Abstract
Schizophrenia and bipolar disorder are associated with different clinical profiles of disturbances in motivation, yet few studies have compared the neurophysiological correlates of such disturbances. Outpatients with schizophrenia (n = 34), or bipolar disorder I (n = 33), and healthy controls (n = 31) completed a task in which the Late Positive Potential (LPP), an index of motivated attention, was assessed along motivational gradients determined by apparent distance from potential rewards or punishments. Sequences of cues signaling possible monetary gains or losses appeared to loom progressively closer to the viewer; a reaction time (RT) task after the final cue determined the outcome. Controls showed the expected pattern with LPPs for appetitive and aversive cues that were initially elevated, smaller during intermediate positions, and escalated just prior to the RT task. The clinical groups showed different patterns in the final positions just prior to the RT task: the bipolar group’s LPPs to both types of cues peaked relatively early during looming sequences and subsequently decreased, whereas the schizophrenia group showed relatively small LPP escalations, particularly for aversive cues. These distinct patterns suggest that the temporal unfolding of attentional resource allocation for motivationally significant events may qualitatively differ between these disorders.
Keywords: Schizophrenia, Bipolar Disorder, Event Related Potentials, Psychological Distance, Motivation
Introduction
Disturbances in motivation are common features of severe mental illnesses, including schizophrenia and bipolar disorder. For example, the negative symptoms of schizophrenia are defined, in large part, by diminished motivation to engage in productive and potentially rewarding activities (Blanchard, Kring, Horan, & Gur, 2011). Descriptions of bipolar disorder, in contrast, often include heightened drive to engage in goal directed or risky activities, and enhanced reactivity to reward-related stimuli, even during euthymic periods (Alloy et al., 2015; Johnson et al. 2012). Although these disturbances have important clinical and functional consequences for both disorders, the temporal dynamics and neurophysiological correlates of these disturbances are poorly understood. Using a translational affective neuroscience approach, this event-related potential (ERP) study assessed engagement of the appetitive and aversive motivational systems in response to cues signaling impending rewards or punishments in individuals with schizophrenia or bipolar disorder.
Grounded in basic animal research, Lang’s neurobiological model of appetitive and aversive motivation postulates that perception of motivationally relevant stimuli initiates a cascade of autonomic, reflexive, and brain responses that promote survival and flourishing (Bradley & Lang, 2007; Lang, Greenwald, Bradley, & Hamm, 1993). Within this framework, the neurophysiological components of emotions are seen as “action dispositions,” i.e., states of heightened attention to motivationally salient environmental stimuli and physiological mobilization, which facilitate adaptive approach and defensive behaviors. Support for the attentional component of this conceptualization comes from laboratory studies of emotional picture viewing with concomitant ERP recording. For example, the Late Positive Potential (LPP), a positive going ERP that begins around 300 ms post-picture onset, is reliably enhanced while viewing pleasant or unpleasant versus neutral pictures (see Hajcak, Weinberg, MacNamara, & Foti, 2011). This emotion-modulated LPP enhancement, which can be sustained for several hundred millliseconds, is conceptualized as an index of motivated attention to emotionally arousing stimuli. This enhanced central nervous system response in humans resembles the heightened vigilance and physiological mobilization observed in animals (Low, Lang, Smith, & Bradley, 2008).
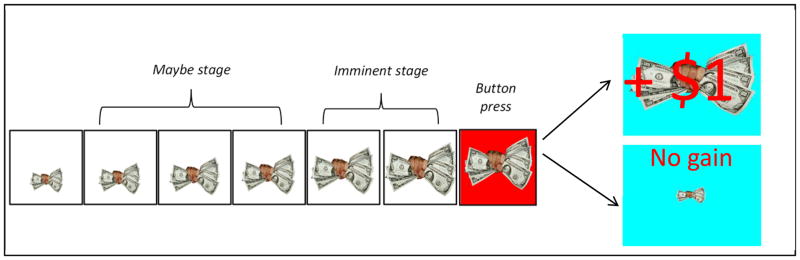
Moving beyond tasks that involve viewing single pictures, Lang’s group developed an ERP motivational gradient paradigm to assess the unfolding cascade of vigilance and action mobilization as a potential threat or reward becomes increasingly imminent (Low et al., 2008). In this paradigm, participants observe a stream of briefly presented neutral (non-looming) pictures on most trials, intended to mimic the repetitive neutral events that occupy most of daily life. Occasionally, however, an appetitive cue (fist full of money) or an aversive cue (hand pointing a gun) is presented and then appears to loom progressively closer (i.e., becomes larger) to the viewer in up to six sequential cue presentations (called cue “positions”). Some sequences terminate early (i.e., before a sixth cue is presented), an analog of real-world scenarios in which potential rewards/punishments never reach the stage of requiring a response. After the sixth and final cue presentation in a sequence, the background color changes and a button press reaction time (RT) task determines the outcome; if the button press is fast enough the participant gains money following reward sequences or avoids losing money following punishment sequences.
In addition to faster reaction times for looming reward/punishment sequences versus looming neutral sequences (a control condition), Low and colleagues found in healthy individuals that the LPP was systematically modulated across three stages of the looming cues: (a) in the “initial” stage (cue position 1) the typical pattern of enhanced LPPs for appetitive and aversive (versus neutral) was found, as in single picture viewing tasks, (b) in the intermediate “maybe” stage (cue positions 2 – 4), LPP responses to appetitive/aversive cues were substantially attenuated, and (c) in the final “imminent” stage (cue positions 5 – 6), LPPs to appetitive and aversive were again enhanced and showed maximal positivity for the cues presented in position 6 just prior to the reaction time task. The paradigm thus allows for an examination of the temporal unfolding of vigilance and action preparation for impending rewards and punishments, and whether participants’ LPPs sufficiently “ramp up” during the final imminent stage.
The current study applied a motivational gradient paradigm to individuals with schizophrenia or bipolar disorder and matched healthy controls. Based on research using conceptually related affective science measures and self-report emotional/motivational trait measures, we expected that the patient groups would show different LPP response patterns from controls, particularly for impending appetitive cues during the “imminent” stage. For bipolar disorder, we expected heightened LPP to appetitive cues. This prediction is based on studies supporting the reward hyperactivity model of bipolar spectrum disorders (Alloy et al., 2015; Johnson et al. 2012). Elevated reports of behavioral activation system sensitivity (closely related to appetitive motivation) are associated with the diagnosis, course, and risk for development of a bipolar spectrum. This theory is also supported by behavioral, electrophysiological, and fMRI studies showing heightened and prolonged reactivity to pleasant/rewarding cues and stimuli among individuals with (even during euthymic periods), or at risk for, bipolar spectrum disorders (e.g., Gruber, 2011; Hassel et al., 2008; Nusslock, Young, & Damme, 2014). For aversive cues, the smaller relevant literature did not lead to a clear directional hypothesis, as behavioral and neurophysiological responses to punishment/unpleasant cues and stimuli more often than not appear normal in bipolar disorder (e.g., Gruber, Hay, & Gross, 2014; Johnson, Gruber, & Eisner, 2007; Nusslock et al., 2012).
For schizophrenia, we expected diminished LPPs to appetitive cues. This prediction is based on evidence that individuals with schizophrenia show decreased anticipatory pleasure (closely related to appetitive motivation) on self-report trait, behavioral, and neurophysiological measures, despite showing intact “in-the-moment” responses to rewarding/pleasant stimuli (Kring & Barch, 2014; Kring & Elis, 2013). As was the case for bipolar disorder, the relevant literature in schizophrenia did not lead to a clear directional prediction for aversive cues. Although patients report elevated behavioral inhibition system sensitivity (closely related to aversive motivation), trait negative affectivity, and negative emotional responses in certain contexts (Cohen & Minor, 2010; Horan, Blanchard, Clark, & Green, 2008; Horan, Wynn, Mathis, Miller, & Green, 2014), they often demonstrate normal psychophysiological and neural responses to unpleasant and punishment-related stimuli (Anticevic et al., 2012; Horan, Wynn, Kring, Simons, & Green, 2010; Kring & Elis, 2013). In addition to evaluating between-group LPP differences, we examined whether LPPs during the experimental task correlated with symptoms.
Methods
Participants
Participants were 67 outpatients with schizophrenia (n=34) or bipolar disorder (n=33) and 31 healthy control subjects. Patients were recruited from outpatient clinics at University of California, Los Angeles (UCLA), the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS), and from local clinics and board and care facilities. Patients met criteria for schizophrenia or bipolar I disorder based on the Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders (First et al., 1996). In the bipolar group, 24 patients had a history of psychotic symptoms. Patients were excluded if they had substance dependence in the past six months, substance abuse in the past month, or an estimated premorbid verbal IQ < 70 based on the Wechsler Test of Adult Reading (Holdnack, 2001). All patients were clinically stable as defined by: no DSM-IV defined mood episodes in the past month, no hospitalizations in the past 3 months, no changes in living situation in the past 2 months, and no medication changes in the past 6 weeks. All the schizophrenia patients were taking antipsychotic medications (31 atypical medications, 3 typical medications), and 20 of the bipolar patients were taking antipsychotic medications (all atypicals). For schizophrenia patients, 1 was taking lithium, 5 were taking anticonvulsants, and 7 were taking antidepressants. For bipolar patients, 11 were taking lithium, 12 were taking anticonvulsants, and 13 were taking antidepressants.
Control participants were recruited through advertisements posted on websites. Controls were excluded if they had a lifetime history of schizophrenia, other psychotic disorder, bipolar disorder, recurrent major depressive disorder, or substance dependence disorder based on the SCID. They were also excluded for substance abuse disorder in the past month. Controls were also administered portions of the SCID for Axis II Disorders (First et al., 1994) and excluded if they met criteria for avoidant, paranoid, schizoid, schizotypal, or borderline personality disorder. They were also excluded for family history of a psychotic or bipolar disorder among first-degree relatives. Additional exclusion criteria for all participants were a history of loss of consciousness for more than one hour, significant neurological disorder, or insufficient fluency in English.
Symptom rating scales administered to the bipolar and schizophrenia groups included the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993) from which the positive symptom subscale and total score were used (Kopelowicz et al., 2008), the Young Mania Rating Scale (YMRS; Young et al., 1978), and the Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960). Negative symptoms were assessed with the Clinical Assessment Interview for Negative Symptoms (CAINS; Kring et al., 2013), which is comprised of two subscales. The Motivation and Pleasure (MAP) subscale includes nine items based on motivation, interest, and emotional experiences, as well as reported engagement in relevant social, vocational, and recreational activities, over the past week. The expression (EXP) subscale includes four items based on interviewer ratings of affective and verbal expression. Each item is rated on a scale from zero (no impairment) to four (severe deficit).
All interviewers were trained through the Treatment Unit of the Department of VA Veterans Integrated Service Network 22 Mental Illness Research, Education, and Clinical Center (MIRECC). Interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items on the SCID and to a minimum kappa of 0.75 – 0.80 for the BPRS, YMRS, and HAM-D (Ventura et al., 1998). For the CAINS, raters completed didactic training sessions with one of the scale developers (WPH), achieved acceptable reliability (ICC < .80) using a library of tapes with gold-standard ratings, and completed at least two co-rated interviews with MIRECC training faculty members. All participants had the capacity to give informed consent and provided written informed consent after procedures were fully explained, in line with procedures approved by the institutional review board at VAGLAHS.
Motivational gradient task
We used a reaction time-dependent monetary gain/loss paradigm closely modeled on Low et al. (2008). Three blocks of trials were administered. Each block involved viewing a continuously presented stream of different pictures. All pictures were presented for 1.5 seconds each with a 0.5-s inter-picture interval (blank screen). Two types of pictures were shown. The first type consisted of non-looming neutral pictures, which were selected from the International Affective Picture System (Lang et al., 2005) and appeared most of the time (160 per block). The second type consisted of looming (i.e., progressively larger) sequences of pictures (i.e., “cues”), which signaled the possibility of an upcoming button press RT task. There were three varieties of cues: (1) appetitive (a picture of fist full of money), (2) aversive (a picture of gun pointed at the participant), and (3) neutral (a picture of a clock).
Within each of the three blocks of trials, each of the three types of cues appeared either: (a) as a single, isolated cue in the far distance (4 times per block), (b) as a looming sequence of four cues of increasing size (4 times per block), or (c) as a looming sequence of six cues of increasing size (8 times per block; see Figure 1). The cue sequences in this task are designed to more validly approximate the typical experience of action preparation. In particular, it is often uncertain whether distant cues signaling potential threats or rewards will actually end up in a direct confrontation with a threat or an opportunity to obtain a reward. The use of different cue sequence lengths is intended to capture this element of uncertainty and task engagement; if all sequences were exactly the same, seeing a particular cue would always lead to a particular response requirement. Consistent with the three conceptually-based stages delineated by Low et al., the “initial stage” was defined as position 1 in the sequence, the “maybe stage” as positions 2 – 4, and the” imminent stage” as positions 5 – 6. During the maybe stage, psychological distance was greater and continuation of the sequence was uncertain (no more than 4 cues may be presented) as compared to the imminent stage. During the later imminent stage (cue positions 5 and 6) the response requirement was certain. These three stages are defined for descriptive purposes to facilitate interpretation of the results; as detailed below, the primary data analyses included each of the six positions in the looming sequences to maximize the temporal precision of the analyses.
Figure 1.
Sample of looming cue sequence for the appetitive condition
To enhance the motivational relevance of the appetitive, aversive, and neutral cues, participants were required to make a button press just after the sixth looming cue appeared. The button press was signaled by a change in the background color (see Figure 1). Within each block, for each type of cue the button press requirement occurred eight times. Sufficiently fast RTs resulted in a $1 reward for appetitive cues and in avoiding a $1 punishment for aversive cues (from an initial $10 allotment). To ensure roughly equivalent numbers of reward/non-reward and punishment/non-punishment outcomes across subjects, a procedure was used to establish individualized RT cut-offs. Initial RT cut-off scores were individually calibrated based on 20 trials of a simple, non-emotional RT task. A 3rd quartile cut-off (i.e., RT at the 75% slowest trial) was used to determine each participant’s individual RT threshold for block 1. To keep the task challenging, the cut-offs were adjusted for blocks 2 – 4. This was accomplished by using each participant’s mean RT for the preceding block as the cut-off for blocks 2-4. Thus, the individually calibrated RT cut-offs were intended to produce comparable levels of reward and punishment outcomes across groups, and thereby facilitate between-group comparisons of ERP’s across the task conditions. After a series of practice trials and a practice block, the three experimental blocks were administered with rest breaks between blocks. After the paradigm was completed, participants received the amount of money they earned in cash.
EEG recording and analysis
Participants had their EEG activity recorded continuously from 64 electrodes based on a modified 10/20 system placed in an electrode cap (Cortech Solutions, USA) and the ActiveTwo BioSemi system (BioSemi, The Netherlands). The signal was preamplified at the electrode with a gain of one; the EEG was digitized at 24-bit resolution with a sampling rate of 1024 Hz. Recordings were taken from the 64 electrodes, and also from two electrodes placed on the left and right mastoids. The electro-oculogram was recorded from four facial electrodes: two 1 cm above and below the left eye, one 1 cm to the left of the left eye, and one 1 cm to the right of the right eye. Each electrode was measured online with respect to a common mode sense electrode that formed a monopolar channel.
Off-line analysis was performed using Brain Vision Analyzer software (Brain Products, Germany). All EEG data were re-referenced to the average of all electrodes and band-pass filtered from 0.1 to 30 Hz. The EEG was segmented for each image beginning 200 ms before each stimulus and continuing for 600 ms post-stimulus onset (total of 800 ms). Each EEG segment was corrected for blinks and eye movements using the method developed by Gratton et al. (1983). Specific channels were rejected in each trial using a semi-automated procedure, with physiological artifacts identified by the following criteria: a step of more than 50 μV between sample points, a maximum difference of less than 0.5 μV within 100-ms intervals, and an amplitude that exceeded 75 μV. Two patients (one schizophrenia, one bipolar disorder) were excluded due to poor ERP data quality (less than 50% artifact free trials) and one bipolar disorder patient was excluded because ERP values differed by more than 3 S.D. from the patient group means. The final samples consisted of 34 schizophrenia, 33 bipolar, and 31 control participants.
We followed the same approach as Low et al. (2008) and focused on the LPP during the 300 – 600 ms period post cue-onset for each of the six cue positions before the required button press. This early period of the LPP is thought to reflect relatively obligatory capture of attention by motivationally salient stimuli, whereas later (slow wave activity occurring after 600 ms) parts of the LPP are instead indicative of the increasing influence of top-down attentional processes (see Weinberg et al., 2012, 2013). ERPs were constructed by separately averaging segments of the three experimental conditions (appetitive, aversive, neutral) using average activity in the 200-ms window prior to the onset of each picture (i.e., blank screen during the ITI) as the baseline. To select electrodes we examined the topographical maps for each group (see Supplemental Figures 1 – 2). Across groups, the LPPs for the appetitive and aversive versus neutral conditions appeared to be maximal in two regions: a set of six parietal electrodes for positions 1 – 4 (P1, Pz, P2, PO3, POz, PO4) and in a more anterior set of six central electrodes for positions 5 – 6 (C1, Cz, C2, CP1, CPz, CP2). Hence, region was entered as a factor in the initial analyses. The LPP was quantified as the mean activity during the 300–600 ms epoch post cue onset in each set of electrodes for each participant.
Statistical analysis
First, demographic and clinical variables were evaluated with one-way ANOVAs or t-tests for continuous variables and with chi-square tests for categorical variables. Second, we examined group differences on behavioral data from the motivational gradient task, including RT (repeated-measures ANOVA) and money earned (one-way ANOVA). Third, the LPP data during the task was initially examined with a Region (2 electrode clusters) X Position (6 cue positions) X Condition (3 levels: aversive, appetitive, control) X Group (3 levels: schizophrenia, bipolar, control) repeated-measures ANOVA using Greenhouse–Geisser epsilon corrections for analyses with more than one degree of freedom. Subsequent ANOVAs and t-tests were used to decompose significant interaction effects. Finally, to examine associations with clinical symptoms, we focused on LPPs during the imminent stage (positions 5 – 6 just before the button press) using Spearman correlation coefficients.
Results
Demographic and clinical data
As shown in Table 1, the groups did not differ on sex, age, race, ethnicity, or parental education. As expected, there were group differences on personal education; schizophrenia patients had lower education levels compared to bipolar and control participants (t’s > 2.13, p’s < .005), who did not differ from each other, t(62) = .75, p > .05. The schizophrenia group also had lower estimated verbal IQs than the bipolar and control groups (t’s > 3.20, p’s < .005), which did not differ from each other, t(62) = 1.58, p > .05. On the CAINS, schizophrenia patients demonstrated higher MAP and EXP negative symptoms than the other two groups (t’s > 2.26, p’s < .05). Bipolar patients also demonstrated higher MAP symptoms, t(62) = 3.64, p < .001, and marginally higher EXP symptoms, t(62) = 1.95, p = .05, compared to controls. On the other symptom scales, schizophrenia patients had higher BPRS total and positive symptoms compared to bipolar patients. The patient groups did not differ on ratings of manic or depressive symptoms. Finally, the schizophrenia group had higher chlorpromzaine dosage equivalents (Andreasen et al. [2010]) than the bipolar group.
Table 1.
Demographic and Clinical Characteristics
| Schizophrenia (N = 34) | Bipolar (N = 33) | Control (N = 31) | Statistic | |
|---|---|---|---|---|
| Sex (% male) | 61.8 | 57.6 | 67.7 | χ2(2,98)= 0.97 |
| Age (SD) | 46.94 (9.91) | 43.55 (11.29) | 46.90 (6.53) | F(2,95)= 1.38 |
| Race (%) | χ2(6,98)= 4.80 | |||
| White | 58.8 | 66.7 | 77.4 | |
| African American | 17.6 | 15.2 | 12.9 | |
| Asian | 14.7 | 6.1 | 3.2 | |
| Other | 8.8 | 12.1 | 6.5 | |
| Ethnicity (% Hispanic) | 20.6 | 18.2 | 16.1 | χ2(4,108)= 2.31 |
| Education (SD) | 12.91 (1.80) | 14.42 (2.15) | 14.81 (1.92) | F(2, 95) = 8.62*** |
| Parental Education (SD) | 13.41 (2.77) | 14.81 (2.93) | 14.40 (2.49) | F(2,95)= 2.03 |
| Estimated Verbal IQ (SD) | 96.9 (10.5) | 104.5 (10.2) | 107.9 (7.4) | F(2,95) = 13.13**** |
| Age of onset (SD) | 21.47 (6.82) | 21.15 (6.78) | t(65)= 0.19 | |
| BPRS Positive (SD) | 1.86 (0.74) | 1.28 (0.28) | t(65)= 4.16**** | |
| BPRS Total (SD) | 39.38 (10.64) | 33.06 (6.44) | t(65)= 2.90** | |
| YMRS (SD) | 4.15 (4.35) | 3.85 (4.97) | t(65)= 0.26 | |
| HAM-D (SD) | 8.65 (6.89) | 6.18 (5.49) | t(65)= 1.62 | |
| CAINS MAP (SD) | 14.15 (5.20) | 10.78 (6.80) | t(65)=2.26* | |
| CAINS EXP (SD) | 5.24 (3.61) | 2.21 (2.82) | t(65)= 3.79**** | |
| Chlorpromazine equivalents (SD) | 452.5 (402.0) | 229.6 (177.7) | t(52)= 2.21* |
Notes: BPRS = Brief Psychiatric Rating Scale; YMRS = Young Mania Rating Scale; HAM-D = Hamilton Depression Rating Scale; CAINS = Clinical Assessment Interview for Negative Symptoms;
p < 0 .05,
p < .005;
p < 0.001.
Behavioral data
For the RT data there was a significant condition effect, F(2,190) = 11.63, p < .001, ηp2 = .11, but non-significant group, F(2,95) = 2.40, p = .10, ηp2 = .05, and interaction effects, F(4,190) = .09, p > .99, ηp2 = .002. As expected, RTs were significantly faster for the appetitive (M = 371.44 ms; SD = 210.23) and aversive (M = 370.55 ms; SD = 237.43) conditions than for the neutral condition (M = 425.08 ms; SD = 225.57) (t’s > 3.75, p’s < .001). RTs did not differ between the appetitive and aversive conditions, t(97) = .08, p = .93. The groups did not differ in the amount of money won, F(2,97) = .81, p = .45. On average, participants made $27.87 (SD = 7.77). Regardless of performance, participants received a minimum of $20. The similar findings across groups indicate that the individualized RT calibration procedures worked well, and resulted in comparable levels of reward and punishment outcomes across groups.
LPP data
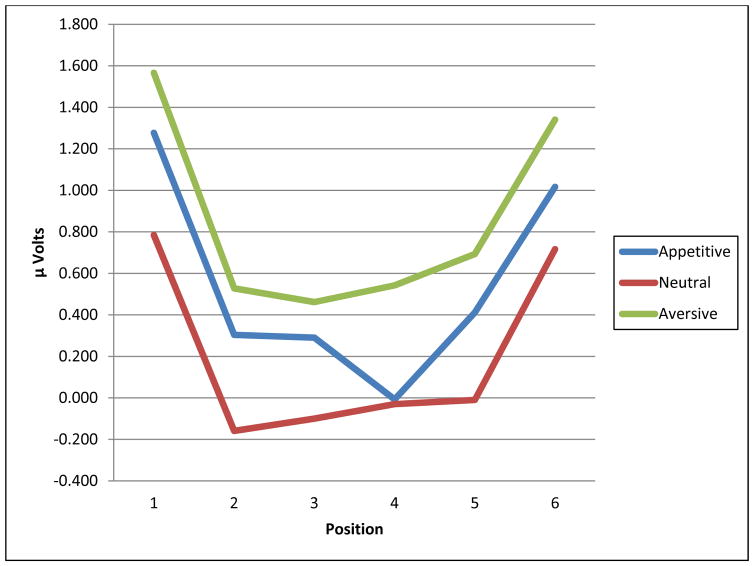
First, we wanted to make sure the paradigm yielded valid data across samples. To do this, we collapsed across group and electrodes and also considered non-linear effects. As shown in Figure 2, the LPPs in the appetitive and aversive conditions were large in the initial stage, decreased during the maybe stage, and increased again during the imminent stage. The quadratic trend across positions was significant, F(1,97) = 58.83, p < .001, ηp2 = .36, and there was a significant condition effect, F(1.95; 189.36) = 48.98, p < .001, ηp2 = .34, indicating LPP differences between each condition (aversive > appetitive > neutral). The position X condition effect was not significant, F(7.87, 763.10) = 1.62, p = .55, ηp2 = .01. The overall consistency of this pattern with previous findings from a similar paradigm (Low et al., 2008) bolsters confidence of the validity of our adapted paradigm.
Figure 2.
Overall Mean LPP Amplitudes Collapsed Across the Three Groups for the Appetitive, Neutral, and Aversive Conditions
Based on the visual inspection of the topographic maps (see Supplemental Figure 1), it appeared that the relevant electrode clusters differed for different stages. In fact, there was a highly significant region X position interaction, F(3.30, 313.19) = 132.10, p < .001, ηp2 = .58 (see Supplemental Table 1 for full results). Hence, it would have been misleading to use a single set of electrodes for all positions. We assigned positions 1-4 to the parietal electrode cluster, and positions 5 and 6 to the central cluster, and thereby eliminated electrode as a factor for subsequent analyses (see Supplemental Figure 2 for grand average waveforms). Instead, we conducted two separate 3-way ANOVAs (position X condition X group) for the two electrode clusters.
LPP at parietal electrodes for positions 1 - 4
Results of a Position (4 levels) X Condition X Group ANOVA are summarized in Table 2. There was a significant main effect for Position reflecting generally higher LPPs in the initial stage that decreased over the maybe stage (linear trend F[1,95] = 90.23, p < .001, ηp2 = .49). There was also a significant main effect for Condition reflecting that LPPs were higher for the aversive than appetitive condition, t(97) = 4.76, p < .001, which was higher than the neutral condition, t(97) = 5.67, p < .001. These main effects were qualified by two significant and relatively subtle interactions (see Figure 3).
Table 2.
LPP Results for (a) Parietal Electrodes: Positions 1 – 4 and (b) Central Electrodes: Positions 5 – 6
| (a) Parietal Electrodes: Positions 1-4
|
(b) Central Electrodes: Positions 5-6
|
||||||
|---|---|---|---|---|---|---|---|
| df | F | ηp2 | df | F | ηp2 | ||
|
|
|
||||||
| Position | (1.77, 168.22) | 63.75 | .40 | (1.00, 95.00) | 130.45**** | .58 | |
| Position X Group | (3.54, 168.22) | .91 | .02 | (2.00, 95.00) | 2.20 | .04 | |
| Condition | (1.95, 185.00) | 58.65**** | .38 | (1.78, 168.80) | 31.39**** | .25 | |
| Condition X Group | (3.89, 185.00) | 2.74* | .06 | (3.55, 168.80) | 1.76 | .04 | |
| Position X Condition | (5.29, 502.14) | 3.4*** | .04 | (1.95, 185.17) | .36 | .004 | |
| Position X Condition X Group | (10.57, 502.14) | 1.17 | .02 | (3.90, 185.17) | 3.02* | .06 | |
| Group | (2,95) | 1.2 | .03 | (2,95) | .42 | .009 | |
Notes:
p < .05,
p < .005;
p < .01
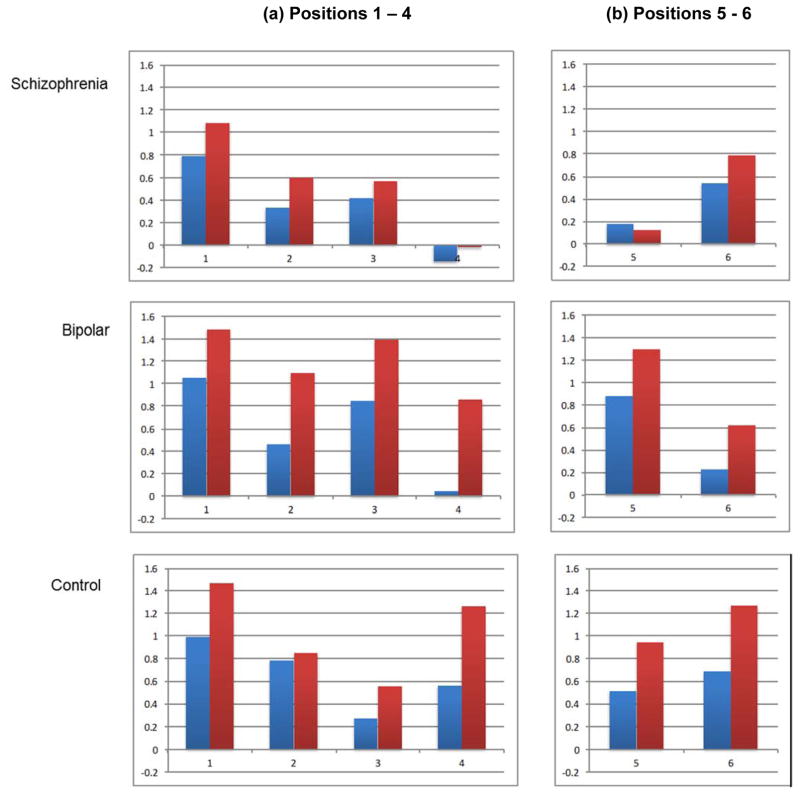
Figure 3.
LPP results for appetitive - neutral cues (blue) and aversive – neutral cues (red) at (a) Positions 1 - 4 (Parietal electrodes) and (b) Positions 5 - 6 (Central electrodes) in the Schizophrenia, Bipolar, and Control Groups. The Y-axis is scaled in microvolts.
First, a significant Position X Condition interaction indicated that LPPs during positions 1 – 3 showed a linear pattern of differences across conditions (aversive > appetitive > neutral; all t’s > 2.44, p’s < .05), but at position 4, LPPs were larger in the aversive condition than in the appetitive and neutral conditions (t’s > 2.97, p’s < .005), which did not significantly differ from each other, t = .74, p > .05. Second, a significant Condition X Group interaction indicated a slightly different pattern of between-condition LPP differences in the bipolar and control groups as compared to the schizophrenia group. For the bipolar and control groups, the three conditions significantly differed from each other (Aversive > Appetitive > Neutral) (bipolar: all t’s > 4.03, p’s < .001; control: all t’s > 2.34, p’s < .05). However, for the schizophrenia group, the Aversive and Appetitive conditions were both higher than the Neutral condition (all t’s > 2.43, p’s < .05), but did not differ from each other, t(33) = 1.69, p > .05. Thus, the schizophrenia group did not show the same distinction between the Aversive and Appetitive conditions seen in the control and bipolar groups during the initial and maybe stages.
LPP at central electrodes for positions 5 - 6
Results of a Position (2 levels) X Condition X Group ANOVA are summarized in Table 2. Significant Position (Position 6 > Position 5) and Condition (aversive > appetitive > neutral) main effects were qualified by a significant Position X Condition X Group interaction. To illustrate the nature of this more complex three-way interaction, we simplified the dependent variables by focusing on two LPP difference scores: (1) aversive minus neutral and (2) appetitive minus neutral. We first conducted within-group analyses using separate 2 (Position: 5 and 6) X 2 (Difference score: Aversive, Appetitive) Repeated-Measures ANOVA’s within each group (see Figure 3). For controls, there was a significant Difference score effect, F(1,30) = 13.24, p < .001, ηp2 = .31, reflecting higher LPP for the Aversive than the Appetitive score. There were no significant Position, F(1,30) 1.37, p > .05, ηp2 = .04, or Position X Difference score interaction, F(1,30) = .29, p > .05, ηp2 = .01, effects.
For the bipolar group, there was a significant Difference score effect, F(1,32) = 8.66, p < .01, ηp2 = .21, reflecting higher LPP for the Aversive than the Appetitive score. There was also a significant Position effect, F(1,32) = 6.78, p < .01, ηp2 = .18, indicating that these LPPs decreased from position 5 to position 6. There was non-significant Position X Difference score interaction effect, F(1,32) = .01, p > .05, ηp2 = .001. For the schizophrenia group, there was a significant Position effect, F(1,33) = 4.48, p < .05, ηp2 = .12, indicating that these LPPs increased from position 5 to position 6. However, there were no significant Difference score, F(1,33) = .32, p > .05, ηp2 = .01, or Position X Difference score interaction F(1,33) = .91, p > .05, ηp2 = .03, effects.
We then conducted between-group comparisons for each of the difference scores using a series of ANOVAs. For position 5, there was a significant group effect for the Aversive difference score, F(2,95) = 6.97, p < .001, ηp2 = .13, indicating lower scores in the schizophrenia group than the bipolar, t(65) = 3.78, p < .001, and control, t(63) = 2.52, p < .05, groups, which did not differ from each other, t(62) = 1.03, p > .05. There were no significant group effects for the position 5 Appetitive score, F(2,95) = 1.86, p > .05, the position 6 Aversive score, F(2,95) = 1.82, p > .05; or position 6 appetitive score, F(2,95) = .91, p > .05.
Symptom correlates
We focused on relations between symptoms and LPP difference scores (appetitive – neutral, aversive – neutral) during the imminent stage within the each patient group (Table 3). The most robust relations were seen at position 5 in the bipolar group: higher LPPs to aversive cues correlated with higher positive, total, and manic symptoms, and higher LPPs to appetitive cues correlated with lower depressive symptoms. At position 6, there was also a significant correlation between higher positive symptoms and lower LPPs to appetitive cues in the bipolar group. Within the schizophrenia group, there was only one significant result: higher positive symptoms significantly correlated with higher LPPs to aversive cues at position 5.
Table 3.
Correlations between LPPs during the imminent stage (positions 5 – 6) and symptom ratings within the bipolar and schizophrenia groups
| Position 5: Appetitive cues | Position 5: Aversive cues | Position 6: Appetitive cues | Position 6: Aversive cues | |||||
|---|---|---|---|---|---|---|---|---|
| Bipolar | Schizophrenia | Bipolar | Schizophrenia | Bipolar | Schizophrenia | Bipolar | Schizophrenia | |
| BPRS Positive | −.05 | .18 | .36* | .41* | −.35* | .14 | −.23 | .22 |
| BPRS Total | −.04 | .07 | .52** | .24 | −.07 | .13 | −.11 | .13 |
| YMRS | −.00 | −.05 | .36* | .11 | −.12 | .19 | −.08 | .07 |
| HAMD | −.39* | .15 | .19 | .13 | −.09 | .16 | −.19 | .10 |
| CAINS MAP | −.09 | .01 | .11 | −.13 | .30 | .15 | .24 | .05 |
| CAINS EXP | .10 | −.12 | −.01 | −.22 | .09 | −.12 | .18 | −.08 |
Notes: BPRS = Brief Psychiatric Rating Scale; YMRS = Young Mania Rating Scale; HAM-D = Hamilton Depression Rating Scale; CAINS = Clinical Assessment Interview for Negative Symptoms;
p < .05,
p < .01.
Supplemental analyses
Supplemental analyses examined whether there were any LPP differences among subgroups of bipolar disorder patients either with vs. without histories of psychosis, or taking vs. not taking antipsychotics. Although the sample sizes for these subgroups are small, there were no systematic differences between bipolar subgroups with regard to history of psychosis or current antipsychotic use (details are presented in Supplemental Tables 2–4 and Supplemental Figure 3).
We also examined whether chlorpromazine equivalent units or estimated verbal IQ related to LPP appetitive and aversive difference scores at each position in the two clinical samples. Neither of these variables showed a systematic relation to LPP levels. For chlorpromazine equivalents, there were no significant correlations within the bipolar or schizophrenia groups; in the combined sample, there was only one negative correlation with the aversive difference score at position 3 (r = −.33, p < .05) and one positive correlation with the appetitive difference score at position 6 (r = .29, p < .05). For verbal IQ, there were also no significant correlations within the schizophrenia or bipolar groups; in the combined sample, there was only one positive correlation between verbal IQ and the aversive difference score at position 5 (r = .27, p < .05). Finally, we examined whether there were differences associated with histories of substance use disorder within the bipolar (16 = with history; 17 = without history) and the schizophrenia (22 = with history; 12 = without history) groups. Although the sample sizes were again small, there were no systematic differences between these bipolar or schizophrenia subgroups (details are presented in Supplemental Tables 5 – 7).
4. Discussion
This study found abnormal neural reactivity patterns during a motivational gradient task in bipolar and schizophrenia patients, though the patterns provided only partial support for our hypotheses. In terms of behavioral performance, all three groups displayed comparable RT patterns across experimental conditions and achieved comparable levels of total money earned. These findings indicate that the individual RT calibration procedure worked well, that the groups completed comparable numbers of successful and unsuccessful trials, and that the groups showed comparable levels of task engagement. The comparable behavioral performance findings facilitate interpretation of between-group ERP comparisons; any significant group differences are not simply attributable to relatively greater exposure to unfavorable outcomes (i.e., not winning money on appetitive trials, not losing money on aversive trials) during the paradigm.
All three groups also showed generally similar overall LPP patterns during the initial and maybe stages, though the schizophrenia group did not show the same clear distinction between the Aversive and Appetitive conditions seen in the other groups. More pronounced group differences emerged during the imminent stage for both appetitive and aversive cues, with the bipolar group showing LPPs to appetitive and aversive cues that peaked earlier in the sequence and subsequently decreased prior to the RT task, whereas the schizophrenia group showed somewhat smaller LPPs to both types of cues that increased prior to the RT task. Thus, the current findings suggest that the time course of attentional resource mobilization for motivationally salient events qualitatively differs between individuals with bipolar disorder and schizophrenia.
Findings for Bipolar Disorder
Our hypothesis was that the bipolar group would show hyperactivity to appetitive cues based on the reward hyperactivity model of bipolar spectrum disorders (Alloy et al., 2015; Johnson et al. 2012). We found no evidence for elevated LPPs in this group to either appetitive or aversive cues during the initial or maybe stages. The bipolar and control groups showed comparable initial LPP elevations for the appetitive and aversive conditions as compared to the maybe stage, and similarly higher LPPs for aversive than appetitive cues across the initial and maybe stages.
The bipolar group did, however, show a unique pattern during the imminent stage, when LPPs to both appetitive and aversive cues significantly decreased from positions five to six. This differs from the pattern in the control group, which showed LPPs to aversive and appetitive cues that did not significantly differ across positions, and showed a tendency toward increasing from position five to six. Although the bipolar group’s relatively elevated LPPs to appetitive cues at position five could be viewed as partly consistent with the reward hyperactivity model, they showed similar responses for aversive cues. This elevated response to aversive cues is at odds with the normal neurophysiological responses to punishment or unpleasant stimuli found in most studies in euthymic patients (Gruber et al., 2014; Johnson et al., 2007; Nusslock et al., 2012b), though a few studies have found, for example, amygdala hyperactivity in tasks involving faces expressing fear (see Townsend & Altshuler, 2012).
Overall, the bipolar group’s pattern suggests a relatively subtle disturbance in the affective chronometry of motivational responding. Affective chronometry refers to the temporal dynamics of emotional responding, including emotional response profiles in anticipation of, during exposure to, and following the offset of motivationally salient stimuli (Davidson, 2003; Davidson, Jackson, & Kalin, 2000). In the current study, the bipolar groups’ LPP amplitudes appeared to peak relatively early in the sequences of looming cues, a pattern that is consistent with the impulsivity associated with this disorder (Reddy et al., 2014; Saddichha & Schuetz, 2014). Since enhanced LPPs are believed to reflect increased attentional resource allocation to motivationally significant events (Hajcak, et al., 2011), the relatively earlier increase in LPP in the bipolar group could indicate suboptimal approach and avoidance behavior whereby mobilization for potential action happens before it is warranted.
It is unclear why we did not find evidence of LPP hyperactivity during the initial or maybe stages for the bipolar group. An important consideration is whether certain clinical characteristics of our bipolar sample impacted the results. Supplemental analyses indicated that the bipolar groups’ results were not systematically related to the presence versus absence of psychosis histories or current antipsychotic use, though the subsamples in these analyses were small. Further, in both clinical samples, there were no systematic relations between ERPs and antipsychotic dosages, estimated verbal IQ, or substance use disorder histories. Regarding the clinical state of our sample, although none of the bipolar patients was experiencing an episode of mania/hypomania or depression (or psychotic symptoms), the sample did display some variability in mood and general psychiatric symptoms. For example, higher depressive symptoms correlated with lower LPPs at position 5, consistent with prior studies showing associations between depression and altered reward processing in bipolar disorder (e.g., Chase et al., 2013; Fletcher et al., 2013). Correlational analyses also revealed that higher mood and general symptoms were associated with larger LPPs for aversive cues at position five. There appears to be a complex relationship between clinical status and reward processing in bipolar disorder (Alloy, Nusslock, & Boland, 2015; Nusslock, et al., 2014), and the current findings must be interpreted in the context of this particular sample’s clinical characteristics.
Findings for Schizophrenia
Our hypothesis that the schizophrenia group would show diminished LPPs to appetitive cues received minimal support. As in the other two groups, the schizophrenia sample showed a pattern of relatively larger LPPs to appetitive and aversive cues in the initial compared to the maybe stage. However, they did not show the significantly higher overall LPPs to aversive versus appetitive cues across these stages that was present in the other groups. This finding is at odds with the normal neural and physiological responses to single punishment cues or unpleasant stimuli found in most prior studies (Anticevic, et al., 2012; Horan, et al., 2010; Kring & Elis, 2013).
The schizophrenia group’s LPP response profile also showed notable differences in the imminent stage. In contrast to the bipolar group, LPPs to aversive and appetitive cues significantly increased from position five to six. In addition, mean LPP amplitudes to appetitive and aversive cues were generally lower in the schizophrenia group than the bipolar and control groups during the imminent stage. However, between-group differences only achieved significance for aversive cues at position five. This hypoactivation to aversive cues, as noted above, is at odds with the normal neural and physiological responses to aversive stimuli found in most prior studies (Anticevic, et al., 2012; Horan, et al., 2010; Kring & Elis, 2013). Thus, although the schizophrenia group demonstrated a significant increase in LPPs during the imminent stage, they showed relatively low LPPs during this stage, which were most apparent for aversive cues at position five.
The schizophrenia group’s overall LPP pattern suggests a different disturbance in affective chronometry from that seen in the bipolar group. Whereas the bipolar group appeared to respond too early in the looming sequence, the schizophrenia group did not sufficiently “ramp up” their neural responses just prior to the imperative stimulus, particularly for aversive stimuli. This pattern is broadly consistent with prior studies showing diminished ERPs during anticipation of emotional stimuli, as well as during motor response preparation, in schizophrenia (Karayanidis et al., 2006; Reuter, Herzog, Endrass, & Kathmann, 2006; Wynn, Horan, Kring, Simons, & Green, 2010). Diminished mobilization of approach/avoidance systems is conceptually linked to negative symptoms such as avolition and asociality. However, LPPs during the imminent phase did not show significant relations to negative or most other types of symptoms in the schizophrenia group.
Additional considerations
Some additional aspects of the LPP results warrant further consideration. First, in our healthy control group, the looming sequential picture sequences elicited a similar LPP pattern across the initial, maybe, and imminent stages as found in healthy college students by Low et al. This pattern provides further support for the “distance hypothesis,” which proposes that in humans, as in other animals, neurophysiological responses systematically vary with apparent distance from perceived punishments/threats and rewards to promote adaptive functioning (Low et al., 2008). Second, aversive cues elicited larger LPPs than appetitive cues in control and bipolar samples. This is consistent with Low et al. and considerable other human and animal research indicating that motivation to avoid a loss is greater than motivation to achieve a gain (Kahneman & Tversky, 1979; Kahneman & Tversky, 2000; Miller, 1951).
Our results differed from Low et al. in terms of topography of the LPP across the cue sequences. Whereas they found maximal LPPs in the central-parietal scalp region throughout the task, we found that LPPs were maximal in the central-parietal region during the initial and maybe stages, but then shifted forward to a central-frontal region during the imminent stage. Although it is not clear why the topographies differed across studies, there is some evidence of an “anteriorization” of the LPP from early to later stages of emotional processing in single emotional picture viewing tasks (Foti, Hajcak, & Dien, 2009; Hajcak, et al., 2011; Macnamara, Foti, & Hajcak, 2009). It has been proposed that anteriorization of the LPP may reflect more complex and elaborative processing of emotional stimuli that relies on frontal activation. Speculatively, it is possible that cues in the motivational gradient paradigm engaged more frontal regions during the imminent stage. However, future efforts to replicate this finding and incorporate neuroimaging methods with better spatial resolution is clearly necessary to substantiate this possibility.
Limitations and conclusions
Some limitations of this study should be considered. First, the patients were taking antipsychotic and other types of psychiatric medications at clinically determined dosages. As noted above, we did not observe differential performance in subgroups of bipolar patients based on the presence or absence of antipsychotic use, or any systematic relations with antipsychotic dosage levels in either clinical sample. However, research in larger unmedicated samples is required to definitively examine the impact of medications. Second, the bipolar group included a mixture of patients with and without histories of psychosis, although we did not find any systematic differences between these relatively small subgroups that impacted the results. Third, the patient groups were relatively old, chronically ill, and had residual psychiatric symptoms. Hence, it is unclear whether the same findings would be observed in other samples such as early course or fully euthymic patients. Fourth, the sample sizes may have limited our power to detect additional effects. Although the samples were relatively large for a clinical EEG study and we were able to detect significant interaction effects involving group, larger samples may have revealed, for example, significant LPP increases during the imminent stage in controls or additional between-group differences during the imminent stage. Fifth, we did not measure ERPs during the presentation of the outcomes – i.e., when participants received feedback about whether they won/did not win on appetitive trials and lost/did not lose on aversive trials. This would be a useful variable to evaluate in future studies with this type of task. Finally, the neural generators of the LPP during the motivational gradient task are unknown and further research using complementary methods will be required to address this issue.
There is a long history of interest in whether the diagnostic categories of schizophrenia and bipolar disorder reflect a valid and clinically meaningful distinction (Fischer & Carpenter, 2009). Indeed, recent research has emphasized shared vulnerability factors, candidate genes, neuropathology, and neurocognitive endophenotypes (e.g., Arnone et al., 2009; Lichtenstein et al., 2009; Purcell et al., 2009; Thaker, 2008). The current findings contribute to evidence that there are qualitative differences between these disorders in their emotional response profiles, which can be understood in an affective chronometry framework. Bipolar disorder was associated with earlier responses to looming cues, whereas schizophrenia was associated with diminished escalation of responses to looming cues (particularly aversive) during anticipation of rewards/punishments. Additional published evidence points to differences in other temporal components; bipolar disorder has been associated with heightened in-the-moment, and possibly prolonged, reactivity to pleasant stimuli (Farmer et al., 2006; Gruber, 2011; Gruber, Eidelman, Johnson, Smith, & Harvey, 2011), whereas schizophrenia is associated with normal in-the-moment reactivity to pleasant stimuli but difficulty sustaining these responses (Gard et al., 2011; Kring, Germans Gard, & Gard, 2011). These different profiles provide guidance for research aimed at further understanding and treating the emotional phenotypes associated with these disorders.
Supplementary Material
General Scientific Summary.
Although schizophrenia and bipolar disorder are associated with different clinical profiles of disturbances in motivation, little is known about the neurophysiological correlates of such disturbances. Using an event related potential paradigm to record electrophysiological responses, this study suggests that individuals with schizophrenia or with bipolar disorder show distinct patterns in the temporal unfolding of attentional resource allocation for motivationally significant events.
Acknowledgments
Support for this study came from a NIMH Grants MH091468 (William P. Horan, Ph.D.) and MH065707 and MH43292 (Michael F. Green, PhD). The authors wish to thank Amanda Bender, Michelle Dolinsky, Crystal Gibson, Cory Tripp, and Katherine Weiner for assistance in data collection.
Footnotes
Financial disclosure:
Dr. Green has been a consultant to AbbVie, DSP, Forum, and Takeda, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen and Forum. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
References
- Alloy LB, Nusslock R, Boland EM. The development and course of bipolar spectrum disorders: an integrated reward and circadian rhythm dysregulation model. Annual Review of Clinical Psychology. 2015;11:213–250. doi: 10.1146/annurev-clinpsy-032814-112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulous P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophrenia Bulletin. 2012;38(3):608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the Next Generation of Negative Symptom Assessments: The Collaboration to Advance Negative Symptom Assessment in Schizophrenia. Schizophrenia Bulletin. 2011;37:91–99. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and bipolar II disorders. American Journal of Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia Bulletin. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Darwin and the neural bases of emotion and affective style. Annals of the New York Academy of Sciences. 2003;1000:316–336. doi: 10.1196/annals.1280.014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Farmer A, Lam D, Sahakian B, Roiser J, Burke A, O’Neill NPM. A pilot study of positive mood induction in inter-episode bipolar subjects compared with healthy controls. Psychological Medicine. 2006;36:1213–1218. doi: 10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- Fischer BA, Carpenter WT., Jr Will the Kraepelinian dichotomy survive DSM-V? Neuropsychopharmacology. 2009;34:2081–2087. doi: 10.1038/npp.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher K, Parker G, Manicavasagar V. Behavioral Activation System (BAS) differences in bipolar I and II disorder. Journal of Affective Disorders. 2013;151:121–128. doi: 10.1016/j.jad.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Gard DE, Cooper S, Fisher M, Genevsky A, Mikels JA, Vinogradov S. Evidence for an emotion maintenance deficit in schizophrenia. Psychiatry Research. 2011;187(1–2):24–29. doi: 10.1016/j.psychres.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: taking stock and moving forward. Emotion. 2013;13(3):359–365. doi: 10.1037/a0032135. [DOI] [PubMed] [Google Scholar]
- Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clinical Psychology & Psychotherapy. 2011;18(5):356–365. doi: 10.1002/cpp.776. [DOI] [PubMed] [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology. 2011;120:956–961. doi: 10.1037/a0023667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Hay AC, Gross JJ. Rethinking emotion: cognitive reappraisal is an effective positive and negative emotion regulation strategy in bipolar disorder. Emotion. 2014;14:388–396. doi: 10.1037/a0035249. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. The Oxford Handbook of Event-Related Potential Components. Oxford: Oxford University Press; 2011. pp. 441–472. [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie L. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman LA, Raila H, Chiao JY, Gruber J. Positive Emotion Regulation and Psychopathology: A Transdiagnostic Cultural Neuroscience Approach. Journal of Experimental Psychopathology. 2013;4(5):502–528. doi: 10.5127/jep.030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack HA. Wechsler Test of Adult Reading: WTAR. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin. 2008;34:856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Hajcak G, Wynn JK, Green MF. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychological Medicina. 2013;43(11):2377–2391. doi: 10.1017/S0033291713000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. Journal of Abnormal Psychology. 2010;119:18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Mathis I, Miller GA, Green MF. Approach and withdrawal motivation in schizophrenia: an examination of frontal brain asymmetric activity. PLoS One. 2014;9(10):e110007. doi: 10.1371/journal.pone.0110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Gruber J, Eisner L. Emotion in Bipolar Disorder. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. pp. 123–150. [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Kahneman D, Tversky A. Choices, values, and frames. New York: Cambridge University Press; 2000. [Google Scholar]
- Karayanidis F, Nicholson R, Schall U, Meem L, Fulham R, Michie PT. Switching between univalent task-sets in schizophrenia: ERP evidence of an anticipatory task-set reconfiguration deficit. Clinical Neurophysiology. 2006;117:2172–2190. doi: 10.1016/j.clinph.2006.06.716. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. European Neuropsychopharmacology. 2014;24(5):725–736. doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annual Review of Clinical Psychology. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. Journal of Abnormal Psychology. 2011;120(1):79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: emotional arousal in threat and reward. Psychological Science. 2008;19(9):865–873. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9(4):531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- Miller NE. Comments on theoretical models illustrated by the development of a theory of conflict behavior. Journal of Personality. 1951;20:82–100. doi: 10.1111/j.1467-6494.1951.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, LaBarbara EJ, Klein C. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar adults. Bipolar Disorders. Bipolar Disorders. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Young CB, Damme KS. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behavior Research and Therapy. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll C, Laing J, Mason O. Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clinical Psychology Review. 2014;34(6):482–495. doi: 10.1016/j.cpr.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, Green MF. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2014;39(2):456–463. doi: 10.1038/npp.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Herzog E, Endrass T, Kathmann N. Brain potentials indicate poor preparation for action in schizophrenia. Psychophysiology. 2006;43:604–611. doi: 10.1111/j.1469-8986.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Saddichha S, Schuetz C. Is impulsivity in remitted bipolar disorder a stable trait? A meta-analytic review. Comprehensive Psychiatry. 2014;55(7):1479–1484. doi: 10.1016/j.comppsych.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. Neural correlates of reward processing in schizophrenia – relationship to apathy and depression. Schizophrenia Research. 2010;118:154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophrenia Bulletin. 2013;39:872–883. doi: 10.1093/schbul/sbs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak NT, Green MF, Wynn JK, Proudfit GH, Altshuler L, Horan WP. Perceived Emotional Intelligence in Schizophrenia and Bipolar Disorder: Clinical and Functional Correlates. Schizophrenia Research. 2015;162:185–195. doi: 10.1016/j.schres.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophrenia Bulletin. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disorders. 2012;14:326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Swart M, van der Velde J, Pijnenborg G, Wiersma D, Bruggeman R, Aleman A. Neural correlates of emotion regulation in patients with schizophrenia and non-affected siblings. PLoS One. 2014;9(6):e99667. doi: 10.1371/journal.pone.0099667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hilgard J, Bartholow BD, Hajcak G. Emotional Targets: Evaluative categorization as a function of context and content. International Journal of Psychophysiology. 2012;84:149–154. doi: 10.1016/j.ijpsycho.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Ferri J, Hajcak G. Interactions between Attention and Emotion: Insights from the Late Positive Potential. In: Robinson M, Watkins E, Harmon-Jones E, editors. Handbook of Cognition and Emotion. New York: Guilford Publications; 2013. pp. 35–54. [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, Green MF. Impaired anticipatory event-related potentials in schizophrenia. International Journal of Psychophysiology. 2010;77:141–149. doi: 10.1016/j.ijpsycho.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.