Abstract
Purpose
Primary Ewing sarcoma of the jaw is rare. The aim of this study was to describe new cases of primary Ewing sarcoma of the jaw and investigate reported prognostic factors of Ewing sarcoma in this series and treatment outcome.
Materials and Methods
Six patients with primary Ewing sarcoma of the jaw were treated at the Memorial Sloan Kettering Cancer Center (MSKCC) from 1992 through 2013. Clinical data, pathology reports, treatment prescribed, treatment regimens, outcome, and follow-up information were reviewed.
Results
Five of 6 patients were female and 5 cases were in the mandible. No patient presented with metastatic disease at diagnosis. All cases were positive for CD99, and 3 patients with genetic confirmation were positive for EWS-FLI1 fusion or EWSR1 gene rearrangement. All patients received induction multiagent chemotherapy and surgical resection and 2 patients received adjuvant radiotherapy. Total (grade IV) or nearly total (grade III) tumor necrosis in 3 of 5 patients (60%) assessed for histologic response to chemotherapy indicated intense sensitivity. All patients were alive and free of disease, with no history of local recurrence, at a median follow-up period of 6.5 years.
Conclusion
Patients with primary Ewing sarcoma of the jaw have a good prognosis and metastasis is an uncommon occurrence at initial presentation.
Keywords: Ewing family of tumors, Ewing sarcoma, Primary Ewing sarcoma, Jaw
Introduction
Ewing sarcoma (ES) is a malignant, small, blue, round cell tumor of uncertain origin. It is the second most common bone malignancy in children after osteosarcoma and can occur in young adults.1 The Ewing family of tumors (EFT) encompasses classic ES, atypical (large cell variant) ES, adamantinoma-like variant, extraskeletal ES, primitive neuroectodermal tumor (PNET), and Askin tumor (small, blue, round cell tumor of the thoracopulmonary region).2 These tumor types possess similar genetic (EWSR1-related fusions) and histologic features.2
ES is most common in Caucasian patients, but rare in other racial groups and has a male predilection. ES usually occurs in the long bones and pelvis.2, 3, 4 Although uncommon in the jaws,5 ES at this site is most likely to occur in the posterior mandible.6 Patients typically present with loose teeth, toothache, gingival swelling, dental abscess, jaw pain, jaw swelling, paresthesia, or fever.3, 6 Radiographically, ES usually presents as an ill-defined osteolytic lesion.6
The prognosis of ES of other anatomic sites depends strongly on the stage and presence of metastases, with one fourth of patients having metastases at initial presentation.7, 8 Five-year survival for localized disease ranges from 65 to 75%, whereas patients with metastatic disease have a 5-year survival rate lower than 30%.8 Other prognostic factors include patient’s age, tumor size, anatomic site, and histologic response after chemotherapy.9, 10, 11 This report describes 6 cases of primary ES of the jaw, detailing the rate of metastases at initial diagnosis, survival rate, and other prognostic factors in ES.
Materials and Methods
This retrospective study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSKCC [New York, NY]). From 1992 through 2013, 6 cases of primary EFT of the jaw were treated at this institution. The following clinical data were reviewed: gender, age at diagnosis, race, anatomic site, tumor size, initial clinical presentation, radiographic findings, stage and presence of metastasis at time of diagnosis, pathology reports (immunohistochemical, cytogenetic results, status of resection margins, and histologic response to chemotherapy),10 treatment (initial chemotherapy, radiation therapy, and surgical resection), outcome, and follow-up information.
All patients underwent the following evaluation for metastatic disease: bone marrow biopsy examination, computed tomography of the chest, and whole-body technetium bone scanning. MSKCC’s ES treatment guidelines include the use of standard multiagent induction chemotherapy medications, such as vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide, to decrease tumor size and prevent the development of new and metastatic tumors. Then, surgery is performed to remove the tumor. Depending on where the tumor is located, radiation therapy might be used. Patients with high risk of recurrence are often placed on irinotecan and temozolomide medications.12
Overall survival (OS) and local control (LC) were calculated. OS was calculated from the date of diagnosis to the date of death of any cause. LC was defined as any local recurrence or metastases of disease after therapy was instituted.
Results
Six patients (5 female and 1 male; age range, 4 to 20 yr; median age, 8 yr) were diagnosed with primary ES of the jaw. All patients were Caucasian. Five cases involved the mandible (4 in the posterior region and 1 in the anterior region) and 1 case involved the maxilla. Tumor size ranged from 2.5 to 5.3 cm. No patient had metastatic disease at diagnosis. Patients presented with symptoms ranging from tooth pain, nonresolving jaw pain, gingival swelling, jaw swelling, loose teeth, nasal congestion, and protruding mass. Radiographically, ES presented as expansile osteolytic lesions in 5 cases (Figure 1, Figure 2, Figure 3) and as an odontogenic cyst in 1 case. A summary of clinical features of patients with primary ES of the jaw is presented in Table 1.
Figure 1.
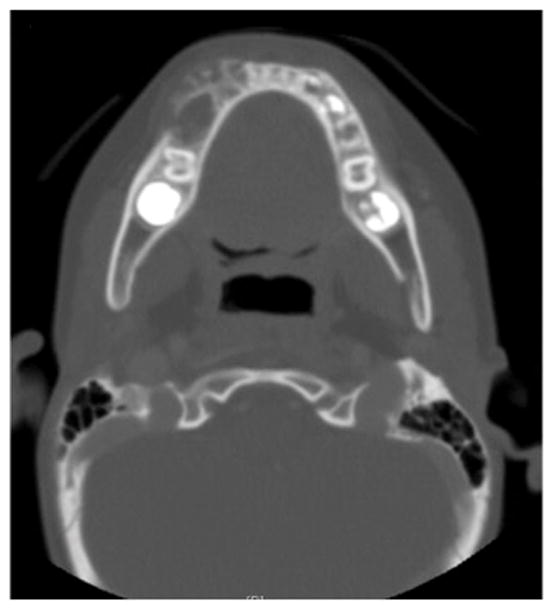
Axial view of computed tomography scan shows an expansile osteolytic lesion associated with cortical disruption, Case 4
Figure 2.

Axial view of computed tomography scan shows an expansile osteolytic lesion involving the anterior mandible, Case 5
Figure 3.

Axial view of computed tomography scan shows an expansile osteolytic lesion of the maxilla, Case 6
Table 1.
Clinical features of patients with primary Ewing sarcoma of the jaw
| Case | Sex | Age | Site | Size of lesion (cm) | Clinical presentation | Radiographic presentation | Metastasis at time of diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | F | 4 | Mandible | 2.5 x 2.0 | Gum swelling followed by a hard swelling in the jaw | Cyst identified on panoramic radiograph | No |
| 2 | F | 12 | Mandible angle | N/A | Loose tooth | Expansile osteolytic lesion with permeation of inner cortex | No |
| 3 | F | 8 | Mandible angle | 2.8 x 1.7 | Non-resolving jaw pain initially diagnosed as dental abscess; later accompanied by high fevers | Osteolytic lesion with cortical breakthrough | No |
| 4 | F | 8 | Mandible | 5.3 x 3.3 x 3.2 | Facial asymmetry after biting down on candy and hearing a ‘pop’ | Expansile osteolytic lesion associated with cortical disruption | No |
| 5 | M | 5 | Anterior mandible | 5.2 x 4.4 x 3.1 | Loose teeth with difficulty chewing followed by jaw swelling | Imaging showed an expansile osteolytic lesion | No |
| 6 | F | 20 | Maxilla | 3.5 x 2.7 x 2.2 | Nasal congestion followed by loose teeth. On examination there was a large soft tissue mass protruding from the palate | Imaging revealed an expansile osteolytic lesion | No |
N/A – Not available
Histopathologically, all tumors had typical morphologic features composed of small, blue, round cells (Fig 4); 4 cases were classified as classic ES and 2 were classified as PNET. Immunohistochemically, all tumors were positive for CD99 (Fig 5), and 3 cases tested also were positive for friend leukemia integration-1 transcription factor (FLI1). The 2 PNET cases were positive for neuron-specific enolase and synaptophysin. Three cases tested were confirmed cytogenetically, with 2 cases showing EWSR1 gene rearrangements by fluorescence in situ hybridization (FISH; Fig 6), and reverse transcriptase polymerase chain reaction (PCR) detected the presence of EWSR1-FLI1 fusion in 1 case. The 3 remaining cases could not be genetically confirmed because of decalcified tissue sections in the archive that impaired FISH analysis. Results of immunohistochemical and genetic tests are presented in Table 2. In all patients, resection margins were negative for tumor. The histologic response to chemotherapy was assessed in 5 cases; 1 had a complete response with no viable tumor identified (grade IV response), 2 cases had less than 10% of viable tumor (grade III response), and 2 cases had less than 50% of tumor necrosis (grade I response). All patients received induction multidrug chemotherapy, 2 patients subsequently received radiation therapy at the authors’ institution at 45 and 50.4 Gy, and all patients had surgery with reconstruction for all mandibular resections. Follow-up period for all patients ranged from 7 months to 22 years (median, 6.5 yr). All patients were alive and free of disease at the last follow-up visit; the OS and LC rates were 100%. A summary of treatments and outcomes of patients with primary ES of the jaw is presented in Table 3.
Figure 4.

Photomicrograph H&E (x200) shows solid growth of undifferentiated small blue round cells with ill-defined cell borders, Case 5
Figure 5.
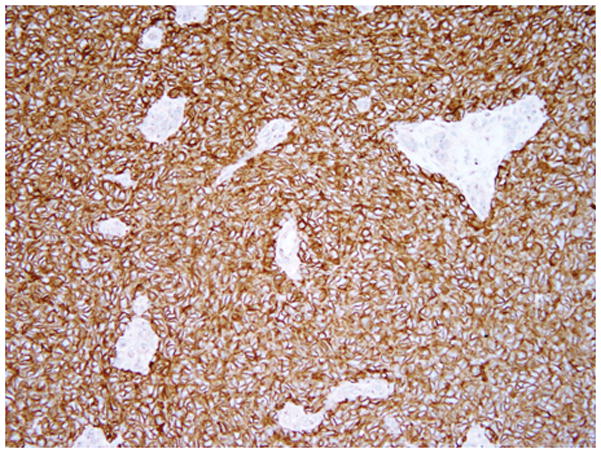
Photomicrograph CD99 (x200) shows diffuse and strong membranous staining (note the internal negative control, blood vessels are negative), Case 5
Figure 6.

The arrow shows the split EWSR1 signals, red, centromeric and green, telomeric. In contrast the uninvolved EWSR1 allele shows a yellow signal (resulting from the fused red and green probes), Case 5
Table 2.
Results of immunohistochemical and genetic test
| Case | Positive immunohistochemical stains | Negative immunohistochemical stains | Genetic confirmation |
|---|---|---|---|
| 1 | CD99 | N/A | ND |
| 2 | CD99, NSE, vimentin | Desmin | ND |
| 3 | CD99, FLI1, vimentin, synaptophysin | NSE, S100, desmin, myogenin | ND |
| 4 | CD99, vimentin | CK7, CK20, AE1/3, LCA, S100, TTF-1, desmin, CD56, chromogranin | EWS-FLI1 fusion by PCR |
| 5 | CD99, FLI1 | CK7, CK20, AE1/3, LCA, synaptophysin, chromogranin, CD56, S100, actin, desmin, EMA, WT1 | EWSR1 rearrangement by FISH |
| 6 | CD99, FLI1 | CD3, TdT | EWSR1 rearrangement by FISH |
NSE – neuron specific enolase, CK – cytokeratin, LCA – leukocyte common antigen, TTF-1 – thyroid transcription factor – 1, TdT – terminal deoxynucleotidyl transferase, EMA – epithelial membrane antigen, WT1 – Wilms tumor 1, N/A – not applicable, ND – not done
Table 3.
Treatment and outcome of patients with primary Ewing sarcoma of the jaw
| Case | Induction chemotherapy | Surgical resection | Radiation therapy (Gy) | Outcome, follow-up time |
|---|---|---|---|---|
| 1 | Multidrug therapy | Mandibulectomy with fibula free flap reconstruction | ND | NED, 22 years |
| 2 | Multidrug therapy | Mandibulectomy with fibula free flap reconstruction and condyle transplant | ND | NED, 7 months (patient returned to native country) |
| 3 | Multidrug therapy | Mandibulectomy with fibula free flap reconstruction | 50.4 | NED, 15 years |
| 4 | Multidrug therapy | Mandibulectomy with fibula free flap reconstruction | ND | NED, 10 years |
| 5 | Multidrug therapy | Mandibulectomy with fibula free flap reconstruction | ND | NED, 2 years |
| 6 | Multidrug therapy | Maxillectomy | 45 | NED, 3 years |
ND – Not done, NED – No evidence of disease
Discussion
Primary ES of the jaw is a relatively uncommon diagnosis. In 1987, the Intergroup Ewing Sarcoma Study reported 10 cases involving the jaw,5 and Allam et al13 reported 15 of 259 cases of primary ES of the jaw during a 22-year period. Qureshi et al14 also identified 26 cases of primary ES of the jaw in their institutional database. A 40-year retrospective study from the University of Florida identified only 3 primary cases involving the jaws.15 In the authors’ institution, primary ES of the jaw accounted for 1.09% (6 of 549) of the total number of ES treated during the same period (1992 through 2013). Further evidence of this condition’s rarity is found in a recent literature review by Stewart et al16 who identified only 11 cases of primary ES of the jaw with genetic confirmation. In the present study, the 3 tested cases were positive for EWSR1-FLI1 fusion or EWSR1 gene rearrangement.
ES of the jaw can present innocuously as a dental abscess or gum or periodontal disease.17, 18, 19 Two of the present cases had a similar presentation: 1 manifested as a dental cyst on panoramic radiograph and another case was initially considered a dental abscess refractory to treatment with medications. In the present series, 5 of 6 patients were female despite the aforementioned male predilection of ES of the jaw.6, 13, 20 In 1 case, ES was located in the anterior mandible, a rare site of occurrence.6, 21
Lymphoblastic lymphoma (especially T cell), rhabdomyosarcoma (solid alveolar type), neuroblastoma, small cell carcinoma, and small cell osteosarcoma share similar morphologic features of malignant, small, round, blue cells with ES, hence the need to differentiate them further immunophenotypically. CD99 and FLI1 are the main immunohistochemical stains used in the identification of ES. However, it is important to note that these stains are not specific for ES. CD99 can be expressed in lymphoblastic lymphoma, rhabdomyosarcoma, small cell carcinoma, and small cell osteosarcoma, whereas FLI1 also can be expressed in lymphoblastic lymphoma and neuroblastoma.22, 23, 24, 25 The adamantinoma-like variant of ES is positive for cytokeratins and has a morphologic appearance of palisaded peripheral cells with nuclear polarization.26, 27 Neuroendocrine markers, such as neuron-specific enolase, S100, and synaptophysin, also show positivity in EFT, particularly the PNET variant.28 The PNET variant usually presents with rosettes (Homer Wright) morphologically. Therefore, identification of EWSR1 rearrangement by FISH or PCR is crucial, but also not specific for ES. The t(11;22)(q24;q12) resulting in EWSR1-FLI1 and t(21;22)(q22;q12) resulting in EWSR1-ERG are detected in 85 and 10% of ES cases, respectively.2 Other rare gene fusions involving EWSR1 fused to ETV1, ETV4, FEV, NFATc2, POU5F1, SMARCA5, PATZ1, or SP3 also have been described.29, 30, 31, 32, 33 EWSR1 gene rearrangement also can be seen in extraskeletal myxoid chondrosarcoma, clear cell sarcoma, hyalinizing clear cell carcinoma, clear cell odontogenic carcinoma, desmoplastic small round cell tumor, and myxoid liposarcoma.34, 35, 36, 37, 38, 39 Thus, corroborating morphologic, immunophenotypic, and genetic features is required for a definitive ES diagnosis.
ES of other anatomic sites has a strong tendency to metastasize, with 25% of cases having metastatic disease at initial presentation.7, 8 Patients with ES and large tumor, older age, pelvic tumor, and poor histopathologic response after induction chemotherapy have a poor outcome.9, 10, 11 Similar to the present report, other series of primary ES of the jaws did not report the presence of metastases at initial presentation.13, 14, 15, 20 In the present study, tumors were smaller than 5.5 cm, in contrast to the study by Allam et al13 in which most tumors were larger than 10 cm and had a lower 5-year OS rate. The rate of intense sensitivity to chemotherapy and negative resection margins in the present patients might have contributed to the high OS rate in this series.
The 5-year OS reported by Qureshi et al14 for ES of the jaw was 87.5% and those reported by Allam et al13 for the mandible and maxilla were 80 and 71%, respectively. Whaley et al15 reported that of 3 patients with jaw involvement, only 1 died of disease at 1.8 years. In the present study, all patients were alive and free of disease at a median follow-up period of 6.5 years, with no history of local recurrence. Two limitations of this study are the small sample and the experience of 1 institution.
In summary, our study shows that ES of the jaw has a better prognosis than ES in other anatomic sites and metastasis is an uncommon occurrence at initial presentation. This can be attributed to early detection of tumor because of ease of visibility and good surgical access. Patients with ES of the jaw should be diagnosed promptly and accurately, so their disease can be managed appropriately.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Alava E, Lessnick LS, Soreson PH. WHO classification of tumours of soft tissue and bone. 4. Lyon: International Agency for Research on Cancer; 2013. Ewing sarcoma; p. 306. [Google Scholar]
- 3.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20:412. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 4.Khoury JD. Ewing sarcoma family of tumors. Adv Anat Pathol. 2005;12:212. doi: 10.1097/01.pap.0000175114.55541.52. [DOI] [PubMed] [Google Scholar]
- 5.Siegal GP, Oliver WR, Reinus WR, et al. Primary Ewing’s sarcoma involving the bones of the head and neck. Cancer. 1987;60:2829. doi: 10.1002/1097-0142(19871201)60:11<2829::aid-cncr2820601139>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Wood RE, Nortje CJ, Hesseling P, Grotepass F. Ewing’s tumor of the jaw. Oral Surg Oral Med Oral Pathol. 1990;69:120. doi: 10.1016/0030-4220(90)90280-6. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med Pediatr Oncol. 2003;40:276. doi: 10.1002/mpo.10240. [DOI] [PubMed] [Google Scholar]
- 8.Gorlick R, Janeway K, Lessnick S, et al. Children’s Oncology Group’s 2013 blueprint for research: bone tumors. Pediatr Blood Cancer. 2013;60:1009. doi: 10.1002/pbc.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Wunder JS, Paulian G, Huvos AG, et al. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80:1020. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 12.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 13.Allam A, El-Husseiny G, Khafaga Y, et al. Ewing’s Sarcoma of the Head and Neck: A Retrospective Analysis of 24 Cases. Sarcoma. 1999;3:11. doi: 10.1080/13577149977811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi SS, Kembhavi S, Bhagat M, et al. Primary non-metastatic Ewing sarcoma of the jaw in children: results of surgical resection and primary reconstruction. J Surg Oncol. 2014;110:689. doi: 10.1002/jso.23698. [DOI] [PubMed] [Google Scholar]
- 15.Whaley JT, Indelicato DJ, Morris CG, et al. Ewing tumors of the head and neck. Am J Clin Oncol. 2010;33:321. doi: 10.1097/COC.0b013e3181aaca71. [DOI] [PubMed] [Google Scholar]
- 16.Stewart BD, Reith JD, Knapik JA, Chi AC. Bone- and cartilage-forming tumors and ewing sarcoma: an update with a gnathic emphasis. Head Neck Pathol. 2014;8:454. doi: 10.1007/s12105-014-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosau M, Baumhoer D, Ihrler S, Kleinheinz J, Driemel O. Ewing sarcoma of the mandible mimicking an odontogenic abscess - a case report. Head Face Med. 2008;4:24. doi: 10.1186/1746-160X-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keshani F, Jahanshahi G, Attar BM, et al. Ewing’s sarcoma in mandibular similar to dental abscess. Adv Biomed Res. 2014;3:62. doi: 10.4103/2277-9175.125841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao BH, Rai G, Hassan S, Nadaf A. Ewing’s sarcoma of the mandible. Natl J Maxillofac Surg. 2011;2:184. doi: 10.4103/0975-5950.94479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakhshi S, Pathania S, Mohanti BK, Thulkar S, Thakar A. Therapy and outcome of primitive neuroectodermal tumor of the jaw. Pediatr Blood Cancer. 2011;56:477. doi: 10.1002/pbc.22615. [DOI] [PubMed] [Google Scholar]
- 21.Ko E, Brouns ER, Korones DN, et al. Primary Ewing sarcoma of the anterior mandible localized to the midline. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e46. doi: 10.1016/j.oooo.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemirli M, Fanburg-Smith JC, Hartmann DP, Azumi N, Miettinen M. Differentiating lymphoblastic lymphoma and Ewing’s sarcoma: lymphocyte markers and gene rearrangement. Mod Pathol. 2001;14:1175. doi: 10.1038/modpathol.3880455. [DOI] [PubMed] [Google Scholar]
- 23.Lumadue JA, Askin FB, Perlman EJ. MIC2 analysis of small cell carcinoma. Am J Clin Pathol. 1994;102:692. doi: 10.1093/ajcp/102.5.692. [DOI] [PubMed] [Google Scholar]
- 24.Folpe AL, Hill CE, Parham DM, O’Shea PA, Weiss SW. Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000;24:1657. doi: 10.1097/00000478-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Devaney K, Abbondanzo SL, Shekitka KM, Wolov RB, Sweet DE. MIC2 detection in tumors of bone and adjacent soft tissues. Clin Orthop Relat Res. 1995;310:176. [PubMed] [Google Scholar]
- 26.Folpe AL, Goldblum JR, Rubin BP, et al. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025. [PubMed] [Google Scholar]
- 27.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing Family Tumors of the Head and Neck: A Pitfall in the Differential Diagnosis of Basaloid and Myoepithelial Carcinomas. Am J Surg Pathol. 2015;39:1267. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter RL, al-Sams SZ, Corbett RP, Clinton S. A comparative study of immunohistochemical staining for neuron-specific enolase, protein gene product 9.5 and S-100 protein in neuroblastoma, Ewing’s sarcoma and other round cell tumours in children. Histopathology. 1990;16:461. doi: 10.1111/j.1365-2559.1990.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Bhargava R, Zheng T, et al. Undifferentiated small round cell sarcomas with rare EWS gene fusions: identification of a novel EWS-SP3 fusion and of additional cases with the EWS-ETV1 and EWS-FEV fusions. J Mol Diagn. 2007;9:498. doi: 10.2353/jmoldx.2007.070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szuhai K, Ijszenga M, de Jong D, et al. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009;15:2259. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi S, Yamazaki Y, Ishikawa Y, et al. EWSR1 is fused to POU5F1 in a bone tumor with translocation t(6;22)(p21;q12) Genes Chromosomes Cancer. 2005;43:217. doi: 10.1002/gcc.20171. [DOI] [PubMed] [Google Scholar]
- 32.Sumegi J, Nishio J, Nelson M, et al. A novel t(4;22)(q31;q12) produces an EWSR1-SMARCA5 fusion in extraskeletal Ewing sarcoma/primitive neuroectodermal tumor. Mod Pathol. 2011;24:333. doi: 10.1038/modpathol.2010.201. [DOI] [PubMed] [Google Scholar]
- 33.Mastrangelo T, Modena P, Tornielli S, et al. A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene. 2000;19:3799. doi: 10.1038/sj.onc.1203762. [DOI] [PubMed] [Google Scholar]
- 34.Stenman G, Andersson H, Mandahl N, Meis-Kindblom JM, Kindblom LG. Translocation t(9;22)(q22;q12) is a primary cytogenetic abnormality in extraskeletal myxoid chondrosarcoma. Int J Cancer. 1995;62:398. doi: 10.1002/ijc.2910620407. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol. 1992;16:411. doi: 10.1097/00000478-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Bilodeau EA, Weinreb I, Antonescu CR, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37:1001. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 37.Albergotti WG, Bilodeau EA, Byrd JK, et al. Hyalinizing clear cell carcinoma of the head and neck: Case series and update. Head Neck. 2014 doi: 10.1002/hed.23902. [DOI] [PubMed] [Google Scholar]
- 38.Kraft S, Antonescu CR, Rosenberg AE, Deschler DG, Nielsen GP. Primary clear cell sarcoma of the tongue. Arch Pathol Lab Med. 2013;137:1680. doi: 10.5858/arpa.2012-0467-CR. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Matsui Y, Higashimoto M, et al. Myxoid liposarcoma-associated EWSR1-DDIT3 selectively represses osteoblastic and chondrocytic transcription in multipotent mesenchymal cells. PLoS One. 2012;7:e36682. doi: 10.1371/journal.pone.0036682. [DOI] [PMC free article] [PubMed] [Google Scholar]


