0447.1991.tb03163.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1746291. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opinion on Therapeutic Targets. 2012;16(11):1097–112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- Makrinioti H, Toussaint M, Jackson DJ, Walton RP, Johnston SL. Role of interleukin 33 in respiratory allergy and asthma. The Lancet Respiratory Medicine. 2014;2(3)(13):226–37. 70261–3. doi: 10.1016/S2213-2600. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani AS, Salmond RJ, Liew FY. Interleukin-33 and the function of innate lymphoid cells. Trends in Immunology. 2012;33(8):389–396. doi: 10.1016/j.it.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured Gene-Environment Interactions in Psychopathology: Concepts, Research Strategies, and Implications for Research, Intervention, and Public Understanding of Genetics. Perspectives on Psychological Science: A Journal of the Association for Psychological Science. 2006;1(1):5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40(3):216–25. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel “Alarmin”? PLoS ONE. 2008a;3(10):1–8. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel “alarmin”? PloS One. 2008b;3(1):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Miller aH, Porter MR, Manatunga a, Gao F, Penna S, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American Journal of Psychiatry. 2001;158(8):1252–7. doi: 10.1176/appi.ajp.158.8.1252. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11481159. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71(4):454–6. doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatrica Scandinavica. 2001;103:226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Devane CL. Pro-inflammatory biomakers in depression: treatment with venlafaxine. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry. 2009;10(4):313–23. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Kastelein Ra. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JH, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. The Journal of Clinical Investigation. 2007;117(6):1595–604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nature Reviews Immunology. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone S, Orino E, Mizuno K, Yano S, Nishioka Y, Haku T, Ogura T. Production of IL-1 and its receptor antagonist is regulated differently by IFN-gamma and IL-4 in human monocytes and alveolar macrophages. The European Respiratory Journal. 1994;7(4):657–63. doi: 10.1183/09031936.94.07040657. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8005245. [DOI] [PubMed] [Google Scholar]
- Springer KW, Sheridan J, Kuo D, Carnes M. The long-term health outcomes of childhood abuse. An overview and a call to action. Journal of General Internal Medicine. 2003;18(10):864–70. doi: 10.1046/j.1525-1497.2003.20918.x. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1494926&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73(2002):1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–28. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Sweeney C. Haplotype Analysis of Common Vitamin D Receptor Variants and Colon and Rectal Cancers. Cancer Epidemiology Biomarkers & Prevention. 2006;15(4):744–749. doi: 10.1158/1055-9965.EPI-05-0814. [DOI] [PubMed] [Google Scholar]
- Wang EH, Hong CJ, Yeh HL, Liou YJ, Yang AC, Liu ME, Tsai SJ. Interleukin-1 alpha (rs1800587) genetic polymorphism is associated with specific cognitive functions but not depression or loneliness in elderly males without dementia. Neuroscience Letters. 2013;556:69–72. doi: 10.1016/j.neulet.2013.09.057. [DOI] [PubMed] [Google Scholar]
- Watson GH, Paxinos C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- Xiao E, Xia L, Ferin M, Wardlaw SL. Intracerebroventricular injection of interleukin-1 stimulates the release of high levels of interleukin-6 and interleukin-1 receptor antagonist into peripheral blood in the primate. Journal of Neuroimmunology. 1999;97(1-2):70–6. doi: 10.1016/s0165-5728(99)00050-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10408981. [DOI] [PubMed] [Google Scholar]
- Xu WD, Zhang M, Zhang YJ, Ye DQ. IL-33 in rheumatoid arthritis: potential role in pathogenesis and therapy. Human Immunology. 2013;74(9):1057–60. doi: 10.1016/j.humimm.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Matsumoto M, Togashi H. Adrenoceptor-mediated enhancement of interleukin-33 production by dendritic cells. Brain, Behavior, and Immunity. 2011;25(7):1427–1433. doi: 10.1016/j.bbi.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Research. 2011;1385:8–17. doi: 10.1016/j.brainres.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Yu JT, Song JH, Wang ND, Wu ZC, Zhang Q, Zhang N, Tan L. Implication of IL-33 gene polymorphism in Chinese patients with Alzheimer's disease. Neurobiology of Aging. 2012;33(5):1014.e11–4. doi: 10.1016/j.neurobiolaging.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chen T, Hong C, Chen H, Tsai S. Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28(6):1182–5. doi: 10.1038/sj.npp.1300172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


