Abstract
Objective
Angiotensin II (AngII)-infusion profoundly increases activity of calpains, calcium-dependent neutral cysteine proteases, in mice. Pharmacological inhibition of calpains attenuates AngII-induced aortic medial macrophage accumulation, atherosclerosis, and abdominal aortic aneurysm in mice. However, the precise functional contribution of leukocyte-derived calpains in AngII-induced vascular pathologies has not been determined. The purpose of this study was to determine whether calpains expressed in bone marrow (BM)-derived cells contribute to AngII-induced atherosclerosis and aortic aneurysms in hypercholesterolemic mice.
Approach and Results
To study whether leukocyte calpains contributed to AngII-induced aortic pathologies, irradiated male LDL receptor −/− mice were repopulated with BM-derived cells that were either wild-type(WT) or overexpressed calpastatin (CAST), the endogenous inhibitor of calpains. Mice were fed a fat-enriched diet and infused with AngII (1,000 ng/kg/min) for 4 weeks. Overexpression of CAST in BM-derived cells significantly attenuated AngII-induced atherosclerotic lesion formation in aortic arches, but had no effect on aneurysm formation. Using either BM-derived cells from calpain-1 deficient mice or mice with leukocyte-specific calpain-2 deficiency generated using cre-loxP recombination technology, further studies demonstrated that independent deficiency of either calpain-1 or -2 in leukocytes modestly attenuated AngII-induced atherosclerosis. CAST overexpression significantly attenuated AngII-induced inflammatory responses in macrophages and spleen. Furthermore, calpain inhibition suppressed migration and adhesion of macrophages to endothelial cells in vitro. Calpain inhibition also significantly decreased hypercholesterolemia-induced atherosclerosis in the absence of AngII.
Conclusions
The present study demonstrates a pivotal role for BM-derived calpains in mediating AngII-induced atherosclerosis by influencing macrophage function.
Keywords: Angiotensin II, calpain, atherosclerosis, macrophages, inflammation
INTRODUCTION
Angiotensin II (AngII), a major bioactive peptide of the renin-angiotensin system, is a critical mediator of aortic diseases including atherosclerosis and abdominal aortic aneurysms (AAA).1, 2 Chronic infusion of AngII into hypercholesterolemic mice promotes atherosclerosis and leads to the development of AAAs.2 AngII-induced atherosclerosis is characterized by an intimal macrophage infiltration that becomes engorged with lipids; whereas AngII-induced AAAs are characterized by small focal regions of macrophage accumulation in the aortic media.3 Systemic deficiency of AngII type 1a (AT1a) receptors completely ablates development of AngII-induced atherosclerosis and AAAs in mice.4 However, identities of key regulators and underlying mechanisms for development of these vascular pathologies remain undefined.
Calpains are calcium dependent intracellular cysteine proteases that tightly regulate their substrate proteins through limited proteolysis.5 The two major isoforms, calpain-1 and -2, are expressed ubiquitously, whereas the other isoforms (e.g. −3, −9) are tissue-specific.5 Activated calpain by calcium causes damage to cells by selectively degrading intracellular proteins, including signaling proteins (eg, cyclin-dependent kinase, protein kinase C)6, 7, cytoskeletal proteins (eg, talin, spectrin)8, 9, and transcription factors (eg, c-Jun, IkB).10–12 Calpains play a critical role in cellular apoptosis through the activation of both caspase-dependent and caspase-independent pathways.13, 14 Calpains are also involved in acute inflammatory processes via the activation of nuclear factor kappa B (NF-kB).15 Since calcium-induced calpain activation is an irreversible reaction, calpains are tightly regulated by calpastatin (CAST), which is an endogenous inhibitor that binds strongly to calpains.16 CAST contains four tandem repeats of a calpain-inhibitory domain, and each CAST molecule is capable of inhibiting more than one calpain molecule.17 Calpains have been implicated to play a critical deleterious role in endothelial dysfunction,18 hypertrophy and fibrosis.19 Previously, we demonstrated that AngII-infusion significantly increased calpain protein and activity in AAA and atherosclerotic lesions by Western blot, an activity assay and immunohistochemistry.20, 21
Recently, using a pharmacological inhibitor and calpain-1 deficient mice, we demonstrated that calpain inhibition significantly attenuated AngII-induced atherosclerosis and AAAs in mice.20, 21 Calpain inhibition was associated with reduced aortic medial macrophage accumulation and inflammation. This suggests that the beneficial effect of calpain inhibition was possibly mediated by modulation of macrophage inflammatory responses in AngII-induced vascular pathologies. However, the functional contributions of leukocyte-derived calpains in AngII-induced vascular pathologies are not known. Moreover, the precise role of calpain isoforms (−1 or −2) needs to be defined.
To elucidate a functional role for leukocyte-derived calpains in AngII-induced atherosclerosis and AAAs, we repopulated irradiated male LDL receptor −/− mice with bone marrow (BM)-derived cells that overexpress CAST, the endogenous inhibitor of calpains. These studies demonstrated that calpain inhibition in leukocytes resulted in decreased atherosclerosis, but not AAAs. Furthermore, using calpain-1 or leukocyte-specific calpain-2 deficient mice, we demonstrated that deficiency of either calpain-1 or -2 in leukocytes modestly, but significantly, attenuated AngII-induced atherosclerosis. Additionally, calpain inhibition significantly modulated inflammatory, migratory, and adhesive properties of macrophages.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Calpains are Present in AngII-induced Atherosclerosis
We demonstrated previously that calpains are activated and increased in aortic atherosclerosis and aneurysmal tissue formed during AngII infusion.20, 21 In this study, we determined the distribution of calpain-1 and -2 in AngII-induced atherosclerosis by immunostaining. Calpain-1 and -2 (Figure 1A, B) immunostaining was most pronounced in regions containing macrophages (Figure 1C). Diffused immunostaining was also observed in the aortic media and adventitia.
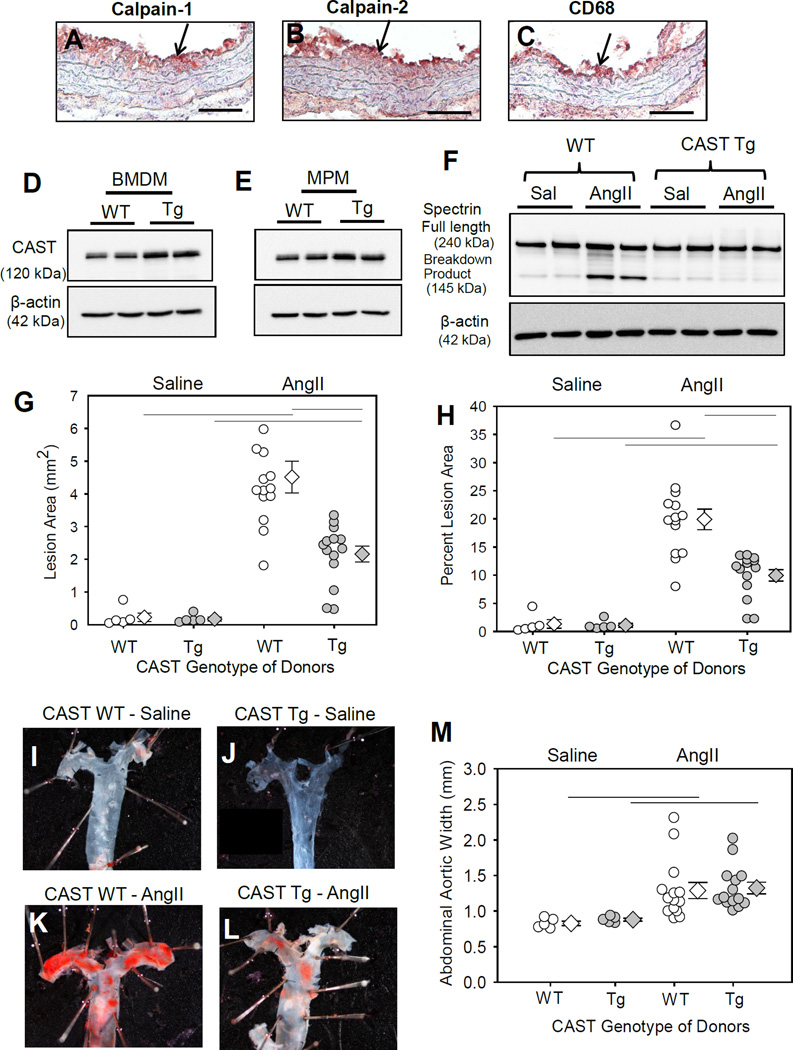
Figure 1. CAST overexpression significantly reduced AngII-induced atherosclerosis.
Serial cross-sections from the ascending aorta of LDL receptor−/− mice infused with AngII were immunostained with anti-mouse calpain-1 / -2 (A, B) and CD68 (C), (red color). Arrow indicates positive immunostaining. CAST protein was detected in BMDM (D) and MPMs (E). F. Spectrin full-length protein and breakdown product was detected in macrophages from saline and AngII infused LDL receptor−/− mice transplanted with WT or CAST-Tg BMs (n=4). Atherosclerotic lesion area was measured on the intimal surface of aortic arch (G,H) (Saline n=5; AngII n=14). I–L represent Oil Red O stained photographs of mouse aortas represent atherosclerotic lesions nearest the mean of each group. (M) Measurements of maximal external width of abdominal aortas (Saline n=5; AngII n=14). White (WT) and gray circles (Tg) represent individual mice, diamonds represent means, and bars are SEMs. Horizontal lines represent significance of P<0.05 (Two-way ANOVA with Holm-Sidak post hoc analysis).
CAST Overexpression in BM-derived Cells Decreased AngII-induced Atherosclerosis
To investigate the role of leukocytic calpains in AngII-induced vascular pathologies, first we used the CAST overexpressing transgenic mice to inhibit activities of both calpain-1 and -2. We confirmed the presence of CAST transgene (CAST-Tg) by PCR using DNA isolated from BM-derived cells (Figure IA in the online-only Data Supplement). Western blot analyses of cell lysates showed a modest ~ 2 fold increase in the abundance of CAST protein in BM (Figure 1D) and peritoneal macrophages (MPMs; Figure 1E) of CAST-Tg mice compared to WT mice. This data confirms the transgenic overexpression of CAST in leukocytes.
To examine the role of leukocyte-derived calpains in AngII-induced aortic pathologies, irradiated LDL receptor −/− mice were repopulated with BM-derived cells from either WT or CAST-Tg mice. Successful repopulation of donor cells was confirmed by CAST genotyping of DNA from BM–derived cells of recipient mice after termination (Figure IB in the online-only Data Supplement). Mice were fed a saturated fat-enriched diet and infused with saline or AngII for 4 weeks. To confirm that the CAST overexpression suppressed calpain activity, we examined the breakdown product of spectrin, a major well known substrate of calpain. Western analyses using protein lysates from macrophages harvested from CAST WT and Tg mice further confirmed increase calpain activity only in WT and not in Tg macrophages as demonstrated by an increased breakdown product of spectrin (Figure 1F). This data clearly suggests a strong inhibition of calpain activity in macrophages by CAST. CAST overexpression in BM-derived cells resulted in a significant decrease (52%; P<0.05) in AngII-induced atherosclerotic lesion areas in aortic arches (WT −20 ± 1.8 % vs Tg −9.5 ± 1.0 %; Figure 1G,H). Examples of Oil Red O stained ex vivo images of represent atherosclerotic lesions nearest the mean of each group are shown in the Figures 1I–L. In addition, CAST overexpression had no effect on body weight, total cholesterol concentrations, or blood cell counts (Table I in the online-only Data Supplement). AngII infusion significantly increased systolic blood pressure in both groups of mice (Table I in the online-only Data Supplement). Furthermore, CAST overexpression in BM-derived cells had no effect on AngII-induced AAA formation (Figure 1M) and aortic rupture (Figure II in the online-only Data Supplement).
Calpain-1 or -2 Deficiency in Myeloid Cells Modestly Reduced AngII-induced Atherosclerosis
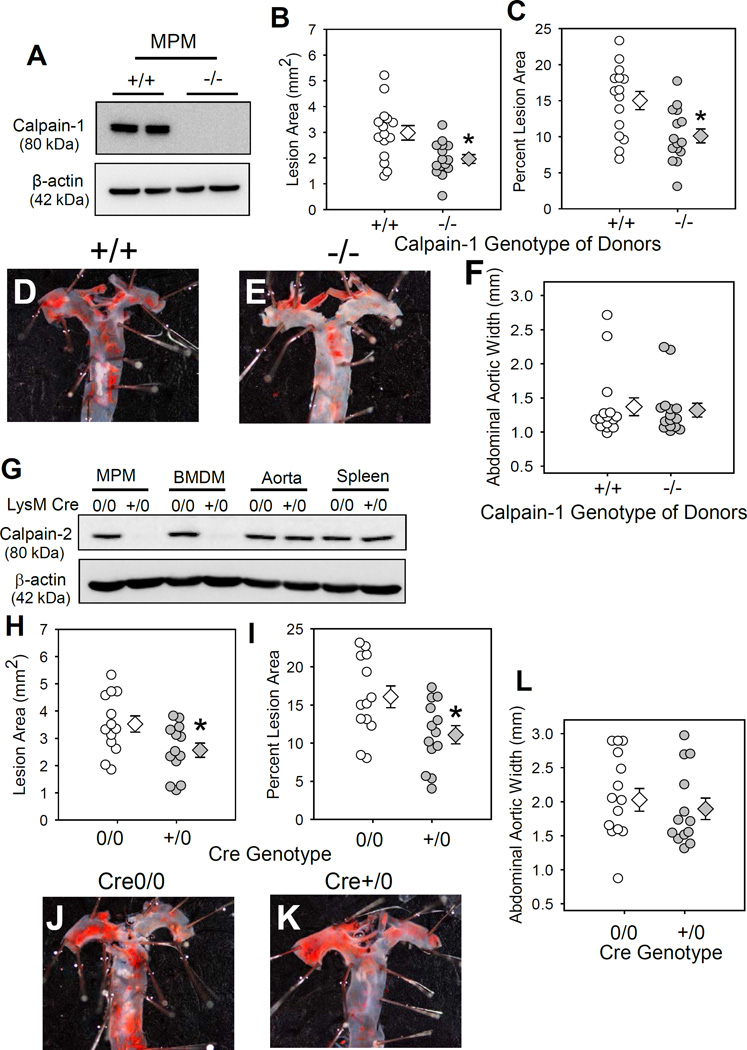
To determine the calpain isoform responsible for attenuating atherosclerosis, irradiated male LDL receptor −/− mice were repopulated with BM-derived cells that were either calpain-1 +/+ or −/−. Calpain-1 genotype was confirmed by PCR (Figure IIIA in the online-only Data Supplement). Successful repopulation of donor cells was confirmed by calpain-1 genotyping of DNA from BM–derived cells of recipient mice after termination (Figure IIIB in the online-only Data Supplement). Since calpain-2 deficient mice are embryonically lethal,22 leukocyte-specific calpain-2 deficient LDL receptor −/− mice were generated using calpain-2 floxed (f/f) and LysM Cre transgenic mice. Both calpain-2f/f and LysM Cre transgenic mice were bred to an LDL receptor −/− background. Female calpain-2 f/f mice were bred with male LysM Cre transgenic mice to yield offspring homologous for the floxed allele and hemizygous for the Cre transgene (Cre+/0). Littermates that were homozygous for the floxed calpain-2 gene, but without the Cre transgene (Cre0/0), were used as control mice. Calpain-2 f/f and Cre genotypes were confirmed by PCR (Figure IV in the online-only Data Supplement). Western blot analyses confirmed depletion of calpain -1 (Figure 2A) and calpain-2 (Figure 2G) in leukocytes.
Figure 2. Calpain-1 or-2 deficiency in myeloid cells significantly attenuated AngII-induced atherosclerosis.
A. Calpain-1 protein in MPMs from calpain-1 +/+ and −/− mice. Atherosclerotic lesion area was measured on the intimal surface of aortic arch (B, C) from LDL receptor−/− mice repopulated with calpain-1 +/+ or −/− BMs (n=16 each). D, E. Photographs of Oil Red O stained mouse aortas representing atherosclerotic lesions nearest the mean of each group. F. Maximal external width of abdominal aortas. G. Calpain-2 protein were detected in cell and tissue lysates from calpain-2 f/f LysM Cre0/0 and +/0 mice. Atherosclerotic lesion area was measured on the intimal surface of aortic arch (H, I) from AngII-infused calpain-2 f/f mice that were either Cre 0/0 or +/0 (n =13). J, K. Photographs of Oil Red O stained mouse aortas representing atherosclerotic lesions nearest the mean of each group. L. Maximal external width of abdominal aortas. White (+/+/Cre 0/0) and gray circles (−/−/Cre +/0) represent individual mice, diamonds represent means, and bars are SEMs. * denotes P<0.05 when comparing +/+/Cre 0/0 vs −/−/Cre +/0 mice (Mann-Whitney Rank sum test).
Mice were fed a saturated fat-enriched diet and infused with AngII for 28 days. Calpain-1 or -2 deficiency in myeloid cells had no effect on body weight, total plasma cholesterol concentrations, or cell counts (Table II and III in the online-only Data Supplement). Calpain-1 or -2 deficiency resulted in a modest, but significant decrease (33 and 31%; P<0.05) in AngII-induced atherosclerosis in aortic arches (Calpain-1: WT −15 ± 1.2 % vs KO −10 ± 0.96 %; Figure 2B,C; Calpain-2 f/f: LysM Cre 0/0 −16 ± 1.4 % vs +/0 −11 ± 1.2 %; Figure 2H,I). Examples of Oil Red O stained ex vivo images of represent atherosclerotic lesions nearest the mean of each group are shown in the Figures 2D,E and 2J,K. Similar to CAST-Tg overexpression (Figure 1N, Figure II in the online-only Data Supplement), calpain-1 or -2 deficiency in myeloid cells had no effect on AngII-induced AAA formation (Figure 2F, 2L) and aortic rupture (Figure V in the online-only Data Supplement).
CAST Overexpression Attenuated Macrophage Accumulation
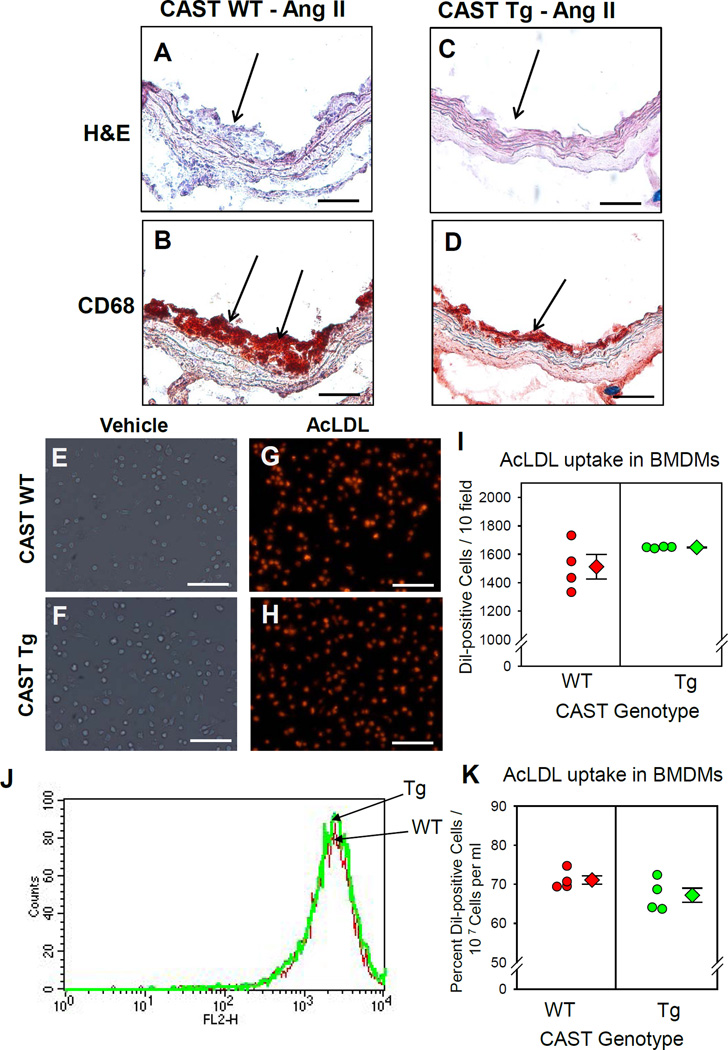
Since macrophage accumulation is one of the earliest events occurring in the development of atherosclerosis, we examined the contribution of leukocytic calpains on macrophage accumulation in AngII-induced atherosclerosis. Histology and immunostaining of AngII-induced atherosclerotic lesions of WT and CAST-Tg mice using H&E stain and an anti-CD68 antibody revealed accumulation of CD68+ macrophages in atherosclerotic lesions (Figure 3A–D). Compared to WT, CAST Tg overexpression in BM-derived cells had less macrophage recruitment to the smaller atherosclerotic lesions.
Figure 3. CAST overexpression significantly reduced macrophage accumulation in atherosclerotic lesions and had no effect on lipid uptake.
Representative atherosclerotic sections (200x magnification) from LDL receptor−/− mice transplanted with WT or CAST-Tg BMs – H&E stain (A,C) and immunostained for CD68 (B,D). Arrows indicate positive staining (red). WT and CAST-Tg BMDMs were incubated with Dil-AcLDL (25 µg/ml) for 24 h. Dil-AcLDL was detected by either fluorescent microscope (E–I) or flow cytometry (J,K). Under fluorescent microscope, Dil-AcLDL positive cells were counted from 10 fields at the power of 100x magnification. Values are represented as mean ± SEM. * denotes P<0.05 when comparing WT vs Tg (Student t test).
In addition to lesional macrophage accumulation, further histological and immunostaining analyses of aortic arches from WT and CAST-Tg mice infused with AngII revealed occurrence of focal elastin layer disruption (Figure VI A, B, I, J in the online-only Data Supplement) associated with the accumulation of CD68+ macrophages (Online Figure 6E, F). However, CAST overexpression attenuated AngII-induced medial elastin layer disruption (Figure VI C, D in the online-only Data Supplement), and medial macrophage accumulation (Figure VI G, H in the online-only Data Supplement). The specificity of immunostaining was verified using appropriate negative controls (Figure VII in the online-only Data Supplement).
CAST Overexpression had no effect on macrophage lipid uptake
To define whether calpains influence macrophage lipid uptake as a mechanism in mediating atherosclerosis, we examined the effect of CAST overexpression on mRNA and protein abundance of ABC transporters and other atherosclerosis-associated genes in macrophages. WT and CAST-Tg BM-derived macrophages (BMDMs) were incubated with either vehicle or acetylated LDL (AcLDL; 25 µg/ml) for 24 or 48 h. Cells were lysed to harvest either mRNA or protein.
mRNA abundance of ABCA1, G1, SRA-1, CD36, ACAT and LXRα were examined by qRT-PCR. The primers used are detailed in Table V in the online-only Data Supplement. AcLDL incubation significantly upregulated mRNA abundance of ABCA1 (24, 48 h), ABCG1 (48 h), LXRα (24, 48 h) and downregulated SRA-1 (48 h) (Figure VIII in the online-only Data Supplement). These alterations in the mRNA abundance of ABCA1, ABCG1, SRA-1, LCAT and LXRα were not influenced by CAST overexpression (Figure VIII in the online-only Data Supplement). Interestingly, CAST overexpression only showed a significant upregulation of CD36 mRNA post 24h AcLDL incubation compared to WT-BMDMs. At the protein level, compared to WT-BMDMs, CAST overexpression showed an increase in ABCA1 protein upon AcLDL incubation. CAST overexpression had no influence on the abundance of ABCG1, CD36 and SRA-1 protein (Figure IX in the online-only Data Supplement).
Next, we examined the effect of CAST overexpression on macrophage lipid uptake. WT and CAST-Tg BMDMs were incubated with either vehicle or fluorescent Dil labeled AcLDL (25 µg/ml) for 24 h. Dil labeled AcLDL was used to trace the AcLDL uptake. Dil-AcLDL uptake was assessed by fluorescent microscopy (Figure 3E–I) and flow cytometry (Figure 3J, K). The cells were washed with ice-cold acid buffer before detection to avoid the contamination of surface sticking AcLDL. Results from both analyses showed that AcLDL uptake was not impaired by calpain inhibition. Therefore, calpain inhibition in macrophages by CAST overexpression had no effect on macrophage lipid uptake.
To further investigate whether AngII-infusion had any effect on atherosclerosis-associated genes in macrophages, CAST WT and Tg mice were infused with either saline or AngII. Peritoneal macrophages were elicited using thioglycollate (1 ml; 3% wt/vol) 72 h prior harvest as described previously.20 mRNA abundance of ABCA1, ABCG1, SRA-1, CD36 and LXRα were examined in peritoneal macrophages. Neither AngII infusion nor CAST overexpression influenced mRNA abundance of these atherosclerosis-associated genes (Figure X in the online-only Data Supplement).
CAST Overexpression Attenuated Macrophage Adhesion and Migration
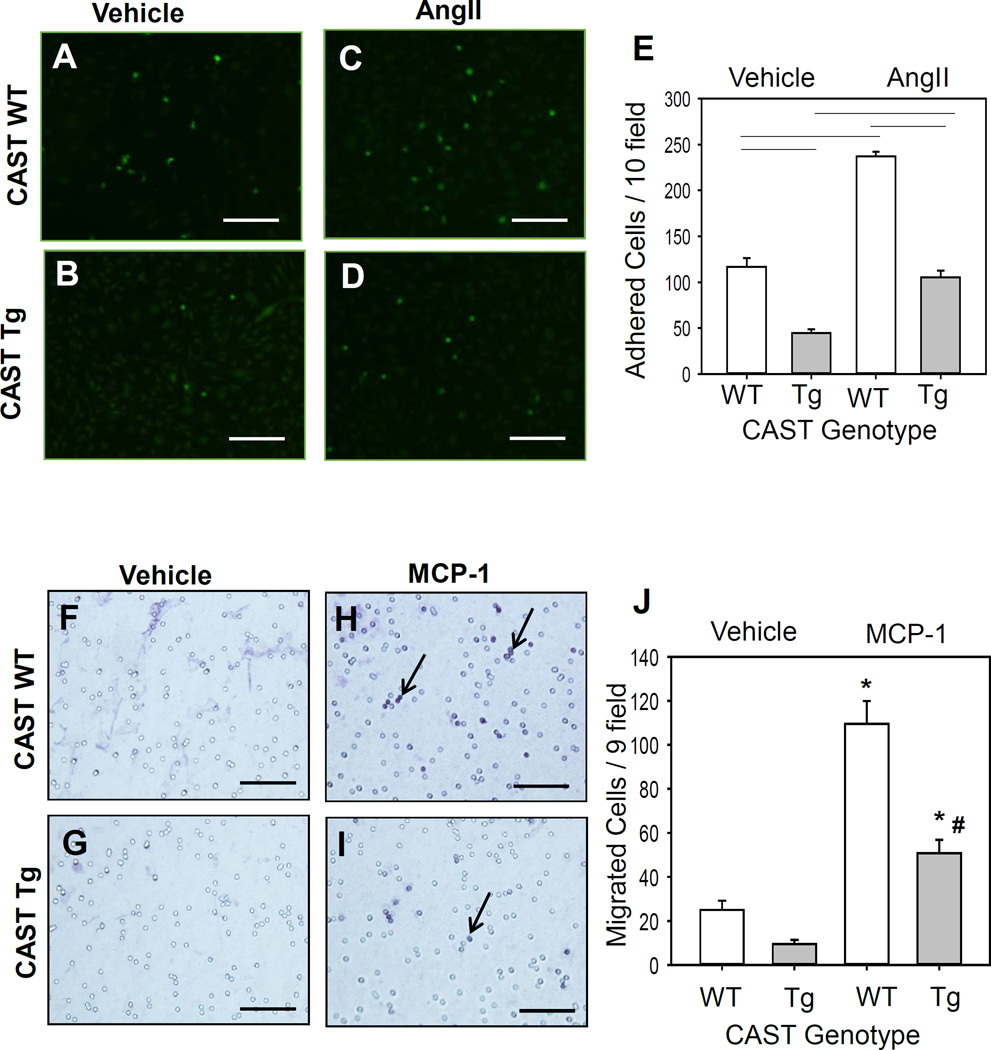
To define potential mechanisms by which calpain influenced macrophage accumulation in atherosclerotic lesions, we investigated whether calpain inhibition influenced functional properties of macrophages such as migration and adhesion to endothelial cells (ECs). The adhesion assay was performed on mouse aortic ECs. The endothelial cell purity was verified using a biotin labeled CD31 antibody by immunohistochemistry (Figure XI in the online-only Data Supplement). The ECs were stimulated with AngII (1 µM) overnight. Co-incubation of BMDMs with stimulated ECs showed that AngII significantly promoted macrophage adhesion to ECs. Compared to WT BMDMs, CAST Tg overexpression significantly suppressed BMDM adhesion to ECs both under basal and AngII conditions (Figure 4A–E). In addition, the effect of CAST overexpression on macrophage migration towards monocyte chemoattractant, MCP-1, was examined by transwell migration assay. MCP-1 strongly stimulated migration of macrophages from WT mice compared to vehicle control, whereas overexpression of CAST significantly reduced MCP-1-induced macrophage migration (Figure 4F–I). Therefore, calpain inhibition in macrophages by CAST overexpression decreased the migration and adhesion abilities of macrophages.
Figure 4. CAST overexpression significantly reduced macrophage adhesion and migration.
A–D: Calcein labeled WT and CAST-Tg BMDMs were added to a mouse aortic endothelial cell monolayer for 60 min. Adhered cells were counted from 10 fields at the power of 100× magnification using a fluorescence microscope and quantified (E) (n=4). F–I: WT and CAST-Tg BMDMs were seeded on transwell filters and lower chambers were filled with media containing either vehicle or MCP-1 (100 µg/mL). Cells that migrated through the membrane stained with H&E and were counted from 9 fields at the power of 200x magnification (J) (n=4). Horizontal lines represent significance of P<0.05. * and # denotes P<0.05 when comparing AngII vs saline and WT vs Tg, respectively (Two-way ANOVA with Holm-Sidak post hoc analysis).
CAST Overexpression Attenuated AngII-induced Inflammation
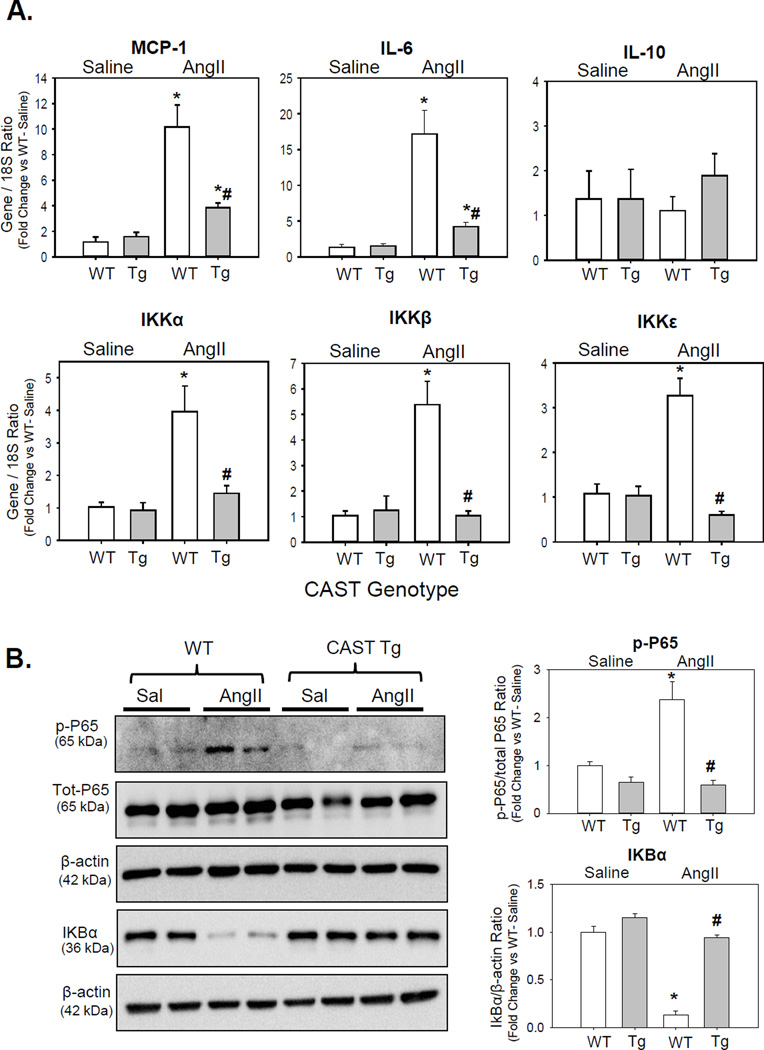
To further investigate the role of calpain on AngII-induced inflammation, WT and CAST-Tg BMDMs were incubated with either vehicle or AngII. AngII incubation failed to show any effect on secretion or induction of MCP-1 (Figure XII A, B in the online-only Data Supplement) or other inflammatory genes (e.g. IL-6) expression from cultured BMDMs at 12 h or 24 h (data not shown). However, BMDMs incubated with positive control LPS showed a strong induction of MCP-1 (Figure XII A, B in the online-only Data Supplement). Since we failed to observe any response of AngII in cultured BMDMs, next we used an in vivo approach in which WT and CAST-Tg mice were infused with either saline or AngII for 7 days. Peritoneal macrophages were elicited using thioglycollate (1 ml; 3% wt/vol) 72 h prior harvest as described previously.20 mRNA or protein were extracted from the MPMs and subjected to qPCR and Western blot analyses. mRNA abundance of MCP-1, inflammatory cytokines (IL-6, IL-10) and NF-kB-related IkappaB kinases (IKKα, β and ε) were examined in peritoneal macrophages and spleen. The primers used are detailed in Table V in the online-only Data Supplement. AngII-infusion significantly increased mRNA abundance of MCP-1, IL-6, IKKs, and had no effect on IL-10 in macrophages (Figure 5A) and spleen (Figure XIII in the online-only Data Supplement). In contrast, CAST overexpression significantly reduced AngII-induced MCP-1, IL-6 and IKKs gene expression.
Figure 5. CAST overexpression significantly reduced AngII-induced MCP-1, IL-6 and NF-kB-dependent inflammatory genes in macrophages.
A. mRNA abundance of MCP-1, IL-6, IL-10, and IKK genes in MPMs from saline and AngII infused LDL receptor−/− mice transplanted with WT or CAST-Tg BMs were analyzed by qPCR (n=4–6). B. Western blot analyses of phospho and total P65, and IkBα protein in MPMs from saline and AngII infused LDL receptor−/− mice transplanted with WT or CAST-Tg BMs (n=4). Values are represented as mean ± SEM. * and # denotes P<0.05 when comparing AngII vs saline and WT vs Tg respectively (Two-way ANOVA with Holm-Sidak post hoc analysis).
IKK-dependent NF-kB activation has been implicated in the development of atherosclerosis.23, 24 Activated NF-kB is also shown to induce inflammatory cytokines such as IL-6 and TNF-α. NF-kB is sequestered in the cytosol and its translocation to the nucleus is prevented by the inhibitor of NF-kB translocation (IkB).25 IκB was also shown to be a target of calpain in selected cell types including macrophages.26–28 In order to investigate the possibility that calpain activation mediates AngII-induced NF-kB activation and IkB degradation, phosphorylation of NF-kB subunit P65 and IkB protein were examined by Western blot. AngII-infusion significantly reduced IkB protein and significantly increased phosphorylation of P65 in WT-MPMs (Figure 5B). In contrast, CAST overexpression significantly prevented AngII-induced IkB protein degradation and P65 phosphorylation in macrophages (Figure 5B).
CAST Overexpression in BM-derived Cells Decreased Hypercholesterolemia-induced Atherosclerosis
In order to understand the role of endogenous calpains in leukocytes on atherosclerosis in the absence of exogenous stimuli, AngII, we examined the role of leukocytic calpains in the development of hypercholesterolemia-induced atherosclerosis in the absence of AngII. Since studies highlighted in Figures 1 and 2 showed that deficiency of Calpain-1 or -2 in leukocytes resulted in a modest decrease (33 and 31%; P<0.05) in AngII-induced atherosclerosis (Figure 2), whereas inhibition of both calpain-1 and -2 by overexpression of its endogenous inhibitor, CAST, significantly decreased (52%; P<0.05) AngII-induced atherosclerotic lesion areas in aortic arches (Figure 1H, I). Based on these observations, we examined the effect of leukocytic calpains on hypercholesterolemia-induced atherosclerosis using CAST overexpression Tg mice instead of calpain-1 or -2 isoform specific deficient mice.
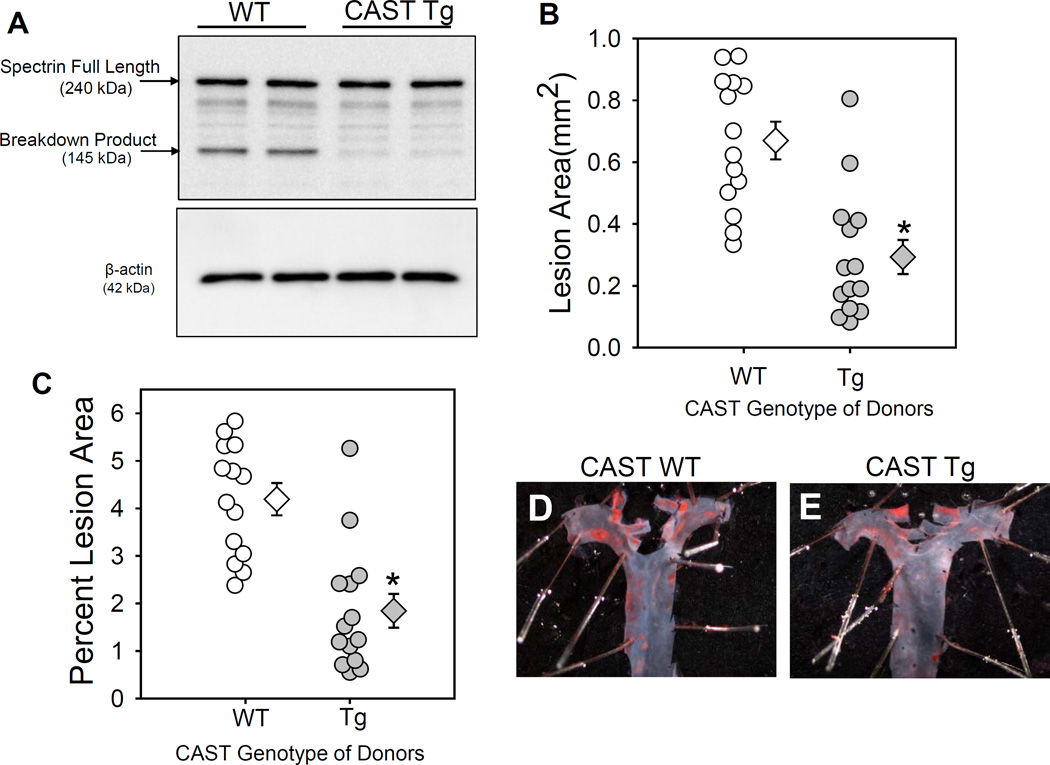
To examine the role of leukocyte-derived calpains on hypercholesterolemia-induced atherosclerosis, irradiated LDL receptor −/− mice were repopulated with BM-derived cells from either WT or CAST-Tg mice. Successful repopulation of donor cells was confirmed by CAST genotyping of DNA from BM-derived cells of recipient mice after termination (Figure IC in the online-only Data Supplement). Mice were fed a saturated fat-enriched diet for 12 weeks. CAST overexpression had no effect on body weight gain, plasma total cholesterol concentrations, or blood cell counts (Table IV in the online-only Data Supplement). CAST overexpression suppressed hypercholesterolemia-induced calpain activity, as demonstrated by decreased breakdown of its known substrate, spectrin, in macrophages harvested from WT and Tg mice fed with high fat diet (Figure 6A). This data clearly suggests a strong inhibition of calpain activity in macrophages by CAST. CAST overexpression in BM-derived cells resulted in a significant decrease (58%; P<0.05) in hypercholesterolemia-induced atherosclerotic lesion areas in aortic arches (WT−4.2 ± 0.3 % vs Tg −1.8 ± 0.3 %; Figure 6B,C). Examples of ex vivo images of Oil Red O stained aortas represent atherosclerotic lesions nearest the mean of each group are shown in the Figures 6D,E.
Figure 6. CAST overexpression significantly reduced hypercholesterolemia-induced atherosclerosis.
A. Spectrin full-length protein and breakdown product was detected in macrophages from high fat diet fed LDL receptor −/− mice transplanted with WT or CAST-Tg BMs (n=4). Atherosclerotic lesion area was measured on the intimal surface of the aortic arch (B, C; n=14). D, E represent photographs of Oil Red O stained mouse aortas representing atherosclerotic lesions nearest the mean of each group. White (WT) and gray circles (Tg) represent individual mice, diamonds represent means, and bars are SEMs. * denotes P<0.05 when comparing WT vs Tg mice (Mann-Whitney Rank sum test).
DISCUSSION
Activated calpain has been implicated in AngII-induced aortic pathologies such as atherosclerosis and AAAs. However, the contribution of leukocytic calpains on the development of AngII-induced vascular pathologies are not known. In the present study, we generated 3 different mice models: (i) chimeric LDL receptor −/− mice that overexpress CAST, an endogenous inhibitor of calpains, in BM-derived cells, (ii) chimeric LDL receptor −/− mice that were transplanted with either calpain-1 +/+ or −/− BM cells, and (iii) LDL receptor −/− mice with leukocyte-specific calpain-2 deficiency. Using these 3 different calpain deficient mice models, we examined the role of leukocyte calpains in AngII-induced atherosclerosis and AAAs. Here, we demonstrated that combined inhibition of both calpain-1 and -2 activities significantly reduced AngII-induced atherosclerosis, without influencing AAA formation. The beneficial effect of calpain inhibition on AngII-induced atherosclerosis was associated with the reduction of macrophage accumulation, and inflammation, which attributed to blunted functional properties of macrophages including migration and adhesion. Furthermore, we demonstrated that calpain inhibition also suppressed hypercholesterolemia-induced atherosclerosis in mice.
Overexpression of CAST or calpain-1 or -2 deficiency in leukocytes did not have any effects on AngII-induced blood pressure elevation. Consistent with the current observation, our earlier study of calpain inhibition using BDA-410, or calpain-1 deficiency also showed no effect on AngII-induced increased blood pressure.20, 21 This result is in agreement with an earlier study by Letaverner et al., in which overexpression of calpastatin attenuated AngII-induced cardiac hypertrophy without affecting blood pressure.19 Further, development of AngII-induced AAA and atherosclerosis also shown to be independent of increases in blood pressure.29
Calpain inhibition in BM-derived cells by CAST overexpression significantly reduced AngII-induced atherosclerosis. In agreement, depletion of AT1a receptors in BM-derived cells, not endothelial or SMCs, partially reduced AngII-accelerated atherosclerosis.4 Our present study clearly demonstrated that AngII promoted calpain activity in macrophages, made evident by increased breakdown product of spectrin, a well-known substrate of calpain. Furthermore, calpain inhibition in BM-derived cells reduced both AngII-induced and hypercholesterolemia-induced atherosclerosis without affecting plasma cholesterol concentrations. In addition, calpain inhibition had no influence on macrophage lipid (Ac-LDL) uptake under in-vitro conditions. Upon lipid-loading, CAST overexpression showed an increase in the protein abundance of ABCA1, whereas it had no influence on mRNA and protein abundance of ABCG1 and other atherosclerosis-associated genes (e.g. SR-A1, CD36) in macrophages. Macrophage ABCA1 transporter deficiency is shown to promote atherosclerosis in mice by increasing plaque inflammation30 and suppressing cholesterol efflux pathways.31, 32 Pharmacological inhibition of calpain or mutation of calpain binding PEST sequence in ABCA1 showed that calpain degrades ABCA1 by proteolysis.33–35 The observed beneficial effect of CAST Tg overexpression on AngII / hypercholesterolemia -induced atherosclerosis may be due to improved cholesterol efflux from macrophages rather than lipid uptake. These current findings suggest that calpain activation promotes atherosclerosis by influencing functional properties of macrophages such as migration, adhesion, and subsequent inflammation without influencing macrophage lipid uptake. However, further studies are warranted to understand whether calpain has any role on ABCA1-mediated macrophage cholesterol efflux and/or reverse cholesterol transport in promoting atherosclerosis. Interestingly, the combined inhibition of calpain-1 and -2 by CAST overexpression showed a strong reduction in atherosclerosis over independent depletion suggesting a synergistic role of calpain-1 and -2 in reducing atherosclerosis. However, calpain inhibition in leukocytes had no influence on AngII-induced AAA formation. In agreement, previous studies showed that BM transplantation using AT1a receptor deficient mice had no effect on AngII-induced AAA development.4 Our earlier studies clearly demonstrated that whole body calpain inhibition attenuated AngII-induced AAAs suggesting that calpain derived from vessel wall cells are involved in AAA formation.20, 21
Calpain inhibition reduced AngII-induced pro-inflammatory gene expression such as IKKs, MCP-1, and IL-6 in macrophages. Furthermore, calpain inhibition in macrophages impaired migration and adhesion properties, which may have contributed to decreased AngII-induced atherosclerosis36, 37 In addition, calpain inhibition suppressed AngII-induced NF-kB activation (P65 phosphorylation) and prevented AngII-induced IkB degradation in macrophages. IKK-dependent NF-kB activation has been implicated in the development of atherosclerosis.23 Recently, LDL receptor −/− mice with macrophage-specific IKKβ deficiency had less hypercholesterolemia-induced atherosclerosis.24 NF-kB translocation inhibitor, IκB, was also shown to be a target of calpain in selected cell types including macrophages.25–28 In support, CAST deficiency in intestinal macrophages also induced NF-kB translocation to the nucleus.27 Further, deletion of calpain-4, the common small subunit of calpain-1 and -2 in cardiomyocytes resulted in suppressed NF-kB activity followed myocardial infarction in mice.15 NF-kB activation plays an important role in promoting expression of various proinflammatory factors (e.g. MCP-1, IL-6), which mediate inflammatory responses.38, 39 MCP-1 and IL-6 play important roles in aortic medial macrophage recruitment and inflammation in mice. Mice deficient with CCR2, the cognate receptor of MCP-1, have attenuated AngII-induced atherosclerosis and AAAs.40 In addition, in the elastase-induced AAA model, MCP-1 deficient mice displayed a strong reduction in macrophage infiltration.41 Further, MCP-1 deficient mice also showed significant reduction in hypercholesterolemia-induced atherosclerosis.42 Mice deficient in IL-6 had reduced AngII-induced aortic dissection and inflammation partially by suppressing MCP-1 mediated macrophage recruitment.43 In the present study, the suppressed NF-kB activation may result in decreased downstream events such as induction of inflammatory cytokines, MCP-1 and IL-6. This may contribute to decreased migration, adhesion and inflammation of CAST overexpressing macrophages. In line, the expression of AngII-induced MCP-1, IL-6, and NF-kB P65 phosphorylation were significantly decreased in CAST overexpressing macrophages. This reduction in macrophage inflammation, in addition to, impaired macrophage migration and adhesion upon CAST overexpression may well correlate with the observed reduction of macrophage infiltration into AngII-induced atherosclerotic lesions in the aortic arch.
Since CAST overexpression had no effect on changes in plasma cholesterol concentrations and macrophage lipid uptake, our results suggest that an inhibitory effect on macrophage inflammation, migration and accumulation may be the key mechanism by which calpain inhibition attenuates AngII-induced atherosclerosis. Thus, it is postulated that AngII-induced calpain activation promotes NF-kB activation, resulting in the induction of proinflammatory cytokines - MCP-1 and IL-6, which in turn stimulates macrophage migration and adhesion, thereby causing atherosclerosis. However, future studies are required to further understand the mechanisms through which calpain regulates functional properties of macrophages under inflammatory conditions.
In summary, we demonstrated that inhibition of leukocyte derived-calpains resulted in decreased atherosclerosis, which was associated with reduced macrophage inflammation, migration and adhesion. These results suggest that inhibition of calpain activity may offer a new therapeutic target to reduce atherosclerosis.
Supplementary Material
SIGNIFICANCE.
Pharmacological inhibition of calcium dependent cysteine protease, calpains, decreases angiotensin II (AngII)-induced atherosclerosis, AAAs, and medial macrophage accumulation in LDL receptor −/− mice, independent of plasma cholesterol concentrations. This finding suggests that calpain inhibition exerts an antiatherogenic effect mediated by modulation of inflammatory responses in atherosclerotic plaques. Here, we evaluated this hypothesis by investigating the contribution of macrophage-derived calpains to development of AngII-induced aortic pathologies. Using leukocyte-specific calpain deficient mice, we demonstrated that (i) leukocyte-derived calpains play a critical role in the development of AngII-induced atherosclerosis; (ii) both calpain-1 and -2, the major ubiquitous isoforms, play a synergistic role in accelerating atherosclerosis; (iii) inhibition of calpain activity in macrophage reduces macrophages migration, adhesion to endothelial cells and accumulation in atherosclerotic lesions. These results suggest that targeted inhibition of calpain activity may offer a new therapeutic direction to reduce atherosclerosis.
Acknowledgments
We thank Dr. Alan Daugherty for his helpful suggestions and discussions of this project.
SOURCES OF FUNDING
The study was supported by a Beginning Grant-in Aid (13BGIA14560001), Scientist Development Grant (14SDG18740000) from the American Heart Association, a Pilot award from the DRC at Washington University (5P30DK020579), an Early Investigator Grant from the Marfan Foundation and by the National Institutes of Health (Grants P20GM103527, R01HL89517). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Haruhito A. Uchida belongs to endowed Department of Chronic Kidney Disease and Cardiovascular Disease, Okayama University by Chugai Pharmaceutical, MSD, Boehringer Ingelheim and Kawanishi Holdings Inc.
Non-standard Abbreviations and Acronyms
- AngII
angiotensin II
- AAA
abdominal aortic aneurysm
- BM
bone marrow
- BMDM
bone marrow-derived macrophage
- CAST
calpastatin
- IKK
inhibitor of kappa B kinase
- IL-6
interleukin 6
- IL-10
interleukin 10
- LDL
low-density lipoprotein
- MPM
mouse peritoneal macrophage
- MCP-1
monocyte chemoattractant protein-1
- Tg
transgene
Footnotes
DISCLOSURES
The other authors report no conflicts.
REFERENCES
- 1.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the at1a receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 4.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient at1a receptors are required to initiate angiotensin ii-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 5.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Minegishi S, Takano J, Plattner F, Saito T, Asada A, Kawahara H, Iwata N, Saido TC, Hisanaga S. Calpastatin, an endogenous calpain-inhibitor protein, regulates the cleavage of the cdk5 activator p35 to p25. Journal of neurochemistry. 2011;117:504–515. doi: 10.1111/j.1471-4159.2011.07222.x. [DOI] [PubMed] [Google Scholar]
- 7.Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase c upregulates intercellular adhesion molecule-1 and leukocyte-endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011;31:289–296. doi: 10.1161/ATVBAHA.110.217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bate N, Gingras AR, Bachir A, Horwitz R, Ye F, Patel B, Goult BT, Critchley DR. Talin contains a c-terminal calpain2 cleavage site important in focal adhesion dynamics. PLoS One. 2012;7:e34461. doi: 10.1371/journal.pone.0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobeissy FH, Liu MC, Yang Z, Zhang Z, Zheng W, Glushakova O, Mondello S, Anagli J, Hayes RL, Wang KK. Degradation of betaii-spectrin protein by calpain-2 and caspase-3 under neurotoxic and traumatic brain injury conditions. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-jun and c-fos, by calpain. FEBS Lett. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 11.Carillo S, Pariat M, Steff AM, Roux P, Etienne-Julan M, Lorca T, Piechaczyk M. Differential sensitivity of fos and jun family members to calpains. Oncogene. 1994;9:1679–1689. [PubMed] [Google Scholar]
- 12.Fenouille N, Grosso S, Yunchao S, Mary D, Pontier-Bres R, Imbert V, Czerucka D, Caroli-Bosc FX, Peyron JF, Lagadec P. Calpain 2-dependent ikappabalpha degradation mediates cpt-11 secondary resistance in colorectal cancer xenografts. The Journal of pathology. 2012;227:118–129. doi: 10.1002/path.3034. [DOI] [PubMed] [Google Scholar]
- 13.Kerbiriou M, Teng L, Benz N, Trouve P, Ferec C. The calpain, caspase 12, caspase 3 cascade leading to apoptosis is altered in f508del-cftr expressing cells. PLoS One. 2009;4:e8436. doi: 10.1371/journal.pone.0008436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS, Green DR. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- 15.Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC, Peng T. Deficiency of capn4 gene inhibits nuclear factor-kappab (nf-kappab) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480–27489. doi: 10.1074/jbc.M112.358929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wendt A, Thompson VF, Goll DE. Interaction of calpastatin with calpain: A review. Biol Chem. 2004;385:465–472. doi: 10.1515/BC.2004.054. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Hoffmann FW, Norton RL, Hashimoto AC, Hoffmann PR. Selenoprotein k is a novel target of m-calpain, and cleavage is regulated by toll-like receptor-induced calpastatin in macrophages. J Biol Chem. 2011;286:34830–34838. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scalia R, Gong Y, Berzins B, Freund B, Feather D, Landesberg G, Mishra G. A novel role for calpain in the endothelial dysfunction induced by activation of angiotensin ii type 1 receptor signaling. Circ Res. 2011;108:1102–1111. doi: 10.1161/CIRCRESAHA.110.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin ii-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian V, Uchida HA, Ijaz T, Moorleghen JJ, Howatt DA, Balakrishnan A. Calpain inhibition attenuates angiotensin ii-induced abdominal aortic aneurysms and atherosclerosis in low-density lipoprotein receptor-deficient mice. J Cardiovasc Pharmacol. 2012;59:66–76. doi: 10.1097/FJC.0b013e318235d5ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian V, Moorleghen JJ, Balakrishnan A, Howatt DA, Chishti AH, Uchida HA. Calpain-2 compensation promotes angiotensin ii-induced ascending and abdominal aortic aneurysms in calpain-1 deficient mice. PLoS One. 2013;8:e72214. doi: 10.1371/journal.pone.0072214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano J, Mihira N, Fujioka R, Hosoki E, Chishti AH, Saido TC. Vital role of the calpain-calpastatin system for placental-integrity-dependent embryonic survival. Mol Cell Biol. 2011;31:4097–4106. doi: 10.1128/MCB.05189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanters E, Pasparakis M, Gijbels MJJ, Vergouwe MN, Partouns Hendriks I, Fijneman RJA, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MPJ. Inhibition of nf-kappa b activation in macrophages increases atherosclerosis in ldl receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SH, Sui Y, Gizard F, Xu J, Rios-Pilier J, Helsley RN, Han SS, Zhou C. Myeloid-specific ikappab kinase beta deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2869–2876. doi: 10.1161/ATVBAHA.112.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins ND. Integrating cell-signalling pathways with nf-kappab and ikk function. Nature reviews. Molecular cell biology. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 26.Shumway SD, Maki M, Miyamoto S. The pest domain of ikappabalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Rose AH, Hoffmann FW, Hashimoto AS, Bertino P, Denk T, Takano J, Iwata N, Saido TC, Hoffmann PR. Calpastatin prevents nf-kappab-mediated hyperactivation of macrophages and attenuates colitis. Journal of immunology. 2013;191:3778–3788. doi: 10.4049/jimmunol.1300972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Demers LM, Vallyathan V, Lu Y, Castranova V, Shi X. Impairment of nf-kappab activation and modulation of gene expression by calpastatin. American journal of physiology. Cell physiology. 2000;279:C709–C716. doi: 10.1152/ajpcell.2000.279.3.C709. [DOI] [PubMed] [Google Scholar]
- 29.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Ang ii infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of atp-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of abca1 and abcg1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of abca1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama S, Arakawa R, Wu CA, Iwamoto N, Lu R, Tsujita M, Abe-Dohmae S. Calpain-mediated abca1 degradation: Post-translational regulation of abca1 for hdl biogenesis. Biochim Biophys Acta. 2012;1821:547–551. doi: 10.1016/j.bbalip.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A pest sequence in abca1 regulates degradation by calpain protease and stabilization of abca1 by apoa-i. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez LO, AgerholmLarsen B, Wang N, Chen WG, Tall AR. Phosphorylation of a pest sequence in abca1 promotes calpain degradation and is reversed by apoa-i. Journal of Biological Chemistry. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 36.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 37.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe-/- mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donadelli R, Abbate M, Zanchi C, Corna D, Tomasoni S, Benigni A, Remuzzi G, Zoja C. Protein traffic activates nf-kb gene signaling and promotes mcp-1-dependent interstitial inflammation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36:1226–1241. doi: 10.1053/ajkd.2000.19838. [DOI] [PubMed] [Google Scholar]
- 39.Rego D, Kumar A, Nilchi L, Wright K, Huang S, Kozlowski M. Il-6 production is positively regulated by two distinct src homology domain 2-containing tyrosine phosphatase-1 (shp-1)-dependent ccaat/enhancer-binding protein beta and nf-kappab pathways and an shp-1-independent nf-kappab pathway in lipopolysaccharide-stimulated bone marrow-derived macrophages. Journal of immunology. 2011;186:5443–5456. doi: 10.4049/jimmunol.1003551. [DOI] [PubMed] [Google Scholar]
- 40.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin ii infusion promotes ascending aortic aneurysms: Attenuation by ccr2 deficiency in apoe-/- mice. Clin Sci (Lond) 2010;118:681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Ren J, Morgan S, Liu Z, Dou C, Liu B. Monocyte chemoattractant protein-1 (mcp-1) regulates macrophage cytotoxicity in abdominal aortic aneurysm. PLoS One. 2014;9:e92053. doi: 10.1371/journal.pone.0092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 43.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.