Abstract
Deregulation of developmental signaling pathways such as Wnt/b-catenin and NOTCH are commonly observed in different cancers. A normal wnt pathway is essential for development and tissue homeostasis to preserve a normal balance between the differentiation and proliferation. PYGO2 is the main transcription factor of wnt pathway, while Msi1 is one of the wnt inhibitors. In this study we assessed the correlation between Msi1 and PYGO2 mRNA expression using Real time polymerase chain reaction in 48 esophageal squamous cell carcinoma (ESCC) patients. Although, there was not any significant correlation between the levels of Msi1 and PYGO2 mRNA expression, we observed a significant correlation between the Msi1 and PYGO2 overexpressed cases and depth of tumor invasion (p = 0.05). In conclusion, despite the role of these markers in tumor depth of invasion there is not any feedback between Msi1 and PYGO2 gene expression in ESCC.
Keywords: Wnt pathway, NOTCH pathway, Cancer stem cell, Tumor, Translation, Suppressor, Metastasis, Expressional analysis
Introduction
Esophageal squamous cell carcinoma (ESCC) is the 5th and second-leading cause of cancer-related deaths in the world and northeast of Iran, respectively (Ferlay et al. 2010; Gholamin et al. 2009). Although multimodalitic treatments are progressed in recent years, patients have poor prognosis with a 5-year survival rate of <42 % (Rice et al. 2009). Mostly, such tumors are diagnosed at advanced stages of tumor cell progression (stage III) and therefore the majority of patients show distant metastasis of tumor cells through the body making it not suitable for the esophagectomy (Headrick et al. 2002). Although, it is believed that different factors including the lifestyle and accumulation of genetic mutations are involved in ESCC progression, the cancer stem cell hypothesis is also under debate as the hallmark of ESCC biology, in which a small subpopulation of stem like cells with self renewal capacity are in charge of tumor progression and metastasis (Ben-Porath et al. 2008; Zhang 2013).
Deregulation of developmental pathways such as Wnt/b-catenin, NOTCH and Sonic Hedgehog are commonly observed in different cancers (Moghbeli et al. 2013a, b, 2014a, b). A controlled Wnt pathway is essential for development and tissue homeostasis to preserve a normal balance between cell differentiation and proliferation. Canonical Wnt/b-catenin pathway is activated through a complicated process in which, cytoplasmic b-catenin remains unphosphorylated and its nucleus accumulation activates the LEF/TCF/PYGO2 complex. In this cascade, PYGO2 upregulates the Wnt target genes through recruitment of b-catenin/BCL9 to their promoters (Townsley et al. 2004). It has been shown that the PYGO2 overexpression is correlated with the ovarian (Popadiuk et al. 2006) and breast cancers (Andrews et al. 2007) tumorigenesis. Therefore, it would be expected to see a factor in normal cells which is in charge of its inactivation.
It has been demonstrated that Numb and Dickkopf3 act as suppressors of Notch (Guo et al. 1996) and Wnt signaling pathways (Glazer et al. 2008; Wang et al. 2008). respectively. However, Musashi1 (Msi1) in cancer stem cells is able to suppress such suppressors leading to activation of NOTCH and Wnt pathways. Msi1 as an important RNA-binding protein (RBP), exerts this regulatory role through binding to a specific sequence in 3′ UTR of target mRNAs leading to translational inhibition (Battelli et al. 2006; Imai et al. 2001). In this process, Msi1 binds to the poly A binding protein (PABP) as an antagonist of the eukaryotic translation initiation factor 4G (eIF4G) resulting in inhibition of 80S ribosome subunit assembly (Kawahara et al. 2008). Besides the signalling pathways which are targets for the Msi1, p21WAF-1 as a cell cycle regulator and wnt inhibitor is also one of the main targets of Msi1 (Okano et al. 2005). Msi1 expression has been studied in different cancers (Gotte et al. 2008; Schulenburg et al. 2007; Shu et al. 2002; Toda et al. 2001) and we have also reported the role of Msi1 and PYGO2 in ESCC progression previously (Moghbeli et al. 2013a, b, 2014a, b). Having considered the importance of Wnt pathway in ESCC progression, we aimed to assess the probable correlation between PYGO2 and Msi1 in ESCC tumorigenesisas well as their probable mutual correlation with the clinicopathological features of the patients.
Materials and methods
Tissue samples
This study was done enrolling fourty eight ESCC patints who were undergone the esophagectomy at Omid, Qaem, and Imam Reza Hospitals of Mashhad University of Medical Sciences. Tumor and margin normal tissues were transferred to the RNA later solution (Qiagen, Hilden, Germany) following the mincing after surgery and stored at −20 C until the mRNA extraction. Histopathologic examination in all of cases was performed to verify that all of the tumor tissues have at least 70 % tumor cells Wittekind and Oberschmid (2010). The patients filled and confirmed the informed consent forms which were approved by the ethic committee of Mashhad University of Medical Sciences (No. 921202).
RNA extraction, cDNA synthesis, and quantitative RT-PCR
RNA extraction from fresh tumor/margin-normal tissues and cDNA synthesis (oligo-dT method) were performed using the RNAeasy Mini kit (Qiagen, Hilden, Germany) and First-Strand Synthesis kit (Fermentas, Lithuania), respectively, as described before (Moghbeli et al. 2013a, b, 2014a, b). Comparative relative Real Time PCR was done using SYBR Green method (GENETBIO, Korea) and Stratagene Mx-3000P real-time thermocycler (Stratagene, La Jolla, CA) to assess the level of Msi1 and PYGO2 mRNA expression (the primer sequences are presented previously (Moghbeli et al. 2013a, b, 2014a, b)). All the reactions were performed in triplicates and the thermal profile included an initial denaturation step of 95 C for 10 min followed by 45 cycles at 94 C (30 s), 62 C (30 s) and 72 C (30 s). To normalize the data we used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a normalizer (Moghbeli et al. 2013a, b, 2014a, b). More than two fold decrease and more than two fold increase in the level of mRNA expression in tumor versus normal tissues were considered as under and overexpression, respectively, wherease the fold changes between −2 and +2 was considered as normal expression.
Statistical analysis
Spearman’s q and Pearson v-squared tests were used to assess the statistical correlations between Msi1 and PYGO2 expression. Moreover, probable correlations between the clinicopathological futures of tumors and levels of PYGO2/Msi1 mRNA expressions were evaluated through the ANOVA and t test. A P value < 0.05 was considered to be statistically significant. All the statistical analyzis were done using SPSS 16.0 (SPSS, Chicago, IL).
Results
Study population
Fourty eight ESCC patients were recruited in this study comprising the 20 (41.7 %) females and 28 (58.3 %) males. All the cases were examined histologically to find the percentage of tumor cells in every tumor bulk, and all the tumors which had less than 70 % of tumor cells were excluded from the study. Moreover, lack of receiving chemo-radio therapeutic treatments was also considered as prerequisites of tissue recruitment. Age range of patients was between 30 and 83 years with mean age of (61.85 ± 12.33 years). The range of tumor sizes was between 1.5 and 12 cm with a mean size of 4.23 ± 1.91 cm. Most of tumors (27/48, 56.2 %) were located in middle esophagous while the other cases were in lower part. Twenty two of 48 (45.8 %) patients had invasion of tumor cells to the proximal lymph nodes. The majority of cases (31/48, 64.5 %) and (40/48, 83.3 %) were moderately differentiated and T3 tumor depth of invasion to adventitia, respectively. Furthermore, despite one sample which was in pathological tumor stage of I, all of tumors were in stages of II/III. Clinicopathological features of patients are mentioned in Table 1.
Table 1.
Correlation between level of PYGO2/Msi1 mRNA expression and clinicopathological features of ESCC patients
| Total | Msi1 overexpression | PYGO2 overexpression | Msi1/PYGO2 overexpression | P Value | |
|---|---|---|---|---|---|
| Patients | 48 | 20(41.7 %) | 17(35.4 %) | 5(10.4 %) | |
| Mean age (mean ± SD) | 61.85 ± 12.33 | 58.75 ± 3.19 | 63.41 ± 2.49 | 57.20 ± 3.22 | |
| Size (mean ± SD) | 4.23 ± 1.91 | 4.25 ± 0.51 | 3.49 ± 0.40 | 2.90 ± 1.29 | |
| Sex | 0.696 | ||||
| Male | 28(58.3 %) | 13(65 %) | 11(64.7 %) | 4(80 %) | |
| Female | 20(41.7 %) | 7(35 %) | 6(35.3 %) | 1(20 %) | |
| Location | 0.704 | ||||
| Lower | 21(43.8 %) | 8(40 %) | 9(52.9 %) | 2(40 %) | |
| Middle | 27(56.3 %) | 12(60 %) | 8(47.1 %) | 3(60 %) | |
| Grade | 0.747 | ||||
| P.D | 8(16.7 %) | 3(15 %) | 4(23.5 %) | 1(20 %) | |
| M.D | 31(64.6 %) | 13(65 %) | 10(58.8 %) | 4(80 %) | |
| W.D | 9(18.8 %) | 4(20 %) | 3(17.6 %) | - | |
| Lymph node | 0.672 | ||||
| Yes | 22(45.8 %) | 8(40 %) | 7(41.2 %) | 1(20 %) | |
| No | 26(54.2 %) | 12(60 %) | 10(58.8 %) | 4(80 %) | |
| Stage | 0.711 | ||||
| I/II | 29(60.4 %) | 13(65 %) | 10(58.8 %) | 4(80 %) | |
| III/IV | 19(39.6 %) | 7(35 %) | 7(41.2 %) | 1(20 %) | |
| Depth of tumor invasion (T) | 0.050 | ||||
| T1 | 1(2.1 %) | 1(5 %) | 1(5.9 %) | 1(20 %) | |
| T2 | 7(14.6 %) | 1(5 %) | 2(11.8 %) | - | |
| T3 | 40(83.3 %) | 18(90 %) | 14(82.4 %) | 4(80 %) |
Levels of Msi1/PYGO2 mRNA expression in ESCC patients
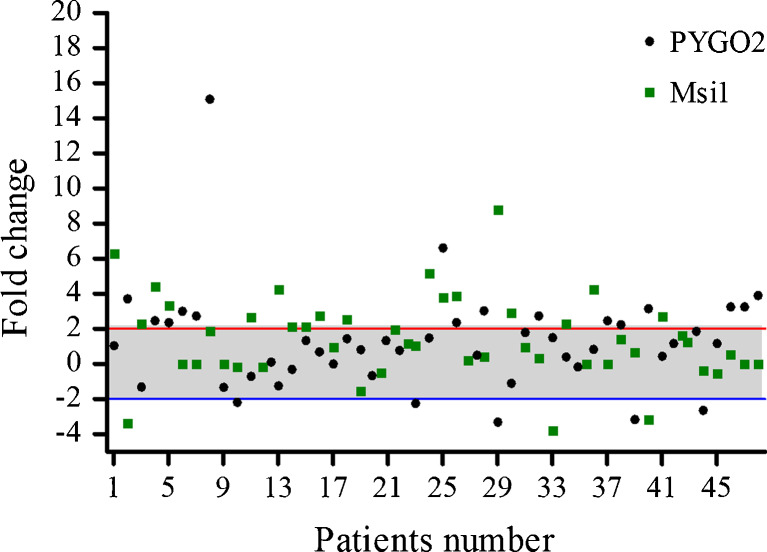
Msi1 and PYGO2 mRNA expressions were assessed in the tumor tissues in comparison with their corresponding normal tissues using the comparative relative real time PCR. Seventeen out of 48 cases (35.4 %) and 20 out of 48 samples (41.7 %) had PYGO2 and Msi1 overexpression, respectively. Total range of fold changes were between −3.8 and 8.8 (mean ± SD: 1.41 ± 2.35 fold changes) for the Msi1, and −3.31 to 15.10 (mean ± SD: 1.27 ± 2.86 fold changes) for the PYGO2 (Moghbeli et al. 2013a, b, 2014a, b). Scatter plot illustrates the levels of PYGO2 and Msi1 mRNA expression via the fold changes (Fig.1). The level of PYGO2 mRNA expression was meaningfully higher in the Msi1 and PYGO2 positive cases in comparison with the only PYGO2 overexpressed tumors (5.78 ± 2.47 vs. 2.94 ± 0.17, fold change), showing that despite the lack of a significant correlation between these markers, Msi1 may be an inducer for the PYGO2 expression. Furthermore, as the Fig.1 shows, an outlayer fold change of 15.10 was detected in one samplewith Msi1 overexpression.
Fig. 1.
Scatter plot represent descriptive analysis of relative gene expression of Msi1 and PYGO2 in ESCC patients. The red and blue lines mention to the thresholds for the over and under expression, respectively. The grey area shows the cases with normal Msi1 and PYGO2 mRNA expression
Clinicopathological features and PYGO2 and Msi1 mRNA expression
A significant correlation was observed between levels of Msi1 mRNA expression and sex (p = 0.05), in which levels of Msi1 expression in males were higher than females with mean fold of 2.00 vs 0.78 (Moghbeli et al. 2013a, b, 2014a, b). In this report, twenty seven out of 48 (56.3 %) cases had shown over expression in at least one of these markers whereas, 16/48 (33.3 %) patients had normal expression in both of these markers. There was not any significant correlation between the levels of PYGO2 and Msi1 mRNA expression (p = 0.202). Only 5/48 (10.4 %) patients had concomitant overexpression of PYGO2 and Msi1. Although there was not any significant correlation between Msi1 and PYGO2 overexpressed tumors and grade of tumor, the majority of these cases (4/5, 80 %) were moderately differentiated (p = 0.747). Msi1 and PYGO2 ovrexpressed tumors were scattered almost equally in lower and middle part of esophagous. Most of such cases were in T3 tumor depth of invasion and without any lymph node metastasis (4/5, 80 %). Levels of PYGO2 mRNA expression in PYGO2 and Msi1 overexpressed cases in T3 tumor depth of invasion was significantly higher than Msi1 overexpressed cases in T3 (6.61 ± 3.00 vs 3.23 ± 0.46, fold change) (p = 0.050). Most of PYGO2 and Msi1 overexpressed cases were in pathological tumor stages of I/II (4/5, 80 %). In the case of sex, four out of 5 cases with PYGO2 and Msi1 overexpression were males. The PYGO2 and Msi1 overexpressed patients had the lowest mean age (mean ± SD: 57.20 ± 3.22 years old), which is meaningfully lower than cases with normal expression of these markers and also the total mean age. Moreover, the Msi1 and PYGO2 over expressed tumors were meaningfully smaller than the other cases with mean size of (mean ± SD: 2.90 ± 0.58 cm). Generally, patients with Msi1 overexpression had lower ages in comparison with the patients with PYGO2 overexpression (mean ± SD: 59.27 ± 4.17 vs. 66.00 ± 3.03 years old). In the case of tumor sizes also Msi1 overexpressed tumors were bigger than PYGO2 overexpressed cases (mean ± SD: 4.70 ± 0.61 vs. 3.73 ± 0.51 cm). Among the patients, two cases with Msi1 underexpression and PYGO2 overexpression was seen. Besides, there was also a case with Msi1 overexpression and PYGO2 underexpression. In the case of tumor grade, there were different aspects among the levels of Msi1 and PYGO2 expression and levels of tumor differentiation in which levels of PYGO2 mRNA expression was higher in poorly differentiated tumors in comparison with the well differentiated ones (mean ± SD: 2.40 ± 0.80 vs. 0.70 ± 0.81, fold change). In contrast with the PYGO2, poorly differentiated tumors had lower levels of Msi1 mRNA expression in comparison with the well differentiated tumors (mean ± SD: -0.22 ± 1.05 vs. 2.41 ± 1.06, fold change). Levels of Msi1 and PYGO2 mRNA expression in T3 tumor tissues were meaningfully higher than samples with T2 tumor depth of invasion (1.48 ± 0.39 vs. 0.55 ± 0.49) and (1.42 ± 0.48 vs. 0.24 ± 0.72).
Discussion
There are complex correlations among different factors which are involved in ESCC tumorigenesis. As we have shown recently, there was a positive correlation between the PYGO2 as a wnt pathway transcription factor and EGFR as a wnt target gene growth factor receptor, in ESCC progression. In this positive feedback EGFR was one of the targets for the PYGO2 and it acts as a stimulator for the PYGO2 function through the stabilization of cytoplasmic b-catenin (Moghbeli et al. 2013a, b, 2014a, b). Regarding this fact that the Msi1 is activator of the wnt and NOTCH signaling pathways through the suppression of related inhibitors such as DKK3 and Numb, we expected to have a probable feedback also between the Msi1 and PYGO2. Therefore, to explore this hypothesis that whether the Msi1 is one of the targets for the PYGO2 in wnt signaling, we assessed the levels of PYGO2 and Msi1 mRNA expression in ESCC patients for the first time. Although we expected to see a positive correlation between these factors, only five cases showed over expression of these markers. The results demonstrated that there is not any significant correlation between these markers. However, our data showed that the level of PYGO2 is interestingly higher in the PYGO2 and Msi1 cases, referring the probable positive role of Msi1 in PYGO2 expression. Considering the strict association between signaling pathways in tumorigenesis process, it would be expcted that the Msi1 is activated through the other pathways. Although the Msi1 is one of the main regulators of wnt pathway, it seems that its regulation is not depending on the wnt signaling pathway in ESCC. Therefore, in a higher point of view we may conclude that among different developmental pathways such as Wnt, NOTH and Shh, which are directly related to the tumor progression and metastasis, there is a grading seanrio in which some pathways are placed in higher grades regarding their regulatory importance to regulate other pathways. Despite this fact that accurate mechanisms of Msi1 regulation is unclear, it was shown that Msi1 is one of the NOTCH pathway target genes (Pasto et al. 2014). Regarding our recent study about the positive role of PYGO2 in ESCC progression and metastasis, we expected to see the Msi1 and PYGO2 overexpressesd tumors among the higher stage cases, while, most of them (4/5, 80 %) were in tumor stages of I/II. Generally, considering this fact that ESCC initiation and progression is a multistep process in which in every step different factors and pathways are involved, it would be expected that Msi1 activation is important in ESCC progression in primary stages of tumor. Levels of Msi1 mRNA expression in tumors with the lower stages were greater than tumors with higher stages (1.66 ± 0.45 vs. 1.02 ± 0.52, fold change). Moreover, based on the role of Msi1 in hypothesis as a wnt activator, only 5 out of 20 cases (25 %) with Msi1 overexpression had also PYGO2 overexpression interestingly, which is in contrast with our mentioned expected.
In conclusion, although cytoplasmic factor Msi1 is upstream of the PYGO2 cascade of gene regulation, this report has illustrated its overexpression is not a warrant for the wnt activation in ESCC patients. From another point of view, Msi1 is not one of the targets of wnt pathway because only (5/20, 25 %) of PYGO2 overexpressed cases had also Msi1 overexpression. It seems that there is not any direct correlation between Msi1 and PYGO2 in ESCC progression and metastasis.
Acknowledgments
This work was supported by a grant from the Vice Chancellor for Research at Mashhad University of Medical Sciences, and was part of a Ph.D. student’s dissertation, No. 921202.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Andrews PG, Lake BB, Popadiuk C, Kao KR. Requirement of pygopus 2 in breast cancer. Int J Oncol. 2007;30:357–363. [PubMed] [Google Scholar]
- Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol Cell Neurosci. 2006;31:85–96. doi: 10.1016/j.mcn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, Sotoudeh M, Rajabi-Mashhadi MT, Forghani MN, Farrokhi F, Abbaszadegan MR. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg. 2009;33:1439–1445. doi: 10.1007/s00268-009-0070-y. [DOI] [PubMed] [Google Scholar]
- Glazer RI, Wang XY, Yuan H, Yin Y. Musashi1: a stem cell marker no longer in search of a function. Cell Cycle. 2008;7:2635–2639. doi: 10.4161/cc.7.17.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schuring AN, Kiesel L. Increased expression of the adult stem cell marker musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–329. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of numb and notch. Neuron. 1996;17:27–41. doi: 10.1016/S0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Headrick JR, Nichols FC, 3rd, Miller DL, Allen MS, Trastek VF, Deschamps C, Schleck CD, Thompson AM, Pairolero PC. High-grade esophageal dysplasia: long-term survival and quality of life after esophagectomy. Ann Thorac Surg. 2002;73:1697–1702. doi: 10.1016/S0003-4975(02)03496-3. [DOI] [PubMed] [Google Scholar]
- Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–3900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Imai T, Imataka H, Tsujimoto M, Matsumoto K, Okano H. Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. J Cell Biol. 2008;181:639–653. doi: 10.1083/jcb.200708004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghbeli M, Abbaszadegan MR, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, Forghanifard MM. Association of PYGO2 and EGFR in esophageal squamous cell carcinoma. Med Oncol. 2013;30:516. doi: 10.1007/s12032-013-0516-9. [DOI] [PubMed] [Google Scholar]
- Moghbeli M, Moghbeli F, Forghanifard MM, Garayali A, Abbaszadegan MR. Cancer stem cell markers in esophageal cancer. Am J Cancer Sci. 2013;2:37–50. [Google Scholar]
- Moghbeli M, Forghanifard MM, Aarabi A, Mansourian A, Abbaszadegan MR. Clinicopathological sex- related relevance of Musashi1 mRNA expression in esophageal squamous cell carcinoma patients. Pathol Oncol Res. 2014;20:427–433. doi: 10.1007/s12253-013-9712-3. [DOI] [PubMed] [Google Scholar]
- Moghbeli M, Moaven O, Memar B, Raziei HR, Aarabi A, Dadkhah E, Forghanifard MM, Manzari F, Abbaszadegan MR. Role of hMLH1 and E-cadherin promoter methylation in gastric cancer progression. J Gastrointest Cancer. 2014;45:40–47. doi: 10.1007/s12029-013-9548-9. [DOI] [PubMed] [Google Scholar]
- Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein musashi-1 in stem cells. Exp Cell Res. 2005;306:349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Pasto A, Serafin V, Pilotto G, Lago C, Bellio C, Trusolino L, Bertotti A, Hoey T, Plateroti M, Esposito G, Pinazza M, Agostini M, Nitti D, Amadori A, Indraccolo S. NOTCH3 signaling regulates MUSASHI-1 expression in metastatic colorectal cancer cells. Cancer Res. 2014;74:2106–2118. doi: 10.1158/0008-5472.CAN-13-2022. [DOI] [PubMed] [Google Scholar]
- Popadiuk CM, Xiong J, Wells MG, Andrews PG, Dankwa K, Hirasawa K, Lake BB, Kao KR. Antisense suppression of pygopus2 results in growth arrest of epithelial ovarian cancer. Clin Cancer Res. 2006;12:2216–2223. doi: 10.1158/1078-0432.CCR-05-2433. [DOI] [PubMed] [Google Scholar]
- Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, Scott WJ, Swisher SG, Watson TJ, Blackstone EH. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1–8. doi: 10.1111/j.1442-2050.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- Schulenburg A, Cech P, Herbacek I, Marian B, Wrba F, Valent P, Ulrich-Pur H. CD44-positive colorectal adenoma cells express the potential stem cell markers musashi antigen (msi1) and ephrin B2 receptor (EphB2) J Pathol. 2007;213:152–160. doi: 10.1002/path.2220. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Saito T, Watanabe H, Ito JI, Takeda H, Okano H, Kawata S. Expression of the Musashi1 gene encoding the RNA-binding protein in human hepatoma cell lines. Biochem Biophys Res Commun. 2002;293:150–154. doi: 10.1016/S0006-291X(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Toda M, Iizuka Y, Yu W, Imai T, Ikeda E, Yoshida K, Kawase T, Kawakami Y, Okano H, Uyemura K. Expression of the neural RNA-binding protein Musashi1 in human gliomas. Glia. 2001;34:1–7. doi: 10.1002/glia.1034. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Thompson B, Bienz M. Pygopus residues required for its binding to legless are critical for transcription and development. J Biol Chem. 2004;279:5177–5183. doi: 10.1074/jbc.M309722200. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and notch pathways. Mol Cell Biol. 2008;28:3589–3599. doi: 10.1128/MCB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind C, Oberschmid B. TNM classification of malignant tumors 2010: general aspects and amendments in the general section. Pathologe. 2010;31(333–334):336–338. doi: 10.1007/s00292-010-1301-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]